Abstract
In this study, a pot experiment was piloted in a greenhouse to evaluate the potential of Celosia argentea var. cristata L. for tolerating/accumulating heavy metals in synthetic wastewater in the presence of Pseudomonas japonica and organic amendment, i.e., moss and compost. Two-week-old seedlings were transferred to pots, and after 4 weeks, the bacterial strain was inoculated, then watered with synthetic wastewater for 5 weeks and harvested after 9 weeks. After harvesting, physiological and biochemical parameters, as well as metal contents of plants, were quantified. The results indicated highest growth and biomass production in moss- and compost-associated plants while highest metal uptake has been found in the presence of P. japonica and synthetic wastewater–irrigated plants. Synthetic wastewater–irrigated plants have shown highest Pb uptake of 2899 mg kg−1 DW, while with P. japonica in soil those plants have shown highest Cd, Cu, Ni, and Cr uptake of 962, 1479, 1042, and 956 mg kg−1 DW, respectively. The production of antioxidant enzymes, i.e., catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), and glutathione-s-transferase (GST), was high in P. japonica–amended plants because of increased uptake of metals. It is concluded that moss and compost have improved growth while P. japonica improved metal accumulation and translocation to aerial parts with little involvement in plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last few decades, our environment is under serious threat, due to intense anthropogenic activities (Khan et al. 2016a). A wide variety of pollutants, including heavy metals, have been released into the natural environment which are posing severe ecological, nutritional, and health effects to the ecosystem, among which heavy metals have acquired considerable attention due to their significant environmental and health concerns. They are ubiquitous, non-biodegradable, persistent, and highly toxic even at low concentrations; their entry into the body poses a potential health risk to humans (Arshad et al. 2017). Due to their toxic properties, they often face strict regulations, so they need to be removed from the environmental compartments.
Several physical and chemical methods are currently being used for the remediation of heavy metals from soil. These processes are costly, involve extensive labor, and cause permanent changes in soil properties and microbial diversity, and sometimes generate secondary pollutants. In recent decades a biological method, i.e., phytoremediation has emerged as an eco-friendly and cost-effective technique that makes the use of plants and their associated microbes for environmental cleaning and restoration purposes. It has been used effectively for various contaminants that might be organic or inorganic in nature, especially for metals (Zhong et al. 2019). It uses the idea that some plants, having a natural ability to grow on metal-rich soils, develop the resistance and capacity to accumulate high levels of metals in internal tissues without showing toxicity effects (Lajayer et al. 2019).
In some cases, this technique faces limitations in the field due to the type and availability of heavy metals. Its acceptance relies on the ability of plants to tolerate and accumulate high concentrations of heavy metals along with higher biomass production (Zhong et al. 2019). In view of practical applications, a key option is the use of rhizospheric microbes having numerous benefits for plants, including growth promotion. Further, many of these microbial strains have strong ability to tolerate contaminants and contribute towards the tolerance and accumulation of heavy metals by plants (Boechat et al. 2017).
The availability of heavy metals to roots is a crucial factor in the process of phytoextraction. Plant’s ability to take up, accumulate, and translocate heavy metals in aerial parts of plants is a crucial factor towards phytoextraction (Boechat et al. 2017). Microbes can enhance the availability of heavy metals in soil solution through the process of solubilization and mobilization; hence, metal uptake by plants increases. Plants and these microbial species co-evolved with each other in a very complex mechanism resulting in a large number of associations between them. Plant bacterial interaction can be divided into three categories: rhizospheric, endophytes, and epiphytes. Plant growth–promoting rhizobacteria (PGPR) include those communities that are present inside the rhizospheric zone and help in plant growth and tolerance. About 2–5% of rhizospheric bacteria are known as PGPR (Nadeem et al. 2017). These PGPR can potentially enhance or reduce metal availability by changing the solubility, availability, and transport of heavy metals. It can also alter the nutrient availability by changing soil pH, releasing chelators, P solubilization, or redox changes. Microorganisms can also enhance the availability of metals with the production of siderophores and low-molecular-weight organic acids and decrease metal mobility through precipitation and reduction mechanisms (Boechat et al. 2017). Until now, several plants have been studied for their remediation potential, but little information is available regarding ornamental plants. Ornamental plants are abundant and can monitor atmospheric contamination. It is essential to investigate these plants, as if they turned out to be effective accumulator and hyperaccumulators, and their application will have more practical advantages if they can be used in combination with microbial species (Liu et al. 2017). The comparison of phytoextraction potential of various terrestrial ornamental plants is provided in Supplementary Table 1.
The use of wastewater to irrigate edible crops and contamination of soil with heavy metals has become a global concern for animals and human health. The condition is further worsened due to the increase in industrialization and urbanization. Also, there are many studies which reported the variety of heavy metals in the soil, which becomes more complex and challenging to tackle (Mishra et al. 2019). Therefore, we explored the growth and heavy metal accumulation properties of Celosia argentea var. cristata L. with Pseudomonas japonica and selected soil amendments (peat moss and compost). C. argentea also known as cockscomb belongs to family Amaranthaceae. It is found commonly in Africa, South America, and some parts of Asia. The plant can grow in full sun as well as in the shade and need acidic sandy/loamy soil.
Recently more and more ornamental plants are analyzed and identified for the potential of heavy metal removal. Until now, very few studies have been reported for C. argentea potential for heavy metal extraction. According to preliminary investigations, C. argentea var. cristata has strong accumulation properties for Pb (González-Valdez et al. 2016). So, in this study, C. argentea is used in combination with P. japonica for accessing the potential of phytoextraction of heavy metals. Another main characteristic of this study is the use of five different heavy metals as the soil has many different heavy metals in real conditions instead of single heavy metal. Celosia argentea has an additional advantage that it has esthetic properties due to its eye-catching red, orange, and yellow flowers.
The objectives of this study were (i) to evaluate the effect of P. japonica on the uptake of Cu, Cd, Cr, Pb, and Ni by C. argentea; (ii) to demonstrate the role of peat moss and compost on the growth of C. argentea; and (iii) to study the effect of peat moss and compost on the mobilization of heavy metals in the soil.
Materials and methods
Plant materials and growth conditions
Seeds of C. argentea were purchased from local seed supplier (Ewan Garden, Islamabad, Pakistan). Seeds were sown in pots containing sterilized sand, watered with half-strength Hoagland’s nutrient solution, and incubated in a growth chamber at 22 °C with a photoperiod of 8:16, day:night. Uniform height seedlings were collected at four leaves stage (4 weeks old) and transferred to pots (one plant per pot) containing sterilized agricultural soil.
Experimental design
The plastic pots were filled with 350 ml of soil. The soil was prepared by mixing agriculture soil and sand in 2:1. Compost and peat moss were added with the ratio 5% (v/v) with the total soil. Details of all treatments used in experiments are provided in Table 1. Previously isolated and characterized bacterial strain Pseudomonas japonica, also used by Arshad et al. (2017), was used for the preparation of bacterial cell suspension (108 cells ml−1 in 10 mM potassium phosphate buffer saline (PBS), pH 7.4), as done by Khan et al. (2016b). P. japonica treatment was developed by adding by adding 10 ml of the bacterial suspension in pot and were left for 2 weeks for acclimation. After 2 weeks of inoculation of the bacterial strain, pots were watered with synthetic wastewater. The composition of synthetic wastewater is given in Table 2.
Plant harvesting
Plants were watered with 13 ml of synthetic wastewater daily (twice a day) and harvested after 3 weeks of exposure. After harvesting plant, physiological parameters (fresh and dried weight of roots, shoots, leaves, and flowers, length of shoot and root, total leaves per plant, and leaf area) were noted. Root and shoot lengths were measured in centimeters using a scale. The root of every plant was measured from the basal portion of the stem to the tip of the longest root. The shoot was measured from the starting point of the stem to the uppermost leaf. The leaf area was determined by using image processing software, Image J (Yahmed et al. 2016). Some fresh plant material was stored at − 80 °C for stress injury and enzyme activities. The plant material was dried at 50 °C in an oven for 72 h until constant weight is achieved. These samples were weighed to obtain dry weight.
Estimation of root tolerance index
The root tolerance index was measured to calculate the ability to plant to grow on metal-contaminated soil using Eq. 1 (Yang et al. 2015).
Stress injury assay
Chlorophyll content was measured in terms of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents spectrophotometrically as described by Arnon (1949). These pigments were extracted from 40 mg of fresh biomass of leaves. Absorbance at 663, 645, and 470 nm against blank of pure acetone was used to calculate these contents using equations proposed by Lichtenthaler (1987).
Lipid peroxidation was assessed in terms of malondialdehyde (MDA) by using thiobarbituric acid (TBA) as explained by Davenport et al. (2003). The absorbance was measured at 450, 532, and 600 nm. The final concentration was calculated using the equation described by Michael and Krishnaswamy (2011).
where Vt = 0.001 L; W = 0.1 g
Electrolyte leakage (EL) was evaluated by using electrical conductivity meter of Cyberscan PC 510 by Eutech instrument (Khan et al. 2019). Two readings of electrolyte leakage were measured according to the method used. Final results were evaluated by using this formula:
where EC1 = primary electrical conductivity and EC2 = secondary electrical conductivity.
Estimation of antioxidant enzymes
Enzyme extract was prepared from leaves. For each treatment, 0.1 g of leaf was homogenized in pre-chilled mortar and pestle with 1 ml of ice-cold potassium phosphate buffer (50 mM, pH 7.4 containing 0.5 mM EDTA). The extract was centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was collected and stored in ice-cold condition for determination of antioxidant enzymes and H2O2 content (Venkatachalam et al. 2017).
For hydrogen peroxide (H2O2) determination, the method of Khan et al. (2019) with little modification was used. The absorbance was recorded at 410 nm, and an extinction coefficient of 0.28 μM−1 cm−1 was used for computing the H2O2 content.
Catalase (CAT) activity was evaluated by the procedure of Maehly and Chance (1954). Two reaction mixtures A and B were used. The absorbance was measured at 240 nm after 1 min, and the extinction coefficient of 39.4 mM−1 cm−1 was used to compute the concentration of CAT.
Ascorbate peroxidase (APX) activity was measured by the protocol of Chen and Asada (1989) with little modification. The absorbance was measured at 240 nm after 3 min, and the extinction coefficient of 2.8 mM−1 cm−1 was used to calculate APX activity.
Guaiacol peroxidase (GPX) activity was evaluated by following the method of Upadhyaya et al. (1985). The absorbance was recorded at 420 nm after 1 min. The activity of GPX was calculated using an extinction coefficient of 26.6 mM−1 cm−1.
Glutathione-s-transferase production was measured by following the method of Xu et al. (2016). The absorbance was measured at 340 nm at 120 s. The following equation measured GST production:
where ΔOD represents the change in absorbance at 340 nm min−1, V is the volume of the reaction mixture, E is the absorption coefficient equal to 9.6 mM−1 cm−1, and L is the cuvette’s length in centimeters. In this experiment, for the spectrophotometry, UV-9200/VIS-7220G, Rayleigh spectrophotometer was used for all measurements.
Plant samples preparation for metals analysis
Dried plant samples were used for metal analysis using acid digestion method. Fifty milligrams of dried samples were taken and processed through grinding. After grinding, 3 ml concentrated HNO3 was added and swirled gently then 3 ml of H2O2 and 0.5 ml of concentrated HCl was added. Hot plate was set at temperature 150 °C, and samples were placed for 10 min with 10-min hold for cooling. After cooling, they were filtered using Whatman filter paper no. 42 and the final volume was adjusted up to 25 ml (Estefan et al. 2013). Digested samples were used for metal analysis using atomic absorption spectrometry (AAS), Perkin Elmer, AAS-700, to assess the uptake of metals in different plant compartments.
Statistical analysis
Analysis of variance (ANOVA) was done using IBM, SPSS version 2.1. They were compared through Duncan’s test with significant differences noted at p < 0.05, and significantly different values were shown with different letters. All experiments were conducted in triplicates.
Results
Plant growth parameters
Table 3 demonstrates the leaf count, leaf area, root length, shoot length, and fresh and dried weight of roots, shoots, leaves, and flowers of C. argentea under different treatments, i.e., peat moss, compost, and P. japonica. With the addition of organic amendments, there was evidence of increasing plant growth. In terms of leaf count, plants with moss + inoculum (M+I), compost, and moss exhibited the highest leaf number per plant (i.e., 23, 21, and 20, respectively). Moss + compost treatment plants produced 18 leaves while bacterial strain–amended plants have shown the lowest number of leaves but still significantly higher as compared to CW (Table 3).
The maximum leaf area has been found with moss, compost, and strain (M+C+I) combination, i.e., 1.34 cm2. Plants with peat moss and inoculum (1.29 cm2) have shown the highest leaf area as compared to compost with inoculum, i.e., 0.96 cm2 (Table 3).
Compared to controls, M+C+I amended plants showed 6% and 22% increase in root and shoot length. The probability of heavy metals to reduce plant growth by changing physiological and biochemical processes is well known. P. japonica does not have a significant impact on root and shoot length as compared to CW. The combination of moss and compost has shown the second highest shoot length, i.e., 20% more in contrast to controls. The compost showed a 2% increase in root length as compared to controls. Peat moss in combination with inoculum showed 21.7-cm shoot while 6.5-cm root length, while C+I showed 5.6-cm root and 19.5-cm shoot length. The CW-treated plants showed significantly reduced plant growth as compared to plants watered with regular water (UW) (Table 3).
Maximum root, shoot, leaves, and flower weights have been noted in the case of M+C+I. Moss and compost alone or in combination have a significant impact on the plant growth as compared to bacterial inoculum and CW (Table 3).
Biochemical parameters and stress injury
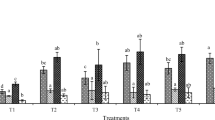
Photosynthetic pigments are considered a sensitive indicator of stress. Photosynthetic pigments were measured in all the treatments in terms of chlorophyll a, chlorophyll b, and carotenoids by using Eqs. 2, 3, and 5, respectively. Total chlorophyll content was found highest in M+C+I as compared to UW controls. There was a significant reduction in photosynthetic pigments production in CW-irrigated controls. The second highest chlorophyll contents were found in the case of M, M+C, and M+I. Low chlorophyll content was noted in the case of C, I, and C+I which were lower than UW (Fig. 1).
Effects of heavy metals on photosynthetic pigments of C. argentea L. after 9 weeks of the growth period, UW (plants irrigated with uncontaminated water), CW (plants irrigated with contaminated synthetic wastewater), M (peat moss), C (compost), and I (bacterial inoculum). Different alphabets on each bar represent statistically significant differences between each studied parameter, with “a” representing significantly highest value followed by the later alphabets at significance level p < 0.05. Each bar is a mean of 3 values ± SE
Lipid peroxidation results due to both biotic and abiotic stresses were calculated using Eq. 6. Heavy metals have been reported to cause lipid breakdown in stressed plant parts as shown by the results of this experiment. Plants that were treated with CW have shown the highest stress factor statistically. Plants amended with C+I resulted in 61% decreased MDA content as compared to CW-treated plants. Compost treatment showed 59% less lipid peroxidation in contrast to CW. P. japonica–amended plants have also demonstrated significantly reduced lipid peroxidation as compared to CW (Fig. 2a).
Effects of heavy metals on (a) lipid peroxidation (in terms of MDA content), (b) electrolyte leakage, and (c) H2O2 content after 9 weeks of growth period, UW (plants irrigated with uncontaminated water), CW (plants irrigated with contaminated synthetic wastewater), M (peat moss), C (compost), and I (bacterial inoculum). Different alphabets on each bar represent statistically significant differences between each studied parameter, with “a” representing significantly highest value followed by the later alphabets at significance level p < 0.05. Each bar is a mean of 3 values ± SE
Electrolyte leakage (EL) was assessed to evaluate the integrity of plant cells by using Eq. 7. Highest EL was recorded in the case of CW. There was no significant difference in EL of plants amended with compost and M+I, while bacterial strain treatment individually had shown 8% reduction in EL content. UW-irrigated plants had shown a 19% reduction in electrolyte content as compared to CW (Fig. 2b).
H2O2 content was found maximum in CW-treated plants. In the case of UW, 33%, less H2O2 content was recorded. The strain amended plants showed 9% less H2O2 content as compared to UW but significantly higher as compared to other amendments. All the other amendments alone or in combination showed similar H2O2 content (Fig. 2c).
Antioxidant enzyme activities
The activities of CAT, APX, GPX, and GST in C. argentea in selected treatments are presented in Table 4. Maximum activities of CAT, APX, GPX, and GST with values of 0.04, 0.46, 0.007, and 0.075, respectively, were recorded in the leaves in CW treatment. GST activity was calculated using Eq. 8. Enzyme activities reduced with the addition of organic amendments. Maximum reduction was detected in the case of M+C and M+C+I amendment as compared to CW. In the case of bacterial inoculum, the activities were significantly low, but the differences were not too significant. ANOVA summary for parameters studied of C. argentea is provided in Supplementary Table 2.
Root tolerance index
Root tolerance index (RTI) was measured to estimate the capability of C. argentea to grow on metal-contaminated soil with different amendments using Eq. 1. The CW-treated controls and inoculum-amended treatments showed the lowest percentage of RTI while the treatments with peat moss and compost showed high RTI. The highest RTI percentage was found in M+C+I. The root tolerance index of all the treatments is given in Table 5.
Plants’ metal content
Different treatments showed the different distribution of heavy metals in roots, shoots, leaves, and flowers. CW-irrigated plants showed highest Pb uptake with the following trend: root (1207) > shoot (896) > flower (554) > leaf (242 mg kg−1 DW). Bacterial strain–amended plants have shown second highest uptake with the following trend: flower (778) > leaf (574) > root (392) > shoot (213 mg kg−1 DW). Plants with M+C have shown the lowest Pb content in all plant tissues of C. argentea. There was no significant effect of bacterial strain in the presence of amendments on Pb uptake in plants. But there was a difference in translocation pattern with aerial parts having more Pb content (Fig. 3a).
Effect of different amendments on metal uptake of C. argentea L. (a) Lead, (b) cadmium, (c) copper (d), nickel, and (e) chromium in root, shoot, leaf, and flower after 9 weeks of exposure to heavy metals containing synthetic effluent, UW (plants irrigated with uncontaminated water), CW (plants irrigated with contaminated synthetic wastewater), M (peat moss), C (compost), and I (bacterial inoculum). Different alphabets on each bar represent statistically significant differences between each plant compartment, with “a” representing significantly highest value followed by the later alphabets at significance level p < 0.05. Each bar is a mean of 3 values ± SE
Regarding the other four metals, Cd, Cu, Ni, and Cr, bacterial strain–amended plants have shown higher uptake and translocation to the aerial parts. In the case of Cd, plants have an overall content of 962 mg kg−1 in the case of inoculum. Its distribution follows the following pattern: leaf (398) > flower (328)> root (167) > shoot (69 mg kg−1 of DW) (Fig. 3b). Copper showed an overall content of 1479 mg kg−1 with the following pattern: flower (598) > leaf (437) > root (319) > shoot (125 mg kg−1 of DW) in the case of inoculum (Fig. 3c). Nickel had 1042 mg kg−1 content with the following pattern: flower (399) > leaf (290) > root (193) > shoot (160 mg kg−1 of DW) in the presence of inoculum (Fig. 3d). The overall Cr content was 956 mg kg−1 DW with the following distribution pattern: leaf (328) > flower (392) > root (167) > shoot (69 mg kg−1 of DW) in the presence of P. japonica (Fig. 3e).
The second highest content of Cu, Cd, Cr, and Ni was found in CW-irrigated plants having overall Cd of 570 mg kg−1, Cu of 1234 mg kg−1, Ni of 996 mg kg−1, and Cr of 570 mg kg−1 by DW (Fig. 3b–e). The pattern of distribution was as follows: (i) Cd, root > shoot > leaf > flower (Fig. 3b); (ii) Cu, root > flower > shoot > leaf (Fig. 3c); (iii) Ni, flower > root > shoot >leaf (Fig. 3d); Cr, root > shoot > leaf > flower (Fig. 3e). Moss and compost have shown significantly reduced metal uptake and accumulation, and Pseudomonas japonica in combination with moss and compost showed no effect. The ANOVA summary of heavy metal uptake and compartmentalization in C. argentea is given in Supplementary Table 3.
Discussion
Plant growth is dependent on the quality of the soil. The present study showed that plant biomass of controls treated with synthetic wastewater and plants inoculated with P. japonica decreased significantly under selected heavy metal stress. This finding is consistent with the previous studies that heavy metals affect the growth of plants. The growth of 2 genotypes of Salix, i.e., Salix matsudana Koidz. (A42) and Salix psammophila C. (A94), decreased due to the exposure and accumulation of Cd and Pb (Xu et al. 2019). The plant biomass of treatments with organic amendments, i.e., compost and peat moss, were found high as compared to controls. Organic amendments not only improve the quality of soil as they provide nutrients to plants, but also improve the microbial abundance. Moss and compost are two types of amendments that were used in this experiment, and both amendments have resulted in improved plant growth as compared to non-amended soil. Mishra et al. (2019) studied the effect of different soil amendments and microorganisms on the bioavailability of metals (Zn, Cd, and Pb) and biomass of Indian Mustard. The results revealed that the green manure–treated plants showed the highest shoot biomass, while the lowest shoot biomass with controls.
On the other hand, the highest root biomass and seed production were found in green manure and Pseudomonas sp. This is in line with the outcomes of the present study where M+C+I showed the highest root and shoot lengths, i.e., the indicator of better growth. It was attributed to the improved soil texture, soil organic carbon, enzymatic, and microbial activity with the addition of amendments. The application of green tea amendment (GTA) and oil cake amendment (OCA) significantly enhanced the quality of soil and decreased the bioavailable fraction of Cd in soil, therefore alleviated Cd stress to Pakchoi cabbage which leads to the highest biomass of aboveground parts with GTA and OCA treatments as compared to controls (Yang et al. 2018). Moss has acidic properties as compared to compost that has usually neutral or alkaline pH. If the soil has alkaline pH, moss application will result in pH reduction (Yang et al. 2018). Moss does not compact over time while compost does. So moss provides better aeration and moisture content for more extended periods. The improvement in plant growth as a result of the addition of moss and compost can be attributed to increased decomposition of organic matter and mineralization of nutrients. Addition of compost enhances the soil water holding capacity and soil quality due to the improvement of nutrients in the soil and hence increases the growth of the plants (Nadeem et al. 2017). Peat moss enhances the plant growth with an increase in water retention capability of soil due to its enhanced capillary forces and cation exchangeability. As compared to other growth substrates, peat moss has the highest moisture retention ability. Stanislawska-Glubiak et al. (2015) reported 2–2.5-fold grain, 2–3-fold more roots, and 1.2–1.6-fold higher growth of other aerial parts in maize with moss-amended soils, while compost loses its nutritional value over time, so it needs replenishment. The reduction in metal availability in the presence of peat moss can be attributed to various sorption mechanisms, including complexation, surface adsorption, ion exchange, and adsorption complexation. The most common among them is the ion exchange mechanism. Peat moss contains surface-charged particles that attract opposite-charged metal ions in the ion exchange mechanism (Yang et al. 2018). The removal of the adsorbed metal ion depends on the strength of adsorption.
In contrast to ion exchange, complexation binds metal ions more strongly. Therefore, the metals binding with electrostatic attractions and ion exchange are more likely on the surface of the peat moss to take place. Compost involves three mechanisms, including adsorption and complexation, redox reactions, and precipitation for reducing the availability of heavy metals to plants. Pseudomonas japonica individually did not improve plant growth. The reason for not showing enhanced plant growth can be the high level of metal accumulation and hence the stress on the plant body. Plants without any amendments showed less growth because of low nutrient content in plain soil and high metal stress in plant body due to the accumulation of metals in C. argentea.
The chlorophyll content is sometimes used as a visual indicator to measure the environmental stress on the plant body to see plant illness and photosynthetic productivity. In the present study, chlorophyll content was found low in inoculum-amended treatments and contaminated water treatments, a clear sign of reduced growth and enhanced metal stress. On the other hand, peat moss and compost-amended plants showed increased chlorophyll content that indicates enhanced growth of the plant and reduced metal stress. The contradictory parts of the data are perhaps because of different heavy metals, which may compete in the soil. Another critical factor is the production of organic acids, playing an essential role in the soil nutrient availability, mobilization of metals, ecology, and productivity. These organic acids can be originated from a range of sources including root exudates, microorganisms, decomposition of organic matter, and may reflect plant response to biotic and abiotic stresses. They have a strong capability to dissolve insoluble fractions of heavy metals by acidification, chelation, and cation exchange reactions. So, acidification and chelation mechanisms facilitate the mobility and availability of metals (Adeleke et al. 2017). Organic acids increase the mobility of metals in soil by reducing the pH of the soil and the formation of complexes with the metals. Organic acids released from plant roots affect the heavy metals in soil by increasing the bioavailability, reducing sorption, and mobilizing the metals. Bacillus, Pseudomonas, Enterobacter, and Rhizobium are some of the microbes producing organic acids (Adeleke et al. 2017). P. japonica enhanced the mobility of heavy metals in soil due to the release of organic acids and increased metal uptake by the plant. In a study, Typha latifolia has shown to uptake increased concentrations of Pb and Zn due to the release of oxalic acids by roots (Man et al. 2016). Bao et al. (2011) studied Cd hyperaccumulator Solanum nigrum L. and concluded that the soil solution was acidic due to the release of organic acids such as oxalic, malic, and citric acids in the surrounding environment, and high content of Cd was found in Solanum nigrum tissues.
Phytohormones play a significant role in plant growth, development, and survival. Phytohormones include indole-3-acetic acid or auxin (IAA), abscisic acid (ABA), ethylene (ET), cytokinin (CK), and gibberellin (GA). Plant hormones such as strigolactone (SL), brassinosteroid (BR), jasmonic acid (JA), and salicylic acid (SA) are recently identified that are vital for the growth and development of plants (Sytar et al. 2018). Phytohormones play an essential role in signaling and defense against heavy metal stress. They maintain and support the plant against abiotic and biotic stresses. ABA plays a crucial role in seed development and dormancy during different stages in the plant life cycle. It is considered necessary for combatting several environmental stresses. The concentration of ABA is known to increase in plant tissues after the exposure of heavy metals, suggesting the involvement of ABA in the stimulation of defense mechanism in response to heavy metals (Bücker-Neto et al. 2017). IAA is the multifunctional plant hormone playing an essential role in the development of plants both under normal and stressed conditions. The concentration of IAA has been found to increase under heavy metal stress. The enhanced level of IAA has been linked to the reduced growth of a plant which is associated with the change in hormonal balance under heavy metal stress. The endogenous application of auxins in maize plant under Pb stress increases the metal uptake and translocation in 60-day-old plants. It also helps to increase the growth of the plant (Hadi et al. 2010). In another study, Tandon et al. (2015) found that the application of two natural auxins and a synthetic auxin enhanced the phytoremediation efficiency in wetlands and non-wetland plants in water bodies. Ethylene was also found to involve in various developmental processes in seedlings, including leaf abscission, senescence, and fruit ripening. Studies have shown the contribution of ethylene in defense against heavy metal stress. The increase in production of ethylene was found in plants exposed to heavy metals at toxic levels (Khan et al. 2015a, b). In the present study, C. argentea was found to have the ability of tolerance and accumulation of five different metals which can be associated with the production of phytohormones.
The elevated level of metals in contaminated soil initiates the oxidative damage by disturbing the production of reactive oxygen species (ROS). The ROS comprising O2−, H2O2, HO2−, OH−, and RO− are very reactive and lethal resulting in damage to cellular components like proteins, lipids, and DNA leading to cell death. Antioxidant enzymes and non-enzymatic species tackle this disturbance. Under steady conditions, they provide sufficient protection, and their ability increases under stress conditions (Khan et al. 2019). In this study, antioxidant enzymes, including CAT, APX, GPX, and GST, were used as physiological biomarkers of heavy metal stress to the plant body. Due to low metal uptake in the presence of moss and compost, the enzyme activities lowered in moss- and compost-amended treatments.
Usually, CAT is found in peroxisomes and mitochondria. It defenses the plant from stress through the decomposition of H2O2 into H2O and O2 through energy-proficient mechanism. APX has an extensive defensive mechanism against stress. It has five isoenzymes that play a vital role in the conversion of H2O2 into H2O with the help of oxidation of ascorbate to monodehydroascorbate. The enhanced levels of antioxidant enzymatic activities, including SOD, APX, CAT, and POD, were reported in Tanacetum parthenium L. when exposed to 0–70 μM Cd and Cu (Hojati et al. 2017). In a study, the enhanced activity of antioxidant enzymes (CAT, SOD, and POX) was reported with the addition of bacterial strain, i.e., Pantoea sp. ZNP-5, under the influence of Cu stress (Singh and Jha 2018). GST is responsible for combating various biotic and abiotic stresses. An increase in the activity of GST has been reported in leaves and roots of P. sativum when exposed to Cd (Dixit et al. 2001). The activity of GPX increased in the Lupinus luteus L. (when grown on soil artificially contaminated with Cd and soil from metallurgical industry) up to ten times the activity on the uncontaminated soil (Jaskulak et al. 2019). According to Zhong et al. (2019), CAT activity of P. laciniata increased until the concentration of Cd reaches 50 mg kg−1 and decreased with further increase in Cd concentration. Khan et al. (2019) found significantly higher antioxidant enzyme activities (CAT, POX, GST, APX, and SOD) in Petunia hybrida L. when exposed to various heavy metals (Cd, Cr, Cu, Ni, and Pb) and the activity of enzymes increases with the concentration of heavy metals. Under Cd stress, the SOD content increased significantly in R. communis with the increase in cadmium concentration and slightly enhanced the CAT content. The Zn treatment markedly enhances the POD and SOD content, and Cu treatment enhances CAT activity to some extent in leaves of R. communis (Wang et al. 2018). The plants’ exposure to heavy metals aggravates responses of antioxidant enzymes which help plants against oxidative damages, but the extent of response is linked to plant species, metal type, and its intensity.
Heavy metal stress to plants generally results in enhanced production of reactive oxygen species (ROS) which are reactive in comparison to molecular oxygen and are highly toxic to plant tissues. The overproduction of reactive oxygen species and free radicals can often damage the biomolecules like proteins, DNA, and lipids. Heavy metal tolerance of plants has been associated with efficient antioxidant protection system by many researchers (Khan et al. 2019). Plants have evolved efficient and complex non-enzymatic and enzymatic antioxidant defense mechanisms to cope with the toxic effects of free radicals. Total antioxidant capacity is the cumulative capacity of the components of plant extracts to scavenge the free radicals. It has been used to determine the antioxidant capacity of various biological samples. It is used to investigate the overall antioxidant capacity of the plants against stress such as heavy metals. Total antioxidant capacity of Salvia officinalis L. grown on soil contaminated with Zn, Cd, Pb, and Cu was measured and compared with non-polluted control soil. The results indicate the high production of H2O2, MDA, oxidized (GSSG) glutathione forms, reduced (GSH), dehydroascorbate, and ascorbate in heavy metal–treated soil. The researchers speculate that the neutralization of H2O2 is a non-enzymatic process (Stancheva et al. 2010).
The metallothionein (MT) are metal-tolerant genes and are expressed in the plants when exposed to abiotic stress like salt, oxidative stress, heat, heavy metals, and drought. The MT genes always express in specific tissues and organs; for example, MT1 express in roots, MT2 in leaves, MT3 in fruit, and other MTs in the seeds. Liu et al. (2015) revealed the involvement of OsMT2c in the enhancement of tolerance to Cu and increased reactive oxygen species (ROS) activity in Oryza sativa. ShMT1 and ShMT2 are the two MT genes from ectomycorrhizal fungus S. himalayensis which are inducible in response to copper and cadmium stress and play a significant role in detoxification of Cu and Cd (Kalsotra et al. 2018). HMA genes are known to express in heavy metal–stressed plants. HMA-associated genes encoding bivalent cation transporters are overexpressed in the shoots and roots of Noccaea caerulescens and Arabidopsis halleri; both are Cd and Zn hyperaccumulators. HMA2 expression in the A. thaliana, rice, wheat, and barley is linked with the translocation of Cd and Zn. HMA3 is utilized in the detoxification of metal in vacuole by Cd sequestration while HMA4 behaves as a physiological agent at the time of metal hyperaccumulation. Moreover, HMA4 and HMA2 play a significant role in the metal translocation from root to shoot (Chaudhary et al. 2018).
Root tolerance index (RTI) indicates the ability of the plant to survive against the stress such as heavy metals. The increased RTI specifies the better growth of plant and tolerance against the heavy metals. The results of this study indicate that the addition of compost and peat moss significantly enhanced the RTI higher than 100%, i.e., the indicator of better growth. On the other hand, the RTI of inoculum-amended treatments and contaminated water–treated plants was found less than 100%, which indicates the high metal stress for these treatments. It can be due to high accumulation of metals by these treatments. Similar results have been reported by Marzilli et al. (2018) where the tolerance index of Populus alba clone Villafranca under Cd (5, 50, and 250 μM) and Cu (5, 50, 250, and 500 μM) stress decreased with the increasing concentration of metal stress. It is reported that the bacterial strains, i.e., K. intermedia, C. murliniae, and K. oxytoca, enhance the bioavailable fraction of cadmium and lead and, in turn, increase the uptake of metals by M. deeringiana in contrast to controls without bacterial inoculation (Boechat et al. 2017). The total lead uptake in plants without bacterial strain was a bit higher than bio-augmented plants, but the concentration was not significantly different from each other. There is a possibility that P. japonica did not play a significant role in solubility of lead in soil and did not contribute towards Pb availability for the plant to uptake. The higher accumulation of Cu in P. japonica–inoculated plants can be attributed to the metal mobilization in soil solution by bacterial strain. Similar findings were reported by Singh and Jha (2018), that in the wheat plant, the uptake of Cu was enhanced by inoculation with bacterial strain ZNP-5. The concentration of Cu was found highest in roots and shoots of the wheat plant. The phytoextraction ability of Solanum nigrum plant enhanced with the addition of bacterial strain Serratia sp. RSC-14 as compared to uninoculated controls (Khan et al. 2015a, b). The uptake and accumulation of Cr enhanced in sunflower when inoculated with Pseudomonas sp. CPSB21 strain (Gupta et al. 2018). As evident from this study, the bioavailability of metals in soil could be enhanced with bioaugmentation by increasing the available fractions in the soil solution. The bacterial strain can solubilize metals from non-soluble phases and increase its availability for plant uptake. Therefore, the enhanced uptake and accumulation of Cd, Cr, Cu, and Ni in plant parts is attributed to the more soluble fraction of metals in soil solution due to bacterial inoculation.
Plants have evolved mechanisms for the uptake of essential nutrients from the environment. The transport mechanisms in the plasma membrane for uptake and translocation of nutrients includes (i) proton pumps (that involves ATPases to consume energy and create electrochemical gradients); (ii) co-transporters and anti-transporters (these are proteins for utilization of electrochemical gradients to initiate the active uptake of ions); and (iii) channels (that are proteins involved in facilitation of transportation of ions into the cell). The uptake of nutrients is often accompanied by the absorption of metals when they are present in ionic form in the soil solution. Evapotranspiration of water through leaves of the plants also serves as a pump for the uptake of metals. It plays an active role in the transport of metals to shoots. Mostly, the microorganisms in the rhizosphere of the plant help in mobilization of metal ions and increase the bioavailable fraction for increased uptake by the plant. Overall, in our study, with the addition of bacterial strain, the translocation of all five metals to aerial parts is apparent. It can be concluded that P. japonica contributes to the enhanced metal translocation in C. argentea.
Nutrient availability and rhizosphere pH
The nutrient availability in the soil is essential for the proper growth of plants, which is exceedingly dependent on the pH of the soil. The pH of soil also determines the availability of toxic elements in soil. At low pH, the soil is acidic, and the growth of plants is limited by excessive Al and Mn ions, and low availability of nutrients, such as P, Mg, K, Ca, and Mo. On calcareous or alkaline soils, the growth of plants is generally limited due to the low bioavailability of micronutrients like Zn, Fe, Cu, and Mn (Khan et al. 2019). The nutrient availability can be enhanced by the addition of amendments to the soil. Compost has acidic to slightly basic pH, and peat moss has acidic pH, which helps to increase the nutrient availability in the soil solution and hence the growth of plants. Ding et al. (2019) observed the effect of pH and uptake of nutrients in six genotypes of Lupinus in hydroponics at pH 5, 6.5, and 8. The results indicate the deficiency of P, at pH 8 for all the Lupinus genotypes. The lateral root growth, uptake, and translocation of Ca, K, Mn, and P was reduced at high pH for all the sensitive species. It proves that the high pH of the soil is responsible for unavailability of nutrients in the soil, and the pH needs to be slightly acidic for the availability of the nutrients. Soil amendments such as compost, biochar, and peat moss can be used to enhance the quality and fertility of heavy metal–stressed soil.
Perspectives of future research and practical applications
The use of ornamental plants has excellent potential for phytoextraction of heavy metals from the environment. It is the emerging technology with more eco-friendly perspective as compared to conventional techniques. Phytoremediation of heavy metals at industrial scale largely depends on several factors including the right plant species with the production of high biomass, proper growth, and tolerance in the presence of various environmental pollutants including heavy metals (Zhang et al. 2018). We can enhance the remediation ability of ornamental plants through some modifications in soil physical or chemical properties, microbial characteristics, or genetic changes of ornamental plants (Lajayer et al. 2019).
In this study, we have chosen a bacterial strain Pseudomonas japonica to check its effect on the phytoextraction ability of an ornamental plant, Celosia argentea, in terms of multiple heavy metal uptake. It is remarkable to study other ornamental plants to recognize more tolerant species among them. Moreover, the growth of plant species in combination with endophytes and rhizospheric bacteria and other soil conditioners needs some consideration. It is because the plant-microbe association can significantly enhance the growth of the plant in the stressed situation and metal phytoextraction as well (Arshad et al. 2017).
The results from our study can be utilized and applied practically on a large scale for the removal of specific heavy metals from the soil as well as from the treated wastewater, which usually contains heavy metals. Celosia argentea is the non-edible plant, so there are rare chances for the contamination of the food chain. Ornamental plants can also be used to extract medicinal oil and aromatic substances from the plant tissues and flowers (Lajayer et al. 2019). There is no study previously reported regarding the contamination of perfume or oil obtained from flowers grown on heavy metal–contaminated sites. Although the growth will be affected by heavy metal stress, it can be compensated by cutting some aerial parts of the plant and allowing growing new leaves to reduce the heavy metal buildup and load in the whole plant. It is a logical, economical way for developing countries where the government is unable to provide incentives to agriculture sector with polluted land. Therefore, income from sales of cut flower from ornamental plants may add to the resources of local people to treat the contaminated soil. Also, the use of ornamental plant will beautify the environment in real.
Celosia argentea can accumulate five different heavy metals from the contaminated soil at the same time. Such results can help the environmental engineer to choose C. argentea for the phytoextraction of soil containing a mix of heavy metals. This plant is perennial and shows high biomass when grown in metal-contaminated sites. Our study also indicates higher translocation level for all five heavy metals from roots to aboveground parts of the plant, which is most important for the practicality of the phytoremediation process. It also can grow well in metal-contaminated soil, thus making it an important candidate for the treatment of contaminated land. The natural esthetic properties of C. argentea also make phytoremediation a more attractive method for cleanup purposes. The practicality of phytoremediation depends on the availability of pollutants in the soil, amount of biomass of plant, translocation of metals, and the metal tolerance of the plant. The combination of the plant with microorganisms is beneficial for remediation of heavy metals in terms of cost and success for in situ implementation in various environments. Microbes enhance the availability of metals in the soil to increase the uptake of metals by plant body.
Phytoremediation applies for shallow contamination to the depth where the roots of plants can penetrate and interact with metals. Phytoremediation is the low-cost, in situ approaches that remove pollutants from the soil without disturbing its present biological activity, soil structure, and fertility. It is a more sustainable method as it is driven by solar energy. Phytoremediation needs to remove the plants from agriculture land and wildlife because they would be contaminated with pollutants, and once loaded, they need to be disposed of properly. Plants can be disposed of easily through various methods including pyrolysis, anaerobic digestion, incineration, acid extraction, gasification, extraction of essential oil, and fibers from plants (Dzantor 2007). The challenges faced in applying the phytoremediation practice to the practical application are enormous, which could be the reason why field utilization of the approach has yet to be recognized more than two decades, after the opinion was established. Investigation of various case studies has shown enlightening directions and issues rising through the application of phytoremediation practices. Participation of all stakeholders including scientists, environmentalists, and civil engineers, and enterprises and accountable authorities, with right approach to decision-making, will result in positive outcomes in provision of sustainable results from cleanup missions and practical employment of more environmentally friendly technologies with close to zero emissions that is important in a scope of minimizing climate change problems (Koptsik 2014).
Future research
-
1.
There is limited knowledge available about the uptake of heavy metals by ornamental plants at the cellular level and needs further investigation.
-
2.
There is a lack of data related to enzymatic and non-enzymatic detoxification pathways, so it needs to be studied in depth in the future.
-
3.
Also, the mechanism of heavy metal uptake by the ornamental plant is poorly understood, and there is a need to study the phenomenon to get a better understanding about the metal detoxification and sequestration mechanism inside the plant body.
-
4.
The role of synthetic and organic chelating agents including EGTA, DTPA, DTA, beneficial microbial amendments, and development of transgenic ornamental plants could be studied to improve the metal uptake ability (Lajayer et al. 2019).
-
5.
Further, the role of plant-microbe association is also needed to be investigated with reference to the ornamental plants. The microorganism must be able to improve the plant growth and the tolerance/accumulation of heavy metals.
Conclusions
The use of C. argentea can not only remediate the environment contaminated with heavy metals but also have esthetic properties. Celosia argentea with P. japonica enhanced the uptake of Cd, Cu, Ni, and Cr. C. argentea with P. japonica showed enhanced translocation of metals, while moss and compost have resulted in reduced heavy metal uptake by C. argentea. Heavy metals enhanced the production of antioxidant enzymes in CW treatments. With these results, C. argentea can be used in polishing of treated wastewater containing heavy metals, and hence the environmental impact of the industry can be reduced and move towards sustainability.
References
Adeleke R, Nwangburuka C, Oboirien B (2017) Origins, roles, and the fate of organic acids in soils: a review. S Afr J Bot 108:393–406
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Arshad M, Khan AH, Hussain I, Anees M, Iqbal M, Soja G, Linde C, Yousaf S (2017) The reduction of chromium (VI) phytotoxicity and phytoavailability to wheat (Triticum aestivum L.) using biochar and bacteria. Appl Soil Ecol 114:90–98
Bao T, Sun T, Sun L (2011) Low molecular weight organic acids in root exudates and cadmium accumulation in cadmium hyperaccumulator Solanum nigrum L. and non hyperaccumulator Solanum lycopersicum L. Afr J Biotechnol 10:17180–17185
Boechat CL, Giovanella P, Amorim MB, de Sá ELS, de Oliveira Camargo FA (2017) Metal-resistant rhizobacteria isolates improve Mucuna deeringiana phytoextraction capacity in multi-metal contaminated soils from a gold mining area. Environ Sci Pollut Res 24:3063–3073
Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Biol 40:373–386
Chaudhary K, Agarwal S, Khan S (2018) Role of phytochelatins (PCs), metallothioneins (MTs), and heavy metal ATPase (HMA) genes in heavy metal tolerance. In: Mycoremediation and Environmental Sustainability. Springer, pp 39–60
Chen G-X, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Davenport SB, Gallego SM, Benavides MP, Tomaro ML (2003) Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annuus L. cells. Plant Growth Regul 40:81–88
Ding W, Clode PL, Lambers H (2019) Effects of pH and bicarbonate on the nutrient status and growth of three Lupinus species. Plant Soil:1–20
Dixit V, Pandey V, Shyam R (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot 52:1101–1109
Dzantor EK (2007) Phytoremediation: the state of rhizosphere ‘engineering’ for accelerated rhizodegradation of xenobiotic contaminants. J Chem Technol Biotechnol 82:228–232
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa Region. 89:170–176
González-Valdez E, Alarcón A, Ferrera-Cerrato R, Vega-Carrillo HR, Maldonado-Vega M, Salas-Luévano MÁ (2016) Seed germination and seedling growth of five plant species for assessing potential strategies to stabilizing or recovering metals from mine tailings. Water Air Soil Pollut 227:1–10
Gupta P, Rani R, Chandra A, Kumar V (2018) Potential applications of Pseudomonas sp.(strain CPSB21) to ameliorate Cr6+ stress and phytoremediation of tannery effluent contaminated agricultural soils. Sci Rep 8:4860
Hadi F, Bano A, Fuller MP (2010) The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 80:457–462
Hojati M, Modarres-Sanavy SAM, Enferadi ST, Majdi M, Ghanati F, Farzadfar S, Pazoki A (2017) Cadmium and copper induced changes in growth, oxidative metabolism, and terpenoids of Tanacetum parthenium. Environ Sci Pollut Res 24:12261–12272
Jaskulak M, Rorat A, Grobelak A, Chaabene Z, Kacprzak M, Vandenbulcke F (2019) Bioaccumulation, antioxidative response, and metallothionein expression in Lupinus luteus L. exposed to heavy metals and silver nanoparticles. Environ Sci Pollut Res 16:1–13
Kalsotra T, Khullar S, Agnihotri R, Reddy MS (2018) Metal induction of two metallothionein genes in the ectomycorrhizal fungus Suillus himalayensis and their role in metal tolerance. Microbiology 164:868–876
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015a) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Khan AR, Ullah I, Khan AL, Park GS, Waqas M, Hong SJ, Jung BK, Kwak Y, Lee IJ, Shin JH (2015b) Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp. RSC-14 inoculation. Environ Sci Pollut Res 22:14032–14042
Khan AHA, Tanveer S, Anees M, Muhammad YS, Iqbal M, Yousaf S (2016a) Role of nutrients and illuminance in predicting the fate of fungal mediated petroleum hydrocarbon degradation and biomass production. J Environ Manag 176:54–60
Khan AHA, Anees M, Arshad M, Muhammad YS, Iqbal M, Yousaf S (2016b) Effects of illuminance and nutrients on bacterial photo-physiology of hydrocarbon degradation. Sci Total Environ 557:705–711
Khan AHA, Butt TA, Mirza CR, Yousaf S, Nawaz I, Iqbal M (2019) Combined application of selected heavy metals and EDTA reduced the growth of Petunia hybrida L. Sci Rep 9:4138
Koptsik G (2014) Problems and prospects concerning the phytoremediation of heavy metal polluted soils: a review. Eurasian Soil Sci 47:923–939
Lajayer BA, Moghadam NK, Maghsoodi MR, Ghorbanpour M, Kariman K (2019) Phytoextraction of heavy metals from contaminated soil, water, and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut Res 26:1–17
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu J, Shi X, Qian M, Zheng L, Lian C, Xia Y, Shen Z (2015) Copper-induced hydrogen peroxide upregulation of a metallothionein gene, OsMT2c, from Oryza sativa L. confers copper tolerance in Arabidopsis thaliana. J Hazard Mater 294:99–108
Liu J, Xin X, Zhou Q (2017) Phytoremediation of contaminated soils using ornamental plants. Environ Rev 26:43–54
Maehly A, Chance B (1954) Methods of biochemical analysis by Glick D. Interscience, New York
Man YB, Chung AKC, Wong MH (2016) Changes in low molecular weight organic acids and antioxidative enzyme activities of wetland plants under metal stresses. Environ Eng Manag J 15:1657–1663
Marzilli M, Di Santo P, Palumbo G, Maiuro L, Paura B, Tognetti R, Cocozza C (2018) Cd, and Cu accumulation, translocation and tolerance in Populus alba clone (Villafranca) in autotrophic in vitro screening. Environ Sci Pollut Res 25:10058–10068
Michael PI, Krishnaswamy M (2011) The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ Exp Bot 74:171–177
Mishra R, Datta SP, Annapurna K, Meena MC, Dwivedi BS, Golui D, Bandyopadhyay K (2019) Enhancing the effectiveness of zinc, cadmium, and lead phytoextraction in polluted soils by using amendments and microorganisms. Environ Sci Pollut Res 26:1–12
Nadeem SM, Imran M, Naveed M, Khan MY, Ahmad M, Zahir ZA, Crowley DE (2017) Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J Sci Food Agric 97:5139–5145
Singh RP, Jha PN (2018) Priming with ACC-utilizing bacterium attenuated copper toxicity, improved oxidative stress tolerance, and increased phytoextraction capacity in wheat. Environ Sci Pollut Res 25:33755–33767
Stancheva I, Geneva M, Hristozkova M, Markovska Y, Salamon I (2010) Antioxidant capacity of sage grown on heavy metal-polluted soil. Russ J Plant Physiol 57:799–805
Stanislawska-Glubiak E, Korzeniowska J, Kocon A (2015) Effect of peat on the accumulation and translocation of heavy metals by maize grown in contaminated soils. Environ Sci Pollut Res 22:4706–4714
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A (2018) Phytohormone priming: a regulator for heavy metal stress in plants. J Plant Growth Regul 38:739–752
Tandon S, Kumar R, Parsana S (2015) Auxin treatment of wetland and non-wetland plant species to enhance their phytoremediation efficiency to treat municipal wastewater. J Sci Ind Res 74:702–707
Upadhyaya A, Sankhla D, Davis TD, Sankhla N, Smith B (1985) Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol 121:453–461
Venkatachalam P, Priyanka N, Manikandan K, Ganeshbabu I, Indiraarulselvi P, Geetha N, Muralikrishna K, Bhattacharya RC, Tiwari M, Sharma N, Sahi SV (2017) Enhanced plant growth promoting the role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol Biochem 110:118–127
Wang S, Zhao Y, Guo J, Liu Y (2018) Antioxidative response in leaves and allelochemical changes in root exudates of Ricinus communis under Cu, Zn, and Cd stress. Environ Sci Pollut Res 25:32747–32755
Xu P, Han N, Kang T, Zhan S, Lee KS, Jin BR, Li J, Wan H (2016) SeGSTo, a novel glutathione S-transferase from the beet armyworm (Spodoptera exigua), involved in detoxification and oxidative stress. Cell Stress Chaperones 21:805–816
Xu X, Yang B, Qin G, Wang H, Zhu Y, Zhang K, Yang H (2019) Growth, accumulation, and antioxidative responses of two Salix genotypes exposed to cadmium and lead in hydroponic culture. Environ Sci Pollut Res 26:19770–19784
Yahmed JB, de Oliveira TM, Novillo P, Quinones A, Forner MA, Salvador A, Froelicher Y, Mimoun MB, Talon M, Ollitrault P, Morillon R (2016) A simple, fast and inexpensive method to assess salt stress tolerance of aerial plant part: Investigations in the mandarin group. J Plant Physiol 190:36–43
Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L (2015) Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int J Environ Res Public Health 12:15100–15109
Yang Z, Liu L, Lv Y, Cheng Z, Xu X, Xian J, Zhu X, Yang Y (2018) Metal availability, soil nutrient, and enzyme activity in response to the application of organic amendments in Cd-contaminated soil. Environ Sci Pollut Res 25:2425–2435
Zhang X, Li M, Yang H, Li X, Cui Z (2018) Physiological responses of Suaeda glauca and Arabidopsis thaliana in phytoremediation of heavy metals. J Environ Manag 223:132–139
Zhong L, Lin L, Liao M, Wang J, Tang Y, Sun G, Liang D, Xia H, Wang X, Zhang H, Ren W (2019) Phytoremediation potential of Pterocypsela laciniata as a cadmium hyperaccumulator. Environ Sci Pollut Res 26:13311–13319
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Iqbal, A., Mushtaq, M.U., Khan, A.H.A. et al. Influence of Pseudomonas japonica and organic amendments on the growth and metal tolerance of Celosia argentea L.. Environ Sci Pollut Res 27, 24671–24685 (2020). https://doi.org/10.1007/s11356-019-06181-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06181-z