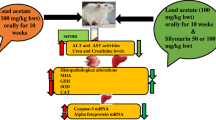
Abstract
The present study evaluated the possible ameliorative efficacy of selenium nanoparticles (SeNPs) on AlCl3-induced hepatorenal injury in rats. Animals were randomly divided into four groups (n = 6): group 1, the control; group 2, received SeNPs (0.4 mg/kg b.wt) for 21 days; group 3, injected with three doses of AlCl3 intraperitoneally (30 mg/kg/body weight) every 5 days; group 4, received SeNPs for 7 days prior to AlCl3 and then received SeNPs concurrently with AlCl3 for the following 14 days. It was observed that AlCl3 increased the levels of AST, ALT, ALP, LDH, total bilirubin, creatinine, urea, uric acid, and MDA significantly; as well as the reduction in the levels of GSH, SOD, GPx stores in comparison with the control group. These biochemical alterations were accompanied and confirmed by the lesion appeared in histological sections in addition to the increase in the expression of caspase-3 and the decrease of the Bcl-2expression. Treatment with SeNPs ameliorates the hepatorenal dysfunction, replenishes the endogenous antioxidant system, downregulates the expression of caspase-3, and upregulates the expression of Bcl-2. This hepatorenal ameliorative role may be due to the ability of SeNPs to equilibrate the oxidant/antioxidant system besides its ability to attenuate apoptosis process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum is the third abundant metallic element on earth; it constitutes about 8% of the total mineral components of the earth’s crust. Recently, increased attention to the biotoxicity of aluminum is considered due to its availability (Al Eisa and Al Nahari 2016). Aluminum is used as food additive, aluminum pots for cooking that deliberate about 20% of it, drinking water with a concentration of 0.2 mg/L, water-purifying agent, can bottles, aluminum foil paper, and in cosmetics as antiperspirant (AFSSAPS 2011). Therefore, human beings are highly susceptible to aluminum toxicity as it may accumulate in the liver and kidney causing hepatorenal toxicity due to its high availability (Tahari et al. 2016). Also, aluminum may deregulate reactive oxygen species (ROS) and apoptosis via stimulation of the pro-oxidant properties of iron and copper resulting in mitochondrial dysfunction and subsequent oxidative deterioration of macromolecules and releasing of cytochrome C from the mitochondria (Al-Olayan et al. 2015; Zahedi-Amiri et al. 2019). Therefore, neutralization and scavenging of free radicals may consider a candidate strategy to get rid of aluminum toxicity. Selenium (Se) is a vital micronutrient that plays a critical role in human health; it has an anti-carcinogenic, anti-aging, antioxidant, and anti-muscular dystrophy role besides its presence in oxido-reductase selenoenzymes (Shalby et al. 2017; Dawood et al. 2019; Khurana et al. 2019). Sodium selenite is the most abundant form of selenium supplement, but its toxicity limits its usage (Urbankova et al. 2018). Further, elemental selenium in the redox state of zero is not soluble and generally considered to be biologically inert (Bunglavan et al. 2014); thereby, there is an urgent demand to improve selenium efficacy and minimize its toxicity. Selenium nanoparticles (SeNPs), especially the red color form, have distinctive biological activities than inorganic selenium compounds and selenomethionine. It is more stable, soluble, and has higher surface activity, subsequently has high bio-catalytic and antioxidant activities in addition to its ability to suppress toxicity (Al-Quraishy et al. 2015; Shalby et al. 2017; Urbankova et al. 2018). Despite the above-mentioned characteristics of SeNPs, its effect on hepatotoxicity, nephrotoxicity, apoptosis, and oxidative insult induced by AlCl3 has not been investigated. Therefore, the present investigation evaluates the potential ameliorative role of SeNPs on AlCl3-induced hepatorenal injury in a rat model.
Materials and methods
Preparation of selenium nanoparticles (SeNPs)
Red SeNPs were prepared according to Bai et al. (2017) with some modification. Briefly, sodium selenite (Na2SeO3) was reduced with ascorbic acid. Briefly, sodium selenite (100 mM) and ascorbic acid (50 mM) were prepared. The ascorbic acid was added drop-wisely to Na2SeO3 under magnetic stirring (1000 rpm) at room temperature to form light orange color, then left to cool with stirring for 30 min. The obtained solution containing SeNPs was lyophilized and stored at room temperature (Zhang et al. 2004).
Characterization of selenium nanoparticles (SeNPs)
The crystal phase structure of prepared SeNPs was characterized using X-ray diffraction (XRD) with Cu-Ka radiation of wavelength 1.54060 Å in the range of 2θ° = 10–80. The size and morphology of the SeNPs were analyzed by high-resolution transmission electron microscopy (HR-TEM) (A JEOL-2100). TEM sample was prepared by diluting and dispersing the powder SeNPs with de-ionized water and a drop of the suspension was applied onto carbon film on copper grids (Figs. 1 and 2).
Animals
Twenty-four male albino rats (Rattus norvegicus) weighing 170 ± 20 g were obtained from breeding animal house preservation at King Khalid University, Saudi Arabia. Each 6 animals were housed in one cage and fed ad libitum in a 12 h light/12 h dark cycle at 23 ± 2 °C room temperature. The experimental protocols using the animals were conducted in accordance with the regulatory laws regarding experimental ethics of animal use and collecting permits, Institute of Animal Care and Use Committee, King Khalid University, KSA.
Experimental design and treatment
After 7 days of accommodation period, male rats were divided into 4 groups each contains 6 rats as follows:
Group 1 (control group): rats fed normal diet and water for 21 days.
Group 2 (SeNPs group): rats received 0.4 mg SeNPs/kg b.wt, p.o for 21 days (Rezaei-Kelishadi et al. 2017).
Group 3 (aluminum chloride (AlCl3) group): rats were injected three doses of AlCl3 (30 mg/kg/body weight) every 5 days intraperitoneally, followed by distilled water to complete 21 days (Tahari et al. 2016).
Group 4: (AlCl3 + SeNPs group): rats received SeNPs for 7 days prior to AlCl3 and then received SeNPs concurrently with AlCl3 for another 14 days.
Sample collection
After 24 h of last dosing, rats were euthanized after fasting state. The blood samples were collected and allowed to coagulate at room temperature and centrifuged at 3000 rpm for 15 min. The obtained sera were stored at − 20 °C for hepatic and renal function analyses. Further, the liver and kidneys were immediately removed, placed in ice-cold isotonic saline, and dissected into 2 parts. One part is fixed in neutral buffered formalin for histological examination and immunohistochemistry, and the other part was minced into small pieces then homogenized with 10% 0.1 M Tris-HCl buffer (pH 7.4) and centrifuged, and the supernatant was separated and stored at − 80 °C for the estimation of oxidative/antioxidative markers.
Determination of liver and kidney functions
Liver and kidney function tests were determined in serum, including aspartate aminotransferase (AST), alanine aminotransferase (ALT) (Reitman and Frankel 1957), alkaline phosphatase (ALP) (Belfield and Goldberg 1971), albumin (Doumas et al. 1971), lactate dehydrogenase (LDH) (Asha and Radha 1985), total bilirubin (Walter and Gerade 1970); creatinine (Bartels et al. 1972), urea (Fawcett and Scott 1960), and uric acid (Barham and Trinder 1972). All parameters were estimated spectroscopically as the manufacturer’s instruction of Biodiagnostic, Spectrum and Diamond companies.
Determination of oxidative/antioxidative markers
The oxidant/antioxidant status was evaluated in liver and kidney tissue homogenates through determination of malondialdehyde, MDA (Ohkawa et al. 1979); reduced glutathione, GSH (Beutler et al. 1963); superoxide dismutase, SOD (Nishikimi et al. 1972); and glutathione peroxidase, GPx (Paglia and Valentine 1967).
Histological examination
Small pieces of the liver and kidneys from each animal in the control and treated groups were fixed in neutral buffered formalin for 24 h. After routine processing, tissue blocks were sectioned into 4-μm slices, stained with hematoxylin and eosin for histological examination with light microscopy.
Immunohistochemical analyses of apoptotic markers in hepatic tissue
Immunohistochemical analyses for Bcl-2 and caspase-3 were carried out according to conventional methods and compared among the different experimental groups. Liver sections (4 μm) were deparaffinized and then boiled in declare to expose antigen sites. Sections were incubated overnight at 4 °C with a 1:200 dilution of each of anti-Bcl-2 and anti-caspase 3 antibodies in phosphate-buffered saline (PBS). Following removal of the primary antibodies and repetitive rinsing with PBS, slides were incubated with a 1:500 dilution of biotinylated secondary antibody. Bound antibodies were detected with avidin-biotinylated peroxidase complex. After appropriate washing in PBS, slides were counterstained with hematoxylin. All sections were incubated under the same conditions with the same concentration of antibodies and at the same time, so the immunostaining was comparable among the different experimental groups.
Statistical data analysis
The SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA), was used for the data analyses to compare between different groups and assess the efficacy of CP against AlCl3 hepatorenal toxicity. Statistical analyses were performed by analysis of means between all groups using one-way ANOVA followed by Tukey post hoc test. Results were expressed as mean ± SEM and dissimilar superscript letters of the same column of tables or even histograms symbolize a significant difference at P < 0.05.
Results
Characterization of selenium nanoparticles (SeNPs)
The XRD pattern of the prepared SeNPs matches very well with that of the standard selenium powder confirming the formation of selenium nanoparticles. The present data has few sharp peaks at 2θ° = 23, 29, 41, and 43. The well-seen peaks could be assigned to the crystalline planes 100, 101, 110, and 102, the trigonal phase of selenium nanoparticles. The lattice constants were a = 4.363 A and c = 4.952 A which were in agreement with the literature value (JCPDS File No. 06-0362). Further, the TEM image indicates that the SeNPs were in nanoscales.
Selenium nanoparticles alleviate aluminum chloride hepatic toxicity
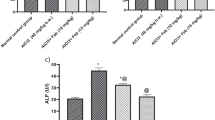
As shown in Table 1, it was observed that AlCl3 induced significant increases (P < 0.05) in the levels of AST, ALT, ALP, LDH, and bilirubin and non-significant change in albumin concentration in comparison with the data obtained for the control group. However, it was observed that AlCl3 rats treated with SeNPs showed significant hepatic markers modulation (P < 0.05) in contrast to the untreated AlCl3 group. Notably, there were significant changes (P < 0.05) between SeNPs and the control groups regarding AST and ALP only.
Selenium nanoparticles attenuate aluminum chloride renal toxicity
No marked change in the values of the creatinine and urea levels between the SeNPs and control rat groups (Table 2). On the other hand, creatinine, urea, and uric acid concentrations increased significantly (P < 0.05) in the AlCl3-injected group compared with the control group. However, the AlCl3 group treated with SeNPs exhibited an ameliorative effect by significant decrease (P < 0.05) in the levels of creatinine, urea, and uric acid in comparison with the untreated AlCl3 group.
Selenium nanoparticles inhibit oxidative damage of aluminum chloride
It was observed that AlCl3 evoked hepatic and renal oxidative insult in the experimental rat groups. This was evidenced by a significant elevation (P < 0.05) in the MDA content accompanied by a significant reduction (P < 0.05) in the contents of GSH, SOD, and GPx compared with the control group. Conversely, a significant reduction (P < 0.05) was recorded in the MDA level in addition to a significant elevation (P < 0.05) in GSH and SOD contents in hepatic and renal tissue homogenates of the AlCl3 + SeNPs group in comparison with the AlCl3-injected group. Also, treatment of AlCl3 with SeNPs showed the ability of SeNPs to increase hepatic and renal GPx, which was significant (P < 0.05) only in liver tissue when compared with AlCl3-injected rats (Fig. 3).
Effect of selenium nanoparticles on aluminum chloride-induced hepatic and renal oxidative insult in rats. Each value represents mean of six rats ± SEM. Similar letters of the same column are not significantly different and dissimilar letters of the same column were significantly different (P < 0.05). AlCl3, aluminum chloride; SeNPs, selenium nanoparticles
Histological and immunohistochemistry observations
In the present study, it was observed that control as well as SeNPs-injected rats showed normal appearance of liver tissue (Fig. 4A and B). While, AlCl3-injected rats showed a marked loss in the hepatocytes cord missed its normal arrangement, degenerated hepatocytes and severe necrosis (Fig. 4C). The following SeNPs treatment showed more or less restoration of these alterations to normal liver tissue (Fig. 4D). Immunostaining showed that the control and SeNPs rat groups expressed normal staining of caspase-3 and Bcl-2 (Fig. 5A and B). Conversely, AlCl3 showed enhancement of apoptosis as it overexpress caspase-3 apoptotic protein and low expression of Bcl-2 (Fig. 5C). Also, SeNPs treatment ameliorated the AlCl3-induced hepatotoxicity manifested by downregulation of caspase-3 staining with upregulation of anti-apoptotic Bcl-2 (Fig. 5D).
Photomicrographs of the liver tissue of the four rat groups stained by hematoxylin and eosin. (A) The control group showed normal hepatic architecture; (B) the selenium nanoparticle group (SeNPs) showed normal appearance of liver tissue; (C) the aluminum chloride group (AlCl3) showed degenerated hepatocytes (DH), necroticarea (*); (D) the aluminum chloride-selenium nanoparticle treated group (AlCl3 + SeNPs) displayed more or less restoration of liver tissue to normal architecture. DH, degenerated hepatocyte; H, hepatocytes; K, Kupffer cell; S, sinusoids. × 40
Immunohistochemistry staining of liver of different groups showing Caspase-3 and Bcl-2 expression. (A) The control group showed no apoptosis; (B) the selenium nanoparticle group (SeNPs) showed normal staining of liver tissue; (C) the aluminum chloride group (AlCl3) showed severe apoptosis indicated by more caspase-3 positive hepatocyte and few Bcl2-positive hepatocytes; (D) the aluminum chloride-selenium nanoparticle treated group (AlCl3 + SeNPs) displayed very less caspase-3 positive hepatocyte and high number of BCl-2 positive cells. ×40
Regarding renal tissue, histological sections of the control and CP rat groups showed normal architecture (Fig. 6A and B). The kidney section of the AlCl3-intoxicated group showed degeneration and tubular necrosis (Fig. 6C), while AlCl3-injected rats and treated with SeNPs showed improvement to their renal architectures (Fig. 6D).
Photomicrographs of hematoxylin and eosin staining kidneys of all studied groups. (A) The control group showed normal renal tissue architecture; (B) the selenium nanoparticle group (SeNPs) showed normal renal tissue appearance with normal Bowman’s capsule and renal tubules; (C) the aluminum chloride group (AlCl3) showed degenerated renal tissue, inflammatory infiltration, and necrotic tubules (*); (D) aluminum chloride-selenium nanoparticle treated group (AlCl3 + SeNPs) displayed more or less normal feature of renal tissue. BC, Bowman’s capsule; G, glomerulus. × 40
Discussion
Nowadays, the excessive exposure to aluminum increases the risk on humans affecting various tissues (Klotz et al. 2017). Selenium is a powerful antioxidant; however, it may be toxic after severe accumulation (Horký 2014; Fernández-Llamosas et al. 2016); thus, there is an urgent demand to develop another form of selenium to diminish its elemental toxicity. Thus, the current study was conducted to evaluate the possible ameliorative effect of selenium nanoparticles (SeNPs) against AlCl3-induced hepatorenal toxicity.
The present study revealed that injection of AlCl3 modifies the following hepatic markers: AST, ALT, ALP, LDH, bilirubin, albumin markedly, relative to values obtained from control rats; this coincide with the studies of Ige et al. (2017) and Adedosu et al. (2018). The increased levels of AST, ALT, and ALP in serum may be attributed to the release of these enzymes from the liver to the bloodstream, where ALP increase in blood as a result of hepatic degeneration which is confirmed by histological and immunohistochemical staining (Al Eisa and Al Nahari 2016; Ahmed and Hamaad 2018). The exposure of rats to AlCl3 provokes nephrotoxicity which may be due to the increased levels of creatinine and urea in serum which reflect glomerular/tubular damage confirmed by the histological examination and kidney abnormalities in agreement with Yakubu and Musa (2012); Vijayaprakash et al. (2013).
According to Zahedi-Amiri et al. (2019), aluminum in the body produces free radicals and increases the hepatooxidative status through its ability to uptake the iron; thus, oxidative stress may contribute the AlCl3 toxicity. It affects the permeability of cell membranes resulting in cellular disintegration producing the peroxidation of lipids in the intracellular membranes (Taus et al. 2013). This interpreted the increasing MDA contents in liver and kidney tissues and also emphasizes the releasing of aminotransferases from damaged hepatocytes. Further, the present study recorded a decrease of antioxidant molecules (GSH, SOD, GPx) in hepatic and renal tissue. The reduced SOD may be attributed to the interaction of aluminum with superoxide radical anion forming aluminum superoxide semi-reduced radical ions (Exley 2004). Actually, SOD represents the first defense against superoxide anions as it converts the superoxide anion into hydrogen peroxide which is then converted to water by CAT and GPx at the expense of GSH. Therefore, the increased MDA may be interpreted herein by an inhibition of SOD, GSH, and GPx activities.
Interestingly, the present results showed that treatment of AlCl3-injected rats with selenium nanoparticles (SeNPs) significantly ameliorates liver and kidney function markers and decreased hepatic and renal MDA contents as well as increased all antioxidative parameters compared with the AlCl3-injected rats. These findings are in parallel with Shalby et al. (2017) and Urbankova et al. (2018). These findings support that SeNPs were considered a promising agent that can protect against the adverse effects of AlCl3. Increasing values of AST and ALP observed in the selenium nanoparticle (SeNPs) group may be due to sodium selenite, the source of prepared SeNPs, that has toxic effect at the cellular level; also Kumar et al. (2018) suggested that selenium and nanoselenium causing some deleterious at acute exposure histologically. However, SeNPs administrated to aluminum-injected rats were found to significantly replenish antioxidant biomarkers near to normal values. Actually, glutathione peroxidase is a Se-dependent enzyme; thereby, supplementation with SeNPs may enhance the enzyme and raise its content and subsequently reverse oxidative damage to hepatic and renal tissues. The current study assumed that SeNPs may have the ability to maintain structural integrity of the liver which prevent continuous lipid peroxidation and hence prevent the leakage of hepatic enzymes. This assumption is in harmony with Shalby et al. (2017) who revealed that SeNPs have superior effect than selenium to stimulate the antioxidant molecules. SeNPs may acquire its powerful antioxidant property from its smaller size and large surface area that facilitate the exposure of more atoms to free radicals for the electron exchange; thereby, SeNPs have potent scavenging activity to multiple free radicals (Shalby et al. 2017). Further, this assumption ascribed by the ability of SeNPs to increase the level of Bcl-2 (anti-apoptotic protein) and inhibit caspase-3 activity (apoptotic enzyme) was observed in the hepatic tissue of the AlCl3-SeNPs rat group. This may interpret the decreasing level of serum transaminases in AlCl3-SeNPs-treated rats, as Bcl-2 counteracts lipid peroxidation and hence reserves hepatocyte membrane. Finally, the present study supposed that SeNPs may modulate superoxide anion, hydrogen peroxide, Bcl-2, and caspase-3 and this may be the possible mechanism of its hepatorenal protective role.
Conclusion
The present study revealed that selenium nanoparticles (SeNPs) represent a promising candidate agent to AlCl3 hepatorenal toxicity via its membrane-stabilizing property. This was exhibited by biochemical, histological, and immunohistological examination. The signaling pathway of hepatorenal ameliorative effect of SeNPs may be related to its ability to downregulates MDA and other ROS which can suppress cytochrome C and subsequently caspase-3 along with the upregulation of GSH, SOD, GPx, and Bcl-2.
References
Adedosu OT, Adeleke GE, Alao TA, Ojugo NE, Akinsoji OE (2018) Effects of vitamin E administration on certain biochemical and antioxidants indices in some rat tissues treated with aluminium chloride. Ann Res Rev Biol 27(4):1–13. https://doi.org/10.9734/ARRB/2018/42582
AFSSAPS (2011) Agence française de sécurité sanitaire des produits de santé, Évaluation du risquelié à l’utilisation de l’aluminiumdans les produitscosmétiques. Rapport d’expertise pp, 164
Ahmed NM, Hamaad FAM (2018) Protective effects of Açai in combination withvitamin C against aluminum-induced toxicity in rat liver. J Biol LifeSci 9(1):1–16. https://doi.org/10.5296/jbls.v9i1.11670
Al Eisa R, Al Nahari H (2016) Protective effect of royal jelly against the liver toxicity caused by aluminum chloride (AlCl3) in adult male rats. Adv Environ Biol 10(3):113–127
Al-Olayan EM, El-Khadragy MF, Abdel Moneim AE (2015) The protective properties of melatonin against aluminium-induced neuronal injury. Int J Exp Pathol 96(3):196–202. https://doi.org/10.1111/iep.12122
Al-Quraishy S, Dkhil MA, Abdel Moneim AE (2015) Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine 29(10):6741–6756. https://doi.org/10.2147/IJN.S91377
Asha S, Radha E (1985) Effect of age and myocardial infarction on serum and heart lactic dehydrogenase. Exp Gerontol 20(1):67–70
Bai K, Hong B, He J, Hong Z, Tan R (2017) Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int J Nanomedicine 12:4527–4539. https://doi.org/10.2147/IJN.S129958
Barham D, Trinder P (1972) Enzymatic colorimetric method for determination of uric acid in serum plasma and urine. Analyst 97:142–146
Bartels H, Böhmer M, Heierli C (1972) Serum creatinine determination without protein precipitation. Clin Chim Acta 37:193–197
Belfield A, Goldberg D (1971) Colorimetric determination of alkaline phosphatase activity. Enzyme 12:561–566
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bunglavan SJ, Garg AK, Dass RS, Shrivastava S (2014) Effect of supplementation of different levels of selenium as nanoparticles/sodium selenite on blood biochemical profile and humoral immunity in male Wistar rats. Vet World 7:1075–1081. https://doi.org/10.14202/vetworld.2014.1075-1081
Dawood MAO, Koshio S, Zaineldin AI, Van Doan H, Moustafa EM, Abdel-Daim MM, Angeles Esteban M, Hassaan MS (2019) Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol Biochem 45(1):219–230
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31(1):87–96
Exley C (2004) The pro-oxidant activity of aluminum. Free Radic Biol Med 36:380–387
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13(2):156–159
Fernández-Llamosas H, Castro L, Blázquez ML, Díaz E, Carmona M (2016) Biosynthesis of selenium nanoparticles by Azoarcus sp. CIB Microb Cell Fact 15(1):109. https://doi.org/10.1186/s12934-016-0510-y
Horký P (2014) Influence of increased dietary selenium on glutathione peroxidase activity and glutathione concentration in erythrocytes of lactating sows. Ann Anim Sci 14(4):869–882. https://doi.org/10.2478/aoas-2014-0056
Ige SF, Adeniyi MJ, Iyalla GO (2017) Allium cepa mitigates aluminum chloride-induced hepatotoxicity in male Wistar rats. J Biomed Sci 6(4,27):1–4. https://doi.org/10.4172/2254-609X.100071
Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812
Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H (2017) The health effects of aluminum exposure. Dtsch Arztebl Int 114(39):653–659. https://doi.org/10.3238/arztebl.2017.0653
Kumar N, Krishnani KK, Singh NP (2018) Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ Sci Pollut Res Int 25(9):8914–8927. https://doi.org/10.1007/s11356-017-1165-x
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues bythiobarbituric acid reaction. Anal Biochem 95(2):351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Rezaei-Kelishadi M, Ghasemi A, Abdolyosefi NN, Zamani-Doabi S, Ramezani M, Changizi-Ashtiyani S, Rahimi A (2017) Effects of selenium nanoparticles on kidney and liver functional disorders in streptozotocin-induced diabetic rats. Physiol Pharmacol 21:155–162
Shalby AB, Abd El-Maksoud MD, Abdel Moneim AE, Ahmed HH (2017) Antifibrotic candidates of selenium nanoparticles and selenium in the experimental model. J Appl Pharm Sci 7(9):191–198. https://doi.org/10.2147/IJN.S43691
Tahari FZ, Lablack M, Hamadouche NA, Tahari Z, Aoues A (2016) Protective effect of Haloxylonsali cornicum on hepatic and renal functions of Wistar rats exposed to aluminium. Afr J Biotechnol 15(9):293–302. https://doi.org/10.5897/AJB2015.15037
Taus N, Farraj M, Tănase S, Mironescu A, Boicu M, Necula V, Taus L (2013) Aluminium – a chemical neurotoxic agent. Bull Transilvania Univ Braşov Ser VI Med Sci 2(55):1–8
Urbankova L, Horky P, Skladanka J, Pribilova M, Smolikova V, Nevrkla P, Cernei N, Lackova Z, Hedbavny J, Ridoskova A, Adam V, Kopel P (2018) Antioxidant status of rats’ blood and liver affected by sodium selenite and selenium nanoparticles. PeerJ 28(6):e4862. https://doi.org/10.7717/peerj.4862
Vijayaprakash S, Langeswaran K, Kumar SG, Revathy R, Balasubramanian MP (2013) Nephro-protective significance of kaempferol on mercuric chloride-induced toxicity in Wistar albino rats. Biomed Aging Pathol 3:119–124. https://doi.org/10.1016/j.biomag.2013.05.004
Walter M, Gerade H (1970) A colorimetric method for determination bilirubin in serum and plasma. Micro Chem J 15:231–236
Yakubu MT, Musa IF (2012) Liver and kidney functional indices of pregnant rats following the administration of the crude alkaloids from Sennaalata (Linn.Roxb) leaves. Iran J Toxicol 6(16):615–625
Zahedi-Amiri Z, Taravati A, Hejazian LB (2019) Protective effect of Rosa damascena against aluminum chloride-induced oxidative stress. Biol Trace Elem Res 187(1):120–127. https://doi.org/10.1007/s12011-018-1348-4
Zhang SY, Zhang J, Wang HY, Chen HY (2004) Synthesis of selenium nanoparticles in the presence of polysaccharides. Mater Lett 58(21):2590–2594. https://doi.org/10.1016/j.matlet.2004.03.031
Funding
This study was financially supported by the Deanship of Scientific Research at King Khalid University through research group project under grant number R.G.P.1–56–39.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocols using the animals were conducted in accordance with the regulatory laws regarding experimental ethics of animal use and collecting permits, Institute of Animal Care and Use Committee, King Khalid University, KSA.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Kahtani, M., Morsy, K. Ameliorative effect of selenium nanoparticles against aluminum chloride-induced hepatorenal toxicity in rats. Environ Sci Pollut Res 26, 32189–32197 (2019). https://doi.org/10.1007/s11356-019-06417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06417-y