Abstract
The present study estimated the ability of four aquatic macrophytes (Eichhornia crassipes (Mart.) Solms, Ludwigia stolonifera (Guill. & Perr.) P.H. Raven, Echinochloa stagnina (Retz.) P. Beauv. and Phragmites australis (Cav.) Trin. ex Steud.) to accumulate Cd, Ni and Pb and their use for indicating and phytoremediating these metals in contaminated wetlands. Three sites at five locations in the Kitchener Drain in Gharbia and Kafr El-Sheikh Governorates (Egypt) were selected for plant, water and sediment sampling. The water in the Kitchener Drain was polluted with Cd, while Pb and Ni were far below the maximum level of Pb and Ni in the irrigation water. In comparison to the other species, P. australis accumulated the highest concentrations of Cd and Ni, while E. crassipes accumulated the highest concentration of Pb in its tissues. The four species had bioaccumulation factors (BAFs) greater than one, while their translocation factors (TFs) were less than 1 for most heavy metals, except Cd in the leaf and stem of E. stagnina and L. stolonifera, respectively, and Ni in the stem and leaf of E. stagnina. The BAF and TF results indicated that the studied species are suitable for phytostabilizing the studied heavy metals, except Ni in E. stagnina and Cd in L. stolonifera, which are suitable for phytoextracting these metals. Significant positive correlations were found between the investigated heavy metals in the water or sediment and the plant tissues. Their high BAFs, with significant proportional correlations, supported the potential of these species to serve as bioindicators and biomonitors of heavy metals in general and in the investigated metals specifically.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic macrophytes are good candidates for phytoremediation processes due to their potential for accumulating high heavy metal concentrations from sediment and water (Fawzy et al. 2012). Because of their potential to uptake pollutants, they can be used in environmental impact assessments, such as in situ water quality assessments (Galal and Shehata 2014). They play an important role in wetland geochemistry because they are the principal bioaccumulators of heavy metals through passive and active absorption (Vodyanitskii and Shoba 2015). Regarding their biomass, macrophytes are the predominant organisms in highly productive littoral ecosystems such as coastal areas and wetlands (Brix and Schierup 1989). Macrophytes are able to maintain aquatic ecosystems and can take up toxic metals via their roots from sediments and water; different plants tend to transfer these metals from the root to the shoot to varying degrees (Kassaye et al. 2016).
Aquatic pollution can be investigated by analysing aquatic plants, water and sediments (Dummee et al. 2012). Valipour and Ahn (2016) and Rezaniaa et al. (2016) suggested that a plant species used for phytoremediation should be native and adaptable to different habitats and have a fast growth rate, high biomass yield, well-developed root system and high tolerance and capability to accumulate pollutants in its aerial tissues. Aquatic macrophytes have high remediation potential for micro-nutrients because of their general fast growth and high biomass production. They are natural filters that mitigate pollutants transported by water and are considered an effective, low-cost, clean-up option for improving the quality of surface water; in addition, they have been globally utilized in the last decades to clean polluted water (Bello et al. 2018; Bonanno 2013; Eid et al. 2019; Galal et al. 2018). In addition, they are the primary recipients of wetland heavy metals; thus, remediation of these metals using macrophytes (phytoremediation) is an effective strategy (Ahmad et al. 2014; Caselles-Osorio et al. 2017; Sarwar et al. 2017).
Heavy metal pollution in aquatic ecosystems resulting from anthropogenic activities, such as urbanization and industrialization, can lead to global problems; in addition, this pollution can transfer through the food chain and pose serious health hazards due to its mobility and solubility (Yan et al. 2017). Heavy metals cannot be degraded by chemical or microbial processes to reduce their toxicity over time (Eid and Shaltout 2014). They are toxic when present in high concentrations and can accumulate in the environment, and they can pollute and degrade environmental resources (Bonanno 2013). Generally, heavy metals inhibit most life processes, but their inhibition potential depends on several factors, such as their concentration, degree of oxidation and ability to form complexes (Szyczewski et al. 2009). The main sources of excessive heavy metal concentrations are anthropogenic activities, industrial wastewater, sewage, fossil fuel combustion, fertilizers and atmospheric deposition (Zhang et al. 2011).
Heavy metal contamination is a common environmental problem worldwide (Garbisu and Alkorta 2003) and is a serious threat to agriculture, aquatic ecosystems and human health (Gupta et al. 2010; Ashraf et al. 2019). Remediation requires coordinated action among the scientific community, the general public and diverse authorities (Saleh et al. 2019). Inadequate treatment of municipal, agricultural and industrial wastewater increases wetland pollution by discharging substantial quantities of nutrients and heavy metals into surface waters, which, in turn, increases the proliferation of algal blooms, depletes oxygen, kills fish and results in dead wetlands (Caselles-Osorio et al. 2017).
Heavy metal accumulation by aquatic macrophytes depends mainly upon several factors, such as plant species since the efficiency of a plant species for accumulating metals is evaluated by either plant accumulation or translocation factors of these metals from soil to plant (Ghazi et al. 2019). The efficiency of a phytoremediation process relies on the selection of suitable plant species for a specific environment (Duman et al. 2007). Therefore, detailed baseline information on the accumulation properties of aquatic macrophytes can help in the selection of appropriate macrophytes for wetland phytoremediation systems (Eid and Shaltout 2014). As Harun et al. (2008) studied the site-related effects of some macrophytes in the remediation of heavy metal contaminations, the present study investigates the capabilities of four aquatic macrophytes: two floating (Eichhornia crassipes (Mart.) Solms and Ludwigia stolonifera (Guill. & Perr.) P.H. Raven) and two emergent (Echinochloa stagnina (Retz.) P. Beauv. and Phragmites australis (Cav.) Trin. ex Steud.), for Cd, Ni and Pb accumulation and their efficient use as phytoremediators for these metals in an aquatic ecosystem (Kitchener Drain, Egypt). Our hypothesis is that the heavy metal accumulation capabilities and potential to serve as bioindicators and biomonitors for heavy metal contamination differ among these four aquatic macrophytes.
Materials and methods
Study area
Kitchener Drain (the width of the drain ranges from 40 to 53 m, its depth is 5 to 6 m, its length is 69 km, and its discharge ranges from 20 to 80 m3 s−1; Aitta et al. 2019) is one of the largest drains in the Nile Delta. This drain emerges in Gharbia Governorate in the middle part of the Nile Delta and extends to the north in Kafr El-Sheikh Governorate. It was constructed to transfer agricultural drainage water from the delta lowlands to the Mediterranean Sea (El-Amier et al. 2017). It is considered one of the most severely polluted drains in Egypt that has caused significant economic, social and environmental hazards. Pollution of the Kitchener Drain emanates from four main sources: (i) domestic wastewater (poorly treated and/or untreated) from numerous villages, (ii) uncontrolled municipal solid waste disposed along the banks and into the drain, (iii) industrial wastewater discharge and (iv) fertilizers and pesticides discharged from the agricultural drainage system (El-Amier et al. 2017).
Sampling design
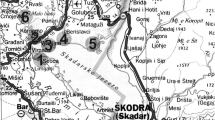
Four aquatic macrophytes, two floating (E. crassipes and L. stolonifera) and two emergent (E. stagnina and P. australis), were selected for the present study. Three sites (50 m from each other) were selected in each of five locations (Fig. 1, Table 1) along the Kitchener Drain at Gharbia and Kafr El-Sheikh Governorates. Locations were selected along the Kitchener Drain that represented substantial stands of the study species. At each site, three composite water, sediment and healthy plant (displaying no signs of stress) samples (9 samples per site) were collected. Samples of E. crassipes were collected from two locations (n = 18) because it was recorded only in two locations (locations 3 and 4), while those of P. australis were gathered from four locations (n = 36) (locations 1, 2, 3 and 4); those of L. stolonifera and E. stagnina were collected from the five locations (n = 45). All samples (plants, water and sediment) were collected during the first week of July 2018. Plant samples were transferred to the laboratory in plastic bags, washed with running tap water to remove debris and then separated into the different tissues as follows: E. crassipes (roots and leaves), P. australis (belowground roots and rhizomes and aboveground stems and leaves), L. stolonifera and E. stagnina (roots, stems and leaves). The different plant tissues were oven dried at 65 °C for 3 days until constant weight for chemical analysis. Plant species were identified according to Boulos (2000, 2005). Moreover, three composite samples of the surface water (1 l each) from each site were collected for chemical analysis. Three composite samples of the sediment from the profile (0–20 cm depth) were also collected using a stainless steel grab sampler and then dried and sieved using a 2-mm sieve.
Plant analysis
Plant samples from the different tissues of each of the four aquatic macrophytes were collected from each site, homogenized by grinding in a metal-free plastic mill and then sieved through a 2-mm mesh size sieve. A 1-g sample of each plant tissue was digested in a 20-ml tri-acid mixture of HNO3:H2SO4:HClO4 (5:1:1, v/v/v) until a transparent colour appeared; then, the mixture was filtered and completed to 25 ml with double de-ionized water following the procedure of Lu (2000). The concentrations of Cd, Ni and Pb were determined using an atomic absorption spectrophotometer (Shimadzu AA-6300; Shimadzu Co. Ltd., Japan).
Water and sediment analyses
For the sediment samples, diethylene-triamine-penta-acetic acid solution (DTPA) was used for the extraction of Cd, Ni and Pb (APHA (American Public Health Association) 1998). Water samples were filtered using Whatman nylon membrane filters (pore size 0.45 μm, diameter 47 mm) for dissolved heavy metal analysis (APHA (American Public Health Association) 1998). The concentrations of Cd, Ni and Pb in the water and sediment samples were determined using an atomic absorption spectrophotometer following the standard methods outlined by Allen (1989).
Quality assurance and control
The instrumental settings and operational conditions were set in accordance with the manufacturers’ specifications. A certified reference material (SRM 1573a, tomato leaves) was used for verification of heavy metal determination accuracy. The reference material was digested and analysed by the same methods as those applied to the tissue samples of the study plants. The digestion and measurement of heavy metals was performed in triplicate. De-ionized water was used throughout the study. Cleaned glassware and analytical grade reagents were properly used. Blank reagents were used to correct the instrument readings. The variation coefficient of replicate analysis was determined for different measurements to calculate analytical precision. To calibrate the system, standard solutions with known concentrations of different metals were used. Accuracy was estimated by comparing the measured concentration with the certified value and then expressed as a percentage. The recovery rates were 94.8% for Cd with a relative standard deviation (RSD) = 4.9%, 103.7% for Ni (RSD = 9.0%) and 96.0% for Pb (RSD = 5.3%). The detection limits for the heavy metals (μg l−1) were 0.05 for Cd, 0.2 for Ni and 0.1 for Pb.
Data analysis
Before performing analysis of variance (ANOVA), the data for the plant, water and sediment parameters were tested for their normality of distribution and homogeneity of variance, and the data were log-transformed when necessary. One-way ANOVA was applied to test the significant variations in the water and sediment variables (at P < 0.05) between the different sampling locations. To provide an overview of the water and sediment heavy metals in the Kitchener Drain, the mean value of all locations was presented. Tukey’s HSD test (P < 0.05) was used to assess significant differences between concentrations among the five locations. In addition, three-way ANOVA was also applied to test the significance of variation in the heavy metal concentrations in the different tissues of the study plants. The simple linear correlation coefficient (r) was calculated to assess the type of relationship between the estimated water and sediment heavy metals and the corresponding metals in the plant tissues. All statistical analyses were performed using Statistica 7.1 software (Statsoft 2007).
The bioaccumulation factor (BAF) is a useful parameter for evaluating the potential of plants to accumulate heavy metals. The translocation factor (TF) shows the ability of a plant to translocate heavy metals from its roots to its rhizomes, stems or leaves. The BAF and TF were determined according to Eid et al. (2019) as follows:
where Csediment/water is the heavy metal concentration in the sediment and water for emergent and floating plants; and Croot, Crhizome, Cstem and Cleaf are the heavy metal concentrations (mg kg−1) in the roots, rhizomes, stems and leaves, respectively. Tukey’s HSD test (P < 0.05) was used to assess the significant differences between the BAFs and TFs among the four plant species and between the three heavy metals.
Results
Water heavy metals
The chemical analysis of the water in the Kitchener Drain showed significant variations in the concentrations of Cd, Ni and Pb among the different study locations (Fig. 2). The concentration of water Cd ranged between 4.7 μg l−1 in location 1 and 28.2 μg l−1 in location 4, with a mean concentration of 12.3 μg l−1, while that of water Ni ranged between 32.3 μg l−1 and 38.6 μg l−1 in locations 5 and 1, respectively, with an average of 35.9 μg l−1. The maximum concentration of water Pb (250.1 μg l−1) was recorded in location 4, while the minimum (2.9 μg l−1) was recorded in location 5, with an average concentration of 139.6 μg l−1. Notably, the concentration of water heavy metals in the Kitchener Drain was in the order of Pb > Ni > Cd.
The concentrations of Cd, Ni and Pb in the water from the five locations in the Kitchener Drain in the Nile Delta, Egypt. Vertical bars indicate the standard errors of the means (n = 9). F-values represent one-way ANOVA, degree of freedom = 4, ***: P < 0.001. Different letters are significantly different at P < 0.05 according to Tukey’s HSD test
Sediment heavy metals
The heavy metals analysis of the sediments in the Kitchener Drain had essentially the same trend as the water heavy metals, with significant variations among the study locations (Fig. 3). The highest concentrations of Cd and Pb (4.1 and 83.4 mg kg−1, respectively) were recorded in location 4, while the lowest concentrations (2.3 and 18.4 mg kg−1) were recorded in locations 1 and 5, respectively, with average concentrations of 2.8 and 56.6 mg kg−1. However, the highest concentration of sediment Ni (68.7 mg kg−1) was recorded in location 2, and the lowest concentration (42.9 mg kg−1) was in location 5, with an average concentration of 59.6 mg kg−1. Generally, the concentration of heavy metals in the sediment occurred in the following order: Ni > Pb > Cd.
The concentrations of Cd, Ni and Pb in the sediment from the five locations in the Kitchener Drain in the Nile Delta, Egypt. Vertical bars indicate the standard errors of the means (n = 9). F-values represent one-way ANOVA, degree of freedom = 4, *: P < 0.05, ***: P < 0.001. Different letters are significantly different at P < 0.05 according to Tukey’s HSD test
Plant heavy metals
The statistical data of the three-way ANOVA indicated significant variations among the different locations, species and tissues, as well as the intercept of locations × species, locations × tissues, species × tissues and locations × species × tissues (Table 2). The Cd concentration was affected by location, species and tissues as well as the intercept of these factors; Ni concentration was significantly affected by all factors except locations × tissues, and Pb was significantly affected by location and the intercept of locations × species. The analysis of heavy metals in the different tissues of these four plant species indicated that the study species accumulated higher concentrations of all heavy metals (except Cd in E. stagnina) in their roots rather than in the shoots (Fig. 4). P. australis accumulated higher concentrations of Cd and Ni than that in the other species, while E. crassipes accumulated the highest concentration of Pb in their tissues. The highest concentrations of Cd and Ni (57.5 and 109.0 mg kg−1, respectively) were recorded in the roots of P. australis, while the lowest concentrations (22.3 and 82.8 mg kg−1, respectively) were accumulated in the leaves of E. crassipes. In addition, the highest concentration of Pb (277.4 mg kg−1) was recorded in the roots of E. crassipes, while the lowest (244.8 mg kg−1) was recorded in the stems of L. stolonifera. Generally, the study species had mean accumulated heavy metal concentrations in the following orders: P. australis > E. stagnina > L. stolonifera > E. crassipes for Cd; P. australis > E. crassipes > L. stolonifera > E. stagnina for Ni; and E. crassipes > P. australis > E. stagnina > L. stolonifera for Pb. The heavy metal concentrations in the tissues of the study plants had the same trend (i.e. Pb > Ni > Cd) of water heavy metals.
Tissue variation in the concentrations of Cd, Ni and Pb in the four plant species grown in the Kitchener Drain in the Nile Delta, Egypt. Vertical bars indicate the standard errors of the means (E. stagnina: n = 45, E. crassipes: n = 18, L. stolonifera: n = 45, and P. australis: n = 36). F-values represent the three-way analysis of variance, df: degree of freedom, *: P < 0.05, **: P < 0.01, ***: P < 0.001, and ns: not significant (i.e. P > 0.05). Cd: Fsite = 51.2***, df = 4; Fspecies = 5.0**, df = 3; Ftissue = 8.4***, df = 3; Fsite × species = 3.3**, df = 8; Fsite × tissue = 7.9***, df = 11; Fspecies × tissue = 9.8***, df = 5; Fsite × species × tissue = 11.3***, df = 15. Ni: Fsite = 323.3***, df = 4; Fspecies = 10.9***, df = 3; Ftissue = 62.5***, df = 3; Fsite × species = 7.2***, df = 8; Fsite × tissue = 1.3ns, df = 11; Fspecies × tissue = 9.8***, df = 5; Fsite × species × tissue = 5.2***, df = 15. Pb: Fsite = 106.5***, df = 4; Fspecies = 3.6*, df = 3; Ftissue = 1.7ns, df = 3; Fsite × species = 5.3***, df = 8; Fsite × tissue = 0.9ns, df = 11; Fspecies × tissue = 0.7ns, df = 5; Fsite × species × tissue = 0.7ns, df = 15
Bioaccumulation and translocation factors
The statistical evaluation of the BAF of heavy metals, from the sediment to the roots, and the TF, from the roots to the rhizomes, stems and leaves, showed significant variations among the different investigated heavy metals and the different species (Table 3). The four study species had BAFs greater than one for the investigated heavy metals. P. australis had the highest BAF for Cd and Pb (23.58 and 7.95, respectively), while L. stolonifera had the highest BAF for Ni (2.01). Moreover, the lowest BAF for Cd (9.15) was recorded in L. stolonifera, while those of Ni and Pb (1.64 and 5.70) were recorded in E. crassipes. The study species were arranged in descending order according to their BAF as follows: P. australis > E. crassipes > E. stagnina > L. stolonifera for Cd; L. stolonifera > P. australis > E. stagnina > E. crassipes for Ni; and P. australis > E. stagnina > L. stolonifera > E. crassipes for Pb. However, the TF of heavy metals from the roots to the shoots showed that Cd was translocated to the leaves and stems (TF: 1.59 and 1.17) of E. stagnina and L. stolonifera, respectively, while Ni was transferred to the stems and leaves of E. stagnina with TFs of 1.30 and 1.37, respectively. However, the TF of Pb did not exceed one for any of the study species.
Plant-water-sediment correlations
The results of the simple linear correlation coefficient between heavy metals in the plant tissues and the sediment and water are represented in Table 4. The Cd concentration in the water was significantly proportional (n = 45; P < 0.05) to its concentration in the stem of E. stagnina. However, the concentration of Ni in the sediment and water was negatively correlated with its concentration in the tissues of the study species, except E. crassipes (n = 18; P < 0.05) roots and leaves (r = 0.803 and 0.801, respectively), which were positively correlated with the Ni water concentration. Moreover, significant positive correlations were observed between the Pb concentration in water and its concentration in the leaves, stems and roots of E. stagnina (r = 0.523, 0.499 and 0.553, respectively); roots of E. crassipes (r = 0.494); leaves, stems and roots (n = 45; P < 0.05) of L. stolonifera (r = 0.603, 0.608 and 0.594, respectively) and rhizomes (n = 36; P < 0.05) of P. australis (r = 0.369). However, sediment Pb was positively correlated with the roots and rhizomes of P. australis (r = 0.359 and 0.371, respectively). Importantly, the concentration of certain heavy metals in the plant tissues may have significantly increased or decreased with the increase in another metal in the water or sediment, such as water Cd with Ni and Pb in the roots of E. stagnina (r = 0.477 and 0.391, respectively) and sediment Ni with Cd in the roots and stems of L. stolonifera (r = − 0.609 and − 0.581, respectively).
Discussion
The concentration of Cd (12.3 μg l−1) in the water samples from the five locations in the Kitchener Drain exceeded the maximum level of Cd (10.0 μg l−1) in irrigation water stated by Rowe and Abdel-Magid (1995). In contrast, the concentrations of Ni and Pb (35.9 and 139.6 μg l−1, respectively) in the water samples from the five locations in the Kitchener Drain were far below the maximum level of heavy metals in the irrigation water (200.0 μg l−1 for Ni and 5000.0 μg l−1 for Pb) reported by Rowe and Abdel-Magid (1995). Similarly, the sediment Cd content (2.3–4.1 mg kg−1) in the locations in the Kitchener Drain exceeded the safe range (0.01–1.1 mg kg−1), while Pb (18.4–83.4 mg kg−1) and Ni (42.9–68.7 mg kg−1) concentrations were within the safe ranges (2.0–200.0 mg kg−1 for Pb and 0.5–100.0 mg kg−1 for Ni) (Kabata-Pendias 2011; Nagajyoti et al. 2010; Samecka-Cymerman and Kempers 2001).
Generally, the concentrations of heavy metals in aquatic macrophytes varied considerably according to the type of species and their tissues as well as to the type of heavy metal (Bragato et al. 2006). Comparing the four species in the present study, P. australis accumulated the highest concentrations of Cd and Ni, while E. crassipes accumulated the highest concentration of Pb in its tissues. This variation in heavy metal accumulation may be due to the different growth forms of these species (Kassaye et al. 2016). The concentrations of Pb in the different tissues of E. stagnina, E. crassipes and P. australis were approximately three-fold higher than the concentrations in the corresponding species in the canals and drains of the middle Nile Delta as reported by Shaltout et al. (2009) and in Lake Burullus as reported by Eid and Shaltout (2014). Moreover, the Cd and Pb concentrations in the tissues of L. stolonifera were higher, and the Ni concentration was lower than those recorded by Abu-Ziada (2007) and Galal et al. (2019) on the same species. Notably, the study species accumulated high concentrations of Cd, Ni and Pb, exceeding the safe range for normal plants (Allen 1989; Nagajyoti et al. 2010). The Cd, Ni and Pb concentrations in the P. australis tissues, the Ni and Pb concentrations in the E. stagnina tissues and the Cd concentration in the L. stolonifera tissues in the Kitchener Drain were higher than the range of concentrations recorded for the same species in some natural and constructed wetlands, while the Ni and Pb concentrations in the L. stolonifera tissues and the Cd, Ni and Pb concentrations in the E. crassipes tissues (except leaf tissues) were lower than the average concentrations in mining habitats worldwide (Table 5). The variations among the results obtained in the current investigation and the reported results by other researchers might be related to sampling time, pollution levels, physico-chemical characteristics of water at the sampling sites, and the analytical methods used in the digestion of plant materials (Du Laing et al. 2003, 2009; Kabata-Pendias 2011).
The study species accumulated higher concentrations of all heavy metals (except Cd in E. stagnina) in their roots than in their shoots. This result coincided with that in Abu-Ziada (2007), Galal et al. (2019) and Mganga et al. (2011) for L. stolonifera; that in Eid et al. (2019), Kamari et al. (2017), Pandey (2016) and Carrión et al. (2012) for E. crassipes; and that in Bonanno et al. (2017), Ahmad et al. (2014), Eid and Shaltout (2014), Bonanno and Lo (2010), Duman et al. (2007) and Abdel-Shafy et al. (1994) for P. australis (Table 5). According to Ahmad et al. (2014), the accumulation of heavy metals in plant roots may be an exclusion strategy because the root is not a photosynthetic organ, and this may increase plant tolerance to toxic concentrations of these metals. In aquatic macrophytes, the compartmentalization strategy for heavy metals is common, and plants sequester high concentrations of metals in their belowground tissues to protect against the harmful effects of metal toxicity (Bonanno et al. 2017). Similarly, Eid and Shaltout (2014) and Rezania et al. (2019) found that P. australis can be used to immobilize some metals, such as Cd and Pb, and store them in its belowground tissues. Moreover, Saleh et al. (2019) stated that L. stolonifera is an efficient aquatic macrophyte for remediating wastewater containing toxic metals, such as Pb, Cd and Cr; in addition, it can be used as a bioindicator for Cd and Pb in contaminated water (Mganga et al. 2011). Furthermore, the concentrations of Cd, Ni and Pb were significantly higher in the leaves than in the stems of the study species because metals are mainly accumulated in leaf vacuoles (Du Laing et al. 2009).
Bioaccumulation factors (BAFs) and translocation factors (TFs) can be used to estimate a plant’s potential for phytoremediation (Eid et al. 2019). The study species had BAFs of more than one for Cd, Ni and Pb. P. australis had the highest BAF for Cd and Pb, while L. stolonifera had the highest BAF for Ni. However, the TFs of the heavy metals from the roots to the different plant tissues were generally lower than one. These results were consistent with the results of several studies on aquatic macrophytes (e.g. Baldantoni et al. 2009; Olivares-Rieumont et al. 2007; Pandey 2016; Kamari et al. 2017; Saha et al. 2017); according to their studies, metal concentrations are generally higher in roots than in shoots. The sequestration of metals in the cellular compartments (e.g. central vacuoles) is a tolerance strategy of aquatic macrophytes (Weis and Weis 2004), and roots are the main tissue for heavy metal uptake by these plants (Fawzy et al. 2012). According to Bello et al. (2018), plants with a high BAF value and low TF value could be suitable for phytostabilization, but plants with BAF and TF values both greater than 1 could be used for phytoextraction. Thus, the study species are suitable for phytostabilization of the tested heavy metals, except E. stagnina for Ni and L. stolonifera for Cd, which are suitable for phytoextraction of these metals.
Globally, more than 500 plant species are known as heavy metal hyper-accumulators, and the majority of them hyper-accumulate As, Cu, Hg, Ni and Pb (Baker et al. 2000). Based on the BAF data, the study species may be considered hyper-accumulators for Cd, Ni and Pb. Similar results were reported for P. australis (Cicero-Fernández et al. 2016; Rezania et al. 2019) and E. crassipes (Eid et al. 2019). However, Ahmad et al. (2014) documented that one of the most empirical and important criteria of hyper-accumulators is that the plants should withstand very high heavy metal concentrations (> 1000 mg kg−1 for Pb and Ni and 100 mg kg−1 for Cd). Therefore, the critical concentrations of the investigated metals in the different tissues of the study plants were less than the required values, indicating that these plants are accumulators rather than hyper-accumulators of these heavy metals. The study species were arranged in a descending pattern according to their BAF as follows: P. australis > E. crassipes > E. stagnina > L. stolonifera for Cd; L. stolonifera > P. australis > E. stagnina > E. crassipes for Ni; and P. australis > E. stagnina > L. stolonifera > E. crassipes for Pb. Bonanno and Lo (2010) attributed the high accumulation potential, particularly in the roots and rhizomes of P. australis, to the presence of large intercellular spaces of the cortical tissue.
The present study recorded significant correlations between the concentrations of Cd, Ni and Pb in the water and sediment and plant tissues. For example, significant proportional correlations were found between Cd in the water and the E. stagnina stems; Ni in the water and Cd in E. crassipes roots and leaves; and Pb in the water and E. crassipes roots; L. stolonifera leaves, stems and roots and P. australis rhizomes. These correlations indicated that the study species can potentially be used as bioindicators and biomonitors of heavy metals in general and the investigated metals in particular. Several studies support this conclusion, according to which heavy metal concentrations in the different plant tissues increased with increasing concentrations in the environment (Eid and Shaltout 2014). Moreover, Eid et al. (2012) reported that plants with strong correlations with environmental heavy metal concentrations are considered potential bioindicators and biomonitors of these metals.
Phytoremediation is an efficient technique for removing heavy metals from the water and sediment, and this technique is broadly accepted worldwide due to its intrinsic environmentally friendly characteristics, performance and feasibility (Salawu et al. 2018; Saleh et al. 2019). Qian et al. (1999) determined the following criteria for good heavy metal accumulators: (i) able to accumulate high concentrations of a metal in its harvestable tissues, (ii) able to grow rapidly and (iii) have a well-developed root system. Consequently, the study species of the present study could be regarded as good accumulators for the studied heavy metals. The rapid growth and reproduction, as well as the well-developed underwater and belowground tissues, of the study species make them powerful candidates for phytoremediation (Abubakar et al. 2014). Overall, to minimize the chances of attaining lethal concentrations, the phytoremediation efficiency of these species could be ameliorated by periodically harvesting them from the remediated locations. The harvested plants could be converted to ash and packed in a safe place, and the accumulated heavy metals could also be recovered for economic purposes (Eid et al. 2019).
Conclusion
The water in the Kitchener Drain was polluted with Cd, while Pb and Ni were far below the maximum level of Pb and Ni in the irrigation water. Aquatic macrophytes accumulated high concentrations of these heavy metals in their roots within the toxic range. In comparison to the other species, P. australis accumulated the highest concentrations of Cd and Ni, while E. crassipes accumulated the highest concentration of Pb in its tissues. The species can be arranged in the following order according to their accumulation potential: P. australis > E. stagnina > L. stolonifera > E. crassipes for Cd; P. australis > E. crassipes > L. stolonifera > E. stagnina for Ni; and E. crassipes > P. australis > E. stagnina > L. stolonifera for Pb. The BAFs and TFs indicated that the study species are suitable for phytostabilizing the investigated heavy metals, except Ni in E. stagnina and Cd in L. stolonifera, which are suitable for phytoextracting these metals. The high BAF of the study species render them good phytoremediator candidates. In addition, the present study recorded significant correlations between the concentrations of Cd, Ni and Pb in the water and sediment and the plant tissues. Such correlations indicate that the study species reflect the cumulative influences of environmental pollution from the water and sediment, thereby suggesting their potential use in biomonitoring and bioindicating the investigated metals. Based on our results, by using manual or mechanical harvesting, which have been used in Egypt for the study species, these species could be used as an environmentally friendly filter for the extraction of heavy metals to reduce the pollution load reaching the drainage and/or irrigation canals. The harvested materials could be used as substrate for biogas production or carbonization to make charcoal or could be burned to ash and packed in a safe place. The accumulated heavy metals could also be recovered for commercial use if desired.
References
Abdel-Shafy H, Hegemann W, Teiner A (1994) Accumulation of metals by vascular plants. Environ Manag Health 5:21–24
Abubakar MM, Ahmad MM, Getso BU (2014) Rhizofiltration of heavy metals from eutrophic water using Pistia stratiotes in a controlled environment. J Environ Sci Toxicol Food Technol 8:1–3
Abu-Ziada ME (2007) Ecological studies on the aquatic macrophytes II: Ludwigia stolinefera (Guill. & Perr.) P.H. Raven. Pak J Biol Sci 10:2025–2038
Agunbiade FO, Olu-Owolabi BI, Adebowale KO (2009) Phytoremediation potential of Eichornia crassipes in metal-contaminated coastal water. Bioresour Technol 100:4521–4526
Ahmad SS, Reshi ZA, Shah MA, Rashid I, Ara R, Andrabi SMA (2014) Phytoremediation potential of Phragmites australis in Hokersar wetland: a Ramsar site of Kashmir Himalaya. Int J Phytoremediat 16:1183–1191
Aitta A, El-Ramady H, Alshaal T, El-Henawy A, Shams M, Talha N, Elbehiry F, Brevik EC (2019) Seasonal and spatial distribution of soil trace elements around Kitchener Drain in the Northern Nile Delta, Egypt. Agriculture 9:1–25
Allen SE (1989) Chemical analysis of ecological materials. Blackwell Scientific Publications, London
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Ashraf S, Ali Q, Ahmad Z, Ashraf S, Naeem H (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol Environ Saf 174:714–727
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biochemical resource for phytoremediation of metal-polluted soils. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soil and water. Lewis, Boca Raton, pp 85–107
Baldantoni D, Ligrone R, Alfani A (2009) Macro- and trace-element concentrations in leaves and roots of Phragmites australis in a volcanic lake in Southern Italy. J Geochem Explor 101:166–174
Bello AO, Tawabini BS, Khalil AB, Boland CR, Saleh TA (2018) Phytoremediation of cadmium-, lead- and nickel-contaminated water by Phragmites australis in hydroponic systems. Ecol Eng 120:126–133
Bonanno G (2013) Comparative performance of trace element bioaccumulation and bio- monitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol Environ Saf 97:124–130
Bonanno G, Lo GR (2010) Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic 10:639–645
Bonanno G, Borg JA, Di Martino V (2017) Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: a comparative assessment. Sci Total Environ 576:796–806
Boulos L (2000) Flora of Egypt, volume two. Geraniaceae-Boraginaceae. Al Hadara Publish, Cairo
Boulos L (2005) Flora of Egypt, volume four. Monocotyledons (Alismataceae-Orchidaceae). Al Hadara Publish, Cairo
Bragato C, Brix H, Malagoli M (2006) Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin. ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environ Pollut 144:967–975
Brix H, Schierup HH (1989) The use of aquatic macrophytes in water pollution control. Ambio 18:101–107
Carrión C, Ponce-de León C, Cram S, Sommer I, Hernández M, Vanegas C (2012) Potential use of water hyacinth (Eichhornia crassipes) in Xochimilco for metal phytoremediation. Agrociencia 46:609–620
Caselles-Osorio A, Vegab H, Lancherosa JC, Casierra-Martíneza HA, Mosquera JE (2017) Horizontal subsurface-flow constructed wetland removal efficiency using Cyperus articulatus L. Ecol Eng 99:479–485
Cicero-Fernández D, Peña-fernández M, Expósito-camargo JA, Antizar-ladislao B, Peña-fernández M, Expósito-camargo JA, Cicero-fern D (2016) Role of Phragmites australis (common reed) for heavy metals phytoremediation of estuarine sediments. Int J Phytoremediat 18:575–582
Du Laing G, Tack FMG, Verloo MG (2003) Performance of selected destruction methods for the determination of heavy metals in reed plants (Phragmites australis). Anal Chim Acta 497:191–198
Du Laing G, Van de Moortel AMK, Moors W, De Grauwe P, Meers E, Tack FMG, Verloo MG (2009) Factors affecting metal concentrations in reed plants (Phragmites australis) of intertidal marshes in the Scheldt estuary. Ecol Eng 35:310–318
Duman F, Cicek M, Sezen G (2007) Seasonal changes of metal accumulation and distribution in common club rush (Schoenoplectus lacustris) and common reed (Phragmites australis). Ecotoxicology 16:457–463
Dummee V, Kruatrachue M, Trinachartvanit W, Tanhan P, Pokethitiyook P, Damrongphol P (2012) Bioaccumulation of heavy metals in water, sediments, aquatic plant and histopathological effects on the golden apple snail in Beung Boraphet reservoir, Thailand. Ecotoxicol Environ Saf 86:204–212
Eid EM, Shaltout KH (2014) Monthly variations of trace elements accumulation and distribution in above- and below-ground biomass of Phragmites australis (Cav.) Trin. ex Steudel in Lake Burullus (Egypt): a biomonitoring application. Ecol Eng 73:17–25
Eid EM, Shaltout KH, El-Sheikh MA, Asaeda T (2012) Seasonal courses of nutrients and heavy metals in water, sediment and above- and below-ground Typha domingensis biomass in Lake Burullus (Egypt): perspective for phytoremediation. Flora 207:783–794
Eid EM, Shaltout KH, Moghanm FS, Youssef MS, El-Mohsnawy E, Haroun SA (2019) Bioaccumulation and translocation of nine heavy metals by Eichhornia crassipes in Nile Delta, Egypt: perspectives for phytoremediation. Int J Phytoremediat 21:821–830
El-Amier YA, Zahran MA, Gebreil AS, Abd El-Salam EH (2017) Anthropogenic activities and their impact on the environmental status of Kitchener drain, Nile Delta, Egypt. J Environ Sci 46:251–262
Fawzy MA, Badr NE, El-Khatib A, Abo-El-Kassem A (2012) Heavy metal biomonitoring and phytoremediation potentialities of aquatic macrophytes in River Nile. Environ Monit Assess 184:1753–1771
Galal TM, Shehata HS (2014) Evaluation of the invasive macrophyte Myriophyllum spicatum L. as a bioaccumulator for heavy metals in some watercourses of Egypt. Ecol Indic 41:209–214
Galal TM, Eid EM, Dakhil MA, Hassan LM (2018) Bioaccumulation and rhizofiltration potential of Pistia stratiotes L. for mitigating water pollution in the Egyptian wetlands. Int J Phytoremediat 20:440–447
Galal TM, Al-Sodany YM, Al-Yasi HM (2019) Phytostabilization as a phytoremediation strategy for mitigating water pollutants by the floating macrophyte Ludwigia stolonifera (Guill. & Perr.) P.H. Raven. Int J Phytoremediat 25:1–10. https://doi.org/10.1080/15226514.2019.1663487
Garbisu C, Alkorta I (2003) Basic concepts on heavy metal soil bioremediation. Eur J Min Proc Environ Protect 13:58–66
Ghazi SM, Galal TM, Husein KH (2019) Monitoring water pollution in the Egyptian watercourses: a phytoremediation approach. LAP LAMBERT Academic Publishing, Saarbrücken
Gupta S, Satpati S, Nayek S, Garai D (2010) Effect of wastewater irrigation on vegetables in relation to bioaccumulation of heavy metals and biochemical changes. Environ Monit Assess 165:169–177
Harun NH, Tuah PM, Markom NZ, Yusof MY 2008 Distribution of heavy metals in Monochoria hastata and Eichornia crassipes in natural habitats. Int Conf Environ Res Technol 550–553
Kabata-Pendias A (2011) Trace elements in soils and plants, Fourth edn. Taylor & Francis Group, Boca Raton
Kamari A, Yusof N, Abdullah H, Haraguchi A, Abas MF (2017) Assessment of heavy metals in water, sediment, Anabas testudineus and Eichhornia crassipes in a former mining pond in Perak, Malaysia. Chem Ecol 33:637–651
Kassaye YA, Skipperud L, Einset J, Salbu B (2016) Aquatic macrophytes in Ethiopian Rift Valley lakes: their trace elements concentration and use as pollution indicators. Aquat Bot 134:18–25
Lu RK (2000) Methods of inorganic pollutants analysis. In: Soil and agro-chemical analysis methods. Agricultural Science and Technology Press, Beijing
Mganga N, Manoko M, Rulangaranga Z (2011) Classification of plants according to their heavy metal content around North Mara Gold Mine, Tanzania: implication for phytoremediation. Tanz J Sci 37:109–119
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Olivares-Rieumont S, Lima L, De la Rosa D, Graham DW, Columbie I, Santana JL, Sánchez MJ (2007) Water hyacinths (Eichhornia crassipes) as indicators of heavy metal impact of a large landfill on the Almendares River near Havana, Cuba. Bull Environ Contam Toxicol 79:583–587
Pandey VC (2016) Phytoremediation efficiency of Eichhornia crassipes in fly ash pond. Int J Phytoremediat 18:450–452
Qian JH, Zayed A, Zhu YL, Yu M, Terry N (1999) Phytoaccumulation of trace elements by wetland plants: III. Uptake and accumulation of ten trace elements by twelve plant species. J Environ Qual 28:1448–1455
Rezania S, Park J, Rupani PF, Darajeh N, Xu X (2019) Phytoremediation potential and control of Phragmites australis as a green phytomass: an overview. Environ Sci Pollut Res 26:7428–7441
Rezaniaa S, Taib SM, Md Din MH, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599
Rowe DR, Abdel-Magid IM (1995) Handbook of wastewater reclamation and reuse. CRC Press, Boca Raton
Saha P, Shinde O, Sarkar S (2017) Phytoremediation of industrial mines wastewater using water hyacinth. Int J Phytoremediat 19:87–96
Salawu MO, Sunday ET, Oyelola H, Oloyede B (2018) Bioaccumulative activity of Ludwigia peploides on heavy metals-contaminated water. Environ Technol Innov 10:324–334
Saleh HM, Aglan RF, Mahmoud HH (2019) Ludwigia stolonifera for remediation of toxic metals from simulated wastewater. Chem Ecol 35:164–178
Samecka-Cymerman A, Kempers AJ (2001) Concentrations of heavy metals and plants nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. Sci Total Environ 281:87–98
Sarwar N, Imran M, Shaheen MR, Ishaq W, Kamran A, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Shaltout KH, Galal TM, El-Komy TM (2009) Evaluation of the nutrient status of some hydrophytes in the water courses of Nile Delta, Egypt. Aust J Bot 2009:862565
Statsoft (2007) Statistica version 7.1. Statsoft Inc, Tulsa
Szyczewski P, Siepak J, Niedzielski P, Sobczyński T (2009) Research on heavy metals in Poland. Pol J Environ Stud 5:755–768
Valipour A, Ahn YH (2016) Constructed wetlands as sustainable ecotechnologies in decentralization practices: a review. Environ Sci Pollut Res 23:180–197
Vodyanitskii YN, Shoba SA (2015) Biogeochemistry of carbon, iron, and heavy metals in wetlands (analytical review). Moscow Univ Soil Sci Bull 70:89–97
Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30:685–700
Yan P, Xia JS, Chena YP, Liu ZP, Guoa JS, Shen Y, Zhangc CC, Wang J (2017) Thermodynamics of binding interactions between extracellular polymeric substances and heavy metals by isothermal titration microcalorimetry. Bioresour Technol 232:354–363
Zhang WH, Tong LZ, Yuan Y, Huang H, Qiu RL (2011) Metal mobility and fraction distribution in a multi-metal contaminated soil chemically stabilized with different agents. J Hazard Toxic Radioact Waste 15:1–11
Funding
This work was supported by the Deanship of Scientific Research at King Khalid University under grant number R.G.P. 1/94/40.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eid, E.M., Galal, T.M., Sewelam, N.A. et al. Phytoremediation of heavy metals by four aquatic macrophytes and their potential use as contamination indicators: a comparative assessment. Environ Sci Pollut Res 27, 12138–12151 (2020). https://doi.org/10.1007/s11356-020-07839-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07839-9