Abstract
The increased use of pesticides is the origin of multiple damages to the environment and to humans; thus, the search for new strategies to reduce or even protect the toxic effects caused by these synthetic products became a necessity. In this context, our study attempted to evaluate the protective effects of fennel essential oil (FEO), the main essential oil extracted from Faeniculum vulgare Mill., a plant with aromatic, flavorful, and medicinal uses, against toxicity induced by an insecticide—triflumuron (TFM)—in human carcinoma cells (HCT116). Our methodological approach consists of the cytotoxicity assay starting with the cell viability test, the ROS generation, the malondialdehyde (MDA) production, the DNA fragmentation, and the measurement of some antioxidant enzymes activities such as catalase (CAT) and superoxide dismutase (SOD). Also, we measured the mitochondrial transmembrane potential. The outcome of the current study showed clearly that after 2 h of HCT 116 cell pretreatment with FEO, there were increase in cell viability, reduction in ROS generation, and modulation in CAT and SOD activities induced by TFM. In the same manner, significant decreases in MDA levels were found. Mainly, the results indicated a perceptible decrease in DNA damages and a significant reduction in the mitochondrial membrane potential loss. Our work demonstrates that FEO can be an important protector against toxic effects induced by TFM in HCT 116 cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive use of pesticides in the agricultural sector can cause chemical contamination leading to the loss of plant and animal lives (Ralph et al. 1996). Indeed, pesticides can pose problems for human health since pesticide residues are frequently detected in different compartments of the environment (surface water, groundwater, soil, etc.) and in products intended for human and animal food (Cerejeira et al. 2003; Yuan et al. 2014).
Numerous epidemiological studies suggest that pesticides may be involved in the induction of cancer, neurodegenerative diseases, fertility, and reproductive disorders (Akoto et al. 2015; Yoshida et al. 2015; Zhang et al. 2016). That is why it is necessary to look for new products with higher selectivity for the targets organisms and lower toxicity for human and for the environment. Insect growth regulators (IGRs) are among these new compounds, as they act on the growth and the development of insects. In fact, IGRs were introduced following the appearance of insect resistance to pyrethroids and organophosphate insecticides (Belinato et al. 2013).
Among these IGRs, we find the class of benzoylureas (disrupting molt) which interferes with the insect’s exoskeleton causing eventually its death (Tunaz and Uygun 2004).
Benzoylurea insecticides are widely used for crops protection, especially fruits and vegetables. Technically, they could be classified such as insecticide of fourth generation having high selectivity, low acute toxicity for mammals, and high biological activity (Tomsej and Hajglov 1995). In this context, our interest in the current study will be focused on triflumuron (TFM), a benzoylurea insecticide commonly used in the world.
Triflumuron ((2-chloro-[[[N-4-trifluoromrthoxy)phenyl]amino]carbonyl]; TFM) equally known as Starycid SC 480 (Batra et al. 2005) is used in order to protect crops, human, and animal health against diseases caused by some insects vectors (Van der Oost et al. 2003; Konradsen 2007; Winkaler et al. 2007; Alavanja 2009; Lowden et al. 2007).
Regarding the molecular level, TFM acts by inhibiting the synthesis of chitin which is a linear polymer composed of N-acetyl-glucosamine (Merzendorfer and Zimoch 2003). Thus, by blocking the transport of N-acetylglucosamine through the epithelial membrane, TFM acts as a general stressor, making the insect more susceptible to malformations and disease, for example, facilitating the entry of pathogenic fungi into insects by weakening the insect’s cuticle (Irigaray et al. 2003).
Almost the majority of TFM toxic effects have been realized on insects. Indeed, TFM induced high mortality levels, delayed the development and inhibited molting in Rhodnius prolixus (Mello et al. 2008). In addition, TFM reduced larval density and caused inhibition of the emergence of adult mosquito larvae (Batra et al. 2005).
In addition to this, the treatment of Aedes albopictus with TFM inhibits the hatching of eggs. Also, abnormal morphology of the eggshell has developed (Suman et al. 2013). Furthermore, TFM acts on the offspring of insects, especially at the time of pupal formation. Like most IGRs, TFM causes malformations of pupae of treated females which die at the hatching moment or a few days later (Ouédraogo 1998) and causes embryo death (Itard 1986). It causes as well high rates of abortion in treated insects (Langley 1995).
However, TFM can cause toxic effects on non-target organisms, especially on aquatic organisms and invertebrates. For example, in rats, dogs, and rabbits, the administration of this compound causes spasms and skin and respiratory irritation resulting in sneezing and in several eye damages (Waller and Lacey 1986; EFSA 2011). Repeated administration of TFM is also known to cause hemolytic anemia (Tasheva and Hristeva 1993) and reproductive toxicity (Suman et al. 2013).
Because of TFM toxic effects on humans, animals, and environment, we focused our interest on antioxidant sources of molecules, as the fennel essential oil (FEO). Fennel is the commercial name of Foeniculum vulgare. It is an Apiaceae plant that is characterized by its medicinal properties. Indeed, this plant can be used to treat gastrointestinal and respiratory disorders (Agarwal et al. 2008). The essential oil extracted from this plan is known as FEO which is used in many sectors such as cosmetic, pharmaceutical, and perfume industries. Besides that, it is used as an additive in the preparation of food (Tinoco et al. 2007). Many studies have shown the important effects of FEO in the treatment of some human diseases, due to its medicinal properties such as a diuretic, anti-inflammatory, analgesic, antioxidant (Gross et al. 2002) antiseptic, sedative, and stimulant activities (Tinoco et al. 2007; He and Huang 2011).
The antioxidant properties of this substance prompt us to study its protective effects against TFM-induced oxidative damages in human cell Carcinoma (HCT 116 cells).
Materials and methods
Chemicals
Triflumuron, fennel essential oil, and pyrogallol were purchased from Sigma–Aldrich (St. Louis, MO, USA). 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), cell culture medium (RPMI 1640), fetal calf serum (FCS), phosphate-buffered saline (PBS), trypsin-EDTA, penicillin and streptomycin mixture, and L-glutamine (200 mM) were from GIBCO-BCL (UK). 2,7-Dichlorofluorescein diacetate (DCFH-DA) was supplied by Molecular Probes (CergyPontoise, France). Low melting point agarose (LMA) and normal melting point agarose (NMA) were purchased from Sigma (St. Louis, MO). All other chemicals used were of analytical grade.
Cell culture and treatment
RPMI 1640 was used for the culture of human carcinoma cells (HCT 116). This medium is supplemented with 10% FBS, 1% L-glutamine (200 mM), 1% of mixture penicillin (100 IU/mL), and streptomycin (100 g/mL), at 37 °C with 5% CO2. TFM was dissolved in DMSO and the FEO was dissolved in absolute ethanol (with a percentage that does not exceed 1% to avoid possible toxixity of absolute ethanol). The different concentrations of TFM (50 to 600 μM) in the presence or absence of the preventive substance (FEO) were added to the cell medium. Thus, the HCT 116 cells are pretreated for 2 h by FEO (1, 1.5, and 2% (v/v)) before their exposure to the TFM.
Cell toxicity assay (MTT assay)
The MTT assays (a tetrazolium salt reduction assay) measure if there are metabolic disorders in cell and more precisely in the mitochondria (Mosman 1983). For this, HCT 116 cells were seeded in 96-well plates at 2.5 × 104 cells/well and were treated with the FEO at 1, 1.5, and 2% (v/v) and different concentrations of TFM for 24 h at 37 °C. Wells containing untreated cells served as a negative control. After treatment, cells were incubated with the MTT solution for 3 h. At the end of the incubation, formed formazan crystals were dissolved in dimethyl sulfoxide and absorbance read at 570 nm using a microplate reader spectrophotometer (BioTek, Elx800). The results were expressed as the percentage of MTT reduction relative to the absorbance’s measured from negative control cells. All assays were performed in triplicate.
Reactive oxygen species determination and oxidative stress status
DCFH-DA is a fluorochrome that enters living cells and will be deacetylated by cell esterases into a non-fluorescent compound DCFH. The latter will be oxidized, in the presence of reactive oxygen species, into a fluorescent compound DCF (Cathcart et al. 1983; Le Bel et al. 1992; Chen and Wong 2009). The determination of free radicals is determined following the seeding of the cells in multi-well plates (96 wells) at a ratio of 2.104 cells/well. After 24 h, the cells were incubated for 30 min with 20 μM DCFH-DA and were then treated with FEO (1, 1.5, and 2% (v/v)) combined or not with the TFM (IC50 = 400 μM) and incubated for 24 h. Fluorescence was measured using a fluorometer (Biotek FLx800) with an excitation wavelength of 485 nm and an emission wavelength of 525 nm.
Protein extraction
HCT 116 cells (106 cells/well) were seeded in 6-well plates at 37 °C for 24 h. Then, we incubated cells for 24 h with 1, 1.5, and 2% (v/v) of FEO and with TFM at 400 μM. A wash step with cold PBS was performed, and then cells were recuperated using a lysis buffer after a 30-min incubation period in ice. Centrifugation allowed obtaining cell extracts which were dosed using BioRad protein assay in order to determine protein concentrations in each sample (Bradford 1976).
Lipid peroxidation evaluation
To measure the level of MDA produced by the cells, 7.5 105 cells were plated on 6-well plates. The treatment of cells with both TFM (400 μM) and FEO (1, 1.5, and 2% (v/v)) was carried out for 24 h at 37 °C. Thus, cells were recovered in PBS and incubated in the lysis buffer for 1 h. Centrifugation allowed the obtention of cell lysates, which were mixed with 200 μL of KCl (1.15% w/v), 100 μL of SDS (8.1% w/v), 750 μL of acetic acid (20%; pH 3.5 v/v), and 750 μL of thiobarbituric acid (0.8% w/v). The mixture is then incubated for 2 h at 90 °C. A cooling step for 10 min is necessary before adding a volume of 2.5 ml of n-butanol-pyridine (15:1/ v/v). The mixture obtained is then vortexed until the organic phase became up. Finally, a centrifugation allowed isolating the supernatant whose absorbance was read at 546 nm (Ohkawa et al. 1979).
Measurement of catalase (CAT) activity
To determine the activity of this enzyme, 780 μL of phosphate buffer (100 mM; pH 7.0) is mixed with 20 μL of proteins extracts and 200 μL of H2O2 (20 mM). Then the absorbance was measured at 240 nm over a period of 3 min (Aebi 1984) at 37 °C. Using a molar extinction coefficient of 0.04/mM/cm, the calculus of this activity was effected and results were expressed as mmol/min/mg protein.
Measurement of superoxide dismutase (SOD) activity
SOD is enzyme that catalyzes the disproportionation of the superoxide anion into oxygen and dihydrogen peroxide. The assay of this activity is based on the ability of SOD to inhibit the autooxidation of pyrogallol by SOD. To do this, 970 μL of Tris HCl (50 mM; pH 8.5), 10 μL of pyrogallol (24 mM), and 20 μL of protein extract are introduced into a curve, and the measurement of the optical density was effected at 440 nm for an interval of 3 min at 37 °C. In order to calculate the SOD activity, the amount of protein that inhibits the pyrogallol oxidation at 50% is determined. Results are expressed as U/mg protein (Marklund and Marklund 1974).
Mitochondrial membrane potential (MMP) assay
Rhodamine-123 is a fluorescent agent used to measure transmembrane mitochondrial potential (Debbasch et al. 2001). For this, cells, already seeded in a 96-well plate, were incubated with 1, 1.5, and 2% (v/v) of FEO in the presence or the absence of TFM for 24 h. A washing step with 100 μL of PBS was primordial before putting 100 μL of rhodamine 123 (1 μM). Then, the plate was incubated for 15 min at 37 °C. Finally, the supernatant was discarded and was replaced by 150 μL of PBS. Thus, a fluorimeter is used to determine the uptake of rhodamine 123. The results were expressed as the percentage of uptaken rhodamine fluorescence relative to the fluorescence measured from negative control cells.
DNA damage assessed by the comet assay
The DNA damage was quantified by using the comet test, which is an electrophoretic nuclei technique with a fluorescent agent, which allows the detection of DNA breaks on isolated cells after their inclusion in an agarose gel. To perform this test, cells were seeded in 6 well plates at 7.5 × 105 cells/well. After 24 h, cells were treated with 1, 1.5, and 2% (v/v) of FEO and TFM at 400 μM. After recovering the cells in PBS, they were mixed with 60 μL of low melting agarose (1.2%, w/v) and the solution was pretreated on coded slides and already covered with normal agarose (1%, w/v). Then slides were held in lysis buffer for 1 h at + 4 °C. Then, an electrophoresis step (30 min, 25 v, and 300 mA) (BioRad) made it possible to migrate DNA in an electric field. Finally, a neutralization step with Tris buffer was applied for all the slides for 15 min. At the time of DNA damage count, the slides were stained with ethidium bromide (20 mg/mL) and visualized by a fluorescence microscope. The DNA damage was classified into four classes according to the length of the DNA smear (Collins et al. 1996).Thus, a total score was calculated according to the following equation: (% of cells in class 0 × 0) + (% of cells in class 1 × 1) + (% of cells in class 2 × 2) + (% of cells in class 3 × 3) + (% of cells in class 4 × 4).
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The analysis parameters were tested for homogeneity of variance and normality, and they were found to be normally distributed. The data were therefore analyzed using a one-way analysis of variance (ANOVA) with a post hoc Tukey–Kramer test to identify significance between groups and their respective controls. In all cases, p < 0.05 was considered statistically significant.
Results
Cell viability determination
The exposure of HCT 116 cells to different concentration of TFM ranging from 100 μM to 1 mM for 24 h was established in order to determine the cell viability using MTT assay. Our results showed that TFM causes a decrease in the cell viability in a dose-dependent manner and with IC50 around 400 μM (Fig. 1a). For FEO, no cell toxicity was indicated. The pretreatment of HCT cells with 1, 1.5, and 2% (v/v) FEO, 2 h before the TFM treatment decrease significantly the cytotoxicity induced by TFM (Fig. 1b) (*p < 0.05 and **p < 0.01 vs. TFM alone).
a Cytotoxic effect of TFM and FEO on HCT116 cells. Cells were treated with TFM alone or combined with FEO for 24 h. Cell viability was determined using the MTT assay and expressed as percentages of viability. Values are significantly different (p < 0.05) from control. FEO reduces TFM-induced cytotoxicity in HCT116. Cells were pretreated for 2 h with FEO (1, 1.5, and 2%) before TFM treatment for 24 h (400 μM). b Cell viability was determined using MTT assay. Data are expressed as the mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01 vs. TFM alone
Inhibition of ROS generation
Figure 2 shows the effect of FEO on ROS generation induced by TFM on human intestine cells. The level of the ROS produced by cells in the presence or the absence of TFM and FEO was measured according to the fluorescence of DCF. Indeed, in the presence of H2O2 in the intracellular medium, DCFH was oxidated to fluorescent DCF. Our results indicated that the level of ROS production increased from 5300 ± 500 in the control to 15,600 ± 800 in cells treated with TFM at 400 μM. Using different concentrations of 1, 1.5, and 2% (v/v) of FEO, the intracellular ROS produced by TFM was completely abolished. Indeed, with the highest concentration of FEO, we showed that the level of ROS generation was around 4500 ± 200 (Fig. 2) (##p < 0.01 vs. control, **p < 0.01 and ***p < 0.001 vs. TFM alone).
Effects of FEO on TFM-induced ROS generation. HCT116 cells were pretreated with FEO (1, 1.5, and 2%) for 2 h before TFM treatment for 24 h (400 μM). The relative intracellular ROS production was evaluated by recording the fluorescence of DCF, the product of DCFH oxidation mainly by H2O2. Data are expressed as the mean ± SD of three separate experiments. ##p < 0.01 vs. control, **p < 0.01 and ***p < 0.001 vs. TFM alone
Effect of FEO on TFM-induced lipid peroxidation
To determine lipid peroxidation, we measured the rate of MDA which is an ultimate fragment of membrane lipid degradation. Our findings indicated that using TFM alone (400 μM), the level of MDA increased from 0.3 ± 0.03 (μmol/mg of proteins) in the control cells to 0.8 ± 0.03 (μmol/mg of proteins) in cells treated with TFM. The use of FEO at 1, 1.5, and 2% decreased respectively the level of MDA to 0.64 ± 0.03, 0.51 ± 0.04, and 0.38 ± 0.04 (μmol/mg of proteins) compared to the TFM-treated cells (Fig. 3). This decrease was of concentration-dependent manner (###p < 0.001 vs. control, *p < 0.05 and **p < 0.01 vs. TFM alone).
Effects of FEO on TFM-induced lipid peroxidation. HCT116 cells were pretreated with FEO (1, 1.5, and 2%) for 2 h before TFM treatment for 24 h (400 μM). The peroxidation of lipids was recorded by measuring the accumulation of MDA. Data are expressed as the mean ± SD of three separate experiments. ###p < 0.001 vs. control, *p < 0.05 and **p < 0.01 vs. TFM alone
Effect of FEO on antioxidant enzymes activities
The change in the level of antioxidant enzymes can be considered as a marker of oxidative stress. Indeed, SOD catalyzes the dismutation of the highly reactive superoxide anion from oxidative stress to H2O2, which can further be decomposed to water and oxygen by CAT or Gpx. Figure 4a, b shows the measurement of the activities of two antioxidant enzymes that are SOD and CAT.
Effects of FEO on catalase (a) and superoxide dismutase activities (b). HCT116 cells were pretreated with FEO (1, 1.5, and 2%) for 2 h before TFM treatment for 24 h (400 μM). Data are expressed as the mean ± SD of three separate experiments. ###p < 0.001 vs. control, *p < 0.05 and **p < 0.01 vs. TFM alone
Using TFM at 400 μM in HCT116 cells, we found a significant increase in the activities of these two enzymes. Indeed, in the untreated cells, CAT activity passed from 6 ± 0.08 to 47 ± 1 mmol/min/mg of proteins (Fig. 4a) in the TFM-treated cells group. Similarly, for the SOD activity, we noted an increase in this activity which passed from 48 ± 5 U SOD/min/mg of proteins in the control group to 127 ± 1 U SOD/min/mg of proteins (Fig. 4b). The pretreatment of cells with 1, 1.5, and 2% (v/v) of FEO reduced these increases in a dose-dependent manner (###p < 0.001 vs. control, *p < 0.05 and **p < 0.01 vs. TFM alone).
Effect of FEO on TFM mitochondrial alterations
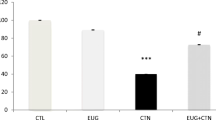
To assess the effect of FEO on the mitochondrial alterations induced by TFM, we incubated cells with rhodamine 123. We noted a significant decrease in the Rh123 in cells treated with TFM (400 μM) alone with a percent of 53% ± 0.5. These findings evidenced that in this case, cells lost their mitochondrial potential. Using FEO, a remarkable restoration in the percentage of Rh123 uptake was evidenced: 59% ± 1%, 78 ± 4%, and 88% ± 0.4% using 1, 1.5, and 2% (v/v) of FEO respectively. Our results demonstrated the protective effect of FEO against mitochondrial alterations caused by TFM (Fig. 5) (##p < 0.01 vs. control, **p < 0.01 vs. TFM alone).
Effects of FEO on TFM-induced loss of mitochondrial transmembrane potential. HCT116 cells were pretreated with FEO (1, 1.5, and 2%) for 2 h before TFM treatment for 24 h (400 μM). The mitochondrial potential was assessed by measuring the uptake of rhodamine 123. Data are expressed as the mean ± SD of three separate experiments. ##p < 0.01 vs. control, **p < 0.01 vs. TFM alone
Effect of FEO on TFM-induced DNA fragmentation
The DNA fragmentation results are illustrated in Fig. 6. We showed that, compared to the control group, the total score increased from 44 ± 1 to 129 ± 6 in cells treated with TFM alone. Using different concentrations of FEO, a significant decrease in the DNA damages was noted: 117 ± 4, 73 ± 2, and 56 ± 4 respectively for 1, 1.5, and 2% (v/v) of FEO (###p < 0.001 vs. control, **p < 0.01 vs. TFM alone).
Discussion
Pesticides, also called plant protection products, are molecules used to protect humans and their environment against attacks caused by harmful vectors and predators (Marutescu and Chifiriuc 2007). Due to their increased use in many areas such as agriculture, industry, and traditional medicine, pesticides cause adverse effects on human health and threaten ecosystems (Farnesi et al. 2012). In this context, several studies have revealed the role of pesticides in the occurrence of numerous pathologies, namely, cancer and neurodegenerative and cardiac disorders (Kland 1988; Juricek and Coumoul 2014; Akoto et al. 2015).
Among those pesticides, we focused our interest on the Insect growth regulators (IGRs) which are a class of pesticide commonly used in agriculture and human and animal health in order to control damages caused by insects (Smith and Wall 1998; and Parween et al. 2001; Zaim and Guillet 2002; WHO 2006).
TFM is one of the most known IGRs which is used to protect human, animal, and crops against insects (Kamsh et al. 1998; Parween et al. 2001). It acts by blocking the synthesis of chitin, the major constituent of the insect cuticle causing consequently its death (Vasuki and Rajavel 1992; Wilson and Cryan, 1997; Amir and Peveling 2004). It is characterized by its several toxic effects in most ecosystems. Also, it can affect the reproductive system of adult females of Rhodnius prolixus causing a high level of mortality in those insects (Henriques et al. 2016). Our previous study showed that TFM was an inductor of oxidative stress in male mice after short-term exposure (Timoumi et al. 2018).
Because of the toxic effects of TFM on the fauna and the flora, we tried to find in our work a natural substance that can reduce and prevent the toxicity induced by TFM. Thus, we worked with fennel essential oil (FEO) that is characterized by its antioxidant, antifungal, and antibacterial proprieties (Al-Amoudi 2017).
Our work aimed to study the protective effects of FEO against toxic effects induced by the insecticide, triflumuron (TFM), using human intestinal cell lines.
Treatment of HCT 116 cells with TFM (400 μM) decreased significantly cell viability by using the MTT assay. However, using FEO, cell death caused by TFM was significantly reduced.
In order to explain the decrease in cell viability induced by TFM, we performed some oxidative stress tests. Indeed, oxidative stress occurs when oxygen-reactive species (ROS) accumulate in cells, leading them to apoptosis (Battisti et al. 2008). ROS are essential intermediates in oxidative metabolism. Nevertheless, when oxidative stress occurs, ROS are generated in excess and consequently may damage cells by oxidizing lipids, disrupting DNA and proteins. Accordingly, we demonstrated that TFM-induced ROS generation and increased MDA levels and DNA damages. As a matter of fact, MDA is an ultimate fragment resulting from degradation of membrane lipids, which is frequently used as a marker of an oxidative state (Cini et al. 1994). The results of this state are the structural destruction in the cell membranes, DNA damages, and apoptotic death (Surapaneni and Venkataramana 2007).
Enzymatic and non-enzymatic antioxidants are cellular defenses having the role of protecting cells against environmental contaminants. These defense systems include catalase (CAT) and superoxide dismutase (SOD). Indeed, in case of stress, the SOD is the first enzyme that manifests. It catalyzes the dismutation of superoxide radicals into oxygen and hydrogen peroxide. The latter in the presence of CAT will be neutralized (Salvi et al. 2007). In our case, SOD, CAT activities, and mitochondrial potential were altered.
Any oxidative stress may be the cause of genotoxicity. Assuredly, in case of a reaction between pesticides and the nuclear DNA, mutagenic and carcinogenic effects may arise. This is why we tried to quantify DNA damage caused by the TFM using the comet assay. Our results indicated the oxidative damages in the DNA level in the studied cells that were exposed to this compound.
These results confirm the share of oxidative stress in the toxicity induced by benzoylurea insecticides and in particular TFM. Moreover, our results showed that TFM is an oxidizing agent in HCT 116 cells, by its ability to increase the production of ROS and to alter the normal functioning of antioxidant enzymes such as CAT and SOD.
However, the use of FEO a natural antioxidant molecule protects against the oxidative stress and the genotoxicity induced by TFM. Indeed, we demonstrated that the use of FEO decreased the generation of ROS and reduced the level of MDA in comparison with the TFM-treated cells.
A modulation in CAT and SOD activities was observed when cells were exposed to FEO, 2 h before their intoxication with the TFM.
By combining the FEO with the TFM, we found an increase in the mitochondrial transmembrane potential accompanied by a decrease in DNA damage when comparing to cells treated with TFM only.
The toxicity study of TFM on cellular and animal models is very limited. However, recently, TFM has been shown to promote metastasis of liver cancer cells (Hep G2) by interfering with hypoxia-inducible factor 1α (Ning et al. 2018).
Moreover, diflubenzuron (DFM), a benzoylurized insecticide as well as TFM, was found to be cytotoxic which decreases cell viability in CHOK1 cells (Bayoumi et al. 2003) and Hep G2 cells (Delescluse et al. 1998) using the MTT assay and the neutral red test. Similarly, the cytotoxicity of DFM has been demonstrated in BALBC/3T3 cells by inducing cellular transformations. Indeed, it increased the lipoperoxydation and activities of CAT, SOD, and Gpx enzymes (Ilboudo et al. 2014).
The investigation of the protective effects of the FEO in vivo has been over-clarified. Indeed, Tripathi et al. (2013) showed that FEO has anticytotoxic and anti-genotoxic effects in mice treated with cyclophosphamide. Thus, they founded that FEO reduced the cytotoxic and the genotoxic effects in mice bone marrow cells. Also, the activities of catalase, SOD, GSH, and the level of MDA measured at the livers of these mice were modulated.
In the same context, FEO was able to prevent hepatorenal damages caused by sodium valproate, a medicine used as an anticonvulsant and mood stabilizer, in albino rats (Al-Amoudi 2017).
It was previously known that fennel extracts are full of many types of polyphenolic compounds (Faudale et al. 2008; Chang et al. 2013) which are characterized by their antioxidant proprieties (Choi and Hwang 2004; Chatterjee et al. 2012). These antioxidant activities are involved in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides (Sofowora 1993; Singh et al. 2006).
A study in human hepatoma cell line (HepG2) showed that estragole, a very common compound found in EFO, is neither cytotoxic nor genotoxic, nor even able to cause apoptosis (Villarini et al. 2014). Mizuno et al. (2015) showed that the FEO can protect neuronal cells (GT 1–7) against the oxidative stress caused by hydrogen peroxide.
In conclusion, our study clearly demonstrates the protective role of fennel essential oil, extracted from fennel, an aromatic plant frequently used in food. In no uncertain terms, FEO is able to protect cultured intestinal cells from toxicity and genotoxicity induced by TFM.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agarwal R, Gupta SK, Agrawal SS, Srivastava S, Saxena R (2008) Oculohypotensive effects of Foeniculum vulgare in experimental models of glaucoma. Indian J Physiol Pharmacol 52:77–83
Akoto O, Oppong-Otoo J, Osei-Fosu P (2015) Carcinogenic and non-carcinogenic risk of organochlorine pesticide residues in processed cereal-based complementary foods for infants and young children in Ghana. Chemosphere 132:193–199
Al-Amoudi WM (2017) Protective effects of fennel oil extract against sodium valproate-induced hepatorenal damage in albino rats. Saudi J Biol Sci 24:915–924
Alavanja MC (2009) Introduction: pesticides use and exposure extensive worldwide. Rev Environ Health 24:303–309
Amir OG, Peveling R (2004) Effect of triflumuron on brood development and colony survival of free-flying honeybee, Apismellifera L. J Appl Entomol 128:242–249
Battisti C, Formichi P, Radi E, Federico A (2008) Oxidative-stress-induced apoptosis in PBLs of two patients with Parkinson disease secondary to alpha-synuclein mutation. J Neurol Sci 267:120–124
Batra CP, Mittal PK, Adak T, Ansari MA (2005) Efficacy of IGR compound Starycide 480 SC (triflumuron) against mosquito larvae in clear and polluted water. J Vector Borne Dis 42:109–116
Bayoumi AE, Pérez-Pertejo Y, Zidan HZ, Balaña-Fouce R, Ordóñez C, Ordóñez D (2003) Cytotoxic effects of two antimolting insecticides in mammalian CHO-K1 cells. Ecotoxicol. Environ. Saf 55:19–23
Belinato TA, Martins AJ, Lima JBP, Valle D (2013) Effect of triflumuron, a chitinsynthesis inhibitor, on Aedes aegypti, Aedes albopictus and Culex quinque fasciatus under laboratory conditions. Parasit Vectors 6:683
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cathcart R, Schwiers E, Ames BN (1983) Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 13:111–116
Cerejeira MJ, Viana P, Batista S, Pereira T, Valério E, Silva A, Ferreira AM (2003) Pesticides in Portuguese surface and ground waters. Water. Res 37(5):1055–1063
Chang S, Bassiri A, Jalali H (2013) Evaluation of antioxidant activity of fennel (Foeniculumvulgare) seed extract on oxidative stability of olive oil. J Chem Health Risks 3:53–61
Chatterjee S, Goswami N, Bhatnagar P (2012) Estimation of phenolic components and in vitro antioxidant activity of fennel (Foeniculumvulgare) and ajwain (Trachyspermumammi) seeds. Adv Biores 3:109–118
Chen T, Wong YS (2009) Selenocysteine induces caspase-independent apoptosis in MCF-7 human breast carcinoma cells with involvement of p53 phosphorylation and reactive oxygen species generation. Int J Biochem Cell Biol 41:666–676
Choi EM, Hwang JK (2004) Anti inflammatory, analgesic and antioxidant activities of the fruit of Foeniculumvulgare. Fitoterapia 75:557–565
Cini M, Fariello RG, Bianchetti A, Moretti A (1994) Studies on lipid peroxidation in the rat brain. Neurochem Res 19:283–288
Collins AR, Dusinska M, Gedik CM, Stetina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104:465–469
Debbasch C, Brignole F, Pisella PJ, Warnet JM, Rat P, Baudouin C (2001) Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest Ophthalmol Vis Sci 42:642–652
Delescluse C, Ledirac N, de Sousa G, Pralavorio M, Lesca P, Rahmani R (1998) Cytotoxic effects and induction of cytochromes P450 1A1/2 by insecticides in hepatic or epidermal cells: binding capability to the Ah receptor. Toxicol Lett 96-97:33–39
EFSA (European Food Safety Authority) Journal (2011) Conclusion on the peer review of the pesticide risk assessment of the active substancetriflumuron 9(1):1941
Farnesi LC, Brito JM, Linss JG, Pelajo-Machado M, Valle D, Rezende GL (2012) Physiological and morphological aspects of Aedesaegypti developing larvae: effects of the chitin synthesis inhibitor novaluron. PLoS. One 7(1):e30363
Faudale M, Viladomat F, Bastida J, Poli F, Codina C (2008) Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. J Agric Food Chem 56:1912–1920
Gross M, Friedman J, Dudai N, Larkov O, Cohen Y, Bar E, Ravid U, Putievsky E, Lewinsohn E (2002) Biosynthesis of estragole and trans-anethole in bit-ter fennel (Foeniculumvulgare Mill. var. vulgare) chemotypes. Changes inSAM: phenylpropene O-methyltransferase activities during development. Plant Sci 162:1047–1053
He WP, Huang BK (2011) A reviews of chemistry and bioactivities of a medicinalspice: Foeniculumvulgare. J Med Plant Res 5:3595–3600
Henriques BS, Genta FA, Mello CB, Silva LR, Codogno TF, Oliveira AF, Genta FA, Marinho LP, Valle D, Lima JB, Feder D, Gonzalez MS, Azambuja P (2016) Triflumuron effects on the physiology and reproduction of Rhodnius prolixus adult females. Biomed Res Int:2016. https://doi.org/10.1155/2016/8603140
Ilboudo S, Edwin F, Virginie R (2014) In vitro impact of five pesticides alone or in combination on human intestinal cell line Caco-2. Toxicol Rep 1:474–489
Irigaray FJSC, Mancebón VM, Pérez-Moreno I (2003) The entomopathogenic fungus Beauveriabassiana and its compatibility with triflumuron: effects on the twospotted spider mite Tetranychusurticae. Biol Control 26:168–173
Itard J (1986) Les glossines ou mouches tsé-tsé. Etude et synthèse de l’IEMVT, 15. Département du Centre de Coopération Internationale en Recherche Agronomique pour le Développement, Paris, France. p 155
Juricek L, Coumoul X (2015) Diet, pesticides and neurological diseases. Notebooks of nutrition and dietetics 49:74–80
Kamsh M, Parween S, Reichmuth C, Akhtar N (1998) Effect of triflumuron on the development of the red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Proceedings of the 7th International Working Conference on Stored Product Protection; October 1998; Beijing, China. pp. 933–939
Kland MJ (1988) Teratogenicity of Pesticides and Other Environmental Pollutants. Studies in Environmental Science 3:315–463
Konradsen F (2007) Acute pesticide poisoning—a global public health problem. Dan Med Bull 54:58–59
Langley PA (1995) Evaluation of the chitin synthesis inhibitor triflumuron for controlling the tsetse Glossina. m. morsitans (Diptera: Glossinidae). Bull Entomol Res 85:495–500
Le Bel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2-,7-dichlorofluorescein as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Lowden S, Gray S, Dawson K (2007) Treatment of natural infestations of the biting louse (Werneckiellaequi) on horses using triflumuron, a benzoylurea derivative insect growth regulator. Vet Parasitol 148:295–300
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Marutescu L, Chifiriuc MC (2017) Molecular mechanisms of pesticides toxicity. New Pesticides and Soil Sensors 393–435.
Mello CB, Mendonça-Lopes D, Feder D, Uzeda CD, Carneiro RM, Rocha MA, Gonzalez MS (2008) Laboratory evaluation of the effects of triflumuron on the development of Rhodnius prolixus nymp. Mem Inst Oswaldo Cruz 103:839–842
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412
Mizuno D, Konoha-Mizuno K, Mori M, Yamazaki K, Haneda T, Koyama H, Kawahara M (2015) An in vitro system comprising immortalized hypothalamic neuronal cells (GT1-7 cells) for evaluation of the neuroendocrine effects of essential oils. Evid Based Complement Alternat Med. https://doi.org/10.1155/2015/343942
Mosman T (1983) Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Ning X, Wang Y, Yan W, Li G, Sang N (2018) Chitin synthesis inhibitors promote liver cancer cell metastasis via interfering with hypoxiainducible factor 1α. Chemosphere 206:231–237
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ouédraogo A (1998) Etude de l’efficacité et de la rémanence de la deltaméthrine et du triflumuron imprégné sur tissu (pour la lutte contre les glossines). Thèse de Doctorat en pharmacie (Diplôme d’état). Faculté de Médecine, de Pharmacie et d’Odonto stomatologie. Université du Mali, Bamako, p 105
Parween S, Faruki SI, Begum M (2001) Impairment of reproduction in the red flour beetle, Triboliumcastaneum (Herbst) (Col., Tenebrionidae) due to larval feeding on triflumuron-treated diet. J Appl Entomol 125:413–416
Ralph S, Petras M, Pandrangi R, Vrzoc M (1996) Alkaline single-cell gel (comet) assay and genotoxicity monitoring using two species of tadpoles. Environ Mol Mutagen 28:112–120
Salvi M, Battaglia V, Brunati AM, La Rocca N, Tibaldi E, Pietrangeli P, Marcocci L, Mondovi B, Rossi CA, Toninello A (2007) Catalase takes part in rat liver mitochondria oxidative stress defense. J Biol Chem 282:24407–24415
Singh G, Maurya S, de Lampasona MP, Catalan C (2006) Chemical constituents, antifungal and antioxidative potential of Foeniculumvulgare volatile oil and its acetone extract. Food Control 17:745–752
Smith KE, Wall R (1998) Suppression of the blowfly Luciliasericata using odour-baited triflumuron-impregnated targets. Med Vet Entomol 12:430–437
Sofowora EA (1993) Medicinal plants and traditional medicine in Africa. John Willey and Sons, Chichester, p 178
Suman DS, Yi W, Anwar L, Bilgrami RG (2013) Ovicidal activity of three insect growth regulator against Aedes and Culex mosquitoes. Acta Trop 128(1):103–109
Surapaneni KM, Venkataramana G (2007) Status of lipid peroxidation glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J Med Sci 61:9–14
Tasheva M, Hristeva V (1993) Comparative study on the effects of five benzoylphenylurea insecticides on haematological parameters in rats. J Appl Toxicol 13:67–68
Timoumi R, Amara I, Neffati F, Najjar MF, El Golli-Bennour E, Bacha H, Abid-Essefi S (2018) Acute triflumuron exposure induces oxidative stress responses in liver and kidney of Balb/C mice. Environ Sci Pollut Res
Tinoco MT, Martins MR, Cruz-Morais J (2007) Antimicrobial activity of Foenicu-lumvulgare Miller essential oil. Rev Ciências Agrárias 30:448–454
Tomsej T, Hajglov J (1995) Determination of benzoylurea insecticides in apples by high-performance liquid chromatography. J Chromatogr A 704:513–517
Tripathi P, Tripathi R, Patel RK, Pancholi SS (2013) Investigation of antimutagenic potential of Foeniculum vulgare essential oil on cyclophosphamide induced genotoxicity and oxidative stress in mice. Drug Chem Toxicol 36:35–41
Tunaz H, Uygun N (2004) Insect growth regulators for insect pest control. Turk J Agric For 28:377–387
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Vasuki V, Rajavel AR (1992) Influence of short time exposure to an insect growth regulator, hexaflumuron, on mortality and adult emergence of vector mosquitoes. Mem Inst Oswaldo Cruz 87:275–283
Villarini M, Pagiotti R, Dominici L, Fatigoni C, Vannini S, Levorato S, Moretti M (2014) Investigation of the cytotoxic, genotoxic, and apoptosis-inducing effects of estragole isolated from fennel (Foeniculum vulgare). J Nat Prod 77:773–778
Waller PJ, Lacey E (1986) The effect of triflumuron (SIR8514) on the free-living stages of sheep nematodes. Vet Parasitol 21(2):119–126
WHO (2006) Pesticides and their application: for the control of vectors and pests of public health importance. 6th. Geneva, Switzerland
Wilson TG, Cryan JR (1997) Lufenuron, a chitin-synthesis inhibitor, interrupts development of Drosophila melanogaster. J Exp Zool 278:37–44
Winkaler EU, Santos TRM, Machado-Neto JG, Martinez CBR (2007) Acute lethal and sublethal effects of neem leaf extract on the neotropical freshwater fish Prochiloduslineatus. Comp Biochem Physiol C 145:236–244
Yoshida M, Inoue K, Takahashi M (2015) Predictive modes of action of pesticides in uterine adenocarcinoma development in rats. J Toxicol Pathol 28(4):207–216
Yuan Y, Chen C, Zheng C, Wang X, Yang G, Wang Q, Zhang Z (2014) Residue of chlorpyrifos and cypermethrin in vegetables and probabilistic exposure assessment for consumers in Zhejiang Province. China Food Control 36(1):63–68
Zaim M, Guillet P (2002) Alternative insecticides: an urgent need. Trends Parasitol 18:161–163
Zhang X, Wu M, Yao H, Yang Y, Cui M, Tu Z, Stallones L, Xiang H (2016) Pesticide poisoning and neurobehavioral function among farm workers in Jiangsu, People’s Republic of China. Cortex 74:396–404
Funding
This study was supported by “Le Ministère Tunisien de l’Enseignement Supérieur et de la Recherche Scientifique.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Timoumi, R., Salem, I.B., Amara, I. et al. Protective effects of fennel essential oil against oxidative stress and genotoxicity induced by the insecticide triflumuron in human colon carcinoma cells. Environ Sci Pollut Res 27, 7957–7966 (2020). https://doi.org/10.1007/s11356-019-07395-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07395-x