Abstract
Fluorene-9-bisphenol (BHPF), a new derivative of bisphenol A (BPA), has been introduced for treatment with estrogen-related tumors, such as endometrial cancer. This study investigated the potential mechanism underlying the action of BHPF against endometrial cancer in vitro. We used the cell counting kit-8 (CCK8) method on Ishikawa cells to screen sub-lethal doses of BHPF and established the optimal concentration at which BHPF influenced the proliferation of Ishikawa cells. Effect of BHPF on cell migration and invasion was investigated by cell scratch assay and transwell assay, respectively. Expression levels of epithelial-mesenchymal transition (EMT)–related proteins were detected by Western blot analysis. BHPF was found to inhibit the proliferation of Ishikawa cells, whose migration and invasion abilities were also reduced. Western blot indicated that BHPF can significantly inhibit the EMT process of Ishikawa cells by blocking transforming growth factor-β (TGF-β) signaling pathway. This is the first report of the effect of BHPF on the biological behavior of endometrial cancer cells and its inhibition of endometrial cancer progression by repressing both endometrial cell proliferation and epithelial-mesenchymal transition, hence suggesting it as a novel anti-cancer drug.

Schematic representation of the molecular basis underlying BHPF treatment. BHPF repressed the EMT process by regulating EMT-related genes, such as E-cadherin, N-cadherin, and vimentin as well as the TGF-β signaling pathway–related genes, including p-Smad2/3 and slug, in a BHPF-dependent manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is widely used in the production of plastic containers and is a known estrogenic chemical (Rahmani et al. 2018). A derivative of BPA, fluorene-9-bisphenol (BHPF), has been reported for its anti-estrogenic activity (Okazaki et al. 2017). In a recent report, Zhaobin Zhang et al. (Patent No. CN1027270470 B) proved BHPF to be an estrogen receptor–related receptor inverse agonist that could effectively inhibit the growth of estrogen receptor–positive cancer cells, such as in breast cancer, ovarian cancer, endometrial cancer, cervical cancer, prostate cancer, and colon cancer, thereby suggesting it as a novel tool to treat advanced or recurrent tumors. However, whether BHPF can inhibit the proliferation of endometrial cancer cells remains unclear, and the probable impact of BHPF on EMT is still to be understood.
Endometrial cancer (EC) is one of the most common malignant tumors of the female reproductive system (Tangjitgamol et al. 2009; Chlebowski et al. 2015); a common type of endometrial cancer is endometrioid adenocarcinoma, which is the most frequent gynecological tumor in developed countries (Ismiil et al. 2007). In 2015, 54,870 new cases of patients were reported in the USA, of whom 10,170 eventually died (Morice et al. 2016). At present, among the patients with gynecologic cancer, in China, the incidence of EC is only lower than that of ovarian cancer, and is ranked in the second place (Li et al. 2018). However, once patients’ endometrial cancer progress into stage III or IV, their 5-year survival rate is less than 30%, accounting for more than half the mortality of patients with EC (Alblas et al. 2018). Current research shows the recurrence rate of endometrial cancer to be still on the rise, and 10 to 15% of early EC cases evolve into recurrent EC (Rose et al. 2000). Most patients with recurrent EC prefer radiotherapy and chemotherapy, despite the poor prognosis (Randall et al. 2006). Therefore, treatment of advanced or recurrent endometrial cancer is extremely important and is still being explored.
The epithelial-mesenchymal transition (EMT) is an important process in tumor invasion, metastasis, and drug-resistance (Campbell 2018; Chen et al. 2018). It is a unique process that is characterized by a transition from polarized immotile epithelial cells to motile mesenchymal cells. The transformation of epithelial cells into interstitial cells can increase their ability of migration (Mariscal et al. 2018); as a result of which, the tumor cells are more aggressive, migratory, and anti-apoptotic. At the same time, they also express more exercise-related proteins, promoting invasion of tumor cells and transporting them to other parts of the body (Suarez-Carmona et al. 2017). Several molecular pathways have been identified in relation to EMT. TGF-β signaling pathway has a predominant function in suppressing the growth of normal epithelial cells (Karlsson et al. 2017; Rodriguez-Salas et al. 2017) and driving the metastatic process in malignant tumor cells. This study investigated the effect of BHPF on the growth of Ishikawa cells and its related mechanism. Overall, the study suggests a new treatment strategy for endometrial cancer.
Materials and methods
Cell lines and cell culture
The human endometrial cancer cell line (Ishikawa) was purchased from the American Type Culture Collection (ATCC) and cultured in a medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen) in a humidified incubator with 5% CO2 at 37 °C.
Cell counting kit-8 cell viability and proliferation assay
BHPF was donated by the College of Urban and Environmental Sciences of Peking University. Cell viability was assessed after seeding 1 × 104 Ishikawa cells in 96-well culture plates in the corresponding media for 24 h prior to BHPF treatment. Cells were then incubated with different concentrations of BHPF (0.5, 1, 5, 10, 20, 50, and 100 μM) and blank serum–free medium for another 24 h. Cell viability and proliferation were detected by cell counting kit-8 (CCK8) (Solarbio Science & Technology Co, Ltd., Beijing, China) according the manufacturer’s protocol; absorbance value was measured at 450 nm. Effect of BHPF was calculated as a percentage of control cell viability, treated with vehicle only.
Migration assay
Migration was assessed by the wound-healing assay. Cells were seeded at 5.0 × 104 cells/well in a 6-well plate until full confluence. After scraping the cell monolayer with sterile micropipette tip, cells were washed with PBS twice and treated with different concentrations of BHPF (0.5 and 5 μM) and blank serum–free medium. The first image of each scratch was acquired at time zero. At 12 and 24 h, each scratch was examined and captured at the same location, and the healed area was measured by Image J software.
Transwell invasion assay
Invasion of tumor cells was assessed in transwell chambers equipped with polyvinylpyrrolidone-free polycarbonated membranes of 8-μm pore and 6.5-mm diameter (Corning Costar Inc., Corning, NY). Cells were seeded onto the upper wells at 2 × 105 cells/well, with various concentrations of BHPF (0, 0.5, and 5.0 μM) for 24 h. The bottom chambers of the transwell were filled with conditioned medium (RPMI-1640 supplemented with 10% FBS, 1% glutamine, and 1% penicillin/streptomycin). After 24-h incubation, cells were fixed with 4% formaldehyde for 10 min at room temperature (22 °C), stained with DAPI (Solarbio Science & Technology Co, Ltd., Beijing, China), and counted under a light microscope (EVOS XL Core).
Western blot analysis
Cells were treated with various concentrations of BHPF (0.5, 1, 5, 10, 20, and 50 μM) for 24 h. After lysing the cells with RIPA buffer supplemented with protease inhibitor cocktail (Roche, Switzerland), proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted using primary antibodies for 12 h at 4 °C. Subsequently, they were treated with appropriate secondary antibodies (shown in Table 1) for 1 h at room temperature (22 °C). The immunoreactive bands were visualized by BIO-RAD ChemiDoc XRS chemiluminescence system (Bio-Rad Inc., CA).
Statistical analysis
The data are expressed as mean ± SEM. Independent t test was used for statistical analysis. P values less than 0.05 (*) or 0.001 (#) were considered statistically significant in the treated group compared with the control group. All statistical analyses were performed by SPSS software (Chicago, IL).
Results
Identification of sub-lethal concentrations of BHPF that interfere with normal cellular processes
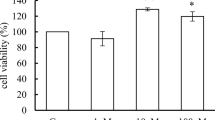
In order to investigate the cytotoxic effects of BHPF on Ishikawa cells, CCK8 assay was performed to determine the sub-lethal doses. After 24-h exposure to increasing doses of BHPF (0 to 20 μM), no toxic effect was observed. However, BHPF significantly affected cell proliferation, inhibiting growth by 60%, at 50 μM (Fig. 1b), thereby suggesting that overall exposure to BHPF at this concentration significantly inhibited ovarian cancer cell proliferation, as demonstrated previously (Patent No. CN1027270470 B).
Screening for the sub-lethal concentrations of BHPF that inhibited the proliferation of Ishikawa cells. a Structural formula of BHPF. b Ishikawa cells were treated with blank serum–free medium as a control, and with BHPF (0.5, 1, 5, 10, 20, 50, and 100 μM) for 24 h, after which cell viability was measured by CCK8 assay. The experiment was repeated five times, and data are reported as the means ± SD. Asterisk (*) indicates P < 0.05 and pound (#) shows P < 0.001, relative to control or as indicated
In the Zhang et al. patent, BHPF significantly inhibited the growth of ovarian cancer cells (OVCAR-3), cervical cancer cells (HeLa), prostate cancer cells (PC3), and endometrial cancer cells (RL95-2). The number of cells in the treatment group was significantly lower than that in the control group, especially when the BHPF concentration was above 50 μM, consistent with our current results. Since significant cell death might complicate the investigation of interaction between BHPF and plasma membrane, the concentration of BHPF in subsequent experiments was selected below the sub-lethal level (50 μM, P < 0.001).
BHPF treatment attenuated cellular migration and invasion
To investigate the interplay between BHPF and biological state, we focused on migration and invasion of cells. First, migration was assessed by wound-healing assay (Bin et al. 2018), and cell motility was observed after 24 h, as shown in Fig. 2a. Migration of the endometrial cancer cells was ameliorated by BHPF, as indicated by the inability of BHPF-treated cells to migrate across the slide. Application of BHPF increased unrecovered wound areas in a time-dependent manner, when compared with those of the control (Fig. 2a). Comparison of cell mobility showed that both 0.5 μM and 5 μM showed significant inhibition, at 20% and 33%, respectively, compared with that in the control group in Fig. 2b (P < 0.05).
BHPF repressed the migration of Ishikawa cells. Cells were treated with blank serum–free medium as a control and BHPF (0.5 and 5 μM) for 24 h and then scratched with a 1-mL micropipette tip. a The images presenting the recovery of wound area were captured at 0 and 24 h, using a microscope at a magnification of ×40. Scale bar, 100 μm. b The percentage of the wound recovery area at each time point was calculated. The experiment was repeated thrice, and data are presented as the means ± SD. Asterisk (*) indicates P < 0.05, relative to control or as indicated
BHPF also inhibited the invasion of Ishikawa cells. To check the altered invasive capacity of cells in response to BHPF treatment, a transwell assay was conducted. Once the cells had been cultured with drug-containing medium for 24 h, the image of Ishikawa cells that moved from the top chamber to the bottom chamber was significantly reduced (Fig. 3a), and the invasion of Ishikawa cells decreased by 40% and 86%, compared with that of untreated cells in Fig. 3b (P < 0.01). The high-BHPF dose group showed significantly inhibited invasion of Ishikawa cells. The effect of BHPF on endometrial cells, as observed in this study, is similar to that of TAM, indicating that its cytotoxicity plays a crucial role. EMT has been elucidated as a crucial mechanism in EC progression (Gu et al. 2018); especially during cell invasion and metastasis, we detected EMT markers in Ishikawa cells. E-cadherin (Mitra et al. 2018), vimentin (Li et al. 2014), and N-cadherin (Shenoy et al. 2016) were selected as EMT markers and detected by Western blotting. Subsequently, we checked the alterations of EMT markers in EC upon BHPF treatment. As shown in Fig. 4, while the epithelial marker E-cadherin increased, the mesenchymal markers N-cadherin and vimentin decreased in ECs upon BHPF treatment, especially at 10 μM (Fig. 4a). These data together suggested that BHPF significantly impairs EMT in ECs, resulting in decreased cell viability and invasiveness, as described above. Collectively, our data revealed the efficacy of BHPF in inhibiting cell proliferation, migration, invasion, and EMT.
BHPF repressed the invasion of Ishikawa cells. Ishikawa cells (2 × 105 cells) were cultured in transwells with the bottom surface covered with fibronectin in each well of a 24-well plate for 24 h. Cells were treated with blank serum–free medium as a control and with BHPF (0.5 and 5 μM). The cells attached to the bottom surface of the transwell were fixed with 10% formalin solution and stained with DAPI. a The stained cells were detected under a microscope and the images were photographed after 24 h. The blue dots are the nuclei. Scale bar, 100 μm. b The number of invading cells was counted, the experiment was repeated thrice, and data are presented as the means ± SD. Pound (#) shows P < 0.001, relative to control or as indicated
Relative expression of proteins. Ishikawa cells were treated with blank serum–free medium as a control as well as with BHPF (0.5, 1, 5, 10, 20, 50, and 100 μM) for 24 h. Total proteins were extracted and analyzed by Western blot. a Band images correspond to E-cadherin, N-cadherin, and vimentin proteins. b The downstream proteins of TGF-β signaling pathway, including Smad2/3, p-Smad2/3, and slug. The experiment was repeated five times, and data are presented as the means ± SD
BHPF inhibits the malignant transformation of endometrial cancer via TGF-β signaling pathway
Based on the role of TGF-β signaling pathway in the progression of endometrial cancer, we postulated that BHPF might exert its proliferation-suppression effect through TGF-β signaling pathway. In a previous study, treatment of Ishikawa cells with a TGF-β signaling inhibitor (SB-431542) had shown it to significantly inhibit proliferation, invasion, and migration of endometrial cancer cells (Muppala et al. 2017; Zhang et al. 2017a), thereby potentially inhibiting the progression of endometrial cancer. To investigate whether BHPF influences the expression of TGF-β in ECs, we conducted Western blot analysis to detect TGF-β expression in Ishikawa cells. In our study, we detected the downstream protein of TGF-β signaling pathway, including the expression levels of Smad2/3 (Klauzinska et al. 2014), p-Smad2/3 (Robertson et al. 2007), and slug (Mallini et al. 2014), after exposing Ishikawa cells to BHPF. We found the level of p-Smad2/3 and slug proteins to be significantly decreased with increasing BHPF concentration (Fig. 4b). Therefore, we tentatively suggested that BHPF inhibits the EMT process of endometrial cancer by repressing the TGF-β signaling pathway in Ishikawa cells.
Discussion
In this study, we described the in vitro inhibition of cell proliferation, invasiveness, migration, and malignancy by BHPF. In addition, we also considered the effect of BHPF on the expression of EMT marker protein and of TGF-β signaling pathway–related proteins. To date, this is the first study to explore these parameters and emphasized that BHPF interferes with the biological characteristics of tumor cells, changing the original protein levels to affect the malignant progression of tumor cells. These findings are consistent with those reported by Zhang et al.
Bisphenol fluorene and its compounds have been developed (Patent No. CN1027270470 B) as an alternative drug to tamoxifen, with potential anti-tumor effects. BHPF is an estrogen receptor and an inverse-agonist of the estrogen receptor–associated receptor. Zhang et al. (2017b) (Patent No. CN1027270470 B) had shown that these compounds can effectively inhibit the growth of estrogen receptor–positive cancer cells. In an in vivo and in vitro experimental study, they have demonstrated that BHPF can lead to irreversible pregnancy outcomes, like low birth weight and even death at early stage (Zhang et al. 2017b). Pregnancy is a special stage at which the human hormonal levels change dramatically. Since BHPF has such toxic side effects on normal physiological processes, it might as well be considered to inhibit estrogen-related tumor cells. In addition, BHPF was found to inhibit the proliferation of human breast cancer cells MCF-7 and human cervical cancer cells HeLa (Patent No. CN102727470), implying the strong anti-estrogenic activity of BHPF. In our study, we selected endometrial cancer cell line (Ishikawa) to investigate the effect of BHPF on EC, since it is a well-differentiated endometrioid adenocarcinoma with positive ER and PR (Menendez et al. 2004). Therefore, we indirectly explained the effect of BHPF on endometrial cancer by studying its effect on Ishikawa cells.
This study is also the first to report that BHPF can inhibit the EMT process of Ishikawa cells by blocking the TGF-signaling pathway. Cancer metastasis is considered to be the main cause of cancer mortality (Mali et al. 2019). At the initial stage of metastasis, EMT plays a crucial role; during which, normal epithelial cells lose their epithelial characteristics and gain migratory behavior, transporting these altered cells away from their epithelial cell population and appearing in vicinal or even more remote location (Brabletz et al. 2018). E-cadherin is a characteristic protein in epithelial cells while vimentin and N-cadherin are present in mesenchymal cells (Bharti et al. 2016). In estrogen-dependent cancer, such as ovarian cancer and breast cancer, anti-estrogenic drug decreases the metastatic potential by inhibiting the migration and invasion of cancer cells (Yamazaki et al. 2015; Nicolini et al. 2016). BHPF has anti-estrogenic properties and can be used to prepare anti-cancer drugs (Patent No. CN1027270470 B). To investigate whether BHPF promotes or inhibits the EMT process in endometrial cancer cells, we examined the marker protein expression of EMT-related genes. Results showed that BHPF inhibits the EMT process, which is consistent with the existing research results (Xin et al. 2019). To explore the underlying mechanisms leading to this phenomenon, we attempted to identify the most common tumor-associated signaling pathway. Previous studies had demonstrated the involvement of TGF in the regulation of EMT, leading to sustained tumorigenesis (Afrin et al. 2018). As is well-known, the ligand, receptor, and intracellular signal transduction molecule, Smad, of TGF-β signaling pathway, constitutes a tumor suppressor pathway. Abnormality in any element of the pathway or effect of the external environment may result in signal transduction disorders leading to tumorigenesis (Afrin et al. 2018). In our study, we detected the downstream protein of TGF-β signaling pathway, including the expression levels of Smad2/3 (Klauzinska et al. 2014), p-Smad2/3 (Robertson et al. 2007), and slug (Mallini et al. 2014), after exposing Ishikawa cells to BHPF; our Western blot analysis found the level of p-Smad2/3 and slug protein to be significantly decreased with increasing BHPF concentration (Fig. 5). These results showed that BHPF could inhibit tumor formation and play an anti-tumor role at the protein level.
Compared with the existing studies on BHPF (Patent No. CN1027270470 B), our study only examined the effect of BHPF on a tumor cell line in vitro, and after gaining consistent results with previous studies, we correlated the anti-tumor effect of BHPF with several of the most common tumorigenesis mechanisms. Under the treatment of BHPF, the expression level of EMT marker protein in tumor cells was found to change correspondingly. At the same time, the expression level of marker protein, downstream of the signaling pathway inducing EMT, was found to be significantly inhibited, thus confirming the hypothesis that BHPF could inhibit tumors. However, the study reports for the first time the mechanism of BHPF in the treatment of endometrial cancer. In order to obtain comprehensive research results, further verification at the gene level and molecular level is required. Considering that BHPF may be used as an anticancer drug, further animal experiments and in vivo studies would be recommended in future for better understanding of the influencing factors.
Conclusion
This study revealed that BHPF could inhibit epithelial-mesenchymal transition of human endometrial cancer cells (Ishikawa), by repressing TGF-β signaling pathway. Based on this mechanism, BHPF significantly attenuated cell viability in terms of proliferation, migration, and invasion, and eventually retarded the malignant progression of EC Ishikawa cells. Collectively, our study shed more light on the interface between BHPF and Ishikawa cells and offered more novel insights into relevant mechanisms of action. BHPF may be used as a drug to prevent malignant tumors and treat cancer. However, further efforts would be required to identify whether this novel finding can be extrapolated to clinical application in endometrial cancer, or in general tumor treatment.
References
Afrin S, Giampieri F, Gasparrini M, Forbes-Hernandez TY, Cianciosi D, Reboredo-Rodriguez P, Zhang J, Manna PP, Daglia M, Atanasov AG et al. (2018) Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnology advances. https://doi.org/10.1016/j.biotechadv.2018.11.011
Alblas M, Velt KB, Pashayan N, Widschwendter M, Steyerberg EW, Vergouwe Y (2018) Prediction models for endometrial cancer for the general population or symptomatic women: a systematic review. Crit Rev Oncol Hematol 126:92–99
Bharti R, Dey G, Mandal M (2016) Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot of IL-6 mediated involvement. Cancer Lett 375(1):51–61
Bin C, Xiaofeng H, Wanzi X (2018) The effect of microRNA-129 on the migration and invasion in NSCLC cells and its mechanism. Exp Lung Res:1–8
Brabletz T, Kalluri R, Nieto MA, Weinberg RA (2018) EMT in cancer. Nat Rev Cancer 18(2):128–134
Campbell K (2018) Contribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasis. Curr Opin Cell Biol 55:30–35
Chen C, Zhao S, Karnad A, Freeman JW (2018) The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11(1):64
Chlebowski RT, Schottinger JE, Shi J, Chung J, Haque R (2015) Aromatase inhibitors, tamoxifen, and endometrial cancer in breast cancer survivors. Cancer 121(13):2147–2155
Gu CJ, Xie F, Zhang B, Yang HL, Cheng J, He YY, Zhu XY, Li DJ, Li MQ (2018) High glucose promotes epithelial-mesenchymal transition of uterus endometrial cancer cells by increasing ER/GLUT4-mediated VEGF secretion. Cell Physiol Biochem 50(2):706–720
Ismiil N, Rasty G, Ghorab Z, Nofech-Mozes S, Bernardini M, Ackerman I, Thomas G, Covens A, Khalifa MA (2007) Adenomyosis involved by endometrial adenocarcinoma is a significant risk factor for deep myometrial invasion. Ann Diagn Pathol 11(4):252–257
Karlsson MC, Gonzalez SF, Welin J, Fuxe J (2017) Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol Oncol 11(7):781–791
Klauzinska M, Castro NP, Rangel MC, Spike BT, Gray PC, Bertolette D, Cuttitta F, Salomon D (2014) The multifaceted role of the embryonic gene Cripto-1 in cancer, stem cells and epithelial-mesenchymal transition. Semin Cancer Biol 29:51–58
Li C, Ma H, Wang Y, Cao Z, Graves-Deal R, Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, Desai K, Gerdes MJ, Solnica-Krezel L, Coffey RJ (2014) Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. J Clin Invest 124(5):2172–2187
Li J, Zhu Q, Yang B, Ning C, Liu X, Luo X, Chen X (2018) Risk factors for ovarian involvement in young and premenopausal endometrioid endometrial cancer patients. Eur J Obstet Gynecol Reprod Biol 222:151–154
Mali AV, Padhye SB, Anant S, Hegde MV, Kadam SS (2019) Anticancer and antimetastatic potential of enterolactone: clinical, preclinical and mechanistic perspectives. Eur J Pharmacol 852:107–124
Mallini P, Lennard T, Kirby J, Meeson A (2014) Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev 40(3):341–348
Mariscal, J., P. Fernandez-Puente, V. Calamia, A. Abalo, M. Santacana, X. Matias-Guiu, R. Lopez-Lopez, A. Gil-Moreno, L. Alonso-Alconada, and M. Abal (2018) Proteomic characterization of epithelial-like extracellular vesicles in advanced endometrial cancer. J Proteome Res
Menendez JA, Oza BP, Atlas E, Verma VA, Mehmi I, Lupu R (2004) Inhibition of tumour-associated fatty acid synthase activity antagonizes estradiol- and tamoxifen-induced agonist transactivation of estrogen receptor (ER) in human endometrial adenocarcinoma cells. Oncogene 23(28):4945–4958
Mitra P, Kalailingam P, Tan HB, Thanabalu T (2018) Overexpression of GRB2 enhances epithelial to mesenchymal transition of A549 cells by upregulating SNAIL expression. Cells 7(8)
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. Lancet 387(10023):1094–1108
Muppala S, Xiao R, Krukovets I, Verbovetsky D, Yendamuri R, Habib N, Raman P, Plow E, Stenina-Adognravi O (2017) Thrombospondin-4 mediates TGF-beta-induced angiogenesis. Oncogene 36(36):5189–5198
Nicolini A, Carpi A, Ferrari P, Biava PM, Rossi G (2016) Immunotherapy and hormone-therapy in metastatic breast cancer: a review and an update. Curr Drug Targets 17(10):1127–1139
Okazaki H, Takeda S, Kakizoe K, Taniguchi A, Tokuyasu M, Himeno T, Ishii H, Kohro-Ikeda E, Haraguchi K, Watanabe K, Aramaki H (2017) Bisphenol AF as an Inducer of Estrogen Receptor beta (ERbeta): Evidence for anti-estrogenic effects at higher concentrations in human breast cancer cells. Biol Pharm Bull 40(11):1909–1916
Rahmani S, Pour Khalili N, Khan F, Hassani S, Ghafour-Boroujerdi E, Abdollahi M (2018) Bisphenol A: what lies beneath its induced diabetes and the epigenetic modulation? Life Sci 214:136–144
Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA, Gynecologic Oncology Group (2006) Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 24(1):36–44
Robertson H, Kirby JA, Yip WW, Jones DE, Burt AD (2007) Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology 45(4):977–981
Rodriguez-Salas N, Dominguez G, Barderas R, Mendiola M, Garcia-Albeniz X, Maurel J, Batlle JF (2017) Clinical relevance of colorectal cancer molecular subtypes. Crit Rev Oncol Hematol 109:9–19
Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee RB (2000) A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 78(2):212–216
Shenoy AK, Jin Y, Luo H, Tang M, Pampo C, Shao R, Siemann DW, Wu L, Heldermon CD, Law BK, Chang LJ, Lu J (2016) Epithelial-to-mesenchymal transition confers pericyte properties on cancer cells. J Clin Invest 126(11):4174–4186
Suarez-Carmona M, Lesage J, Cataldo D, Gilles C (2017) EMT and inflammation: inseparable actors of cancer progression. Mol Oncol 11(7):805–823
Tangjitgamol S, Anderson BO, See HT, Lertbutsayanukul C, Sirisabya N, Manchana T, Ilancheran A, Lee KM, Lim SE, Chia YN, Domingo E, Kim YT, Lai CH, Dali AZ, Supakapongkul W, Wilailak S, Tay EH, Kavanagh J, Asian Oncology S (2009) Management of endometrial cancer in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 10(11):1119–1127
Xin Z, Wu X, Ji T, Xu B, Han Y, Sun M, Jiang S, Li T, Hu W, Deng C, Yang Y (2019) Bakuchiol: a newly discovered warrior against organ damage. Pharmacol Res 141:208–213
Yamazaki R, Inokuchi M, Ishikawa S, Myojo S, Iwadare J, Bono Y, Mizumoto Y, Nakamura M, Takakura M, Iizuka T, Ohta T, Fujiwara H (2015) Tamoxifen-induced ovarian hyperstimulation during premenopausal hormonal therapy for breast cancer in Japanese women. Springerplus 4(425)
Zhang L, Zhou D, Guan W, Ren W, Sun W, Shi J, Lin Q, Zhang J, Qiao T, Ye Y, Wu Y, Zhang Y, Zuo X, Connor KL, Xu G (2017a) Pyridoxine 5’-phosphate oxidase is a novel therapeutic target and regulated by the TGF-beta signalling pathway in epithelial ovarian cancer. Cell Death Dis 8(12):3214
Zhang Z, Hu Y, Guo J, Yu T, Sun L, Xiao X, Zhu D, Nakanishi T, Hiromori Y, Li J, Fan X, Wan Y, Cheng S, Li J, Guo X, Hu J (2017b) Fluorene-9-bisphenol is anti-oestrogenic and may cause adverse pregnancy outcomes in mice. Nat Commun 8(14585)
Acknowledgments
We thank Dr. Zhaobin Zhang for his assistance with the preparation of BHPF samples.
Funding
This work was supported by the Beijing Municipal Science & Technology Commission for capital characteristic clinic project (Grant No. Z141107002514128).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Zhuang, T., Li, F. et al. Fluorene-9-bisphenol inhibits epithelial-mesenchymal transition of human endometrial cancer Ishikawa cells by repressing TGF-β signaling pathway. Environ Sci Pollut Res 26, 27407–27413 (2019). https://doi.org/10.1007/s11356-019-05184-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05184-0