Abstract
The present study was carried out to observe the variation of volatile organic compounds (VOCs) namely benzene, toluene, ethylbenzene, and xylene isomers (BTEX) at three different sites of Delhi, during 2016–2017. Four hourly sampling was carried out day and night separately. Results showed that BTEX concentration was highest in post-monsoon and lowest in monsoon season. Again, daily variation shows that benzene (47%) and toluene (35%) were more during night than day when it was 44% and 33% respectively. Mean concentration of BTEX was observed in following order: ethylbenzene ~ o-xylene < m,p-xylene < toluene < benzene, while overall seasonal variation was observed as follows: post-monsoon > summer > winter > monsoon. Possible emission sources of BTEX were also established through corresponding ratios of individual compounds. Xylene isomers together accounted highest ozone formation potential. The risk assessments of BTEX were carried out in terms of non-cancer (the hazard quotient, HQ) and cancer (the incremental lifetime cancer risk, ILCR) regarding the inhalation exposure only. It was observed that benzene and xylene isomers possessed higher HQs than ethylbenzene and toluene at all sites throughout the study. Again, benzene was found with higher mean ILCR (3.58 × 10−5) than ethylbenzene (1.47 × 10−5).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) comprise a series of compounds, of which the BTEX is ubiquitous pollutants. BTEX refers to benzene, toluene, ethylbenzene, 1,2,4-trimethylbenzene, and xylene isomers (m-xylene, p-xylene, o-xylene). These are known toxic air pollutants and some of them are carcinogen. Sources of these VOCs are gasoline/gasoline-powered vehicles (Hellen et al. 2003), vehicular exhaust (Latella et al. 2005), and other biogenic sources (Guenther et al. 2006). Various anthropogenic activities such as fuel combustion and industrial processes using various kinds of solvents, wood burning, etc. play an important role in the emission of VOCs (Lanz et al. 2008; Demir et al. 2012; Abtahi et al. 2018). Due to the multiple emission sources and ubiquitous nature of VOCs, it can be transported over long distances. It has also been reported that gasoline vehicles emit more VOCs as compared with diesel (Watson et al. 2001). The emission of natural VOCs has also been reported (Guenther et al. 1995). Interestingly, concentration of some VOCs has been observed relatively higher in the indoor environment than outdoor due to the building materials (Missia et al. 2010). Exposure to some BTEX (mainly benzene) can cause acute non-lymphocytic leukemia and a variety of other blood-related disorders in humans (Krewski et al. 2000; Sarkhosh et al. 2012). Benzene, which is a priority pollutant in urban environment, is considered as a genotoxic carcinogen (Hoque et al., 2008). Attention has also been given on study of benzene in food samples (Heshmati et al. 2018). Most of the VOCs in the ambient environment form tropospheric ozone and secondary organic aerosol (SOA), which in turn harm all living creatures and pollute local air quality. There are three major pathways of human exposure to pollutants such as inhalation, ingestion, and dermal (skin) contact. Exposure of VOCs is related to multiple routes (Bruckner et al. 2010). Inhalation plays an important role of enrichment of VOCs in different parts of the body, especially alveoli of lungs. Besides the adverse effects of VOCs on human (de Blas et al. 2012), they are also responsible for carcinogenic effects. According to the International Agency for Research on Cancer (IARC), which is a part of the World Health Organization (WHO), benzene is classified as a group 1 carcinogen (i.e., human carcinogen), ethylbenzenes as a group 2B (i.e., possibly carcinogenic to human), whereas toluene and xylenes are classified as a group 3 (i.e., not classified as human carcinogen) respectively.
In this study, the variation of BTEX at three urban sites in Delhi has been investigated. In India, most of the cities face acute problem of outdoor air pollution with concentration levels significantly exceeding the limits set by the National Ambient Air Quality Standards (NAAQS) under the Central Pollution Control Board, Government of India (CPCB 2009). This puts the lives of Indians living in cities at risk. For instance, it has already been estimated that 3500 cardiovascular mortality occur in Delhi annually due to outdoor air pollution (Gurjar et al. 2010). The high concentration levels of pollutants have been attributed to rapid urbanization, boom in construction activities, increase in number of vehicles, and traffic congestion (Patankar and Trivedi 2011; Hazarika and Srivastava 2017; Hazarika et al. 2017). Besides, studies regarding particulate pollutants in Delhi (Kushwaha et al. 2013a) and other northern cities of India (Pachauri et al. 2013; Kushwaha et al. 2013b), some studies have also been carried out to understand the emission inventories. The emission of particulate pollutants is mainly associated with anthropogenic activities in the Delhi such as industries, vehicular activities, and constructions (Hazarika and Srivastava 2017; Hazarika et al. 2017). Besides particles, emission patterns of VOCs/BTEX have also been investigated by various researchers. A study carried out near the petrol retail distribution centers showed higher concentrations of VOCs in the atmosphere of Delhi (north India) and Mumbai (situated in the west coast of India) mainly due to vehicle refueling (Srivastava et al. 2005). As far as benzene is concerned, higher concentration was observed in urban environments of Delhi by Khillare et al. (2008). They also observed significant variation of concentration before and after the implementation of mandatory compressed natural gas (CNG) fuel in commercial vehicles in Delhi. The standard set by NAAQS for benzene is 05 μg/m3 annual average concentration (CPCB 2009; Govt. of India). However, a number of studies on BTEX and their variations have been carried out by various researchers in the Indian environments (Dutta et al. 2009; Bauri et al. 2016; Masih et al. 2016; Masih et al. 2017) but due to the tremendous growth of vehicular activities and other industrial sources, the air quality of Delhi has been deteriorating day by day. This is why the capital has been recorded as one of the most polluted cities in world. So, to understand the level of BTEX concentrations as a results of vehicular activities this study has been undertaken with an objective to understand the difference between diurnal and nocturnal concentration. We also tried to understand the seasonal and spatial variations.
Material and methods
Study area
Delhi, the National Capital Territory, is also one of the largest cities in India. Delhi is situated at an altitude of 216 m above the mean sea level and spread over an area of approximately 1483 km2, out of which 700 km2 is identified as urban and 783 km2 as rural. Geographically, Delhi is situated 23.38° N and 77.13° S, and divided into three parts such as Delhi ridge (part of Aravali mountain range), the Yamuna river flood plain (rich in alluvial soil), and the Plains (Srivastava et al. 2008; Hazarika et al. 2015, 2019; Hazarika and Srivastava 2017). According to the Indian Metrological Department (IMD; http://www.imd.gov.in), the climate of India is divided mainly into four seasons; summer or pre-monsoon, monsoon or rainy, post-monsoon, and winter. The temperature of Delhi varies from ~ 2 °C in winter to ~ 48 °C in summer. The wind direction of Delhi is mainly west to northwest except during monsoon months (July to September) when it is from south to southwest. The characteristic of winter season is different from other seasons because of temperature inversion and low mixing height. This results to pollutants accumulation in the region.
Sampling sites
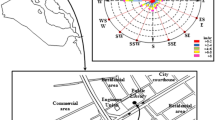
Samplings were carried out at traffic intersection point of three locations in Delhi during August 2016 to June 2017. Samples were taken from Indian Institute of Technology (IIT, Delhi), Mathura Road (near Friends Colony), and Uttar Pradesh (UP)-Delhi border on national highway 24 (NH 24). Samples were collected twice a day from 0900 to 1300 h during daytime and from 2300 to 0300 h during night. Sampling site 1 (S1, traffic intersection point near IIT Delhi) was traffic intersection of Sri Aurobindo Marg and Panchsheel Marg (outer ring road). This place is in the vicinity of a petrol pump and Hauz Khas Metro Station. This site is surrounded by housings, institutional areas, and a commercial center. This area experiences considerable traffic density with different types of vehicular activities both during day and night times. In general, Delhi’s traffic regulations permit enter of heavy vehicles during night, i.e., 2300 to 0500 h. Hence, during daytime, traffic density is much higher than night. In the sampling site 2 (S2), Mathura Road is in the vicinity of highways NH19 and NH44. It experiences very high traffic density. During daytime, small and medium vehicles ply but during night, heavy motor vehicles and trucks ply on this road. This is why number and types of vehicular traffic during night and day times are different. There is a petrol pump in the vicinity. In the Sampling site 3 (S3), Delhi-UP Border is situated on NH24. Comparatively, this place is open and there are some open dumping areas nearby. Similar to other sites, here also only small and middle size vehicles are allowed during day, but during night, both heavy and lighter vehicles are permitted. All the sampling sites covered in this study are shown in Fig. 1.
Sampling and analysis
BTEX samplings were carried out during the day and night times separately for 6 days in each month. Samples were collected using ORBO™-32 charcoal tube (7 cm in length × 6 mm o.d., from Supelco) fitted with a battery-operated pump (Gilian BDX-II Personal Air Sampling Pump 500–3000 cc/min). The air was drawn into the tube at a flow rate of 100 ml min−1 for 4 h during both day (0900 to 1300 h) and night times (2300 to 300 h). Sampler was kept at height of 1.5 m from the ground. In general, this is the respirable height of pedestrian. After sampling, the open sides of ORBO™-32 tubes were closed with Teflon tape to prevent any contaminations and then, wrapped and labeled properly.
In the analytical steps, firstly 2-ml amber-colored glass vial was used for transferring the activated charcoal from the ORBO tube. Afterwards, 1000 μl of CS2 (99% purity with < 0.001% benzene) was used as solvent for extraction, and then, it was put into an ultra-sonicated bath for about half an hour. The analyses of the extracted samples were carried out using gas chromatograph attached with flame ionization detector (GC-FID; Nucon 5700 gas chromatography). Alltech EC-5 capillary fused silica GC column [Alltech part no. 19657 EC-5 30 m × 0.53 mm, ID × 1.2 μm; serial no. 13879; poly(5% phenyl/95% methylpolysiloxane) phase, non-polar], nitrogen (purity 99.999%) as a carrier gas, hydrogen (purity 99.999%) as fuel gas, and zero air (21% oxygen and 79% nitrogen) were used. The column was connected to a flame ionization detector (FID) which was held at maximum temperature 270 °C. The column temperature was set initially at 40 °C for 5 min, ramp at 10 °C min−1 to 130 °C, which was held for 15 min until the temperature reached 270 °C. Analytical procedure was performed as according to the standard methods of the National Institute of Occupational Safety and Health (NIOSH 1003 and 1501) for measuring VOCs (Kumar et al. 2017; Golkhorshidi et al. 2019). The priority VOCs investigated from the collected samples were benzene, toluene, ethylbenzene, m/p-xylene, and o-xylene.
Data analysis
BTEX standard (BTEX mix, 1 × 1 ml, 2000 μg/ml in methanol) used was from Sigma Aldrich. In the analytical processes, the quality assurance and control measures were followed in every step. Calibrations were performed by making five standard solutions of different concentrations. A blank sample was run before and after every three samples. Retention times of five BTEX in standard solution and in the compounds were noted throughout the observed chromatograms. Linear calibrations were done and identification of compounds was carried out by comparing the area and retention times of the compounds with standards. The corresponding calibration curves were observed with R2 > 0.95 for all the BTEX compounds. Limit of detection of the measured compounds was found to vary between 0.2 and 0.4 μg/m3.
Ozone formation potential
The role of BTEX for ozone formation potential (OFP) can be evaluated by applying the maximum incremental reactivity (MIR) scale as per the information provided by Carter (1994). The contribution toward OFP of the individual BTEX can be obtained using the following equation (Hoqu et al. 2008; Garg et al. 2019).
OFP = concentration of BTEX × MIR (1)
Health risk assessments of BTEX
The chronic health risk assessment of the measured BTEX was estimated as per the United States Environmental Protection Agency (USEPA 1997) given below. The hazard quotient (HQ) of an individual compound can be obtained by calculating the chronic daily intake dose (CDI) as follows:
CDI = C × IR × ET × EF × ED/BW × AT (2)
HQ = CDI/RfD (3)
where, C represents the individual concentration of the BTEX in μg/m3; IR is the inhalation rate which is 20 m3/day; ET is the exposure time in h/day which is equivalent to sampling h/day; EF is the exposure frequency days/year which is equivalent to 350 days of year after deducting 15 official leave days; ED is the exposure duration, which is considered 30 years; BW is the body weight while 70 kg is considered as the average of an adult; AT is the average lifetime (i.e., 70 × 365 days); and RfD represents the chronic reference dose of the species (mg/kg/day).
Again, for BTEX, the incremental lifetime cancer risk (ILCR) in terms of inhalation was also calculated as below:
ILCR = CDI × SF (4)
where SF represents slope factor (mg/kg/day)−1 of the individual compounds. In this study, only the inhalation exposure of adults was observed with respect to HQs and ILCR. Similar types of observation of risk assessments on VOCs/BTEX have been done in India (Masih et al. 2016; Kumar et al. 2017; Masih et al. 2017) and abroad (Vilavert et al. 2012;Miri et al. 2016; Kanjanasiranont et al. 2017; Golkhorshidi et al. 2019).
Results and discussion
Diurnal and seasonal variation of BTEX
After investigating day-night variation of BTEX concentration, it was observed that benzene was abundant during post-monsoon season (142.2 μg/m3), and ethylbenzene (10.48 μg/m3) and o-xylene (10.53 μg/m3) were minimum with almost equivalent mean concentrations during day time (Fig. 2a). In this figure, B, T, EB, mpX, and oX represent the compounds benzene, toluene, ethylbenzene, m,p-xylene, and o-xylene respectively. From Fig. 2a, it can be concluded that the concentration of BTEX was found highest during the post-monsoon season followed by summer, winter, and monsoon seasons during day hours. We can infer that toluene was the second most abundant compound after benzene in all the seasons with a maximum and minimum concentrations in post-monsoon and monsoon seasons respectively. The concentration of benzene was found highest followed by toluene, m,p-xylene, o-xylene, and ethylbenzene throughout the seasons during daytime. m,p-Xylene, o-xylene, and ethylbenzene were found to be with significantly lower concentrations than benzene and toluene. It was also noticed that ethylbenzene and o-xylene possessed approximately similar mean concentrations throughout the seasons during day.
Figure 2 b shows the concentration of BTEX during nighttime in different seasons. Similar to the daytime variation, it can be inferred that benzene concentration was highest during all the seasons with maximum and minimum during post-monsoon and monsoon seasons respectively. The variation of BTEX concentration was found in the following order: benzene > toluene > m,p-xylene > o-xylene > ethylbenzene. Here again for all the BTEX compounds, maximum and minimum concentrations were found in post-monsoon and monsoon seasons respectively, whilst the overall variation of BTEX was observed as post-monsoon > summer > winter > monsoon. A major conclusion which can be drawn from Fig. 2a, b is that the concentration of benzene was found highest at all the sites and in all the seasons regardless of day-night hours. This is in consistence with the previous studies, where higher amount of benzene was observed (Rao et al. 1997; Srivastava et al. 2005).
The mean concentration of all BTEX compounds together is plotted in Fig. 2c. From this figure, it is clear that the concentration of BTEX was found in the following order: post-monsoon > summer > winter > monsoon. The distinct pattern of variation of BTEX on the basis of seasons might be due to the different meteorological conditions, anthropogenic activities, and the variability of hydroxyl radical in the environment of Delhi. In the monsoon season, due to rainwash out atmospheric pollutants are removed, reducing the pollutant concentrations in comparison with other seasons. In the summer season, the factors such as high temperature as a result of solar heat may reduce the volatile organic compounds due to photochemical degradation, which may also lead to the formation of other intermediates and simple molecules in the atmosphere (Lai et al. 2013; Kumar et al. 2017). Other possible reason of the lower concentration of BTEX might be associated with the dilution and mixing processes, which in turn results the convections and consequently decrease in the concentration level of volatile compounds (Filella and Peñuelas 2006; Kumar et al. 2017).
Relative abundances of BTEX
The percentage contribution of individual BTEX concentration across all sites during the day hours throughout the study is given in Fig. 3a. During daytime, the percentage contribution of benzene, toluene, ethylbenzene, m,p-xylene, and o-xylene were 44%, 33%, 6%, 10%, and 7% respectively. While Fig. 3b shows that the percentage contribution of individual BTEX concentration across all sites during nighttime. The corresponding compounds during nighttime contributed 47%, 35%, 5%, 8%, and 5% respectively. Among BTEX, benzene was found to be the most abundant species during both the day and night times, while toluene was the second most abundant species. The concentration of benzene and toluene was higher in the nighttime in comparison with daytime, while the concentration of other VOCs, i.e., ethylbenzene, m,p-xylene, and o-xylene were higher during daytime (Fig. 3a, b).
In the present study, variations in BTEX concentrations are observed approximately same as reported in other studies carried out in different parts of India (Srivastava et al. 2005; Srivastava et al. 2006), i.e., higher concentration of benzene in comparisons with toluene. Again, it was also observed that concentration of toluene exceeded benzene in some other parts of Delhi, where isomers of xylenes and ethylbenzene possessed comparatively lower amounts (Hoque et al. 2008; Singh et al. 2016; Kumar et al. 2017). Kumar and Tyagi (2006) observed lower concentrations of benzene and toluene at traffic intersection sites near the Income Tax Office (ITO) in Delhi as compared with the concentrations observed in the present study, but they found higher concentrations at the sites in the vicinity to the petrol pumps and road traffic areas.
Thus, while taking into consideration individual compounds, it has been observed that different studies reflect different results with varying trends of concentrations with respect to the sites and seasons. However, among BTEX, maximum abundance of benzene as observed in the present study is in agreement with some other previous studies (Rao et al. 1997; Srivastava et al. 2005). While some other studies have found toluene as the most abundant compound in other parts of world such as in the city of Hannover, Germany (Ilgen et al. 2001), in the metropolitan zone of Mexico city (Bravo et al. 2002), and in the metropolitan city Ahvaz (Rad et al. 2014).
Benzene and toluene are found in higher concentration than other compounds possibly due to their atmospheric life times and their stability in comparison with xylene. Benzene and toluene have the atmospheric lifetime 12.5 and 2.0 days respectively, while m-xylene’s lifetime is only 7.8 h (Prinn et al. 1987; Tiwari et al. 2010). Though the metrological conditions have an effect on the concentration of BTEX at different sites, but the seasonal characteristics, different sources, availability of OH radical, and insolation play an important role in the formation and destruction of BTEX (Masih et al. 2016). The higher concentrations of BTEX obtained near traffic intersection signify heavy vehicular activities as major factor. Besides vehicular activities (vehicles of Delhi and the nearby areas run on diesel, petrol, and compressed natural gas), other factors such as domestic burning, paints, and petrol pumps significantly contribute to the BTEX.
BTEX variation at different sites
The relative variations of BTEX throughout the sites during both day and night have been provided in Fig. 4a, b. It reveals that the trend of BTEX variation followed almost similar pattern at all the sites. It also shows that site 3 possessed higher BTEX followed by site 1 and site 2 (Fig. 4c). The highest amount of BTEX at site 3 might be due to the presence of various types of vehicles (two, three, and four wheelers) and miscellaneous anthropogenic activities from the nearby bordering areas of two different states (Delhi and Uttar Pradesh). Figure 4 d shows the concentration of BTEX throughout the study period. From this figure, it is clear that concentration of BTEX is found in the following order: benzene > toluene > m,p-xylene > ethylbenzene ~ o-xylene. This is in conformity with some previous studies carried out in Mumbai and Delhi by Rao et al. (1997) and Srivastava et al. (2005) respectively.
Indicator of BTEX emission
The interspecific ratios of BTEX have been used as an indicator of emission sources based on some previous studies (Tiwari et al. 2010; Singh et al. 2016). Though this study was carried out at traffic intersection points or in the areas of vehicular congestions, it is not necessary that all the measured BTEX are emitted from vehicular fuels, but may also be associated with the other man-made activities. Hence, in this study, the major sources of VOCs/BTEX are dominated by vehicular activities, but also have the probability of other anthropogenic activities and the long-range transport. The corresponding ratios (ratio of other VOCs with benzene and ethylbenzene) were observed in order to determine possible sources of BTEX. This difference in ratio mainly occurs due to the differential reactivity of atmospheric volatile compounds with OH radical (i.e., hydroxyl radical). The ratios have been calculated from each site by taking the mean concentrations of both the day and night times across all the seasons and provided in Table 1. The ratios, such as T/B, EB/B, m,pX/B, oX/B, and m,pX/EB represent the corresponding ratios of toluene/benzene, ethylbenzene/benzene, m,p-xylene/benzene, o-xylene/benzene, and m,p-xylene/ethylbenzene respectively, were considered to obtain their emission sources. Noticeably, these ratios are dominated by lower values (< 1) such as for the species T/B (0.74 to 0.83), EB/B (0.11 to 0.14), m,p-X/B (0.19 to 0.22), and o-X/B (0.12 to 0.14), whereas the ratio of m,p-X/EB (1.51 to 1.77) exceeded the unity (Table 1). Except T/B, the corresponding ratios of other species which are primarily < 1 have been found consistent with the previous work carried out in Delhi at different sites such as residential, commercial, industrial, and high traffic density areas (Singh et al. 2016).
It has already been reported that benzene and toluene are the constituents of gasoline, which are emitted into air by vehicular exhaust. Toluene in gasoline and motor vehicle exhaust is found three to four times higher than benzene (Pekey BYılmaz 2011). The unit value (i.e., ~ 1) of T/B indicates the dominant emission from vehicular traffic, while higher value (more than 1) indicates the emission from the nearby pollution sources (Rad et al. 2014). Low value of T/B infers the mixture of aromatic compounds with unleaded gasoline (Gee and Sollars 1998; Elbir et al. 2007). Biomass burning/residential heating and coal combustion can also have significant contribution toward benzene concentration, which is reflected from lower T/B ratio (Elbir et al. 2007). Vehicles running on different fuel types and various categories of industries could also play a role in the variation of T/B ratios (Hoque et al. 2008).
In the environment of Delhi, the ratio of EB/B has already been observed to be < 1 (Singh et al. 2016), but in this study, the corresponding ratio of EB/B has been found to be further lower than by Singh et al. Lower value of X/B ratio was observed at all the sampling sites. X/B ratios with higher and lower values indicate the fresh and aged air mass respectively. This implies aged air masses from vehicular transportations and also their long-range transport (Singh et al. 2016). The activity of benzene is lower than xylenes, which leads to its longer stay in the atmosphere (Niu et al. 2012; Singh et al. 2016). The ratio of m,p-X/EB (1.51 to 1.77) has been found to be in close conformity to the value 1.13 to 1.53 in yet another study carried out in the industrial area of Yokohama, Japan, by Tiwari et al. (2010). But, in general, lower ratios of X/B and X/EB represent the dominance of photochemical reactions (Tiwari et al. 2010; Nelson and Quigley 1983). From the ratios of BTEX, it can be concluded that all the sites were observed to be associated with the traffic emission, photochemical reactions, and other anthropogenic activities such as domestic and biomass burning.
Contribution of BTEX toward ozone formation potential
VOCs play a crucial role in the ozone formation by photochemical reactions. The OFP, which has been calculated using the MIR coefficients, has been used by various researchers to anticipate the potential of individual VOCs toward ozone formation (Hoque et al. 2008; Bauri et al. 2016). In this work, OFP obtained at different traffic congestion points have been presented in Table 2. MIR coefficients used are 0.42, 2.7, 2.7, 8.2, and 6.5 for benzene, toluene, ethylbenzene, m,p-xylene, and o-xylene respectively Carter (1994). The OFPs with respect to different sites are found in the following order: toluene > m,p-xylene > o-xylene > benzene > ethylbenzene. It is also clear from Table 2 that among different sites, site 3 has highest magnitude of OFP. The mean OFP of BTEX compounds are 239.41, 188.93, 98.21, 46.99, and 38.77 for toluene, m,p-xylene, o-xylene, benzene, and ethylbenzene respectively. Overall, xylenes (all the xylene isomers) possessed the highest mean OFP whereas ethylbenzene lowest.
Health risk assessments
The possible risk of BTEX regarding inhalation exposure with respect to the non-cancer (HQ) and cancer risk (ILCR) has been attempted to investigate as per the US EPA guideline. The observed values of HQ and ILCR have been provided in Table 3. The chronic inhalation reference concentration (RfC, in mg/m3) values of the compounds can be obtained from the Integrated Risk Information System (IRIS, www.epa.gov/iris/) and the Risk Assessment Information System (RAIS, www.rais.ornl.gov/). The RfC of benzene, toluene, ethylbenzene, and xylene isomers are 3.00 × 10−2, 5.00, 1.00, and 1.00 × 10−1 respectively. For the estimation of HQ, RfCs were converted to RfD (the inhalation dose, (mg/kg/day)). RfD was calculated using individual RfC of the targeted compounds, inhalation rate, and body weight (i.e., RfD = RfC × inhalation rate/body weight) (www.rais.ornl.gov). Similar types of observation have been made elsewhere (Durmusoglu et al. 2010; Demirel et al. 2014). The average HQs of benzene, toluene, ethylbenzene, m,p-xylene, and o-xylene were found 1.53 × 10−1, 7.26 × 10−3, 5.89 × 10−3, 1.03 × 10−1, and 6.21 × 10−2 respectively (Table 3). It is clear from Table 3 that all the compounds of BTEX at all the sites represent approximately similar order of magnitude of HQs. But among them, HQs of benzene and xylenes are comparatively higher than toluene and ethylbenzene. From these HQs, i.e., < 1, it can be concluded that the individual compounds will not cause any adverse health effects. The hazard index (HI) of BTEX, obtained by adding HQs of the sites 1 to 3, was 3.42 × 10−1, 2.87 × 10−1, and 3.37 × 10−1 respectively. Again, the overall HI, obtained from the average HQs of the individual compounds, was also found to be < 1 (i.e., 3.31 × 10−1).
The ILCR of benzene and ethylbenzene has been observed by various researchers (Dutta et al. 2009; Vilavert et al. 2012; Habeebullah 2015; Masih et al. 2016; Kumar et al. 2017). Compounds with attributable toxicities have been subdivided into three broad categories such as definite, probable, and possible risks depending on the ranges of carcinogenicity (Sexton et al. 2007). In this study, the carcinogenicity associated with benzene and ethylbenzene as a result of inhalation exposure was investigated. Among the observed compounds, benzene and ethylbenzene have the prescribed values of inhalation unit risk (per μg/m3). As per the IARC categorization, these two compounds belong to the group 1 and 2B respectively. The information of unit risk recommended by the Integrated Risk Information System (IRIS;www.epa.gov/iris/) of the US EPA can also be obtained at the Risk Assessment Information System (RAIS; www.rais.ornl.gov). In all the cases from sites 1 to 3, it can be concluded that benzene contributed relatively higher values of ILCR than ethylbenzene (Table 3). Benzene and ethylbenzene were found with the mean ILCR values 3.58 × 10−5 and 1.47 × 10−5 respectively, which are found to be in the recommended ranges (i.e., 10−6 to 10−4). Similar types of results can also be found elsewhere (Demirel et al. 2014; Miri et al. 2016). From these results, it can be concluded that benzene was found to be relatively toxic than ethylbenzene.
Summary and conclusion
This study shows the relative variation of BTEX concentration at three important traffic intersections in Delhi. Out of these one is a two states border (Delhi and Uttar Pradesh) joint. Although many studies are carried out on BTEX in different parts of India but to the best of our knowledge no study so far been done on traffic junctions. Also this study is important from point of view that first time in India study on day and night concentration of VOCs were carried out.
Though various studies carried out on VOCs in the urban residential, commercial, industrial, and traffic intersections, but to understand the emissions of VOCs from vehicles at the traffic intersections sites still await a serious look regarding the cities like Delhi, which is presently the most polluted city in world. So, to understand the pollution scenario in the road traffic points, simultaneous sampling and analyses are required to obtain the air quality and their probable health impacts in the capital region. As far as seasonal variation of BTEX is concerned, it was observed that benzene and toluene had the highest concentrations in all the seasons while the total average concentration of BTEX was found highest during the post-monsoon season followed by summer, winter, and monsoon seasons. Ethylbenzene and xylene isomers were found comparatively higher during day than night times. Site 2 possessed relatively lower concentration of BTEX in comparison with sites 1 and 3. Vehicles run by diesel, gasoline, natural gas, etc. played a major role in the variation of BTEX concentration throughout the sites. Among the BTEX, on an average, benzene and toluene together accounted 77% and 82% during the day and night times. Both together contributed > 78% in ambient air. On the contrary, it was found that the ethylbenzene, m,p-xylene, and o-xylene together possessed lower amount, i.e., 23% and 18% during day and night times respectively. The source identification of BTEX was done using the corresponding ratios of BTEX, which indicated vehicular activities as major emission factor. Except the ratio m,p-X/EB (whose corresponding ratios at sites 1–3 are 1.53, 1.51, and 1.77 respectively), the other interspecific ratios of BTEX were less than unity. Though vehicular emission pre-dominated the BTEX concentration, but other factors such as biomass burning, aged air mass, and photochemical reactions also expected to be responsible factor for its varying concentrations.
The OFP results showed that xylenes (m-, p-, and o-xylenes together) and toluene possessed higher potential than benzene and ethylbenzene. From the observation of non-cancer risk of BTEX, it was found that benzene and xylene isomers possessed higher HQs than ethylbenzene and toluene. But, the HI (i.e., the sum of HQs) was found less than unity at all the sites. Hence, from the HQ, it can be concluded that there was no adverse health risk. Again, benzene and ethylbenzene possessed the corresponding average ILCR values 3.58 × 10−5 and 1.47 × 10−5 respectively.
Abbreviations
- AT:
-
Average Lifetime
- BTEX:
-
Benzene, Toluene, Ethylbenzene and m,p- and o-Xylene
- BW:
-
Body Weight
- CDI:
-
Chronic Daily Intake Dose
- CPCB:
-
Central Pollution Control Board
- ED:
-
Exposure Duration
- EF:
-
Exposure Frequency
- ET:
-
Exposure Time
- GC-FID:
-
Gas Chromatography - Flame Ionization Detector
- HI:
-
Hazard Index
- HQ:
-
Hazard Quotient
- IARC:
-
International Agency for Research on Cancer
- IIT:
-
Indian Institute of Technology
- ILCR:
-
Incremental Lifetime Cancer Risk
- IMD:
-
Indian Metrological Department
- IR:
-
Inhalation Rate
- IRIS:
-
Integrated Risk Information System
- MIR:
-
Maximum Incremental Reactivity
- NAAQS:
-
National Ambient Air Quality Standards
- NH:
-
National Highways
- NIOSH:
-
National Institute of Occupational Safety and Health
- OFP:
-
Ozone Formation Potential
- RAIS:
-
Risk Assessment Information System
- RfC:
-
Inhalation Reference Concentration
- RfD:
-
Chronic Reference Dose
- SF:
-
Slope Factor
- SOA:
-
Secondary Organic Aerosol
- US EPA:
-
United States Environmental Protection Agency
- VOCs:
-
Volatile Organic Compounds
- WHO:
-
World Health Organization
References
Abtahi M, Fakhri Y, Oliveri Conti G, Ferrante M, Taghavi M, Tavakoli J et al (2018) The concentration of BTEX in the air of Tehran: a systematic review-meta analysis and risk assessment. Int J Environ Res Public Health 15(9):1–16
Bauri N, Bauri P, Kumar K, Jain VK (2016) Evaluation of seasonal variations in abundance of BTXE hydrocarbons and their ozone forming potential in ambient urban atmosphere of Dehradun (India). Air Quality, Atmos Health 9(1):95–106
Bravo H, Sosa R, Sánchez P, Bueno E, González L (2002) Concentrations of benzene and toluene in the atmosphere of the southwestern area at the Mexico City Metropolitan Zone. Atmos Environ 36(23):3843–3849
Bruckner JV, White CA, Muralidhara S, Hines C, Dallas CE (2010) Influence of exposure route and oral dosage regimen on 1,1-dichloroethylene toxicokinetics and target organ toxicity. Journal of Pharm ExpTherap 333(2):519–527
Carter WP (1994) Development of ozone reactivity scales for volatile organic compounds. J Air & Waste Manage Assoc 44(7):881–899
Central Pollution Control Board-National Ambient Air Quality Standards (CPCB-NAAQS), 2009
de Blas M, Navazo M, Alonso L, Durana N, Gomez MC, Iza J (2012) Simultaneous indoor and outdoor on-line hourly monitoring of atmospheric volatile organic compounds in an urban building. The role of inside and outside sources. Sci Total Environ 426:327–335
Demir S, Saral A, Ertürk F, Kuzu SL, Goncaloğlu Bİ, Demir G (2012) Effect of diurnal changes in VOC source strengths on performances of receptor models. Enviro Sci Poll Res 19(5):1503–1514
Demirel G, Özden Ö, Döğeroğlu T, Gaga EO (2014) Personal exposure of primary school children to BTEX, NO 2 and ozone in Eskişehir, Turkey: relationship with indoor/outdoor concentrations and risk assessment. Sci Total Environ 473:537–548
Durmusoglu E, Taspinar F, Karademir A (2010) Health risk assessment of BTEX emissions in the landfill environment. Journal Hazard Mat 176(1):870–877
Dutta C, Som D, Chatterjee A, Mukherjee AK, Jana TK, Sen S (2009) Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environ Mon Assess 148:97–107
Elbir T, Cetin B, Cetin E, Bayram A, Odabasi M (2007) Characterization of volatile organic compounds (VOCs) and their sources in the air of Izmir, Turkey. Environ Moni Asses 133(1–3):149–160
Filella I, Peñuelas J (2006) Daily, weekly, and seasonal time courses of VOC concentrations in a semi-urban area near Barcelona. Atmos Environ 40(40):7752–7769
Garg A, Gupta NC, Tyagi SK (2019) Levels of benzene, toluene, ethylbenzene, and xylene near a traffic-congested area of East Delhi. Environ Claims Journal 31:5–15
Gee IL, Sollars CJ (1998) Ambient air levels of volatile organic compounds in Latin American and Asian cities. Chemosphere 36:2497–2506
Golkhorshidi F, Sorooshian A, Jafari AJ, Baghani AN, Kermani M, Kalantary RR et al (2019) On the nature and health impacts of BTEX in a populated middle eastern city: Tehran, Iran. Atmos Pollut Res 10(3):921–930
Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Pierce T (1995) A global model of natural volatile organic compound emissions. Journal Geoph Res: Atmos 100(D5):8873–8892
Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C (2006) Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos Chem Phy 6(11):3181–3210
Gurjar BR, Jain A, Sharma A, Agarwal A, Gupta P, Nagpure AS, Lelieveld J (2010) Human health risks in megacities due to air pollution. Atmos Environ 44(36):4606–4613
Habeebullah TM (2015) Risk assessment of exposure to BTEX in the Holy City of Makkah. Arab Journal Geo 8(2):1155–1162
Hazarika N, Srivastava A (2017) Estimation of risk factor of elements and PAHs in size-differentiated particles in the National Capital Region of India. Air Qua, Atmos Health 10(4):469–482
Hazarika N, Das A, Kamal V, Anwar K, Srivastava A, Jain VK (2019) Particle phase PAHs in the atmosphere of Delhi-NCR: with spatial distribution, source characterization and risk approximation. Atmos Environ 200:329–342
Hazarika N, Jain VK, Srivastava A (2015) Source identification and metallic profiles of size-segregated particulate matters at various sites in Delhi. Environ Monitor Asses 187(9):602–623
Hazarika N, Srivastava A, Das A (2017) Quantification of particle bound metallic load and PAHs in urban environment of Delhi, India: source and toxicity assessment. Sustain Cities Soc 29:58–67
Hellen H, Hakola H, Laurila T (2003) Determination of source contributions of NMHCs in Helsinki (60 N, 25 E) using chemical mass balance and the Unmix multivariate receptor models. Atmos Environ 37(11):1413–1424
Heshmati A, Ghadimi S, Khaneghah AM, Barba FJ, Lorenzo JM, Nazemi F, Fakhri Y (2018) Risk assessment of benzene in food samples of Iran’s market. Food Chem Toxicol 114:278–284
Hoqu RR, Khillare PS, Agarwal T, Shridhar V, Balachandran S (2008) Spatial and temporal variation of BTEX in the urban atmosphere of Delhi, India. Scie the Total Environ 392(1):30–40
Ilgen E, Karfich N, Levsen K, Angerer J, Schneider P, Heinrich J, Begerow J (2001) Aromatic hydrocarbons in the atmospheric environment: part I. indoor versus outdoor sources, the influence of traffic. Atmos Environ 35(7):1235–1252
Kanjanasiranont N, Prueksasit T, Morknoy D (2017) Inhalation exposure and health risk levels to BTEX and carbonyl compounds of traffic policeman working in the inner city of Bangkok, Thailand. Atmos Environ 152:111–120
Khillare PS, Hoque RR, Shridhar V, Agarwal T, Balachandran S (2008) Temporal variability of benzene concentration in the ambient air of Delhi: a comparative assessment of pre-and post-CNG periods. Jour Hazard Mat 154(1):1013–1018
Krewski D, Snyder R, Beatty P, Granville G, Meek B, Sonawane B (2000) Assessing the health risks of benzene: a report on the benzene state-of-the-science workshop. Jour Toxic Environ Health Part A 61(5–6):307–338
Kumar A, Tyagi SK (2006) Benzene and toluene profiles in ambient air of Delhi as determined by active sampling and GC analysis. Jour Sci Indus Res 65:252–257
Kumar A, Singh D, Anandam K, Kumar K, Jain VK (2017) Dynamic interaction of trace gases (VOCs, ozone, and NOx) in the rural atmosphere of sub-tropical India. Air Quality, Atmosphere & Health:1–12
Kushwaha R, Hazarika N, Srivastava A (2013a) Traffic related aerosol exposure and their risk assessment of associated metals in Delhi, India. Intern Jour Environ 2(1):26–36
Kushwaha R, Hazarika N, Srivastava A (2013b) SEM-EDX analysis of size segregated particulate matter in Allahabad located in North India. Inte Jour Adva Rese 1(5):248–255
Lai CH, Chuang KY, Chang JW (2013) Source apportionment of volatile organic compounds at an international airport. Aero Air Qual Res 13:689–698
Lanz VA, Hueglin C, Buchmann B, Hill M, Locher R, Staeheli J, Reimann S (2008) Receptor modeling of C 2–C 7 hydrocarbon sources at an urban background site in Zurich, Switzerland: changes between 1993–1994 and 2005–2006. Atmo Chem and Phy 8(9):2313–2332
Latella A, Stani G, Cobelli L, Duane M, Junninen H, Astorga C, Larsen BR (2005) Semicontinuous GC analysis and receptor modelling for source apportionment of ozone precursor hydrocarbons in Bresso, Milan, 2003. Jour Chrom A 1071(1):29–39
Masih A, Lall AS, Taneja A, Singhvi R (2016) Inhalation exposure and related health risks of BTEX in ambient air at different microenvironments of a Terai zone in North India. Atmos Environ 147:55–66
Masih A, Lall AS, Taneja A, Singhvi R (2017) Exposure profiles, seasonal variation and health risk assessment of BTEX in indoor air of homes at different microenvironments of a Terai province of northern India. Chemosphere 176:8–17
Miri M, Shendi MRA, Ghaffari HR, Aval HE, Ahmadi E, Taban E, Azari A (2016) Investigation of outdoor BTEX: concentration, variations, sources, spatial distribution, and risk assessment. Chemosphere 163:601–609
Missia DA, Demetriou E, Michael N, Tolis EI, Bartzis JG (2010) Indoor exposure from building materials: a field study. Atmos Environ 44(35):4388–4395
Nelson PF, Quigley SM (1983) The m,p-xylenes:ethylbenzene ratio. A technique for estimating hydrocarbon age in ambient atmospheres. Atmos Environ 17:659–662
Niu Z, Zhang H, Xu Y, Liao X, Xu L, Chen J (2012) Pollution characteristics of volatile organic compounds in the atmosphere of Haicang District in Xiamen City, Southeast China. Journal of Environ Monitor 14(4):1144–1151
Pachauri T, Satsangi, A, Singla V, Lakhani A, Kumari, KM (2013) Characteristics and sources of carbonaceous aerosols in PM2.5 during wintertime in Agra, India. Aerosol Air Qual. Res 13(3):977–991
Patankar AM, Trivedi PL (2011) Monetary burden of health impacts of air pollution in Mumbai, India: implications for public health policy. Public Health 125(3):157–164
Pekey BYılmaz H (2011) The use of passive sampling to monitor spatial trends of volatile organic compounds (VOCs) at an industrial city of Turkey. Microch Journal 97(2):213–219
Prinn R, Cunnold D, Rasmussen R, Simmonds P, Alyea F, Crawford A, Fraser P, Rosen R (1987) Atmospheric trends in methylchloroform and the global average for the hydroxyl radical. Science 238:945–9503
Rad HD, Babaei AA, Goudarzi G, Angali KA, Ramezani Z, Mohammadi MM (2014) Levels and sources of BTEX in ambient air of Ahvaz metropolitan city. Air Qua Atmos Health 7(4):515–524
Rao AM, Pandit GG, Sain P, Sharma S, Krishnamoorthy TM, Nambi KSV (1997) Non-methane hydrocarbons in industrial locations of Bombay. Atmos Environ 31(7):1077–1085
Sarkhosh M, Mahvi AH, Zare MR, Fakhri Y, Shamsolahi HR (2012) Indoor contaminants from hardcopy devices: characteristics of VOCs in photocopy centers. Atmos Environ 63:307–312
Sexton K, Linder SH, Marko D, Bethel H, Lupo PJ (2007) Comparative assessment of air pollution-related health risks in Houston. Environ Health Perspect 115:1388–1393
Singh D, Kumar A, Singh BP, Anandam K, Singh M, Mina U, Kumar K, Jain VK (2016) Spatial and temporal variability of VOCs and its source estimation during rush/non-rush hours in ambient air of Delhi, India. Air Quality AtmosHealth 9(5):483–493
Srivastava A, Gupta S, Jain VK (2008) Source apportionment of total suspended particulate matter in coarse and fine size ranges over Delhi. Aerosol Air Qual Res 8(2):188–200
Srivastava A, Joseph AE, Devotta S (2006) Volatile organic compounds in ambient air of Mumbai—India. Atmos Environ 40(5):892–903
Srivastava A, Joseph AE, More A, Patil S (2005) Emissions of VOCs at urban petrol retail distribution centres in India (Delhi and Mumbai). Environ Monit Assess 109(1):227–242
Tiwari V, Hanai Y, Masunaga S (2010) Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Qual, Atmos Health 3(2):65–75
USEPA (1997) Air risk assessment work plan. Air and Radiation Division, Tristate, EPA, Washington
Vilavert L, Nadal M, Figueras MJ, Domingo JL (2012) Volatile organic compounds and bioaerosols in the vicinity of a municipal waste organic fraction treatment plant. Human health risks. EnvironSci Poll Res 19(1):96–104
Watson JG, Chow JC, Fujita EM (2001) Review of volatile organic compound source apportionment by chemical mass balance. Atmos Environ 35(9):1567–1584
Acknowledgments
This study is supported by the Council of Scientific and Industrial Research, India, as research fellowship to DM. The authors acknowledge the anonymous reviewers for improving the quality of this manuscript.
Funding
AS would like to thank UPoE for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehta, D., Hazarika, N. & Srivastava, A. Diurnal variation of BTEX at road traffic intersection points in Delhi, India: source, ozone formation potential, and health risk assessment. Environ Sci Pollut Res 27, 11093–11104 (2020). https://doi.org/10.1007/s11356-019-07495-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07495-8