Abstract
In this study, the protective effects of 50 mg/L and 100 mg/L doses of anthocyanin-rich bilberry extracts (ABE) against the toxicity caused by 20 μM copper(II) chloride (CuCl2) on Allium cepa L. were investigated. Alterations in weight gain, germination percentage, and root elongation were evaluated as physiological parameters while micronucleus (MN), mitotic index (MI), and chromosomal abnormality (CA) frequency were studied as cytogenetic parameters. Oxidative stress indicators such as malondialdehyde (MDA) formation, superoxide dismutase (SOD) activity, and catalase (CAT) activity were analyzed and also damages in root tip meristem cells were determined by cross sections. As a result, it was found that the percentage of germination, weight gain, root length, and MI decreased and the frequency of MN and CAs increased with CuCl2 treatment. CuCl2 exposure caused a significant increase in SOD and CAT activities and MDA levels. A number of anatomical abnormalities and damages were detected in the cross sections of CuCl2-treated roots. On the other hand, ABE applications ameliorated notably all copper-induced damages in a dose-dependent manner. Therefore, the powerful protective potential of ABE against copper-induced toxicity was proven through an extensive study in a popular plant model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) and Cu compounds are widely used in industrial processes, agriculture, and daily life. As a result of its widespread usage in different areas, Cu can be present in high concentrations in the natural environment as well as in human living environments. As a heavy metal, Cu is a persistent environmental pollutant because it cannot be degraded or decomposed (Duruibe et al. 2007). It can be released into the environment by both natural events and anthropogenic activities such as mining, industrial and domestic wastes, agricultural practices, and use of pesticides and antifouling paints (Liu et al. 2009). As a cofactor, Cu is essential for structural and catalytic functions of various enzymes within the biological processes required for growth, development, maintenance, and electron transport systems (Gaetke and Chow 2003; Jomova and Valko 2011). Although it is irreplaceable for many metabolic processes in prokaryotes and eukaryotes, it becomes toxic at high concentrations (Flemming and Trevors 1989). Excessive amounts of CuCl2 may cause cytotoxicity and genotoxicity because it induces growth deceleration and inhibition, cell cycle disorders, oxidative stress, and degradation effects in many organisms, including plants (Franscescon et al. 2018).
The negative and toxic effects of the chemicals that we are frequently exposed to in daily life can be reduced by the use of many natural and antioxidant compounds in the daily diet. Bilberry (Vaccinium myrtillus L.) is a berry of a shrubby plant which is grown in Europe and North America. Medicinal use of bilberry for many different illnesses dates back to early Renaissance (Valentova et al. 2007). Bilberry fruit is one of the richest sources of dietary anthocyanins, a group of water-soluble pigments, with antioxidant, anticancer, and anti-inflammatory properties (Takikawa et al. 2010; Seeram 2008). Nowadays, anthocyanin-rich bilberry extracts (ABE) of different brands can be found in the market for therapeutic purposes. There are a number of studies that focused on the health effects of bilberry and bilberry extracts (Valentova et al. 2007; Karlsen et al. 2010; Takikawa et al. 2010). Previous researchers studied antioxidative and cytoprotective roles of ABE against oxidative damage in several in vitro models (Martin-Aragon et al. 1998; Valentova et al. 2007). However, their protective effects against heavy metal–induced toxicity and genotoxicity have not been understood extensively.
Since cytogenotoxicity studies on experimental animals are expensive and time consuming, alternatives, including plant root experiments, have gained interest (Samuel et al. 2010). Onion (Allium cepa L.) bioassay is considered as an easy and reliable test model for assessing the genotoxicity mainly due to its fast growing roots, high percent of dividing cells, large sized monocentric chromosomes (2n = 16), and responsive genetic material to pollutants (Vesna et al. 1996; Fiskesjo 1997). Therefore, Allium has become a very common biological test material for cytogenetic researches since 1938 (Levan 1938). The validity of A. cepa assay has been well documented through the studies on a number of environmental contaminants such as heavy metals (Seth et al. 2008; Leme and Marin-Morales 2009; Yıldız et al. 2009; Çavuşoğlu et al. 2011; Yalçın et al. 2019). Furthermore, international organizations such as the United Nations Environment Programme (UNEP) and the International Programme on Chemical Safety (IPCS) accepted A. cepa assay as a well-established and functional test method for in situ monitoring of pollutants (Mangalampalli et al. 2018).
In the present study, the toxic effects of CuCl2 and the protective effects of ABE against this toxicity were evaluated in physiological, cytogenetic, biochemical, and anatomical aspects using A. cepa assay in vivo. In order to assess physiological effects, germination percentage, root elongation, and weight gain of bulbs were determined. In addition to that, mitotic index (MI), micronucleus (MN) frequency, and chromosomal abnormalities were utilized to investigate the cytogenetic effects of CuCl2 and ABE on root tips. Malondialdehyde (MDA) levels and the activities of SOD and CAT enzymes were used to screen the oxidative stress on Allium bulbs while the anatomical changes in CuCl2-treated bulbs were observed by microscopic examination of root tip cross sections.
Materials and methods
Materials
Copper(II) chloride dihydrate (CuCl2·2H2O) (Merck) was used for preparation of Cu solutions. A. cepa bulbs and ABE with 25% anthocyanin content (Nexgen Pharma Inc., CA, USA) were obtained commercially from local markets.
Experimental design and treatments
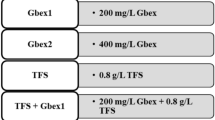
Healthy and equally sized A. cepa bulbs were chosen for this study. The bulbs were divided into six groups and each group consisted of 25 bulbs. After the bulbs were placed in sterilized glass beakers, the groups were treated with the following solutions for 72 h at room temperature in the dark (Table 1).
Determination of germination percentage, root elongation, and weight gain
The germination percentage of the bulbs was calculated using the equation (Eq. 1) given by Atik et al. (2007).
Root elongations were measured with a ruler after germination. Differences between the weight of bulbs before and after 72 h of treatments were accepted as weight gain.
Micronucleus assay and chromosomal aberrations
After 72 h of treatment, A. cepa roots were thoroughly washed with distilled water. Root tips of onions were cut with a razor nearly 0.5 cm long and fixed with Clarke’s solution (3: ethyl alcohol/1: glacial acetic acid) for 2 h. Then, they were washed for 15 min in 96% ethanol before hydrolyzed in 1 N HCl at a temperature of 60 °C for 11 min. Hydrolyzed root tips were stained with 1% acetocarmine solution for 24 h. The root tips were crushed in 45% acetic acid and photographed with a binocular research microscope (Staykova et al. 2005). The same slide was used for calculations of the MN and the MI and for determination of chromosomal aberrations. The total cell number for MN counting was identified according to the criteria set by Fenech et al. (2003). The MI was accepted as the number of cells in mitosis per 1000 observed cells and expressed as percentage.
Anatomical alterations
In order to determine the anatomical alterations, cross sections of the root tips of A. cepa bulbs were taken. Slides were stained using 1% methylene blue and photographed at × 500 magnification with a binocular research microscope.
MDA analysis
At the end of the treatments, MDA contents of A. cepa roots were evaluated with the method suggested by Unyayar et al. (2006). 0.5 g of root material was homogenized by means of an ULTRA-TURRAX homogenizer in 5% trichloroacetic acid (TCA). Collected homogenates were centrifuged at 12,000 rpm for 15 min at room temperature. Equal amounts of 20% TCA–0.5% thiobarbituric acid (TBA) solution and supernatant were mixed in a glass tube. Glass tubes with reaction mixture were boiled at 98 °C for 25 min. After 25 min, the tubes were placed in an ice bath to interrupt the reactions. Chilled mixtures were centrifuged at 10,000 rpm for 5 min. The absorbance of mixtures was measured spectrophotometrically at 532 nm and 600 nm.
SOD and CAT analyses
0.2 g of fresh onion roots was weighted and homogenized with 5 mL of cooled sodium phosphate buffer (50 mM, pH 7.8) for determination of SOD and CAT activities. Homogenates of roots were centrifuged at 10,000 rpm at 4 °C for 20 min. The supernatants were used for SOD and CAT enzyme activity analyses.
The method mentioned by Beauchamp and Fridovich (1971) was used for determination of SOD activity. SOD activity analysis results were presented as units per milligram fresh weight (U/mg FW). The method mentioned by Beers and Sizer (1952) was used for determination of CAT activity. CAT activity was expressed as OD240nmmin/g FW.
Statistical analysis
The statistical analysis of data was performed with SPSS software (SPSS Inc., Chicago, IL, USA). Results were analyzed using Duncan’s test and analysis of variance (ANOVA) test. The data were presented as means and standard deviation (SD), and the statistical significance of difference was taken as less than 0.05.
Results and discussion
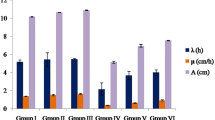
The effects of CuCl2 and ABE treatments on physiological parameters of A. cepa are presented in Table 2 and Fig. 1. It was clearly seen that CuCl2 negatively changed the physiological parameters and caused a decrease in weight gain, root elongation, and germination percentage. The most remarkable toxic effect was observed in the weight gain of the 20 μM CuCl2-treated group, which gained only 1.26 g of total weight at the end of the experimental period.
It was determined that CuCl2 treatment led to statistically marked reductions in both root elongation and germination percentage (p < 0.05). The shortest roots were measured as 5.46 ± 1.60 cm in group IV while the maximum root elongation was determined in the control group as 15.51 ± 2.74 cm. Also, germination percentage decreased upon CuCl2 treatment and the lowest germination was obtained as 48% in group IV.
Doğanlar and Atmaca (2011) reported that uptake and accumulation of heavy metals led to a morphological and a physiological retardation in plants. According to previous studies, negative effect of Cu on root growth is due to its inhibitory function on cell division (Kahle 1993; Doncheva et al. 1996; Posmyk et al. 2009). Furthermore, Geremias et al. (2010) and Yıldız et al. (2009) mentioned a reduction in root elongation in copper-exposed onion bulbs similar to our study. Our results in group V and group VI clearly demonstrated that when ABE are applied together with CuCl2 solution, they ameliorate the negative effects of CuCl2 on growth parameters.
ABE can contain very high anthocyanin amounts along with other phenols, and the healing power of the extract may be associated with its high anthocyanin content (Valentova et al. 2007). Indeed, anthocyanins, as strong antioxidants, are crucial members of defense systems against stresses in different parts of plants including fruits.
The mitotic index is considered as an important parameter since it reflects the frequency of cell division (Liu et al. 2009). MI values of group I, group II, and group III were found as 888.00 ± 19.59, 900.10 ± 20.67, and 910.50 ± 19.98, respectively, without statistical difference (Table 3). MI frequency of CuCl2-treated group IV (518.00 ± 31.32) decreased drastically while the values of group V (582.20 ± 33.74) and group VI (650.70 ± 27.69) improved progressively as a function of increased ABE concentrations. It has already been reported by other researchers that copper and copper salts significantly suppress mitosis and reduce mitotic index of A. cepa (İnceer and Beyazoğlu 2000; Srivastava et al. 2005; Yıldız et al. 2009; Liu et al. 2009). Inhibition of the mitosis in meristem cells of CuCl2-treated A. cepa roots clarified the decrease in root elongation and the weight gain.
Group I, control group; group II, 50 mg/L ABE; group III, 100 mg/L ABE; group IV, 20 μM CuCl2; group V, 50 mg/L ABE+20 μM CuCl2; group VI, 100 mg/L ABE+20 μM CuCl2. 1000 cells were counted for MN frequency. 10,000 cells were counted for MI analysis. The mean values indicated by different superscript letters (a–d) in the same column are significant at p < 0.05
The roots are the first and the most sensitive part of the plants that absorb Cu from soil. Rapid proliferation of meristematic tissues, as apical root tips, offers a highly sensitive way for monitoring the genotoxicity of chemical agents. The MN frequency test and the chromosomal aberration analysis in Allium root meristems have been widely used to detect DNA damage associated with hazardous contaminants (Gupta et al. 2018; Bolsunovsky et al. 2019). The absence of MN and chromosomal aberration in group I, group II, and group III is an undeniable proof that ABE are not genotoxic (Table 3 and Table 4). As expected, the members of CuCl2-treated group IV exhibited a sharp rise in MN abundance, while the increasing doses of ABE in CuCl2 solutions depressed the MN formation gradually in group V and group VI. According to Silveira et al. (2017), the rise in MN and chromosomal aberrations are markers of chromosomal instabilities in cells. Here we proved that CuCl2 triggered a number of chromosomal aberrations in Allium roots, including fragment, sticky chromosome, vagrant chromosome, bridge, unequal chromatin distribution, and abnormal polarization (Fig. 2 and Table 4). These abnormalities, together with an increased MN incidence, are strong evidence for clastogenic and aneugenic damage caused by CuCl2. The most observed chromosomal aberrations were fragment (79.10 ± 9.01) and sticky chromosome (71.00 ± 8.51) formation in group IV. Similar to our results, Gupta et al. (2018) reported that fragment and sticky chromosome were among the most abundant chromosomal aberration types induced by heavy metal exposure in the Allium root meristem. Moreover, sticky chromosomes are accepted as critical signs for cell death risk (Gupta et al. 2018). For all chromosomal damage types, incidence decreased significantly with enhanced ABE doses as seen in group V and group VI. Owing to its mobility and redox potential, Cu has a central function in the generation of active oxygen radicals that attack DNA molecules rapidly and lead to breaks in DNA strands. These breaks are accompanied with modifications of the bases and deoxyriboses of the nucleic acids which then induces severe carcinogenesis in cells (Theophanides and Anastassopoulou 2002). Genoprotective and anticancer effects of bilberry fruits are powerful but very complex. Smeriglio et al. (2014) summarized that these effects are mainly results from anthocyanins that scavenge oxidants, induce the antioxidant response element and phase II detoxification enzymes, and interact directly forming a DNA copigmentation complex. Indeed, our results of MN and chromosomal abnormalities verify the protective role of ABE on genetic material.
Cross sections of CuCl2-treated A. cepa roots were photographed with a research microscope (Fig. 3). Thickened cortex cell wall (Fig. 3a), flattened cell nucleus (Fig. 3b), and cell deformations (Fig. 3c) were the anatomical disorders observed in root meristematic tissues. Thickening of the cortex and squeezed epidermal cell deformations were probably the adaptations by the plant to limit the absorption of excess CuCl2 from the growth medium. Yalçın et al. (2019) stated that plants developed some anatomical adaptations to prevent the transfer of an external toxic agent to inner tissues. On the other hand, flattened cell nucleus may be an irreversible damage caused by toxic CuCl2 doses that plants were unable to overcome.
As a product of membrane lipid peroxidation, MDA content increases when plants experience oxidative stress (Meng et al. 2007). While the MDA contents of Allium roots in group I, group II, and group III were statistically identical, the MDA content in CuCl2-treated group IV was significantly increased compared with the control group (group I) (Fig. 4). On the other hand, as the doses of ABE increased, MDA concentrations of group V and group VI gradually decreased. Our results in MDA levels showed that CuCl2 application causes oxidative stress in Allium plants. Previous studies on copper toxicity showed that this heavy metal resulted in an increase in MDA levels of onion and garlic root membranes (Meng et al. 2007; Vargas et al. 2017). Meanwhile, ABE application remediated oxidative stress originated from CuCl2-oriented. Aly et al. (2019) suggested that various berries had a great level of metal-chelating and radical scavenging potential to prevent oxidative damages. The ability of bilberry fruits to suppress the membrane-destructive oxidative stress is mainly due to their superior phenolic and anthocyanin contents (Sellappan et al. 2002).
Results of SOD and CAT levels on Allium roots were highly correlated with each other (Figs. 5 and 6). SOD and CAT contents of group II and group III were statistically equal to group I. Levels of both enzymes peaked sharply in group IV and reduced with increasing ABE concentrations in group V and group VI. CuCl2, as a crucial cofactor of various metalloproteins including SOD enzyme, plays an important role in radical scavenging in cells. However, in elevated doses, it induces oxidative stress and triggers a series of protective responses in plants (Qin et al. 2016). Enzymatic antioxidant system including the induced activity of SOD and CAT is a vital process in order to scavenge free radicals such as superoxide (O2·−) and hydrogen peroxide (H2O2).
Qin et al. (2016) indicated that the antioxidant enzyme acted as natural guards in ROS defense at low-dosed CuCl2 stress in A. cepa roots. Coordinated function of ABE in Allium roots with regulated enzyme activities reduced the oxidative stress to some extent. Yalçın et al. (2019) stated that antioxidant enzyme activities increased in the case of oxidative stress. In our study, elevated levels of these oxidative stress indicators were in accordance with the frequency of CA which might be among the results of oxidative stress.
Conclusion
In conclusion, restorative and protective abilities of ABE against CuCl2-induced toxicity were investigated comprehensively by using A. cepa bioassay. Although the deleterious effects of CuCl2 stress on organisms have already been known, this is the first study which demonstrated the therapeutic and preventive potential of ABE against such a hazardous and common pollutant in onion roots. Anatomical cross sections of roots clearly exhibited the “struggle” of the plant to block the entrance of CuCl2 to inner tissues. In any case, the data obtained from the other parameters of CuCl2-treated groups proved that the plant could not prevent the intake of the chemical completely. On the other hand, ABE acted as a vital protector to overcome the physiological and genotoxic harms of CuCl2. Indeed, the restorative effects of ABE increased gradually with its increasing doses. Therefore, Allium assay was shown once again to be a reliable method for not only screening the carcinogenic roles of the pollutants but also monitoring the protective potentials of antioxidant agents. Additionally, the functional mechanism of ABE, a popular supplement with a range of health benefits, was revealed efficiently. Our findings provided a reliable basis for further studies that focus on the use of ABE for the prevention of cancer and heavy metal–associated diseases. These kinds of studies on ABE will gain value as the heavy metal pollution increases.
References
Aly A, Maraei R, El-Leel OA (2019) Comparative study of some bioactive compounds and their antioxidant activity of some berry types. Potr S J F Sci 13(1):515–523
Atik M, Karagüzel O, Ersoy S (2007) Sıcaklığın Dalbergia sissoo tohumlarının çimlenme özelliklerine etkisi. Mediterr Agric Sci 20(2):203–210
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bolsunovsky A, Dementyev D, Trofimova E, Iniatkina E, Kladko Y, Petrichenkov M (2019) Chromosomal aberrations and micronuclei induced in onion (Allium cepa) by gamma-radiation. J Environ Radioact 207:1–6
Çavuşoğlu K, Yalçın E, Türkmen Z, Yapar K, Çavuşoğlu K, Çiçek F (2011) Investigation of toxic effects of the glyphosate on Allium cepa. JAS 17:131–142
Doğanlar Z, Atmaca M (2011) Influence of airborne pollution on Cd, Zn, Pb, Cu, and Al accumulation and physiological parameters of plant leaves in Antakya (Turkey). Water Air Soil Pollut 214(1/4):509–523
Doncheva S, Nikolov B, Ogneva V (1996) Effect of copper excess on the morphology of the nucleus in maize root meristem cells. Physiol Plant 96(1):118–122
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN Project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65–75
Fiskesjo G (1997) Allium test for screening chemicals: evaluation of cytological parameters. In: Wang W, Gorsuch JW, Hughes JS (eds) Plants for environmental studies. Lewis Publishers, New York, pp 307–333
Flemming CA, Trevors JT (1989) Copper toxicity and chemistry in the environment: a review. Water Air Soil Pollut 44(1-2):143–158
Franscescon F, Mazon SC, Bertoncello KT, Boligon AA, Sachett A, Rambo CL, Rosemberg DB, Magro JD, Siebel AM (2018) Protective role of jaboticaba Plinia peruviana peel extract in copper-induced cytotoxicity in Allium cepa. Environ Sci Pollut Res 25(35):35322–35329
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicol 189(1-2):147–163
Geremias R, Fattorini D, Fávere VT, Pedrosa RC (2010) Bioaccumulation and toxic effects of copper in common onion Allium cepa L. Chem Ecol 26(1):19–26
Gupta K, Mishra K, Srivastava S, Kumar A (2018) Cytotoxic assessment of chromium and arsenic using chromosomal behavior of root meristem in Allium cepa L. Bull Environ Contam Toxicol 100(6):803–808
İnceer H, Beyazoğlu O (2000) Bakır klorür’ün Vicia hirsuta (L.) SF Gray kök ucu hücreleri üzerine sitogenetik etkileri. Turk J Biol 24:553–559
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicol 283(2-3):65–87
Kahle H (1993) Response of roots of trees to heavy metals. Environ Exp Bot 33(1):99–119
Karlsen A, Paur I, Bøhn SK, Sakhi AK, Borge GI, Serafini M, Erlund I, Laake P, Tonstad S, Blomhoff R (2010) Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr 49(6):345–355
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res Rev Mutat Res 682(1):71–81
Levan A (1938) The effect of colchicine on root mitoses in Allium. Hereditas 24:471–486
Liu D, Jiang W, Meng Q, Zou J, Gu J, Zeng M (2009) Cytogenetical and ultrastructural effects of copper on root meristem cells of Allium sativum L. Biocell 33(1):25–32
Mangalampalli B, Dumala N, Grover P (2018) Allium cepa root tip assay in assessment of toxicity of magnesium oxide nanoparticles and microparticles. J Environ Sci 66:125–137
Martin-Aragon S, Basabe B, Benedi JM, Villar AM (1998) Antioxidant action of Vaccinium myrtillus L. Phytother Res 12:104–106
Meng Q, Zou J, Zou JH, Jiang WS, Liu DH (2007) Effect of Cu2+ concentration on growth, antioxidant enzyme activity and malondialdehyde content in garlic (Allium sativum L). Acta Biol Cracov Ser Bot 49(1):95–101
Posmyk MM, Kontek R, Janas KM (2009) Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol Environ Saf 72(2):596–602
Qin R, Ning C, Björn LO, Li S (2016) Proteomic analysis of Allium cepa var. agrogarum L. roots under copper stress. Plant Soil 401(1-2):197–212
Samuel OB, Osuala FI, Odeigah PG (2010) Cytogenotoxicity evaluation of two industrial effluents using Allium cepa assay. Afr J Environ Sci Technol 4(1):21–27
Seeram NP (2008) Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem 56(3):627–629
Sellappan S, Akoh CC, Krewer G (2002) Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J Agric Food Chem 50(8):2432–2438
Seth CS, Misra V, Chauhan LKS, Singh RR (2008) Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and Comet assay approach. Ecotoxicol Environ Saf 71(3):711–716
Silveira GL, Lima MGF, dos Reis GB, Palmieri MJ, Andrade-Vieria LF (2017) Toxic effects of environmental pollutants: comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178:359–367
Smeriglio A, Monteleone D, Trombetta D (2014) Health effects of Vaccinium myrtillus L.: evaluation of efficacy and technological strategies for preservation of active ingredients. Mini-Rev Med Chem 14(7):567–584
Srivastava R, Kumar D, Gupta SK (2005) Bioremediation of municipal sludge by vermitechnology and toxicity assessment by Allium cepa. Bioresour Technol 96:1867–1871
Staykova TA, Ivanova EN, Velcheva IG (2005) Cytogenetic effect of heavy metal and cyanide in contamined waters from the region of southwest Bulgaria. J Cell Mol Biol 4:41–46
Takikawa M, Inoue S, Horio F, Tsuda T (2010) Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr 140(3):527–533
Theophanides T, Anastassopoulou J (2002) Copper and carcinogenesis. Crit Rev Oncol Hematol 42(1):57–64
Unyayar S, Celik A, Cekic FO, Gozel A (2006) Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis 21:77–81
Valentova K, Ulrichova J, Cvak L, Simanek V (2007) Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem 101:912–917
Vargas JT, Rodríguez-Monroy M, Meyer ML, Montes-Belmont R, Sepúlveda-Jiménez G (2017) Trichoderma asperellum ameliorates phytotoxic effects of copper in onion (Allium cepa L.). Environ Exp Bot 136:85–93
Vesna S, Stegnar P, Lovka M, Toman MJ (1996) The evaluation of waste, surface and ground water quality using the Allium test procedure. Mutat Res 368:171–179
Yalçın E, Uzun A, Çavuşoğlu K (2019) In vivo epiclorohidrine toxicity: cytogenetic, biochemical, physiological, and anatomical evidences. Environ Sci Pollut Res 26:22400–22406
Yıldız M, Ciğerci İH, Konuk M, Fidan AF, Terzi H (2009) Determination of genotoxic effects of copper sulphate and cobalt chloride in Allium cepa root cells by chromosome aberration and comet assays. Chemosphere 75(7):934–938
Funding
The present study was supported financially by the Giresun University Scientific Research Unit (project no. FEN-BAP-A-150219-21).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Macar, O., Kalefetoğlu Macar, T., Çavuşoğlu, K. et al. Protective effects of anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract against copper(II) chloride toxicity. Environ Sci Pollut Res 27, 1428–1435 (2020). https://doi.org/10.1007/s11356-019-06781-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06781-9