Abstract
Industrial hydrodesulfurization method has not been efficient for removal of dibenzothiophene (DBT) from petroleum distillates. Therefore, in this current study, adsorptive desulfurization (investigated in batch mode) was carried out using functionalized carbon nanotubes (FCNTs) to reduce the amount of DBT in a model diesel. Different techniques, such as, scanning electron microscope (SEM) equipped with energy-dispersive X-ray (EDX), were used to check the morphological structure and the elemental compositions of the adsorbent; Fourier transmission infrared (FTIR) was used to check the chemical functionalities of the adsorbent; and nitrogen physisorption at 77 K was used to check the surface area, pore size, and pore volume of the adsorbent. The results show that the FCNTs outperformed the non-functionalized carbon nanotubes (CNTs) during the desulfurization by about 10%, indicating the functionalization did improve the desulfurization performance of the CNTs. The % removal of DBT by the FCNTs and CNTs was 70.48 and 60.88%, respectively. It can be concluded that the acid treatment of CNTs enhanced its surface affinity for DBT, thus contributing to the improved adsorption performance of the adsorbent. The isotherm results show that Freundlich isotherm model described well the mechanism of the adsorption process for both CNTs and FCNTs. In addition, pseudo second-order kinetics describes the behavior of the adsorbents during the adsorption process. The results obtained in this study therefore show that functionalized CNTs could be efficient and potential adsorbent for removal of DBT in petroleum distillate (e.g., diesel), to meet up with the stringent policies regarding emission of sulfur oxides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfur usually exists in the form of organo-sulfur compound in diesel. Sulfur oxides (SOx) released during combustion of fuel have increasingly become a serious environmental challenge resulting in degradation of the environment and formation of acid rain that corrodes metallic materials (Jiang et al. 2003). These have also contributed greatly to health challenges, such as, heart disease and asthma (Sadare et al. 2017). As a result, a good number of countries have enforced policies to minimize the emission of these compounds (Jiang et al. 2003). Therefore, removal of sulfur compound before combustion from transportation fuel has been the interest of researchers over the years. In order to ensure clean fuel combustion and to eradicate environmental degradation (Gawande and Kaware 2018), different techniques of desulfurization, such as, oxidation, biodesulfurization, extraction, hydrodesulfurization, and adsorption, have been employed by researchers for removal of organo-sulfur compound from diesel. Hydrodesulfurization (HDS) is the most widely employed technique in petroleum refineries. It has been discovered to effectively remove organo-sulfur compounds, such as, sulfides, thiols, and disulfides. However, thiophene (TH) and aromatic thiophene derivatives, such as, dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene, are recalcitrant to this technique (Hernández-Maldonado and Yang 2004). In addition, HDS is costly and energy-consuming due to elevated pressure and temperature. Therefore, exploiting a promising and cost-effective method of desulfurization is sorted for, from both environmental and economic considerations. Adsorption has been considered as a promising option for desulfurization of sulfur compounds (e.g., removal of DBT) in petroleum distillates. It has been employed by many researchers because it is relatively cheap and can be conducted at relatively mild temperature and atmospheric pressure (Moosavi et al. 2012; Gawande and Kaware 2018). In addition, regeneration and re-usability of the adsorbents employed in adsorption are promising (Ahmed and Ahmaruzzaman 2015). Researchers have used different kinds of adsorbents, such as, activated carbon (AC) (Zhou et al. 2009), carbon nanotubes (Saleh et al. 2014), zeolite (Xiao et al. 2008), bentonites and montmorillonite clay (Ahmad et al. 2017), and palm kernel shell (Al-Zubaidy et al. 2013), to remove organo-sulfur compounds from transportation oil. Commercial AC, among others, has been widely used as adsorbent for its excellent removal efficiency because of its improved surface area and microstructures (Eddebbagh et al. 2016). However, processing of AC and its use as an adsorbent in desulfurization are cost-intensive (Ahmed and Ahmaruzzaman 2015).
The exceptional properties of CNTs have drawn the attention of researchers for employing them as adsorbent materials. Surface of CNTs is chemically inert with high surface area and strong Van der Waal binding energy. They have more definite and uniform surfaces compared to AC at the atomic scale. CNTs’ adsorption capacity can be quantified directly with the available adsorption sites; however, efficiency of AC depends on the pore diameter distribution in order to quantify its adsorption properties. CNTs have been widely investigated owing to their extremely hollow and porous structures, improved surface area, and strong interaction with organic molecules. Khaled (2015) studied the adsorption performance of MWCNTs and graphene oxide (GO) for removal of TH and DBT from model diesel. Saleh et al. (2014) also investigated a novel nanomaterial of multi-walled carbon nanotubes doped with titania (CNT/TiO2) for the treatment of a model diesel (e.g., DBT). Furthermore, DBT adsorption on CNTs supported on CoMoS/HDS catalyst was investigated by Chen et al. (2004). However, to improve DBT adsorption performance of CNTs, their surface area needs to be modified, and this can be achieved via acid treatment which purifies and induces functional group that improves the adsorption of DBT onto the active adsorption sites of the adsorbent (Saleh et al. 2014). However, a few studies have been conducted on the application of modification of CNT surface by acid treatment for desulfurization of DBT compound in diesel. Therefore, for the first time, this study investigates and reports adsorptive desulfurization of DBT in petroleum distillate (model diesel) using acid-treated and functionalized CNTs (using (H2SO4/KMnO4)) as an adsorbent. Influence of surface modification on the desulfurization performance of the CNTs was equally studied. For better understanding of the adsorption mechanisms of the adsorbent and the interaction that exists with adsorbents and adsorbates, kinetics and thermodynamic studies were carried out as well.
Materials and experimental
Materials
Multi-walled carbon nanotubes (> 98% carbon basis), dibenzothiophene (DBT), and hexane (98% purity) were purchased from Sigma-Aldrich (Pty) Ltd., South Africa, and used in this study.
Experimental
Functionalization of carbon nanotubes
Acid functionalization of CNTs was done in our laboratory. Firstly, the CNTs were purified using solution of concentrated acetic acid and concentrated hydrochloric acid at a ratio of 3:1. The ratio of solid (CNTs) to liquid was 1:100. The solution was stirred on a magnetic stirrer at 400 rpm for 24 h at 80 °C. The CNTs were filtered and washed severally with deionized water until a neutral pH was achieved. The CNTs were dried at ambient temperature for 24 h and then in an oven at 50 °C for 24 h. For the functionalization, a mixture solution containing about 10 g of KMnO4 and 500 mL of 0.5 M H2SO4 was used, and the same operating conditions employed during purification of CNTs were used in the functionalization. The mixture, containing CNTs in the ratio of 1:100, was stirred on a magnetic stirrer using a reflux medium for 24 h. The resulting mixture was washed thoroughly with deionized water until pH of 7 and then filtered. The filtered CNTs were dried at ambient temperature for 24 h and then at 50 °C for 24 h until a constant weight was achieved.
Physico-chemical characterization of CNTs and FCNTs
Fourier transmission infrared (FTIR) spectroscopy, nitrogen physisorption at 77 K, scanning electron microscopy (SEM), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDS), and Raman spectroscopy were used to check the surface chemistry, textural properties, morphology, crystallinity and phases, elemental composition, and chemical information, respectively, of the adsorbents. FTIR analyzer, at a wavenumber range of 500 to 5000 cm−1, was used to check the attachment functional groups on the adsorbent. N2 physisorption experiments were conducted at 77 K on the adsorbents to obtain the following textural properties: surface area, cumulative pore volume, and pore size of the adsorbents. The morphology of the adsorbents was checked using Carl Zeiss Sigma field electronic scanning electron microscope (FESEM). The purity of the CNTs after functionalization was also checked with Raman spectroscopy as suggested elsewhere (Mlanga et al. 2009). Thermogravimetry analyzer (TA SDT Q600 DRYCAL TA) was used to investigate the thermal stability and weight degradation of the samples from 30 to 1100 °C at 10 °C/min under air. About 4.0 ± 0.2 mg of CNT and FCNT samples was weighed and put inside a container and placed on an alumina pan. The data was recorded and plotted automatically.
Desulfurization experiment
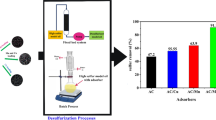
The desulfurization of DBT in model diesel was carried out on two different commercial adsorbents, namely CNTs and FCNTs, in batch mode. Hexane (100 mL) was used as model oil to dissolve 0.1 g of DBT. The solution was stirred until completely dissolved. Different initial DBT concentrations were prepared from this stock solution. About 0.2 g adsorbent was weighed into an Erlenmeyer flask consisting of 20 mL of model oil. The mixture was stirred for 180 min on a rotary shaker at 25 °C and 130 rpm. The operating variables, such as, DBT concentration, adsorption temperature, contact time, and amount of adsorbent, were varied. Samples of desulfurized model diesel were taken for analysis at different time intervals.
Analysis of the treated diesel was done on a gas chromatography–mass spectroscopy (GC–MS, Shimadzu (Japan)) equipped with fused silica column RXi-5MX. The dimension of the column was 0.25 μm in thickness, 0.25 mm in diameter, and 30.0 mm in length. The temperature of the column was set to hold at 90 °C for 2 min and then increase to 300 °C at 10 °C/ min and kept at 300 °C for 30 min. MS-ion source temperature was 200 °C. The pressure was set at 100 kPa with 1.39 mL/min column flow, linear velocity at 43.7 cm/s, purge flow at 3 mL/min, and stop time at 10 min. The injection of the sample (1.0 μL) was through a split mode with a ratio of 100:1 (the ratio indicates that the volume of the sample goes into the sampling loop for analysis versus the volume that was vented into the atmosphere). A pre-determined calibration curve was used to calculate the unknown concentrations of the treated model diesel.
To minimize error and ensure accuracy of the experimental results, all the experiments were done three times. The mean value of the data obtained was used in this study. Adsorption capacity, qe was calculated using the formula in Eq. (1):
where V in L is the volume of the model diesel, m in g is the mass of adsorbent, C0 in mg/L is the initial DBT concentration, and Ce in mg/L is the DBT concentration at equilibrium.
The percentage sulfur (DBT) removal (SR %) efficiency of CNTs and FCNTs was calculated using Eq. (2):
where Cf is the final DBT concentration in the desulfurized model diesel and SR is the sulfur (DBT) removal.
Isotherm studies of the desulfurization using CNTs and FCNTs
Isotherm studies provide comprehensive information on the adsorption mechanism (Wang and Wei 2017). In this study, evaluation of equilibrium data was done using Freundlich and Langmuir isotherm models. Equation (3) represents the linearized form of Langmuir isotherm model.
where b is the Langmuir constants and qo in mg/g is the maximum monolayer coverage capacities. Favorability of Langmuir adsorption process can be determined by separation factor, RL, as expressed in Eq. (4) (Wang and Wei 2017):
where b in L/mg is the Langmuir coefficient and C0 is the initial concentration of adsorbate in mg/L. The RL values in an adsorption process indicate the following:
RL between 0 and 1 means that the adsorption process is favorable
RL > 1 indicates that the adsorption process is unfavorable
RL = 1 indicates that the adsorption process is linear
RL = 0 shows that the adsorption process is irreversible
Freundlich isotherm model can be linearized as shown in Eq. (5):
Kf in mg/g is the Freundlich constants (adsorption capacity) and n is the adsorption intensity.
Kinetic study of the adsorptive desulfurization
Kinetic study on the adsorption process helps to understand the behavior of the adsorbent. Lagergren model (pseudo first-order kinetic) is the most commonly used (Lagergren 1898). The expression in Eq. (6) represents the Lagergren kinetic equation:
Pseudo first-order kinetic equation is given by:
where qt in mg/g is the amount of adsorbate on the adsorbent at time t and k1 in L/min is the pseudo first-order constant.
The linearized form of Ho and McKay’s pseudo second-order kinetics is given in Eq. (8) (Ho and McKay 1999):
where K2 is the pseudo second-order constant.
Thermodynamic study of the adsorptive desulfurization
Equilibrium adsorption constant, Kd, can be determined using the expression in Eq. (9):
Kd values obtained from Eq. (9) can be used to obtain the thermodynamic parameter, ΔG0, using Eq. (10):
where ΔG0 in kJ/mol is the standard free energy, R is the universal gas constant (8.314 Jmol−1 K−1), and T is temperature in Kelvin.
ΔH0 in J/mol is the standard enthalpy which can be determined using the expression in Eq. (11). ΔS0 is the standard entropy (J/K) which can be calculated using Eq. (12):
Results and discussion
Physico-chemical characterization of CNTs and FCNTs
Figure 1 (a) and (b) show the surface morphology of CNTs and FCNTs, respectively. It could be observed that there were agglomerates and bundles of CNTs clustering together before functionalization (see Fig. 1a). However, after functionalization of the CNTs, the bundles are more dispersed, compared to non-functionalized CNTs (Fig. 1b) (Guadarrama-Fernández et al. 2014). This could enhance the surface of the FCNTs for adsorption, therefore improving the adsorption performance of the adsorbent (Ishaq et al. 2017).
Elemental compositions of CNTs and FCNTs before and after adsorption experiments are shown in Table 1. It could be seen from Table 1 that the carbon content in CNTs has been reduced from 97.28 to 90.88% after functionalization, while the oxygen content increased from 2.72 to 9.12%. This could be attributed to oxidation of the CNTs by the acid treatment. The results agree with the observation of Deborah et al. (2015). Furthermore, presence of sulfur was noticed after adsorption experiments for both CNTs and FCNTs. The presence of sulfur in the used adsorbent could be a result of the sulfur-containing compound (DBT) used as model oil in this study. This could be an indication that DBT was adsorbed onto the surface of the adsorbent.
Figure 2 depicts the adsorption–desorption isotherms of the adsorbent. It can be observed that the distance between adsorption and desorption plots was wide indicating pore condensation hysteresis with relatively weak attractive adsorbate–adsorbent interaction.
Table 2 shows the textural properties of the CNTs. It could be seen in the table that after surface modification, the surface area of CNTs decreased from 245.49 to 224 m2/g. This could be attributed to the obstruction of the pores by functional groups attached during the modification of the adsorbent surface by acid treatment. These results are in line with results documented in Fallah and Azizian (2012). The pore volume of CNTs also reduced after the chemical modification (see Table 2). Large pore diameter above 0.7 nm enhances the adsorption of DBTs in the adsorbents by reducing mass transfer resistance and hence increasing the adsorption rate. As indicated in Table 2, there was a decrease in the pore diameter of CNTs after surface modification; nevertheless, pore diameters of both adsorbents are ˃ 0.7 nm, which is essential for enhancing access of DBT into the inner pores of the adsorbents. This observation agrees with literature (Jiang et al. 2003; Seredych et al. 2012).
Figure 3 shows the chemical functionalities of the CNT and FCNT adsorbents. The broad peak at 3425 cm−1 for the FTIR spectra of FCNTs is a characteristic of the O–H stretch of the hydroxyl group (Fig. 3). This can be attributed to the oscillation of carboxyl groups. The overtone region usually contains a prominent band in the region 2200 and 2580 cm−1 (Ngoy et al. 2011). The peak as observed at 1986 cm−1 is related with the stretch mode of carboxylic groups signifying that carboxylic groups are attached as a result of the oxidation of the CNTs by sulfuric acid. It can be deduced that functionalization of the CNTs adsorbent with a hydroxyl group enhanced the adsorption performance of the adsorbent (Sahebian et al. 2015). The adsorption affinity of the DBT compound by FCNTs increased as the number of aromatic rings increased, enhancing the adsorption performance of FCNTs. This could be ascribed to the –OH attachment to the adsorbent surface. The spectrum of the FCNTs shows that the OH group was successfully attached to the surface of the CNT adsorbent after treatment with acid (Le et al. 2013).
Figure 4 shows the Raman spectroscopy of CNTs and FCNTs. The existence of disorder in sp2-hybridized carbon systems results in resonance Raman spectra. This D-mode is as a result of disordered structure of graphene. The D-band peaks are at 1354 and 1381 cm−1 for FCNTs and CNTs, respectively, and this corresponds to distortion of Sp2 crystal structure, while the G-band which arises as a result of the stretching of the C–C bond in graphitic materials and common to all sp2 carbon materials is detected at 1636 and 1635 cm−1 for FCNTs and CNTs, respectively. These bands are associated to the lattice vibration mode of all carbon (graphite) materials with sp2 bonds (Misra et al. 2007). The value of IG/ID ratio of CNTs (1.35) is lower than the IG/ID ratio of FCNT (1.42). This is an indication that purification of carbon nanotubes resulted into fewer defect and higher structural quality of the FCNTs, enhancing the adsorption performance of the adsorbent.
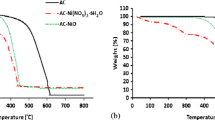
The thermal stability and weight degradation of the CNTs and FCNTs are described in Fig. 5. The TGA determines the percentage weight as a function of temperature. The thermal stability and degradation of the adsorbent which may be used for the desulfurization are significant, in order to approach the real application. Therefore, TGA analysis was carried out to determine the thermal stability of the adsorbent materials used in this study. Initially, from the room temperature to the onset temperature, there was no significant weight loss. The TGA profile shows a horizontal line with minor loss of weight. The first visible weight loss was noticed after a while. This might be as a result of evaporation of moisture in the adsorbent materials. The second stage showed a major degradation of the materials with evident weight loss. Typically, oxidation of amorphous carbon takes place at temperature below 400 °C (Landi et al. 2005). There is an indication from the TGA profile that there was no weight loss below 400 °C; this therefore established that there was no amorphous carbon present in the as-received CNTs (Tetana et al. 2012). The weight loss curves showed that CNT and FCNT degradations occur in a two-step process. There was a slight weight loss observed at 250 °C for FCNTs. This could be due to the oxidation of the functional group, which usually occurs under 400 °C (Silva et al. 2012). The weight drastically decreased with an increasing temperature at 550 °C for both CNTs and FCNTs, which was nearly completed at around 940 °C for CNTs and 900 °C for FCNTs.
Performance evaluation of CNTs and FCNTs during desulfurization
In this study, the parametric effects of operating variables, such as mass of adsorbent, initial DBT concentration, contact time, and temperature, on the adsorption process were investigated. Figure 6 shows the effect of contact time on the adsorption process. The contact time of the adsorbent with the adsorbate was varied from 0 to 180 min, while the operating parameters of the initial DBT concentration (1000 mg/L), volume of model diesel (20 mL), and temperature (298 K) remained constant. The results show that, for the first 10 min, there was rapid uptake of about 13.3 and 30.3% DBT onto the surface of CNTs and FCNTs, respectively. These might be due to availability of many vacant adsorption sites on the surface of the adsorbents at the beginning of the adsorption experiment. The higher percentage of DBT removed by FCNTs at this stage compared to CNTs could be as a result of the functional group attached to the surface of the FCNTs after acid treatment, enhancing the adsorption of DBT from the solution. It could be observed that there was drastic decrease in the adsorption performance between 10 and 50 min. This may possibly be due to reduction in the number of vacant adsorption sites on the surface of the adsorbents. Therefore, equilibrium was reached at 50 min, indicating DBT has fully occupied the available vacant adsorption sites on the surface of the adsorbents. These observations are in line with results reported by Vadivelan and Kumar 2005. The results show that functionalization of CNTs enhanced the adsorptive removal efficiency of the adsorbent.
The effect of temperature on the adsorption of DBT onto CNTs and FCNTs is as depicted in Fig. 7 (a) and (b), respectively. The temperature of the adsorption experiment was varied from 298 to 308 K. It can be inferred that adsorption performance of the adsorbent decreased with increasing temperature (Liu et al. 2017) from 298 to 308 K for both CNT and FCNT adsorbents as the time increased. This behavior is expected since the adsorption process is known to be an exothermic reaction where adsorption is favored at lower temperature. Hence, the highest adsorption performance was obtained at 298 K for both CNTs and FCNTs. Maximum adsorption performances of 60.88, 47.01, and 35.41% were obtained by CNT adsorbent at 298, 303, and 308 K, respectively. In addition, about 70.84, 51.52, and 43.86% percentage DBT removal was achieved by FCNTs at 298, 303, and 308 K, respectively.
Figure 8 depicts the effect of amount of adsorbents on their adsorption performance. The amount of CNTs and FCNTs for desulfurization of model diesel was varied from 0.2 to 1.0 g, while other operating parameters, such as, initial DBT concentration (1000 mg/L), time (60 min), and temperature (298 K), remained the same. The results show that there was increase in the DBT adsorption onto the adsorbent surface as the amount of the adsorbents increases (Daware et al. 2015; Khaled 2015). This could be as a result of the available and accessible vacant adsorption sites and more surface area for the attachment of DBT compound. It was observed that about 70.84 and 60.88% DBT were adsorbed onto the surface of FCNTs and CNTs, respectively when the amount of adsorbents reached 0.8 g. However, additional increase in the adsorbent dosage from 0.8 to 1.0 g for both adsorbents showed no further increase in the adsorptive removal of the DBT. This might be as a result of DBT inhibition diffusion to the surface of the adsorbents (Gong et al. 2009). The result showed that percentage desulfurization performance for FCNTs was higher than that for CNTs. The adsorption capacity of FCNTs (14.17 mg/g) was higher than that of CNTs with adsorption capacity of 12.18 mg/g. The result obtained in this study is favorable in the commercial application of nanotube powder since a higher adsorption capacity can be obtained at a minimum amount (0.8 g) for CNTs and FCNTs. In addition, decrease in percentage adsorption of CNT compared to FCNT powder may result from aggregation of adsorbent which prevented some active adsorbent sites from being exposed for the adsorption of DBT (Jiang et al. 2003). This is an indication that purification of CNTs enhanced the adsorption performance of the adsorbent.
Figure 9 depicts the effect of initial DBT concentration on the adsorption performance of the adsorbents at equilibrium. To determine the effect of initial DBT concentration, in the model diesel, the DBT concentration was varied from 250 to 1000 mg/L, while the adsorbent amount (0.8 g), temperature (298 K), volume of model diesel (20 mL), and contact time (60 min) remained the same. From the results obtained at equilibrium in Fig. 8, it could be observed that as the initial DBT concentration increases from 250 to 1000 mg/L, the amount of DBT adsorbed per unit mass of adsorbent increases. The adsorption capacity of CNTs increased from 3.46 to 15.11 mg/g, as the initial DBT concentration increased from 250 to 1000 mg/L. The same trend was observed for FCNT where adsorption capacity increased from 3.75 to 17.71 mg/g. This observation could be attributed to the developed concentration gradient on the surface of the adsorbent and the DBT solution, resulting from increase in the driving force, as a result of increase in concentration. This also explains the small difference in the amount of DBT adsorbed (qe) of CNTs and FCNTs at lower concentrations than 800 mg/L. These results are in agreement with literature (Fayazi et al. 2015; Ishaq et al. 2017). The results accounted for 60.88 and 70.81% DBT removal for CNTs and FCNTs, respectively.
Isotherm, kinetics, and thermodynamics of CNTs and FCNTs for adsorptive desulfurization
Langmuir and Freundlich isotherms were used to describe the behavior of the CNTs and FCNTs. Table 3 represents the Freundlich isotherm parameters for DBT adsorption onto CNTs and FCNTs. Figure 10 (a) and (b) depict the Langmuir isotherms of FCNTs and CNTs, respectively. Figure 10 (c) and (d) depict the Freundlich isotherms of FCNTs and CNTs, respectively. It can be observed that Freundlich isotherm models describes well the adsorption of DBT onto CNTs and FCNTs. As shown in Fig. 10a, Langmuir isotherms for CNTs and FCNTs disagree with the Langmuir model, therefore not describing well the behavior of the adsorbents. From Table 3, it can be seen that the R2 for CNTs and FCNTs is 0.9802 and 0.9910, respectively, for Freundlich isotherm model (Al-Ghouti et al. 2017). The Freundlich constant for CNTs and FCNTs is 0.0125 and 0.0038, respectively. The values of adsorption intensity, n ˂ 1, for both FCNTs and CNTs indicate favorability of the adsorption process.
Table 4 presents the parameters of pseudo first-order and pseudo second-order of DBT adsorption onto FCNTs and CNTs at 298, 303, and 308 K. Comparing the coefficient of determination (R2) of the kinetic models of CNTs with pseudo first-order, it was observed that the pseudo second-order model was better fitted into the experimental data with higher coefficient of determination (0.9985, 0.9989, 0.9885) than the coefficient of determination (R2) (0.3192, 0.7156, 0.8293) for pseudo first-order model at 298, 303, and 308 K, respectively. Furthermore, the experimental value of qe (12.18 mg/g) obtained for pseudo second-order kinetics is closer to the calculated qe (12.44 mg/g) compared to what were obtained for the experimental qe (12.18 mg/g) and calculated qe (3.14) for the 1st-order kinetics (Table 4). Also, for FCNT adsorbent, the coefficient of determination (R2) was higher in pseudo second-order kinetics (0.9996, 0.9987, 0.9984) compared to lower coefficient of determination (R2) obtained for pseudo first-order (0.5880, 0.5176, 0.7133) at 298, 303, and 308 K, respectively. The model value for qe calculated (14.29 mg/g) was very close in the experimental value obtained for qe (14.17 mg/g) for pseudo second-order kinetics for adsorption of DBT onto FCNTs. However, the model value calculated for qe (23.06 mg/g) is higher than the value obtained in the experiment (14.17 mg/g) for pseudo first-order kinetics of DBT adsorption onto FCNTs. The adsorption rate could be controlled by the movement of DBT in the pores of the CNT and FCNT adsorbents. It could be assumed that there were involvements of chemical interactions in the adsorption process. The results obtained in this study are in agreement with what was reported by Wang and Wei (2017). Lower coefficient of determination values for pseudo first-order could indicate that the adsorption rate is not dependent on concentration factor; however, it is dependent on time as well as concentration. This is in agreement with Kumar and Tamilarasan (2017).
The values of adsorption thermodynamic parameters, standard free energy ΔGo, standard entropy ΔSo, and standard enthalpy ΔHo, for CNT and FCNT adsorbents are shown in Table 5. The negative values of ΔGo and ΔHo are indication that adsorption of DBT onto CNTs and FCNTs is spontaneous and exothermic in nature. Negative ΔSo values for adsorption of DBT process show a decrease of the randomness at the CNT and FCNT solution interface during adsorption. In addition, the results show that the values of ΔSo decreased with increasing temperature while the values of ΔGo increased with increasing temperature. The activation values obtained for both CNTs and FCNTs are 25.34 and 14.13 kJ/mol, respectively. These values are less than 65 kJ/mol; these are indications that adsorption of DBT onto CNTs and FCNTs occurred more readily. In addition, the adsorption processes for both adsorbents could be said to be physical adsorption. This is in agreement with literature (Fei et al. 2017; Saini et al. 2017; Al-Ghouti et al. 2017).
Table 6 presents the results obtained in this study, compared with literature. Nazal et al. (2015) modified the surface of CNTs with 5% aluminum metal. A total of 80% adsorption performance was obtained when initial DBT concentration was 250 mg/L. Comparing this result with that obtained in this study, their modified CNTs outperformed what was obtained for CNTs (60.88%) and FCNTs (70.84%) in this study. This could only be as a result of higher initial DBT concentration used in this study, which is four times higher than what Nazal et al. (2015) used. In addition, 1.5 g of their adsorbent was used, which is almost two times what was used in this study. The result obtained will most likely be better than what was obtained by Nazal et al. (2015), if the same operating conditions are used. Saleh et al. (2014) also conducted an adsorptive desulfurization experiment to remove DBT from a model oil using CNTs modified with titanium oxide. Results showed 65% desulfurization performance using 0.75 g adsorbent. The result obtained in this study for acetic and sulfuric acid-treated CNTs which was 70.88% outperformed the non-functionalized carbon nanotubes used in this study by 5%. This better performance of CNT/TiO2 compared to non-functionalized CNTs could be as a result of the modified surface of their adsorbent. About 87% adsorptive removal of DBT was achieved in an experiment conducted by Khaled (2015) using sulfuric acid treated 1.5 g of CNTs, at initial DBT concentration of 250 mg/L. The time for equilibrium to be reached in their study (160 min) was higher than that reached in this study (60 min). In addition, a lower initial concentration was used which is four times lower than what was used in this study. In addition, about two times amount of adsorbent used in this study was used in Khaled (2015). Therefore, it cannot be concluded that their result is better than what was obtained in this study for both CNTs and FCNTs because of the lower initial concentration used in their study.
It can therefore be concluded that the result obtained from this study can be compared with studies reported in literature. This is the first report on the use of acetic–sulfuric acid-treated CNTs as an adsorbent for treatment of model diesel as far as it could be established. These documented results from this study could offer a platform for more investigations in this area of research.
Conclusions
As has been established in this study, CNTs and FCNTs are promising candidates for adsorption of DBT from model diesel. The following conclusions could be derived from this study:
The acid treatment of CNTs enhanced its surface affinity for DBT, thus contributing to the improved adsorption capacity of the adsorbent. CNTs tend to aggregate together as bundles because of Van der Waals interactions.
Addition of –COOH group on the surface of the adsorbent improved the performance of the adsorbent, even though the surface area was decreased after functionalization. The results showed that functionalized CNTs outperformed the non-functionalized CNTs during the desulfurization by about 10%, indicating functionalization of the CNTs did improve the desulfurization performance of the CNTs. Therefore, the percentage performances of the adsorbents were 70.48 and 60.88%, for FCNTs and CNTs, respectively, at 0.8 g adsorbent dosage, temperature of 25 °C, and maximum contact time of 50 min.
Therefore, the results show that functionalized CNT is an efficient and promising adsorbent for removal of DBT in petroleum distillate, such as diesel, so as to meet up with the stringent policies regarding emission of sulfur oxides. However, there is need for further studies on how to improve the surface area of the FCNTs after the acid treatment. Therefore, the documented results in this study may possibly offer a platform for further investigations in this field of research
References
Ahmad W, Ahmad I, Ishaq M, Ihsan K (2017) Adsorptive desulfurization of kerosene and diesel oil by Zn impregnated montmorollonite clay. Arab J Chem 10(2):S3263–S3269
Ahmed K, Ahmaruzzaman M (2015) Adsorptive desulfurization of feed diesel using chemically impregnated coconut coir waste Md. J Int J Environ Sci Technol 12:2847–2856
Al-Ghouti MA, Al-Degs YS, Issa AA, Al Bakain RZ, Khraisheh MA (2017) Mechanistic and adsorption equilibrium studies of dibenzothiophene-rich-diesel on MnO2-loaded activated carbon: surface characterization. Environ Prog Sustain Energy 00(00). 36(3):903–913. https://doi.org/10.1002/ep.12539
Al-Zubaidy IAH, Tarsh FB, Darwish NN, Abdul Majeed BSS, Al Sharafi A, Chacra LA (2013) Adsorption process of sulfur removal from diesel oil using sorbent materials. J Clean Energy Technol 1(1):66–68
Chen H, Zhou X, Shang H, Liu C, Qiu J, Wei F (2004) Adsorption properties of dibenzothiophene (DBT) on a CNT (carbon nanotube) support as well as on CoMoS/CNT and CoMoO/CNT catalysts. J Nat Gas 13(4):209–217
Daware GB, Kulkarni AB, Rajput AA (2015) Desulphurization of diesel by using low cost adsorbent. Int J Innov Emerg Res Eng 2(6):69–73
Deborah M, Jawahar A, Mathavan T, Dhas KM, Benial AMF (2015) Preparation and characterization of oxidized multi-walled carbon nanotube and glycine functionalized multi-walled carbon nanotubes. Fullerenes Nanotubes Carbon Nanostruct 23(7):583–590
Eddebbagh M, Abourriche A, Berrada M, Zina MB, Bennamara A (2016) Adsorbent material from pomegranate (Punica granatum) leaves: optimization on removal of methylene blue using response surface methodology. J Mater Environ Sci 7(6):2021–2033
Fallah RN, Azizian S (2012) Removal of thiophenic compounds from liquid fuel by different modified carbon cloths. Fuel Processing Technology 93:45–52
Fayazi M, Taher MA, Afzali D, Mostafavi A (2015) Removal of dibenzothiophene using activated carbon/γ-Fe CO nano-composite: Kinetic and themodynamic investigation of the removal process. Anal Bional Chem Res 2(2):73–84
Fei L, Rui J, Wang R, Lu Y, Yang X (2017) Equilibrium and kinetic studies on the adsorption of thiophene and dibenzothiophene onto NiCeY zeolite. RSC Adv 37. https://doi.org/10.1039/c7r00415j
Gawande PR, Kaware JP (2018) Isotherm and kinetics of desulphurization of diesel by batch adsorption studies. Int J Chem Eng Res 10(1):1–16
Gong J, Wang B, Zeng G, Yang C, Niu C, Niu Q, Zhou W, Liang Y (2009) Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J Hazard Mater 164:1517–1522
Guadarrama-Fernández L, Manzo-Robledo A, Calderon-Dominguez G, Martinez-Rivas A, Ortiz-Lopez J, Vargas-Garcia R (2014) Characterization of functionalized multiwalled carbon nanotubes for use in an enzymatic sensor. Microsc Microanal 20:1479–1485. https://doi.org/10.1017/5143192761401304X
Hernández-Maldonado AJ, Yang RT (2004) New sorbents for desulfurization of diesel fuels via ∏-complexation. AICHE J 50(4):791–801
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ishaq M, Sultan S, Ahmad I, Ullah H, Yaseen M, Amir A (2017) Adsorptive desulfurization of model oil using untreated, acid activated and magnetite nanoparticle loaded bentonite as adsorbent. J Saudi Chem Soc 21(2):143–151
Jiang Z, Liu Y, Sun X, Tian F, Sun F, Liang C, You W, Han C, Li C (2003) Activated carbons chemically modified by concentrated H2SO4 for the adsorption of the pollutants from wastewater and the dibenzothiophene from fuel oils. Langmuir 19:731–736
Khaled M (2015) Adsorption performance of multiwall carbon nanotubes and graphene oxide for removal of thiophene and dibenzothiophene in model diesel fuel. Res Chem Intermed 41:9817–9833. https://doi.org/10.1007/s11164-015-1986-5
Kumar M, Tamilarasan R (2017) Kinetics, equilibrium data and modeling studies for the sorption of chromium by Prosopis juliflora bark carbon. Arab J Chem 10:S1567–S1577
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kung. Svenska. Veten. Akad. Handl, 24:1–39
Landi BJ, Cress CD, Evans CM, Raffaelle RP (2005) Thermal oxidation profiling of single-walled carbon nanotubes. Chem Matter 17:6819–6834
Le VT, Ngo CL, Le QT, Ngo TT, Nguyen DN, Vu MT (2013) Surface modification and functionalization of carbon nanotube with some organic compounds. Adv Nat Sci Nanosci Nanotechnol 4:035017. https://doi.org/10.1088/2043-6262/4/3/035017
Liu Y, Liu X, Dong W, Zhang L, Kong Q, Wang W (2017) Efficient adsorption of sulfamethazine onto modified activated carbon: a plausible adsorption mechanism. Sci Rep 7:12437–12449. https://doi.org/10.1038/s41598-017-12805-6
Mlanga SD, Mondal KC, Carter R, Witcomb MJ Coville NJ (2009) The effect of synthesis parameter on the catalytic synthesis of multiwalled carbon nanotubes using Co/CaCO Catalysts. S Afr J Chem 62:67–76
Misra A, Tyagi PK, Rai P, Misra DS (2007) FTIR spectroscopy of multi-walled carbon nanotubes: a simple approach to study the nitrogen doping. J Nanosci Nanotechnol 7:1820–1823
Moosavi ES, Dastgheib SA, Karimzadeh R (2012) Adsorption of thiophenic compounds from model diesel fuel using copper and nickel impregnated activated carbons. Energies:4233–4250
Nazal MK, Khaled M, Atieh MA, Aljundi IH, Oweimreen GA, Abulkibash AM (2015) The nature and kinetics of the adsorption of dibenzothiophene in model diesel fuel on carbonaceous materials loaded with aluminum oxide particles. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.12.003
Ngoy JM, Iyuke SE, Neose WE, Yah CS (2011) Covalent functionalization for multiwalled carbon nanotubes-folic and bound conjugate. J Appl Sci 11(15):2700–2711
Sadare OO, Obazu F, Daramola MO (2017) Biodesulfurization of petroleum distillates—current status, opportunities and future challenges. Environments 4(4):85–104
Sahebian S, Zebarjad SM, Khaki JV, Lazzeri A (2015) A study on the dependence of structure of multi-walled carbon nanotubes on acid treatment. J Nanostruct Chem 5(3):287–293
Saini S, Kumar R, Chawla J, Kaur I (2017) Punica granatum (pomegranate) carpellary membrane and its modified form used as adsorbent for removal of Carmium(II) ions from aqueous solutions. https://doi.org/10.2166/aqua.2017.026
Saleh TA, Siddiqui MN, Al-Arfaj AA (2014) Synthesis of multiwalled carbon nanotubes—titania nanomaterial for desulfurization of model fuel. J Nanomater 940639:1–66
Seredych M, Wu CT, Brender P, Ania CO, Vix-Guterl C, Bandosz TJ (2012) Role of phosphorus in carbon matrix in desulphurization of diesel fuel using adsorption process. Fuel 92:318–326
Silva WM, Ribeiro H, Seara LM, Calado HDR, Ferlauto AS, Paniago RM, Leite CF, Silva GG (2012) Surface properties of oxidized and aminated multi-walled carbon nanotubes. J Braz Chem Soc 5(6):1078–1086
Tetana ZN, Mhlanga SD, Bepete G, Krause RWM, Coville NJ (2012) The synthesis of nitrogen-doped multiwalled carbon nanotubes using an Fe-Co/CaCO3 catalyst. S Afr J Chem 65:39–49
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci 286:90–100
Wang J, Wei J (2017) Selective and simultaneous removal of dibenzothiophene and 4-methyldibenzothiophene using double-template molecularly imprinted polymers on the surface of magnetic mesoporous silica. J Mater Chem A 5:4651–4659
Xiao J, Li Z, Liu B, Xia Q, Yu M (2008) Adsorption of benzothiophene and dibenzothiophene on ion-impregnated activated carbons and ion-exchanged Y zeolites. Energy Fuel 22:3858–3863
Zhou A, Ma X, Song C (2009) Effects of oxidative modification of carbon surface on the adsorption of sulfur compounds in diesel fuel. Appl Catal B Environ 87:190–199
Acknowledgments
OOS acknowledges the financial support provided by L’Oréal–UNESCO Foundation for Women in Science, Sub-Saharan African Fellowship, for her PhD degree program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadare, O.O., Daramola, M.O. Adsorptive desulfurization of dibenzothiophene (DBT) in model petroleum distillate using functionalized carbon nanotubes. Environ Sci Pollut Res 26, 32746–32758 (2019). https://doi.org/10.1007/s11356-019-05953-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05953-x