Abstract
Yarrowia lipolytica (Y. lipolytica) is an oleaginous yeast that can utilize hydrophobic substrates as carbon source to produce single-cell lipids for biodiesel production. This study attempts to increase the lipid accumulation ability of Y. lipolytica by first gradually elevating pure oil substrate concentration during the cultivation and then adding short-chain carbon compounds, such as glucose and sodium acetate, to a culture substance according to the optimal oil concentration. Results showed that Y. lipolytica cultured under 40.0 g L−1 oil concentration showed higher lipids (2.97 g L−1) and lipid content (37.35%, DW) compared with that cultured under 20.0 g L−1, where the corresponding values were 1.91 g L−1 and 24.46%. By contrast, the lipid content of Y. lipolytica increased from 37.35 to 41.50% when the substrate was changed from 40.0 g L−1 pure oil to 5% sodium acetate combined with 95% oil under the same total carbon concentration. However, lipid accumulation did not increase even though 15% sodium acetate or 5% glucose, or 15% glucose was added to the combined substrate. Moreover, the lipid biomodification of Y. lipolytica was evident when it was cultured under the oil concentration of 20.0 g L−1. Therefore, the lipid accumulation of Y. lipolytica can be elevated through the gradient increase of oil concentration and by adding a suitable amount of easily degradable carbon source. Furthermore, the lipid biomodification of Y. lipolytica improves biodiesel quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to the exacerbation of global energy shortage and environmental deterioration, clean and renewable biodiesel has attracted increasing attention (Pandit et al. 2017). However, the high cost of raw materials limited the industrialization of biodiesel production. Therefore, seeking cheap and sustainable raw materials for biodiesel production is necessary (Sahar et al. 2018).
Biodiesel is a fuel produced by the transesterification or esterification reactions of oils with small chain alcohols, such as methanol and ethanol (Živković and Veljković 2018). Raw materials used for biodiesel production commonly include soybean oil, rapeseed oil, algae, microbial oils, animal fats, and waste cooking oils (Chen et al. 2017; Ma et al. 2018a; Qin et al. 2016; Sahar et al. 2018). Thus, biodiesel production may compete with food production if a large quantity of oil crops, such as soybeans and rapeseed, are used to produce biodiesel. Algae can be used to produce biodiesel but remain undeveloped because the process requires high technology and the algae are difficult to enrich (Ma et al. 2018b). Theoretically, the waste cooking oil is a prior raw material to produce biodiesel, due to cheap and easy available. However, it is unstable and contains many impurities that may complicate pretreatment.
Oily wastewater is produced from wide-range industries (Putatunda et al. 2018), such as petroleum refineries, petrochemical, food processing, and textile (Zhang et al. 2018). The treatment methods for oily wastewater commonly include physical methods, such as air floatation (Etchepare et al. 2017), membrane separation (Saki and Uzal 2018), and adsorption; chemical methods, such as chemical flocculation (Zeng et al. 2007; Zhao et al. 2018) and chemical oxidation; and biological treatments, such as activated sludge or biofilter method (Li et al. 2013; Zhao et al. 2018). Physical and chemical methods have a small application range because of their high costs and secondary pollution. Biological treatment is popular because of its low cost and convenient operation (Dumore and Mukhopadhyay 2012; Song et al. 2011). However, microorganisms cannot directly absorb lipids, and the oil seals of lipids affect the activities of the microorganisms and thereby result in a long degradation time. Therefore, identifying specific microorganisms that can efficiently treat oily wastewater and utilize the oil in wastewater is necessary.
Yarrowia lipolytica (Y. lipolytica) is an oleaginous yeast, which exhibits strong adaptability to oily wastewater (Belle and Goossens 2011). Y. lipolytica can not only degrade organic substances for cell growth but also accumulate lipids for further biodiesel production (Katre et al. 2017). However, the mechanism by which the oil concentration of oily wastewater and the culture time of Y. lipolytica affect lipid accumulation and biodiesel titer remains unknown. The hydrolysis of long-chain fatty acids is inevitable in an actual oily wastewater. Therefore, the carbon source of long-chain lipids is always accompanied by the carbon sources of short-chain lipids or easily degradable carbon sources. Currently, the effect of the types and contents of short-chain carbon sources on lipid accumulation by Y. lipolytica is rarely reported. The purpose of this study is to investigate the effect of increased oil concentration in the substrate and the addition of short-chain carbon sources in the oil substrate on the Y. lipolytica lipid accumulation. This research may have great significance for the biodiesel production with Y. lipolytica and oily wastewater.
Materials and methods
Strain and growth conditions
Y. lipolytica was obtained from Shaanxi Institute of Microbiology and maintained on wort agar solid medium at 4 °C. An initial pre-inoculum of cells was grown in yeast extract peptone dextrose (YPD) medium at 28 °C for 24 h. YPD medium was composed of glucose, peptone, and yeast extract with the concentrations of 20.0, 20.0, and 10.0 g L−1, respectively. The Y. lipolytica pre-inoculum was harvested by centrifuging the broth at 4000 rpm for 5 min and washed with distilled water before resuspending in normal saline. The cultivation medium for producing lipids contained the following components (g L−1): KH2PO4, 7.0; Na2HPO4, 2.5; MgSO4·7H2O, 1.5; CaCl2, 0.15; FeCl3·6H2O, 0.15; ZnSO4·7H2O, 0.02; MnSO4·H2O, 0.06; (NH4)2SO4, 1.0; yeast extract, 2.0; and rapeseed oil, 20.0, 30.0, 40.0. Tween 80 and polyethylene glycol 20,000 were added as emulsifiers, and their additions were 10% of the quantity of rapeseed oil (Papanikolaou and Aggelis 2010). Y. lipolytica cells were grown at 28 °C in an incubator shaker (160 rpm) for 168 h. The rapeseed oil used as the main carbon source for Y. lipolytica cultivation had the following composition: myristic acid (C14:0), 0.1%; palmitic acid (C16:0), 5.5%; palmitoleic acid (C16:1), 0.3%; stearic acid (C18:0), 2.2%; oleic acid (C18:1), 65.4%; linoleic acid (C18:2), 26.5%.
Effect of oily carbon source concentrations solely on the lipid accumulation by Y. lipolytica
The yeast suspension was first cultured in 20.0 g L−1 lipid-production medium for 2 days, and then, the yeast strain suitable for this environment was isolated and screened out for 30.0 g L−1 lipid-production medium. Similarly, the screened yeast was cultured in 30.0 g L−1 lipid-production medium for 2 days and isolated and screened out for 40.0 g L−1 lipid-production medium. The acclimation process was finished after the screened yeast was cultured in 40.0 g L−1 lipid-production medium for 2 days. The biomass, lipid content, and biodiesel titer of Y. lipolytica were measured every day during the acclimation process.
Each screened yeast was cultured as yeast suspension, which was inoculated with 10% (v/v) in the different lipid-production media and cultured under 28 °C and 160 rpm for 7 days. The lipid-production medium contained rapeseed oil as the sole carbon source and was composed of 20.0, 30.0, and 40.0 g L−1 rapeseed oil.
Effects of short-chain carbon mixed with oily carbon on the lipid accumulation of Y. lipolytica
Different lipid-production media were prepared according to Table 1, where each carbon source exhibited the same carbon mass and 100% rapeseed oil (40.0 g L−1) was used as the control. The yeast isolated and purified from 30.0 g L−1 rapeseed oil medium was inoculated with 10% (v/v) and cultured (160 rpm) in the following five media at 28 °C for 168 h (7 days). The biomass, lipid content, and biodiesel titer of Y. lipolytica were measured every day during the culture process.
Extraction and determination of the lipids in Y. lipolytica
The mixture of lipid-production medium and Y. lipolytica was collected in a 100-mL centrifuge tube and centrifuged at 9000 rpm for 5 min. The supernatant was then discarded, and the cells were washed with distilled water and the solution of methanol:chloroform (v/v = 1:1) triply in sequence. Finally, Y. lipolytica were dried at 50 °C for 24 h and then ground into powder with a mortar.
Approximately 15 mL of 4.0 M hydrochloric acid was added into a centrifuge tube that contained 1.0 g of dried cells. After the centrifuge tube stood for 20 min, it was placed into boiling water for 30 min. The tube was then quickly transferred to a refrigerator at − 20 °C. When the temperature of the mixture dropped to room temperature, the centrifuge tube was placed into the ultrasonic cell disruptor with 150 W for 10 min under ice bath conditions (work 3 s, interval 4 s).
The lipids were extracted with chloroform and methanol under a volume ratio of 2:1. The mixture was stirred vigorously for 3 min by a flash vortex and then centrifuged using a 5840R centrifuge at 4000 rpm for 15 min. The extraction procedure was repeated thrice, and the chloroform layer was transferred to another centrifuge tube. The equal volume of 0.15% sodium chloride solution was added to this centrifuge tube. The mixture was stirred and centrifuged under the same condition. Finally, the bottom layer of the chloroform solution was transferred to a conical flask, and the chloroform was removed using a rotary evaporator. The residue was purged by nitrogen and placed at room temperature for 24 h before weighing the lipids. The lipid content was the percentage calculated according to the mass of acquired lipids per unit mass of dried Y. lipolytica cells, whereas lipid production referred to the mass of acquired lipids per unit volume of culture medium (Wang et al. 2015).
Biodiesel production from extracted lipids and analysis
The extracted lipids were converted to fatty acid methyl esters (FAMEs) through acid catalysis. These lipids were dissolved into the mixture of 20 mL of hexane and 40 mL of methanol with 5% sulfuric acid. The whole mixture was heated to 55 °C for 24 h. After cooling down, 3 mL of saturated sodium chloride solution was first added into the mixture, and then, the FAMEs were extracted with hexane triply (3 × 50 mL). The mixture was stirred vigorously for 3 min by a flash vortex and then centrifuged using a 5840R centrifuge operated at 3000 rpm for 3 min. The extraction procedure was repeated thrice to recover any biodiesel remaining in the methanol phase. The supernatant was transferred to a separatory funnel. Subsequently, 5 mL of 2% KHCO3 was added to this funnel as washing solution. After washing, the aqueous layer was discarded from the separatory funnel, and the hexane phase was dried by passing it through a Whatman filter paper (110 mm in diameter) that contained anhydrous sodium sulfate. The hexane phase was then collected in a weighed flask for the measurement of volume and FAME concentration.
A 1.5-mL aliquot of the hexane phase was pipetted into a 2.0 Supelco PTFE-lined capped vial for FAME analysis. The FAMEs were tested for composition and content analysis using an American Agilent 6890N gas chromatograph (Wang et al. 2015).
Results and discussion
Effect of oily carbon source concentrations solely on the lipid accumulation of Y. lipolytica
Effect of different oil concentrations on the biomass accumulation of Y. lipolytica
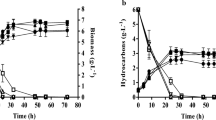
The changes of Y. lipolytica biomass concentration with culture time under different oil concentrations are shown in Fig. 1. The biomass concentration was first increased and then decreased with the culture time, resulting in the maximum biomass concentration at 144 h when different oil concentrations were used as carbon source to culture Y. lipolytica. Compared with 20.0 and 30.0 g L−1 oil concentrations, the biomass concentration was always the largest at 40.0 g L−1 oil concentration when the culture time is constant. Therefore, the maximum biomass concentration was acquired under 40.0 g L−1 oil concentration and 144 h culture time, which was 1.24 and 1.06 times higher than that of the biomass concentration under 20.0 and 30.0 g L−1 oil concentrations, respectively.
Effect of different oil concentrations on lipid quantity of Y. lipolytica
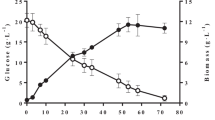
The lipid production changes of Y. lipolytica with culture time under different oil concentrations are shown in Fig. 2.
Figure 2 a shows that a difference in the trend of lipid content during the culture process was observed. The lipid content increased rapidly to 20.10% after 24 h culture time and then fluctuated slightly under 20.0 g L−1 oil concentration, which led to the average lipid content of Y. lipolytica to 24.52% ± 1.96% between 24 and 168 h. By contrast, the lipid percentages of Y. lipolytica increased rapidly to the highest values of 33.20% and 37.40% at 48 h, and then decreased under 30.0 and 40.0 g L−1 oil concentrations, which made the average lipid content of Y. lipolytica 27.70% ± 2.26% and 27.58% ± 1.82% after 48 h. Thus, in terms of the lipid amount that Y. lipolytica accumulated, the optimal condition to acquire the maximum lipid contents in dry cell weight (DCW) was to culture Y. lipolytica for 48 h under 40.0 g L−1 oil concentration.
However, the lipid production per liter of culture was related not only to the lipid content but also to the biomass concentration per unit culture volume. Figure 2 b shows that the change of lipid production per liter culture with time combined the characteristics of Figs. 1 and 2a. The whole process showed an upward trend with the extension of the culture time until the maximum value at 144 h, and then dropped. Therefore, in terms of the lipid amount accumulated per unit volume of the culture medium, the maximum lipid production (3.15 g L−1) was obtained when Y. lipolytica was cultured under 30.0 g L−1 oil concentration for 144 h, which was 1.65 and 1.06 times higher than that of the value under 20.0 and 40.0 g L−1 oil concentrations, respectively.
Commonly, physical methods, such as UV, γ-ray, and X-ray mutagenesis; chemical methods, such as nitrosoguanidine and EMS mutagenesis; and genetic engineering techniques to elevate lipid accumulation of oleaginous microorganisms are present. Sivaramakrishnan et al. improved the lipid accumulation ability of Scenedesmus through UV mutagenesis and H2O2 treatment, by which the biomass of mutant algae increased from 1.90 to 2.40 g L−1, and the lipid content of mutant algae increased from 40 to 55% compared with that of the wild-type algae (Sivaramakrishnan and Incharoensakdi 2017) In addition, high lipid–producing Y. lipolytica was obtained by chemical mutagenesis and cultured with 100.0 g L−1 waste cooking oil medium, where the lipid yield can reach 5.97 g L−1 and was 1.86 times higher than that of wild Y. lipolytica (Katre et al. 2017). In this study, the lipid content of Y. lipolytica increased from 24.46 to 37.35%, and the lipid production per culture volume increased from 1.91 to 2.97 g L−1 when different oil concentrations were used to acclimatize Y. lipolytica. Therefore, increasing the lipid accumulation ability of Y. lipolytica by gradually elevating oil concentrations when oils are used as the only carbon source to adapt Y. lipolytica is a feasible approach.
Effect of different oil concentrations on the titer and composition of biodiesel produced with Y. lipolytica
Effect of different oil concentrations on the titer of biodiesel produced from Y. lipolytica
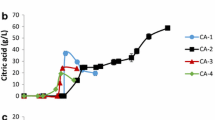
Biodiesel was produced from Y. lipolytica, which was cultured with 20.0 to 40.0 g L−1 rapeseed oil, and the variations of biodiesel titer with culture time under different rapeseed oil concentrations are shown in Fig. 3.
Figure 3 a shows that the change in the trend of biodiesel titer produced from DCW during the culture process was slightly different among different oil concentrations. The titer of biodiesel produced from DCW cultured with 20.0 g L−1 oil concentration increased rapidly to 13.60% in the first 24 h, and then fluctuated slightly. The average titer of biodiesel produced from DCW was 15.71% ± 1.77% after 24 h. However, the titer of biodiesel produced from DCW first increased to the maximum values of 25.30% and 22.20% at 96 h, and then decreased under 30.0 and 40.0 g L−1 oil concentrations. The average titer of biodiesel produced from DCW was 20.14% ± 3.94% and 18.54% ± 2.60% under 30.0 and 40.0 g L−1 oil concentrations, respectively. Therefore, in terms of the titer of biodiesel produced from DCW, the maximum titer of biodiesel from DCW was 25.30% under 30.0 g L−1 oil concentration for 96 h.
By contrast, the biodiesel titer per unit volume culture was related not only to the biodiesel titer from DCW but also to the biomass concentration per unit volume culture. Figure 3 b shows the changes of biodiesel titer per unit volume culture under different oil concentrations. Figure 3 b shows that the variation of biodiesel titer per unit volume culture under different oil concentrations combined the characteristics of Figs. 1 and 3a. The maximum titer of biodiesel produced from per unit culture volume was 1.85 g L−1 under the oil concentration of 30.0 g L−1 for 96 h and 2.08 g L−1 under the oil concentration of 40.0 L−1 for 144 h, respectively, which were evidently higher than the maximum value of 1.35 g L−1 under 20.0 g L−1 oil concentration for 120 h. Therefore, in terms of the biodiesel titer in per unit volume culture, the maximum value of 2.08 g L−1 can be acquired under the oil concentration of 40.0 g L−1 for 144 h.
Effect of different oil concentrations on the composition of biodiesel produced from Y. lipolytica
The composition and percentage of FAMEs in the maximum biodiesel titer acquired from Y. lipolytica cultured under different rapeseed oil are shown in Fig. 4.
Figure 4 shows that the types of FAMEs in biodiesel produced directly from rapeseed oil were similar with that from Y. lipolytica, which contained myristic, palmitic, palmitoleic, stearic, oleic, and linoleic acids. As for the percentage of FAMEs, each kind was very approaching. However, compared with the highest titer of biodiesel produced from Y. lipolytica cultured under 30.0 and 40.0 g L−1 oil concentrations, the composition of biodiesel in the highest titer produced from Y. lipolytica cultured under 20.0 g L−1 oil concentration was slightly different. The linoleic acid (C18:2) content decreased to intracellular 19.10% from extracellular 26.40%, and palmitic acid (C16:0) increased to intracellular 10.60% from extracellular 5.50%. Papanikolaou et al. used a mixture of fatty acids to culture Y. lipolytica and showed that it exhibits evident biomodification for lipid accumulation (Donot et al. 2014; Papanikolaou and Aggelis 2010). They also found that the content of linoleic (C18:2) and oleic acids (C18:1) in Y. lipolytica decreased from extracellular 44.20% and 9.00% to intracellular 22.60% and 3.50% when Y. lipolytica was cultured at a fatty acid mixture concentration of 10.0 g L−1, respectively, whereas stearic (C18:0) and palmitic acids (C16:0) increased from extracellular 22.10% and 13.10% to intracellular 57.30% and 15.60%, respectively (Katre et al. 2017). This result implied that Y. lipolytica can biomodify the lipid but not simply absorb the lipid when the lipid was used as the only carbon source at a certain oil concentration.
The compositions of saturated and unsaturated fatty acids are similar to the various properties of biodiesel. When the content of saturated fatty acids in biodiesel is high, the cetane number is high and the combustion performance is good. The biomodification of Y. lipolytica reduced the content of linoleic acid and increased the content of palmitic acid, which was beneficial to improve the combustion performance of biodiesel. Therefore, Y. lipolytica can absorb the lipid in wastewater and further biomodify the lipids to be the suitable precursor for biodiesel production when the lipid concentration was 20.0 g L−1.
Effects of short-chain carbon mixed with oily carbon on the lipid accumulation of Y. lipolytica
Effect of the addition of short-chain carbon source on Y. lipolytica biomass
Based on the same total concentration of carbon substrate and 40.0 g L−1 rapeseed oil as the control, 5% glucose, 15% glucose, 5% sodium acetate, and 15% sodium acetate were added to a certain quantity of rapeseed oil to form the mixed substrates of 5% glucose + 95% rapeseed oil, 15% glucose + 85% rapeseed oil, 5% sodium acetate + 95% rapeseed oil, and 15% sodium acetate + 85% rapeseed oil, respectively. Variation of Y. lipolytica biomass concentration with time under different mixed substrates is shown in Fig. 5. Figure 5 shows that the biomass concentration of Y. lipolytica cultured with the sole oily carbon source was evidently high compared with all the mixed substrates although the total concentration of carbon substrate was kept the same.
The reason may be because Y. lipolytica can secrete a large amount of lipase in the hydrophobic substance, and lipase can promote the hydrolysis of ester bonds in long-chain triacylglycerols, which are decomposed into fatty acids and glycerol for growth metabolism (Papanikolaou et al. 2007). However, the amount of lipase production in Y. lipolytica cell is directly related to the carbon source used. When glucose is used as carbon source, some intermediate products produced by the metabolic process of glucose exhibit a certain inhibitory effect on the synthesis of lipase (Fickers et al. 2005). Therefore, the Y. lipolytica biomass was decreased after adding glucose to the oil substance. In addition, Y. lipolytica can effectively ferment the medium when sodium acetate was used as the sole carbon source. Y. lipolytica can grow well in a certain range of sodium acetate concentration, but the growth rate decreased when the concentration of sodium acetate exceeded 1% (v/v). The reason was that high concentrations of sodium acetate increased the pH value in the medium, which ceased the growth of Y. lipolytica. Therefore, Y. lipolytica biomass also decreased after the addition of sodium acetate to the hydrophobic substance (Fickers et al. 2005). The results of this study showed that the addition of glucose and sodium acetate, which belong to the easily degraded carbon sources, reduced the Y. lipolytica biomass concentration compared with the single oil carbon source.
Effect of the addition of easily degradable carbon source on the lipid content and lipids of Y. lipolytica
The change of Y. lipolytica lipid content in dried cell and lipid production in the culture medium under different carbon sources is shown in Fig. 6. Figure 6 a shows that the change in the trend of lipid content in DCW during the cultivation process varied greatly. The lipid accumulation of Y. lipolytica under a mixed carbon source of 5% sodium acetate addition was similar to that of Y. lipolytica under 40.0 g L−1 single oil carbon source. Both showed a trend of rapid increase to the maximum and then decreased. The main difference is that the maximum value of mixed carbon source was 41.50% at 72 h, whereas the maximum value of single oil carbon source was 37.35% at 48 h, which caused the average lipid contents of Y. lipolytica 30.12% ± 2.98% and 27.58% ± 1.82% for mixed carbon source of 5% sodium acetate addition and single oil carbon source, respectively. However, under the mixed carbon source with the addition of 5% glucose, 15% glucose, and 15% sodium acetate, the lipid accumulation of Y. lipolytica increased with the culture time and was significantly lower than that of Y. lipolytica cultured with 40.0 g L−1 single oil carbon source. The lipid accumulation of Y. lipolytica was no more than 8% within 96 h for these three mixed carbon sources. Even at 168 h culture time, the lipid accumulation of Y. lipolytica was still approximately 20%. Therefore, in terms of Y. lipolytica lipid accumulation, the maximum lipid content was 41.5%, which was acquired under the mixed carbon source with 5% sodium acetate for 72 h.
Figure 6 b shows the variation of lipid production per unit volume of culture under different carbon sources. The lipids in culture medium per unit volume was the result of lipid content in DCW and biomass per unit volume medium. In addition, the figure also shows that the change in lipids under the conditions of adding different easily degradable carbon sources was the same as that in Fig. 6 a. The lipids of the mixed carbon source with 5% sodium acetate increased rapidly to the maximum value and then decreased. The lipids of the mixed carbon source with 5% sodium acetate were significantly higher than the lipids under the other mixed carbon sources with the addition of easily degradable carbon, but were slightly lower than the lipids under the condition of 40.0 g L−1 single oil carbon source culture. When the mixed carbon source with 5% sodium acetate lasted for 120 h, the lipids reached the maximum value of 2.78 g L−1, which was 11% lower than the maximum value of 3.15 g L−1 under 40.0 g L−1 single oil carbon source culture condition. Therefore, the lipids cultured under the addition of easily degraded carbon source were lower than that of the single oil carbon source under the same total carbon concentration.
Effect of the addition of easily degradable carbon source on biodiesel titer
Biodiesel was produced with Y. lipolytica cultured under the mixed carbon source with 5% sodium acetate and the single carbon source of 40.0 g L−1 oil. The variation of biodiesel titer with culture time under different carbon sources is shown in Fig. 7. Figure 7 a shows that the biodiesel titer from Y. lipolytica cultured with mixed carbon source with 5% sodium acetate was substantially lower than that of Y. lipolytica cultured by single oil carbon source. Unsurprisingly, the biodiesel production per unit volume medium of single oil carbon source (40.0 g L−1) was significantly higher than that of the mixed carbon source with 5% sodium acetate.
The variation of intracellular lipid content and biomass concentration in Y. lipolytica played an important relationship with the carbon source, nitrogen source, and other growth conditions in the medium. When 5% sodium acetate was added, the intracellular lipid content of Y. lipolytica increased but the biomass concentration was reduced compared with the single oil carbon source (40.0 g L−1), which indicating that 5% sodium acetate can promote intracellular lipid accumulation but it is not conducive to Y. lipolytica cell growth and metabolism. Therefore, the lipids and biodiesel titer were not the highest. In addition, the mixed carbon source with the addition of 5% and 15% glucose and 15% sodium acetate was not conducive to biomass increase or lipid accumulation.
De novo and ex novo pathways are two completely different lipid accumulation pathways in Y. lipolytica metabolism. De novo pathway means that Y. lipolytica synthesizes lipids from acetyl-CoA using a hydrophilic substance, which occurs under nitrogen depletion conditions (Liu et al. 2015). Ex novo pathway means that Y. lipolytica using hydrophobic substance accumulates lipids during its growth, which does not depend on nitrogen depletion. The coexistence of easily degradable carbon and hydrophobic substrates in actual oily wastewater is unavoidable. If the easily degradable carbon can be used for growth and metabolism and the hydrophobic substance can be used to accumulate lipids and biomodification, the maximum utilization of carbon sources in oily wastewater was realized. The results of this experiment showed that adding an appropriate amount and type of easily degradable carbon into the hydrophobic substrate could increase the ability of Y. lipolytica lipid accumulation. However, the addition of easily degradable carbon sources decreased the Y. lipolytica biomass, which in turn reduced the biodiesel titer. In view of this, lipid hydrolysis in oily wastewater must be avoided as much as possible when Y. lipolytica was used for biodiesel production during the treatment of oily wastewater to maximize the utilization of lipid resources.
Conclusion
The gradient acclimation of Y. lipolytica with a single oil carbon source can improve lipid accumulation. When the oil concentration in the culture medium increased from 20.0 to 40.0 g L−1, the lipid content of Y. lipolytica increased from 24.46 to 37.35%, and the corresponding lipids increased from 1.91 to 2.97 g L−1. Meanwhile, Y. lipolytica showed an evident biomodification effect on lipids with the oil concentration of 20.0 g L−1, which was beneficial to the production of high-performance biodiesel. In addition, the lipid accumulation ability of the Y. lipolytica was not improved when short-chain carbon compounds were added into the oil substrate. Therefore, the lipid hydrolysis must be avoided as far as possible to maximize the utilization of lipid resources when the oily wastewater was used to produce biodiesel with Y. lipolytica. In summary, Y. lipolytica can utilize and biomodify the lipids of the oily wastewater, which supplied the possibility of biodiesel production and wastewater purification simultaneously. So, this research has great significance to the sustainable development of environment protection and energy recycling.
References
Belle HV, Goossens F (2011) Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur J Lipid Sci Technol 113:1031–1051
Chen YC, Lin DY, Chen BH (2017) Transesterification of acid soybean oil for biodiesel production using lithium metasilicate catalyst prepared from diatomite. J Taiwan Inst Chem Eng 79:31–36
Donot F, Fontana A, Baccou JC, Strub C, Schorr-Galindo S (2014) Single cell oils (SCOs) from oleaginous yeasts and moulds: production and genetics. Biomass Bioenergy 68:135–150
Dumore NS, Mukhopadhyay M (2012) Removal of oil and grease using immobilized triacylglycerin lipase. Int Biodeterior Biodegrad 68:65–70
Etchepare R, Oliveira H, Azevedo A, Rubio J (2017) Separation of emulsified crude oil in saline water by dissolved air flotation with micro and nanobubbles. Curr Microbiol 186:326–332
Fickers P, Nicaud JM, Destain J, Thonart P (2005) Involvement of hexokinase Hxk1 in glucose catabolite repression of LIP2 encoding extracellular lipase in the yeast Yarrowia lipolytica. Curr Microbiol 50:133–137
Katre G, Ajmera N, Zinjarde S, Ravikumar A (2017) Mutants of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil as a biofactory for biodiesel production. Microb Cell Factories 16:176–188
Li Y, Mei H, Fang H (2013) A review of treating oily wastewater. Arab J Chem 265:1913–1922
Liu HH, Ji XJ, Huang H (2015) Biotechnological applications of Yarrowia lipolytica: past, present and future. Biotechnol Adv 33:1522–1546
Ma X, Gao M, Gao Z, Wang J, Zhang M, Ma Y, Wang Q (2018a) Past, current, and future research on microalga-derived biodiesel: a critical review and bibliometric analysis. Environ Sci Pollut Res 25:1–15
Ma Y, Gao Z, Wang Q, Liu Y (2018b) Biodiesels from microbial oils: opportunity and challenges. Bioresour Technol 263:631–641
Pandit PR, Fulekar MH, Karuna MSL (2017) Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ Sci Pollut Res 24:13437–13451
Papanikolaou S, Chevalot I, Galiotou-Panayotou M, Komaitis M, Marc I, Aggelis G (2007) Industrial derivative of tallow: a promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron J Biotechnol 10:425–435
Papanikolaou S, Aggelis G (2010) Selective uptake of fatty acids by the yeast Yarrowia lipolytica. Eur J Lipid Sci Technol 105:651–655
Putatunda S, Bhattacharya S, Sen D, Bhattacharjee C (2018) A review on the application of different treatment processes for emulsified oily wastewater. Int J Environ Sci Technol 16:2525–2536
Qin L, Wang Z, Sun Y, Shu Q, Feng P, Zhu L, Xu J, Yuan Z (2016) Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ Sci Pollut Res 23:8379–8387
Sahar SS, Iqbal J, Ullah I, Bhatti HN, Nouren S, Habib-ur-Rehman NJ, Iqbal M (2018) Biodiesel production from waste cooking oil: an efficient technique to convert waste into biodiesel. Sust Cities Soc 41:220–226
Saki S, Uzal N (2018) Preparation and characterization of PSF/PEI/CaCO3 nanocomposite membranes for oil/water separation. Environ Sci Pollut Res 25:1–12
Sivaramakrishnan R, Incharoensakdi A (2017) Enhancement of lipid production in Scenedesmus sp. by UV mutagenesis and hydrogen peroxide treatment. Bioresour Technol 235:366–370
Song H, Zhou L, Zhang L, Bei G, Wei D, Shen Y, Rui W, Madzak C, Jiang Z (2011) Construction of a whole-cell catalyst displaying a fungal lipase for effective treatment of oily wastewaters. J Mol Catal B-Enzym 71:166–170
Wang Y, Feng S, Bai X, Zhao J, Xia S (2015) Scum sludge as a potential feedstock for biodiesel production from wastewater treatment plants. Waste Manag 47:91–97
Zeng Y, Yang C, Zhang J, Pu W (2007) Feasibility investigation of oily wastewater treatment by combination of zinc and PAM in coagulation/flocculation. J Hazard Mater 147:991–996
Zhang X, Bing Z, Wu Y, Wang T, Qiu J (2018) Preparation and characterization of a diatomite hybrid microfiltration carbon membrane for oily wastewater treatment. J Taiwan Inst Chem Eng 89:39–48
Zhao C, Zheng H, Gao B, Liu Y, Zhai J, Zhang S, Xu B (2018) Ultrasound-initiated synthesis of cationic polyacrylamide for oily wastewater treatment: enhanced interaction between the flocculant and contaminants. Ultrason Sonochem 42:31–41
Živković S, Veljković M (2018) Environmental impacts the of production and use of biodiesel. Environ Sci Pollut Res 25:191–199
Funding
This work was supported by the National Natural Science Foundation of China (No. 21677115) and the Shaanxi Provincial Natural Science Foundation Research Key Project (No. 2016JZ019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chai, B., Wang, Y., Wang, W. et al. Effect of carbon source on lipid accumulation and biodiesel production of Yarrowia lipolytica. Environ Sci Pollut Res 26, 31234–31242 (2019). https://doi.org/10.1007/s11356-019-06249-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06249-w