Abstract
In this paper, we are reporting a simple hydrothermal technique for preparation of MoSe2 nanostructures (nanourchins and nanosheets) using selenium and sodium molybdate as precursors. Samples are characterized by field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), Raman spectroscopy, UV-Vis spectroscopy, Brunauer-Emmett-Teller (BET), and X-ray photoelectron spectroscopy (XPS). The FESEM revealed that the morphology of materials was varying significantly by changing pH value during synthesis. Photocatalytic degradation of anionic dye (MO), cationic dye (MB), and reduction of Cr(VI) into Cr(III) were performed. Nanosheets and nanourchins showed higher photocatalytic activity, enhanced photocatalytic degradation efficiency is correlated with the higher •OH radical concentration, crystallinity of material, and large surface area as evident through XPS, XRD, and BET, respectively. Photocatalysis mechanism along with role of reactive species (•OH and holes) were explained using trapping experiments. Identification of degraded products was carried out using high-performance liquid chromatography (HPLC). Reaction kinetics and reusability of materials were also studied; wherein, it was observed that the materials have reusable properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing number of contaminants such as heavy metal ions, dyes, and organic chemicals in natural water is dangerous to environment and humans. Cr(VI) and dyes are common contaminants which are discharged from industries to water bodies. They are highly dissolvable in water and can cause lung cancer, intestine cancer, kidney damage, asthma, etc. (Ashraf et al. 2019) (Shrivastava 2012; Singh et al. 2017; Toor and Jin 2012). Photocatalysis has been proved to be a capable method to remove water contaminants due to low cost, low energy consumption, and regenerable solution (Bhuyan et al. 2015; Salem et al. 2009; Sharma et al. 2017; Singh et al. 2018). Transition metal dichalcogenides (MoS2, MoSe2, WS2, WSe2) have enticed great attention due to special-layered stable structure, high carrier mobility, direct bandgap, large surface area, and controllable interfaces (Andoshe et al. 2015; Saha et al. 2019; Wang et al. 2018). Among TMD’s, MoSe2 has been proved to be an efficient photocatalyst due to high surface activity, enormous surface area, good chemical stability, and visible-NIR region bandgap (Chu et al. 2016; Narang et al. 2018a; Siddiqui et al. 2018).
There are various methods to synthesize MoSe2 nanostructures (in synthesis section—Table 1, various MoSe2 synthesis methods are summarized) such as solvothermal, molecular beam epitaxy, electrochemical deposition, and chemical vapor deposition (Chu et al. 2016; Jia et al. 2015; Roy et al. 2016; Wang et al. 2016). These methods are complicated or require high temperatures to operate. Hydrothermal is a simple, environmentally friendly, and effective method to synthesize MoSe2 nanostructures. Hydrothermal method provides controlled growth rate and nucleation uniformity, and it is suitable for large scale production (Chaudhary et al. 2018; Narang et al. 2018b). MoSe2 exists in several crystal structures depending on the composition and temperature of the system; these structures exhibit diverse chemical and catalytic properties (Chu et al. 2016; Liu et al. 2015; Siddiqui et al. 2018; Fan et al. 2014).
Photocatalytic degradation process takes place at the interface between photocatalyst surface and pollutant; therefore, it is expected that the photocatalytic activity is correlated with crystalline planes and surface structures. •OH radical is a major reactant responsible for the photocatalytic degradation of pollutants. •OH radicals are formed on MoSe2 surface when holes react with adsorbed water, and electrons react with oxygen-forming superoxide radical which further react and form •OH radicals. Researchers reported that there is a direct correlation between the amount of •OH generation and photocatalytic efficiency (Gągol et al. 2018). Acidic conditions promote the generation of hydroxyl radicals by lowering the recombination reaction of hydroxyl radicals.
It was observed from previously reported results, MoSe2 is a good photocatalyst for cationic dyes such as methylene blue (MB) and rhodamine B (RhB) (Wu et al. 2016). For degradation of anionic dye, surface modification of MoSe2 is required which is possible via doping, synthesizing composite with other suitable materials, and varying pH (Zhou et al. 2018). In this study, nanosheets and nanourchins of MoSe2 were hydrothermally synthesized by varying the pH and comparative photocatalytic study on organic dyes (MB, MO) and heavy metal Cr(VI) were performed. Enhanced photocatalytic efficiency of nanosheets over nanourchins is observed and is correlated with crystallinity, enhanced surface area, and hydroxyl ion concentration. Adsorption studies, role of reactive species, intermediate products using HPLC, and regenerable properties of catalysts were also studied.
Materials
MilliQ deionized water (DI) was used as base-liquid. Selenium Se (99.99%), Central Drug House Pvt. Ltd., sodium molybdate, Na2MoO4 (99.99%), Thermo Fisher Scientific India Pvt. Ltd., hydrazine hydrate, N2H4.H2O (80%), Central Drug House Pvt. Ltd. and acetic acid, CH3COOH (99.5%) from High Purity Laboratory Chemicals Pvt. Ltd. solutions were used in synthesis. Methylene blue (C16H18ClN3S, Fisher scientific), methyl orange (C14H14N3NaO3S, Fisher scientific), potassium iodide (KI, Pallav chemicals and solvents Pvt. Ltd.), and tert-butyl alcohol ((CH3)3COH, Central Drug House Pvt. Ltd.) have been used for photocatalysis.
Synthesis process
Schematic of synthesis process for MoSe2 nanostructures (nanosheets and nanourchins) is shown in Fig. 1. Two solutions are prepared, in the first solution, 0.02 mol of sodium molybdate is dissolved in 50 ml of deionized water (DI) at room temperature and stirred until clear solution is obtained. Separately, second solution is prepared by dissolving 0.04 mol of selenium powder in 10 ml of hydrazine hydrate at room temperature and stirred, to give complete black suspension. Selenium reacts with hydrazine hydrate to yield selenium ions (Se2−) as shown in Eq. (i). Second solution is added dropwise to the first solution and stirred for 20 min. Hydrazine hydrate reduces MoO42− anions from sodium molybdate and produce Mo4+ cations, as shown in Eq. (ii). The resultant dark brown color solution is obtained with pH 12.
MoSe2 nanostructure at pH 5 and pH 8
Acetic acid is added to the as-prepared solution of pure MoSe2 (before transferring to autoclave) to change the pH value of the solution. Solutions at pH 5 and pH 8 were obtained by adding 13 ml and 6 ml of acetic acid, respectively. Finally, dark brown color solutions were transferred to autoclave and placed in furnace at 200 °C for 24 h. In autoclave, reaction between Se2− and Mo4+ takes place to form MoSe2 as shown in Eq. (iii) (Mittal et al. 2019). After 24 h, the autoclaves are allowed to cool till room temperature. Subsequently, obtained solutions were sequentially washed with DI water and acetone, followed by drying at 50 °C for 2 h. Samples prepared at pH 5 and pH 8 are labelled as MSE5 and MSE8, respectively.
Relevant synthesis condition, raw materials, size, and morphology of Mose2 are summarized in Table 1. In the present study, we have reported synthesis of nanosheets and nanourchins morphology of MoSe2 using simple and economic hydrothermal method.
Characterizations
FESEM (Quanta 3D FEG) analysed the morphology of the powdered MoSe2. Crystal structure was analysed using XRD (Rigaku Smart Lab) with Cu Kα radiation of 1.540 Å in the range of 10–80°. UV-Vis spectrophotometer (Cary 100 series, Agilent Technologies) was used to analyse the band gap of the synthesized materials. Raman spectroscopy (HR800 JY, Lab RAM HR) was carried out in the range of 220 to 300 cm−1. XPS (Multiprobe Surface analysis system, Omicron) was used to determine the hydroxyl ion concentration. The surface areas and pore diameter of the materials were analyzed using multipoint BET and Barrett-Joyner-Halenda (BJH) method by nitrogen adsorption–desorption isotherm measurements at 77.4 K using Quantachrome Instruments (version 3.0). The degradation products of MB were analysed by HPLC (Agilent technologies (1260- ∞ series)) using Hiplex X Column with temperature of detector and column at 35 °C and 65 °C, respectively. Deionized water was used as mobile phase with flow rate of 0.6 ml/min.
Results and discussion
Morphology of as synthesized materials was studied using FESEM as shown in Fig. 2. Figure 2a–d showed the SEM images of MSE5 and MSE8 at magnification of 1 μm and 100 nm. MSE5 showed clusters of nanosheets, the pattern is irregular, inner surface is dense, and smooth while the outer surface is composed of multiple folds nanosheets. MSE8 consists of evenly circulated microsphere of diameter ~ 150 nm. The nanourchins were made by the accumulation of several nanospheres which were gathered at one place and giving realization of nanourchins. Thus, significant change in morphology was observed by changing synthesis conditions.
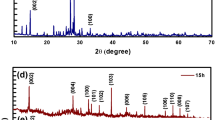
Crystal structure and orientation of MSE5 and MSE8 were observed by XRD diffractograms as shown in Fig. 3a and b, respectively. XRD peaks for MSE5 and MSE8 were observed at 2θ 13.6°, 13.4°; 26.3°, 26.1°; 31.3°, 31.5°; 37.8°, 37.7°; 46.8°,47.1°; 55.8°,55.9°, and 64.5°, 64.3° corresponds to (002), (100), (004), (102), (200), (211), and (204) hkl planes, respectively as confirmed by the JCPDS file no. 77-1715 (Roy et al. 2016; Saha et al. 2019; Tang et al. 2016). As observed from XRD, MSE5 has sharp and intense peaks as compared with MSE8, implying higher crystallinity in MSE5 sample as compared with MSE8. The calculated lattice constants a and c were 3.3 Å and 12.56 Å for MSE5 and 3.3 Å and 12.9 Å for MSE8, which are in good agreement with the earlier results (Zhang et al. 2018).
To further confirm the crystallinity and phase components of MSE5 and MSE8, Raman spectra were recorded as shown in Fig 4a and b, respectively. In the Raman spectrum of MSE5, the peaks were positioned at 239.6 cm−1 and 284.8 cm−1 and of MSE8, peaks positioned at 240.2 cm−1 and 284.9 cm−1, corresponded to A1g and E2g phonon modes of MoSe2, respectively (Ghosh et al. 2016; Kirubasankar et al. 2019). A1g mode is assigned to out of plane and E2g is assigned to in plane optical branch. The Δω between E2g and A1g mode was 45.2 cm−1 and 44.8 cm−1 for MSE5 and MSE8, respectively. It was observed that MSE5 has high intensity as compared with MSE8 which implies more defects in MSE8 than in MSE5.
Band gap of MSE5 and MSE8 was calculated using Tauc’c plot as shown in Fig. 5a and b, respectively. Absorption spectra of MSE5 and MSE8 lie in the visible and IR region. Band gap energies were calculated from plot of (αhʋ)1/2 vs hʋ and found to be 2.0 eV for MSE5 and MSE8, where α is absorption coefficient, defined as a = 2.303 A/t, where “A” is absorbance and t is the thickness of cuvette. It was observed that there is no effect of pH on band gap of MoSe2.
The measurements of N2 adsorption–desorption was performed to calculate the pore diameter, specific surface area, and the pore volume. One hundred milligram of MSE5 and MSE8 were degassed for 6 h at 150 °C before measurement and outgassed for 1 h at 100 °C. The nitrogen adsorption–desorption isotherms and pore size distribution obtained for MSE5 and MSE8 are shown in Fig. 6a and b, respectively. Table 2 represented the BET surface area and pore diameter of MSE5 and MSE8. MSE5 and MSE8 exhibit isotherm of type IV which is characteristic of mesoporous material. MSE8 has narrow pore size distributions than MSE5 and possessed a single peak which confirms uniform size distribution. BET surface area for MSE5 and MSE8 were 78.633 m2 g−1 and 72.978 m2 g−1, respectively. The maximum pore size of MSE5 is 1.6 nm and of MSE8 is 3.5 nm which further confirms the mesoporous nature of MSE5 and MSE8.
XPS study was performed to confirm the oxidation state and calculate the hydroxyl ion concentration of synthesized materials (Khanuja et al. 2007; Khanuja et al. 2008). The general scan as shown in Fig. 7a and b indicated the elemental peaks of Mo, Se, C, and O. The HR-XPS of the Mo 3d core level of MoSe2 are shown in Fig 7c and d, peak at 228.4 eV in MSE5 and 232.4 eV in MSE8 corresponded to Mo 3d5/2 and peak at 231.6 eV in MSE5 and 235.5 eV in MSE8 corresponded to Mo 3d3/2, suggesting Mo4+ characteristics (Zhao et al. 2016). As shown in Fig. 7e and f, Se 3d5/2 and Se 3d3/2 components located at binding energies of 54.2 and 54.7 eV, respectively in MSE5 and 54.8 and 55.3 eV in MSE8, representing an oxidation state of − 2 for Se, thus confirmed the formation of MoSe2 (Bi et al. 2015). The difference in binding energy because of the spin orbital coupling, ∆Eb = Eb (Se 3d3/2) − Eb (Se 3d5/2) was 0.5 eV. The O 1 s spectra are as shown in Fig. 7g and h, peaks at binding energy of 530.1 eV in MSE5 and 530.5 eV in MSE8 corresponded to C=O of MoSe2, peak at 531.8 eV in MSE5 and 531.7 eV in MSE8 corresponded to the C–O–Mo and peak at 533.27 eV in MSE5 and 533.2 eV in MSE8 corresponded to O–OH (Azeez et al. 2018). Effect of pH was observed on surface hydroxyl concentration; percentage of hydroxyl oxygen concentration was 31.3% and 28.2% for MSE5 and MSE8, respectively. Hydroxyl radicals are responsible for degradation of pollutants, and increase in hydroxyl concentration was observed with decrease in pH value. Thus, acidic conditions promote generation of hydroxyl radicals and degradation efficiency by lowering recombination reaction (Zhang et al. 2014).
Figure 8a and b showed the self-photodegradation of MB and MO, respectively. MB and MO was irradiated under xenon arc lamp for 30 min and analyzed using UV-vis spectroscopy at 664 nm and 464 nm for MB and MO, respectively. It was observed that there is no significant change in the MB and MO concentration. This showed that the direct photocatalysis or self-photodegradation was insignificant in the absence of the photocatalyst.
Figure 9 showed degradation efficiency vs time plot for degradation of MB, MO, and reduction of Cr(VI) in the presence of MSE5 and MSE8. Solutions of 1 mg/100 ml of each MB, MO, and Cr(VI) were prepared, and 20 mg of MSE5 and MSE8 were added to each solution and stirred under AM (air mass) 1.5 solar illuminations 100 mW/cm2 from xenon arc lamp under same conditions. One milliliter of solution was taken out from each solution in some interval of time and analyzed using UV-visible spectroscopy. Degradation of MB, MO, and reduction of Cr(VI) into Cr(III) was observed by UV-Vis spectroscopy at characteristic absorbance peak at 664 nm, 464 nm, and 350 nm, respectively. η = 1- [C/C0] * 100 was used to calculate degradation efficiency (η), where C0 denotes absorption of pollutant before irradiation and C denotes adsorption after irradiation at time t. MSE5 degraded 94.7% of MB, ~ 92.6% of MO and ~ 90.7% of Cr(VI) in 45 min of irradiation. MSE8 degraded 78% of MB, 77.8% of MO, and 66.7% of Cr(VI) in Cr(III) in 45 min of irradiation. As evident from the results (Fig. 9), MSE5 showed high photocatalytic efficiency as compared with MSE8 due to (i) enhanced surface area, (ii) high crystallinity, and (iii) large concentration of hydroxyl radicals. Enhanced surface area and large pore size in MSE5 as compared with MSE8 (Table 3) lead to increase in number of active sites, thus slower recombination rate and thereby producing more holes and electrons that help in degradation of adsorbed contaminants present on the surface of photocatalyst (Ashraf et al. 2019). Sample MSE5 has higher crystallinity as compared with MSE8 as evident through sharp and intense peaks observed in MSE5 through XRD and Raman studies (Figs. 3 and 4), resulting in higher degradation efficiency of MSE5 due to favorable charge transport properties. MSE5 has higher concentration of hydroxyl radicals as compared with MSE8 (Fig. 7g, h) which is a major reactant responsible for the degradation of pollutants.
Some of the earlier reported results of photodegradation of pollutants using MoSe2, and its composites are summarized in Table 3. As observed from results, it can be seen that the degradation efficiency of MSE5 and MSE8 are higher for cationic dye, anionic dye as well as organic pollutant than those of other photocatalysts. Hence, MSE5 and MSE8 acted as an excellent photocatalyst.
Figure 10a and b showed C/C0 vs time plot for degradation of MB, MO, and reduction of Cr(VI) in the presence of MSE5 and MSE8. Kinetics of adsorption on MSE5 and MSE8 were investigated by pseudo first order and pseudo second order as shown in Fig. 10.
The pseudo first-order adsorption, pseudo second-order adsorption and intra-particle diffusion model are given by the Eqs. (xiii) and (xiv), respectively.
where Qt and Qe denotes the adsorbed amount at time t and amount adsorbed at equilibrium. k1 and k2 are the rate constant for pseudo first order and pseudo second order, respectively. Values of k1 and k2 were evaluated from the slope of plot ln (Qe − Qt) vs time and plot of t/Qt vs time, respectively.
Kinetic study for MSE5 and MSE8 were found to follow pseudo first and second order adsorption model for MB, which suggested that adsorption process was initially controlled by physisorption followed by chemisorption. For MO and Cr(VI), MSE5 and MSE8 were found to follow pseudo first order adsorption model for MO and Cr(VI), which suggested that adsorption process was controlled by physisorption. In physisorption process, molecules form monolayer adsorption when adsorbed on the surface by valence bond. In chemisorption process, reaction between the surface and the adsorbate takes place. On interaction between the adsorbate molecule and surface of catalyst, in chemisorption process, new interacting electronic bonds, whereas in physisorption process no bond formation take place.
In order to evaluate the reusability of MSE5 and MSE8, reduction of Cr(VI) into Cr(III) was checked for five cycles as shown in Fig. 11. In the first cycle, 92% and 67% of Cr(VI) was reduced using MSE5 and MSE8, respectively. The solutions resultant from the photocatalytic reduction was filtered washed and dried. The dried samples were used for the reduction of new solution of Cr(VI). After 5th cycle, degradation efficiency changed from 92 to 87% in case of MSE5 and 67 to 62% in case of MSE8. Such decrease was due to wastage of photocatalyst during each cycle and can be concluded that both photocatalyst acted as perfect candidate for environment remediation.
Photocatalytic degradation mechanism of MoSe2 is shown in Fig. 12. Photocatalytic reaction initiated when irradiation light has energy equal to or greater than band gap of MoSe2. Electrons from valence band moved to conduction band by absorbing energy from irradiation light, leaving behind a hole and resulting in formation of electron-hole pair. Holes reacts with water to yield hydroxyl radical, and electrons reacts with oxygen to yield superoxide radical which further produce hydroxyl radicals. These radicals react with contaminants and degrade them into harmless or less toxic substances.
Photocatalytic mechanism can be summarized as follows.
-
(a)
Adsorption of photon- and electron-hole pair generation:
-
(b)
Neutralization of OH− groups to produce •OH radicals:
-
(c)
Oxygen ionosorption:
-
(d)
Neutralization of \( \cdotp {O}_2^{-} \) by protons:
-
(e)
Transient hydrogen peroxide formation:
-
(f)
Second reduction of oxygen by H2O2
-
(g)
Decomposition of contaminant by •OH radical
-
(h)
Direct oxidation by holes
In order to investigate the role of the reactive species in photocatalytic degradation mechanism, photocatalytic degradation of MB and reduction of Cr(VI) was performed in the presence of trappers such as t-BuOH for \( {}{}^{\bullet}\mathrm{OH} \)and KI for holes and \( {}{}^{\bullet}\mathrm{OH} \). 1mM in 100ml of t-BuOH and KI were used in the photocatalysis process (Mavinakere Ramesh and Shivanna 2018; Mittal et al. 2019). Effect of radical trappers on the photodegradation efficiency of MSE5 and MSE8 is shown in Fig. 13. Reduction in photocatalytic activity was observed in the presence of trappers, implying the role of \( {}{}^{\bullet}\mathrm{OH} \) in the photodegradation process.
To investigate the degradation products of MB, HPLC was performed at 0 min and 30 min as shown in Fig. 14a and b, respectively. It was indicated from the results that photocatalytic degradation of dye not only caused its decoloration but also degraded the dye molecules. As shown in Fig. 14, intensity of MB peak was decreased, and new peaks appeared which corresponded to degraded products of the dye. Area of MB peak at 5.7 min decreased after 30 min, and new peaks at lower retention time were observed. Intermediates were observed at 2.0, 2.9, 3.2, and 3.5 min were assigned to thionin, azure C (AC), azure A (AA), and azure B (AB), respectively (Alamo-Nole et al. 2013; Ullah et al. 2017). These intermediates were further degraded and finally converted to CO2 and H2O.
Conclusion
Nanosheets and nanourchins morphology of MoSe2 were successfully obtained via hydrothermal method. During the synthesis process, acetic acid plays an important role in modification of the inherent negatively charged MoSe2 surface. The photocatalytic degradation of cationic dye (MB), anionic dye (MO), and reduction of (Cr(VI)) using different morphologies of MoSe2 (nanosheets and nanourchins) were performed and found to have good photocatalytic performance for cationic as well as anionic dye. The superior degradation efficiency of MSE5 (nanosheets) and MSE8 (nanourchins) is due to (i) high crystallinity of material (as observed by XRD and Raman studies), (ii) large pore size (as observed in BET analysis), and (iii) higher concentration of •OH radicals (as observed by XPS studies). The degraded products of MB were examined by high-performance liquid chromatography (HPLC) which confirms the complete degradation of dye. Photocatalysis mechanism along with trapping experiment was explained. Reaction kinetics were studied and found to follow pseudo first- and second-order model. Repeatability of the materials were also studied and found to have reusable properties.
References
Alamo-Nole L, Bailon-Ruiz S, Luna-Pineda T, Perales-Perez O, Roman FR (2013) Photocatalytic activity of quantum dot–magnetite nanocomposites to degrade organic dyes in the aqueous phase. J Mater Chem A 1:5509–5516
Andoshe DM, Jeon J-M, Kim SY, Jang HW (2015) Two-dimensional transition metal dichalcogenide nanomaterials for solar water splitting. Electron Mater Lett 11:323–335
Ashraf W, Fatima T, Srivastava K, Khanuja M (2019) Superior photocatalytic activity of tungsten disulfide nanostructures: role of morphology and defects. Appl Nanosci 1–15. https://doi.org/10.1007/s13204-019-00951-4
Azeez F, Al-Hetlani E, Arafa M, Abdelmonem Y, Nazeer AA, Amin MO, Madkour M (2018) The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci Rep 8:7104
Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Bi E, Chen H, Yang X, Ye F, Yin M, Han L (2015) Fullerene-structured MoSe2 hollow spheres anchored on highly nitrogen-doped graphene as a conductive catalyst for photovoltaic applications. Sci Rep 5:13214
Chaudhary N, Khanuja M, Islam S (2018) Hydrothermal synthesis of MoS2 nanosheets for multiple wavelength optical sensing applications. Sensors Actuators A Phys 277:190–198
Chu H, Liu X, Liu B, Zhu G, Lei W, du H, Liu J, Li J, Li C, Sun C (2016) Hexagonal 2H-MoSe2 broad spectrum active photocatalyst for Cr (VI) reduction. Sci Rep 6:35304
Fan C, Wei Z, Yang S, Li J (2014) Synthesis of MoSe2 flower-like nanostructures and their photo-responsive properties. RSC Adv 4:775–778
Gągol M, Przyjazny A, Boczkaj G (2018) Wastewater treatment by means of advanced oxidation processes based on cavitation—a review. Chem Eng J 338:599–627
Ghosh R, Kim J-S, Roy A, Chou H, Vu M, Banerjee SK, Akinwande D (2016) Large area chemical vapor deposition growth of monolayer MoSe 2 and its controlled sulfurization to MoS2. J Mater Res 31:917–922
Harpeness R, Gedanken A, Weiss A, Slifkin M (2003) Microwave-assisted synthesis of nanosized MoSe2. J Mater Chem 13:2603–2606
Huang J, Liu H, Jin B, Liu M, Zhang Q, Luo L, Chu S, Chu S, Peng R (2017) Large-area snow-like MoSe2 monolayers: synthesis, growth mechanism, and efficient electrocatalyst application. Nanotechnology 28:275704
Jia L, Sun X, Jiang Y, Yu S, Wang C (2015) A novel MoSe2–reduced graphene oxide/polyimide composite film for applications in electrocatalysis and photoelectrocatalysis hydrogen evolution. Adv Funct Mater 25:1814–1820
Jiang Q, Lu Y, Huang Z, Hu J (2017) Facile solvent-thermal synthesis of ultrathin MoSe2 nanosheets for hydrogen evolution and organic dyes adsorption. Appl Surf Sci 402:277–285
Khanuja M, Kala S, Mehta BR, Sharma H, Shivaprasad SM, Balamurgan B, Maisels A, Kruis FE (2007) XPS and AFM studies of monodispersed Pb/PbO core–shell nanostructures. J Nanosci Nanotechnol 7:2096–2100
Khanuja M, Mehta B, Shivaprasad S (2008) Two approaches for enhancing the hydrogenation properties of palladium: metal nanoparticle and thin film over layers. J Chem Sci 120:573–578
Kirubasankar B, Palanisamy P, Arunachalam S, Murugadoss V, Angaiah S (2019) 2D MoSe2-Ni (OH)2 nanohybrid as an efficient electrode material with high rate capability for asymmetric supercapacitor applications. Chem Eng J 355:881–890
Ko YN, Choi S, Park S, Kang YC (2014) Hierarchical MoSe2 yolk–shell microspheres with superior Na-ion storage properties. Nanoscale 6:10511–10515
Liu Y, Zhu M, Chen D (2015) Sheet-like MoSe2/C composites with enhanced Li-ion storage properties. J Mater Chem A 3:11857–11862
Mavinakere Ramesh A, Shivanna S (2018) Visible light assisted photocatalytic degradation of chromium (VI) by using nanoporous Fe2O3. J Mater 2018. https://doi.org/10.1155/2018/1593947
Mittal H, Kumar A, Khanuja M (2019) In-situ oxidative polymerization of aniline on hydrothermally synthesized MoSe2 for enhanced photocatalytic degradation of organic dyes. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2019.02.004
Narang J, Mishra A, Pilloton R, VV A, Wadhwa S, Pundir C, Khanuja M (2018a) Development of MoSe2 nano-urchins as a sensing platform for a selective bio-capturing of Escherichia coli shiga toxin DNA. Biosensors 8:77
Narang J, Singhal C, Khanuja M, Mathur A, Jain A, Pundir C (2018b) Hydrothermally synthesized zinc oxide nanorods incorporated on lab-on-paper device for electrochemical detection of recreational drug. Artif Cells Nanomed Biotechnol 46:1586–1593
Poh SM, Tan SJR, Zhao X, Chen Z, Abdelwahab I, Fu D, Xu H, Bao Y, Zhou W, Loh KP (2017) Large area synthesis of 1D-MoSe2 using molecular beam epitaxy. Adv Mater 29:1605641
Roy A, Movva HCP, Satpati B, Kim K, Dey R, Rai A, Pramanik T, Guchhait S, Tutuc E, Banerjee SK (2016) Structural and electrical properties of MoTe2 and MoSe2 grown by molecular beam epitaxy. ACS Appl Mater Interfaces 8:7396–7402
Saha S, Chaudhary N, Mittal H, Gupta G, Khanuja M (2019) Inorganic–organic nanohybrid of MoS2-PANI for advanced photocatalytic application. Int Nano Lett 1–13. https://doi.org/10.1007/s40089-019-0267-5
Salem MA, Al-Ghonemiy AF, Zaki AB (2009) Photocatalytic degradation of Allura red and Quinoline yellow with Polyaniline/TiO2 nanocomposite. Appl Catal B: Environ 91:59–66
Sharma R, Khanuja M, Islam S, Singhal U, Varma A (2017) Aspect-ratio-dependent photoinduced antimicrobial and photocatalytic organic pollutant degradation efficiency of ZnO nanorods. Res Chem Intermed 43:5345–5364
Shi H, Zhou X, Lin Y, Fu X (2008) Synthesis of MoSe2 nano-flakes modified with dithiophosphinic acid extractant at low temperature. Mater Lett 62:3649–3651
Shrivastava V (2012) Photocatalytic degradation of methylene blue dye and chromium metal from wastewater using nanocrystalline TiO2 semiconductor. Arch Appl Sci Res 4:1244–1254
Siddiqui I, Mittal H, Kohli VK, Gautam P, Ali M, Khanuja M (2018) Hydrothermally synthesized micron sized, broom-shaped MoSe2 nanostructures for superior photocatalytic water purification. Mater Res Exp 5:125020
Singh S, Pendurthi R, Khanuja M, Islam S, Rajput S, Shivaprasad S (2017) Copper-doped modified ZnO nanorods to tailor its light assisted charge transfer reactions exploited for photo-electrochemical and photo-catalytic application in environmental remediation. Appl Phys A 123:184
Singh S, Sharma R, Khanuja M (2018) A review and recent developments on strategies to improve the photocatalytic elimination of organic dye pollutants by BiOX (X=Cl, Br, I, F) nanostructures. Korean J Chem Eng 35:1955–1968
Tang H, Huang H, Wang X, Wu K, Tang G, Li C (2016) Hydrothermal synthesis of 3D hierarchical flower-like MoSe2 microspheres and their adsorption performances for methyl orange. Appl Surf Sci 379:296–303
Toor M, Jin B (2012) Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem Eng J 187:79–88
Ullah AA, Kibria AF, Akter M, Khan M, Tareq A, Firoz SH (2017) Oxidative degradation of methylene blue using Mn3O4 nanoparticles. Water Conserv Sci Eng 1:249–256
Wang B, Ostrikov K, Van Der Laan T, Zheng K, Shao R, Zhu M, Zou S (2016) Growth and photoluminescence of oriented MoSe2 nanosheets produced by hot filament CVD. RSC Adv 6:37236–37245
Wang M, Peng Z, Qian J, Li H, Zhao Z, Fu X (2018) Highly efficient solar-driven photocatalytic degradation on environmental pollutants over a novel C fibers@ MoSe2 nanoplates core-shell composite. J Hazard Mater 347:403–411
Wu Y, Xu M, Chen X, Yang S, Wu H, Pan J, Xiong X (2016) CTAB-assisted synthesis of novel ultrathin MoSe2 nanosheets perpendicular to graphene for the adsorption and photodegradation of organic dyes under visible light. Nanoscale 8:440–450
Wu M-H, Lee J-T, Chung YJ, Srinivaas M, Wu J-M (2017) Ultrahigh efficient degradation activity of single-and few-layered MoSe2 nanoflowers in dark by piezo-catalyst effect. Nano Energy 40:369–375
Zhang J, Nosaka Y, (2014) Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J Phys Chem 118 (20):10824-10832
Zhan J, Zhang Z, Qian X, Xie CWY, Qian Y (1999) Synthesis of MoSe2 nanocrystallites by a solvothermal conversion from MoO3. Mater Res Bull 34:497–501
Zhang Y, Gong Q, Li L, Yang H, Li Y, Wang Q (2015) MoSe2 porous microspheres comprising monolayer flakes with high electrocatalytic activity. Nano Res 8:1108–1115
Zhang C, Chen X, Peng Z, Fu X, Lian L, Luo W, Zhang J, Li H, Wang YH, Zhang D (2018) Phosphine-free synthesis and shape evolution of MoSe2 nanoflowers for electrocatalytic hydrogen evolution reactions. CrystEngComm 20:2491–2498
Zhao X, Sui J, Li F, Fang H, Wang H, Li J, Cai W, Cao G (2016) Lamellar MoSe2 nanosheets embedded with MoO2 nanoparticles: novel hybrid nanostructures promoted excellent performances for lithium ion batteries. Nanoscale 8:17902–17910
Zhou X, Jiang J, Ding T, Zhang J, Pan B, Zuo J, Yang Q (2014) Fast colloidal synthesis of scalable Mo-rich hierarchical ultrathin MoSe2− x nanosheets for high-performance hydrogen evolution. Nanoscale 6:11046–11051
Zhou X, Yao J, Yang M, Ma J, Zhou Q, Ou E, Zhang Z, Sun X (2018) Synthesis of MoSe2/SrTiO3 heterostructures with enhanced ultraviolet-light-driven and visible-light-driven photocatalytic properties. Nano 13:1850038
Acknowledgement
This work has been supported by the Science and Engineering Research Board (SERB), India with grant number ECR/2017/001222. The authors are thankful to Dr. Govind Gupta, CSIR-National Physical Laboratory, New Delhi, India, for XPS measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mittal, H., Khanuja, M. Nanosheets- and nanourchins-like nanostructures of MoSe2 for photocatalytic water purification: kinetics and reusability study. Environ Sci Pollut Res 27, 23477–23489 (2020). https://doi.org/10.1007/s11356-019-06275-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06275-8