Abstract
Sulfur dioxide emissions have been regulated at a global scale; sulfur (S) deposition no longer contributes to soil acidification instead of an alleviation effect in temperate regions; however, it remains unclear whether S deposition still contributes to soil acidification in the tropics. The Pearl River Delta (PRD), South China, has been suffering serious soil acidification, but the contribution of S deposition was ignored because of the regulation of S emission since 2001. Here, we chose the evergreen broadleaf forests, which are the typical forest type at the regional scale in PRD to examine the contribution of S deposition and its characteristics in this acidification, based on an established urban–rural gradient in the range of 260 km. A substantial acidification was evidenced by the significant decline of soil pH from rural to urban sites, with mean pH values decreased by more than 0.60 U through the whole 40-cm depths. However, there was no significant difference in soil pH from 0–10 cm, 10–20 cm, and to 20–40 cm at each site (P > 0.05). Acid-neutralizing capacity (ANC) showed a similar trend to soil pH, with a significant decline along the urbanization gradient and no significant effect of soil depths. Soil sulfate (SO42−), as the most abundant species in ANC, contributed greatly to soil acidification for the whole 40-cm depth, as shown by the significant positive relationships between it with soil pH and base cations. Soils also exhibited the depletion of base cations with low base saturation (< 20%) and the release of Al and Fe. Our research demonstrated that the severe soil acidification in the PRD region has extended to the subsoil level (40-cm depth), and S deposition is still an important driver to this acidification. Therefore, both recovering the acidified soils and controlling the acidifying pollutants, especially S, are particularly difficult in southern China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfur (S) deposition, as an important component of acid deposition, contributes greatly to soil acidification. Sulfate (SO42−) enters soils accompanied by hydrogen ions (H+), and it can be retained in the soil by adsorption, particularly in tropical soils with low pH values (Sokolova and Alekseeva 2008). Thus, the SO42− adsorption capacity of the soil has a great impact on its acidity, and SO42− concentrations always increase with decreasing pH values (McBride 1994). However, the contribution of S deposition to soil acidification has been ignored because S emission has been controlled in most regions, e.g., in Europe (Vestreng et al. 2007), North America (Driscoll et al. 2001), and China (Fang et al. 2013).
Many researches have focused on the changes of acidified soil after the reduction of S emission. The results showed that the acidified soil has experienced a change from negligible alleviation (Gimeno et al. 2001) or continuing acidification (Alewell et al. 2000; Warby et al. 2009) during the early period, to a truly recovered state in recent years (Kirk et al. 2010; Lawrence et al. 2015). The early response of acidified soils was attributed to decreasing atmospheric calcium (Ca2+) and magnesium (Mg2+) inputs accompanied by decreases in sulfur dioxide (SO2) emission (Hedin et al. 1994). Their long-term recovery was explained that decades of atmospheric S deposition can lead to the accumulation of S in forest soils (Driscoll et al. 2001) in the form of SO42− for 30–50 years until being desorbed (Alewell et al. 2000). Consequently, it has taken a long time (more than 30 years) to recover forest soils after the reduction of S emission (Lawrence et al. 2015). This soil acidification was characterized by decreases in pH and ANC, depletion of the nutrient cations (e.g., Ca2+, Mg2+) necessary for plants, and increase in aluminum (Al) mobilization (Driscoll et al. 2001), and so on. However, these researches were performed in temperate regions, not in the tropics.
Compared with the temperate region, the soil in many areas of the tropics is strongly acidic, and nitrogen (N) is not a limiting factor for high N deposition (Galloway et al. 2004; Gu et al. 2015). Thus, SO42− retention and base cation depletion might be more intense in the tropics than in the temperate region, favored by the high temperature and rain conditions of the tropics (Adhikari et al. 2014; Sokolova and Alekseeva 2008). Eventually, the effects of S deposition in this region could be prolonged (for more than 30–50 years) and extended to deeper soil layers than in the temperate region. Furthermore, high N deposition in tropical forests could cause N saturation (Gurmesa et al. 2016), which might strengthen the effects of S deposition on soil acidification (Huang et al. 2015a). Thus, the contribution of S deposition to soil acidification could remain even though S emission reduces, and its drives might be more complex in the tropics than in the temperate region. However, little attention has been paid to the contribution of S deposition to acidified soil in the tropics.
China has controlled S emission since 2001 (Fang et al. 2013), reaching a 75% reduce in S emission after 6 years (Li et al. 2017), but it still has had a rapid increase in N deposition, and becomes the largest producer of reactive N in the world (Gu et al. 2015). Consequently, it has been documented that soil acidification in China’s forests (Tian and Niu 2015), grasslands (Yang et al. 2012), and croplands (Guo et al. 2010) are strongly affected by N deposition but not by S deposition. The Pearl River Delta (PRD) region, South China, is located in the tropics and has had a rapid decrease in SO2 emission since 2005 (EPDGP 2001–2015). But, S deposition was still high with 43 kg S ha−1 year−1 in Guangzhou city in 2010 (Fang et al. 2013). And, S deposition also greatly contributed to the formation of urban “acid islands” via acid deposition in Guangzhou city (Du et al. 2015). In our previous study, substantial soil acidification was observed along an urban–rural gradient in the PRD region, and the contribution of N deposition (> 30 kg N ha−1 year−1, Huang et al. 2015b) to this acidification was confirmed based on the results obtained for pine plantations (Huang et al. 2015c). However, the contribution of S deposition has not been considered. Combing the features of soil and climate in the tropics with the results published in 2015, in which the recovery of acidified forest soils after the reduction of S emission needed a long period in the temperate region (Lawrence et al. 2015), and N-saturated ecosystems could facilitate the positive effects of S deposition on soil acidification in the subtropical region (Huang et al. 2015a), we hypothesize that S deposition still contributes to forest soil acidification in the PRD region, despite the reduction of SO2 emission since 2001.
In order to verify the hypothesis, here, we further carried out the research in the evergreen broadleaf forests in the PRD region. These forests were selected because they are the typical forest types at the regional scale and located at the late stage of forest succession, and the changes in their soil acidification status might be more representative than in other forest types. Moreover, the responses of soil acidity in broadleaf forests to urbanization might be very different from that in pine plantations for there are divergent soil properties (e.g., pH) between in broadleaf forests and in coniferous plantation at the same region especially under high temperature (Hizal et al. 2013).
Methods and materials
Study region and experimental design
The study area is located in Guangdong Province, South China. The climate is warm and humid with annual precipitation ranging from 1566 to 2133 mm and mean annual air temperature from 19.65 to 22.22 °C (Table S1). This region has experienced rapid urbanization since 1978 and showed notable urbanization effects, including the formation of heat islands (Ding et al. 2015) and higher N deposition (34 kg N ha−1 year−1 in urban sites, Huang et al. 2015b) and increased CO2 concentrations in the urbanized than in the non-urbanized areas (Mao et al. 2014). On the contrary, the emission of SO2 has reduced especially after 2007; its emission has nearly cut in half (0.68 million tons) by 2015 compared with 1.29 million tons in 2005, according to annual bulletin of environmental statistics of Guangdong Province (EPDGP 2001–2015). But, S deposition was still high with 43 kg S ha−1 year−1 in Guangzhou city (Fang et al. 2013).This study were carried out on an established urban–rural gradient (Chen et al. 2013; Huang et al. 2015b, c). Four types of locations, namely urban, urban/suburban, suburban/rural, and rural sites, were included in this urban–rural gradient. Fourteen evergreen broadleaf forests were selected, in the range of E111° 54′ 19.78″, N22° 46′ 0.60″ to E115′ 21′ 54.52″, N24° 46′ 40.25″ (Table S1). Huolushan, Maofengshan, and Shunfengshan forests were representative of urban sites; Heshan, Dinghushan (DHS), Guanyinshan, and Xiangtoushan were representative of urban/suburban sites; Heishiding, Shimentai, Yunjishan, and Dachouding were representative of suburban/rural sites; and Huaiji, Dadongshan, and Wuzhishan were representative of rural sites (Fig. 1; Chen et al. 2013).
Location of our study sites in Guangdong Province of southern China. A total of 14 open-field sites were selected along the transect (Cited from Chen et al. 2013)
Evergreen broadleaf forests are predominant in tropical regions, and provide crucial ecosystem services and functions to natural systems and humans, such as biodiversity conservation, carbon sequestration, and climatic regulation. They are very sensitive to acidic inputs from urbanization owing to their properties (e.g., high productivity and highly weathered soils). In the study region, N saturation has been identified in the DHS (urban/suburban) ecosystem (Gurmesa et al. 2016); thus, the responses of evergreen broadleaf forests to urbanization seem to indicate the real states of local forest ecosystems and are helpful to explore the true mechanisms of soil acidification. The evergreen broadleaf forests dominated by Schima superba, a native tree species that is widely distributed in Guangdong Province, were chosen for this study. Their stand ages were between 40 and 60 years, and their stand densities between 600 and 800 trees ha−1 (Table S1). All plots selected for this study were distant from forest edges and had similar slopes and orientations. All sites belonged to the core area of nature reserves, which are unaffected by forest fires, insect infestations, or logging, based on the records of the nature reserves. All forest soils were lateritic red earth (ultisols, according to USDA soil taxonomy).
Sampling collecting and measurement
A diagram was provided to illustrate the experiment work in this study (Fig. 2).
Each forest plot comprised three random subplots (10 × 10 m). Soil was sampled from January to May 2011 using a 10-cm (inside diameter) corer, and three mineral soil layers (0–10, 10–20, and 20–40 cm) were included in each soil sample. Soil samples were mixed thoroughly by hand, passed through a 2-mm sieve after removing roots and stones, and then air-dried for analysis. Soil pH was measured with a glass electrode using a 1:2.5 soil-deionized CO2-free water suspensions (Liu et al. 1996). Soil exchangeable cations, including potassium (K+), sodium (Na+), Ca2+, Mg2+, H+, Al3+, and iron (Fe3+) were determined for calculating soil cation exchange capacity (CEC). K+, Na+, Ca2+, and Mg2+, classified as exchangeable base cations, which were extracted with 1 mol L−1 NH4Ac, and Fe3+ was extracted with 0.1 mol L−1 HCl (Liu et al. 1996), then the concentrations of these cations were all determined using an inductively coupled plasma optical emission spectrometer (Perkin Elmer, USA). Soil samples were extracted with 1 mol L−1 KCl using a 5-g soil: 500 mL KCl solution to determine the total exchangeable acidity (sum of exchangeable H+ and exchangeable Al3+ contents), as well as the exchangeable H+ content. Half of the extract was titrated with 0.02 mol L−1 NaOH solution to determine total exchangeable acidity, and the other half was titrated with 0.02 mol L−1 NaOH after adding 1 mol L−1 NaF to obtain the exchangeable H+ content (Liu et al. 1996). Exchangeable Al3+ content was calculated as the difference between the total exchangeable acidity and the exchangeable H+ content. Soil CEC was calculated as the sum of exchangeable cations (i.e., K+, Na+, Ca2+, Mg2+, H+, Al3+, and Fe3+) on an equivalent basis. Soil base saturation (BS) was calculated as the fraction of the base cations (i.e., K+, Na+, Ca2+, and Mg2+) in the CEC (Mulder and Stein 1994).
Water-soluble ions present in soil samples, i.e., SO42−, nitrate (NO3−), fluoride (F−), chloride (Cl−), K+, Ca2+, Na+, Mg2+, and ammonium (NH4+) were extracted with deionized water (water:soil, 5:1) and then determined by ion chromatography (Metrohm, Switzerland) for calculating soil ANC. Soil ANC was calculated as the difference between the sum of water-soluble acid anions and the sum of water-soluble cations by charge balance (Vogt et al. 2006), that is, ANC = [2(Ca2+) + 2(Mg2+) + (K+) + (Na+) + (NH4+)] − [2(SO42−) + (NO3−) + (Cl−) + (F−)].
Data on atmospheric inorganic N deposition (including NH4+-N and NO3−-N deposition) were obtained from Huang et al. (2015b). Data on soil pH in pine plantation were cited from Huang et al. (2015c). Data on mean annual temperature and mean annual precipitation at each site were collected from the records of local meteorology bureaus, and data on stand densities and tree ages of the 14 forests were obtained from the records of the natural reserve offices at each site. Elevation, longitude, and latitude of each site were recorded by a GPS device (Table S1).
Statistical analysis
One-way analysis of variance (ANOVA) was used to compare the differences among the four types of urbanization sites (urban, urban/suburban, suburban/rural, and rural) and three soil depths (0–10, 10–20, and 20–40 cm) with respect to soil pH, ANC, BS, CEC, exchangeable cations, and soil water-soluble anions (NO3−, SO42−, Cl−, and F−). Univariate analysis was used to determine the interaction effects of urbanization and soil depth on soil pH, ANC, BS, and CEC. Pearson correlation analysis was also performed to examine the relationships among soil pH, ANC, and the concentrations of soil NO3− and SO42−, and between soil NO3− and SO42− concentrations with soil exchangeable K+, Na+, Ca2+, and Mg2+ concentrations. All analyses were conducted using SPSS 13.0 for Windows (SPSS Inc., USA), with statistical significance set at P < 0.05 unless otherwise stated. Displayed values are mean ± standard error of the mean.
Results
Soil pH and ANC
Most soil pH values ranged between 3.6 and 4.5, with few above 5.0, indicating a strongly acidic environment. A significant increase in soil pH was observed from urban to rural sites at 0–10, 10–20, and 20–40 cm depth (P < 0.01; Fig. 3). However, soil pH did not show significant differences across the three soil depths at each site (P > 0.05), and there was no interactive effect of soil depth and urbanization gradient on soil pH (P > 0.05). Soil pH was lower in the urban sites than the rural sites by approximately 0.60 U at each depth (0.64 U at 0–10 cm, 0.68 U at 10–20 cm, and 0.61 U at 20–40 cm, respectively).
Soil pH variation in tropical forests along urban–rural gradient at 40-cm soil depth in the evergreen broadleaved forests of south China. Error bars indicate ± 1 S.E. (N = 3 for urban and rural, N = 4 for urban/suburban and suburban/rural). Different letters indicate significant differences (P < 0.05) between urbanized zones, and same letters indicate no significant differences (P > 0.05) between different urbanization gradients, respectively
Acid-neutralizing capacity showed a significant increase along the urban–rural gradient across the three soil layers (P < 0.05; Fig. 4), which was consistent with the variation in soil pH. Negative ANC was observed in urban and urban/suburban sites at 40-cm depths, and at suburban/rural sites at 10 to 40-cm depths, which was attributed to the high concentrations of soil anions, especially SO42− and NO3−. However, there was no effect of soil depth and no interaction effect of urbanization gradient and soil depth on ANC. SO42− was the most abundant anion at depth of 40 cm, with significantly higher concentrations in urban sites than in the other three types of sites (P < 0.01); NO3−, the next most abundant anion, showed a significant decline in concentration from urban to rural sites (P < 0.05; Fig. 5).
Patterns of acid neutralizing capacity (ANC) from urban to rural at 40-cm soil depth in the evergreen broadleaved forests of south China. Error bars indicate ± 1 S.E. (N = 3 for urban and rural, N = 4 for urban/suburban and suburban/rural). Different letters indicate significant differences (P < 0.05) between urbanized zones, and same letters indicate no significant differences (P > 0.05) between different urbanization gradients, respectively
Patterns of soil water-soluble anions to urbanization gradients in the evergreen broadleaved forests of south China. Error bars indicate ± 1 S.E. (N = 3 for urban and rural, N = 4 for urban/suburban and suburban/rural). Different letters indicate significant differences (P < 0.05) between urbanized zones, and same letters indicate no significant differences (P > 0.05) between different urbanization gradients, respectively
Soil BS and exchangeable cations
Soil BS was very low (< 20%) and was even below 10% except in urban sites. It exhibited a significant decline from urban to urban/suburban and then to suburban/rural sites, but showed no further decline in rural sites (P < 0.05; Fig. 6). No effect of soil depth and no interaction effects of the urbanization gradient with soil depth were observed on BS. CEC showed significant responses to the urbanization gradient (P < 0.01), but was not greatly impacted by soil depth. No interaction effects of the urbanization gradient or soil depth were observed on either soil BS or CEC.
Patterns of soil base saturation (BS) to urbanization gradient in the evergreen broadleaved forests of south China. Error bars indicate ± 1 S.E. (N = 3 for urban and rural, N = 4 for urban/suburban and suburban/rural). Different letters indicate significant differences (P < 0.05) between urbanized zones, and same letters indicate no significant differences (P > 0.05) between different urbanization gradients, respectively
The concentrations of soil base cations (i.e., Ca2+, Mg2+, K+, and Na+) at 40-cm depths showed a decreasing trend from urban to urban/suburban and then to suburban/rural sites, but did not decline further in rural sites. The ions Al3+ and H+ accounted for about 72–90% of the total exchangeable cations, while Fe3+ only accounted for 2–6% of total cations. At 40-cm depth, the mean concentrations of Fe3+ were higher in urban sites than in urban/suburban sites, and accompanied by lower mean concentrations of Al3+ in urban sites than in urban/suburban sites (Fig. 7).
Patterns of soil exchangeable cations to urbanization gradients in the evergreen broadleaved forests of south China. Error bars indicate ± 1 S.E. (N = 3 for urban and rural, N = 4 for urban/suburban and suburban/rural). Different letters indicate significant differences (P < 0.05) between urbanized zones, and same letters indicate no significant differences (P > 0.05) between different urbanization gradients, respectively
Relationships of soil pH with soil properties and atmospheric factors
Soil pH was negatively correlated with the concentrations of soil SO42− and NO3− (at 40-cm soil depths, P < 0.01), while concentrations of soil NO3− and SO42− were positively correlated (at 0–10 cm, P = 0.08; at 10–20 and 20–40 cm, P < 0.001). The ANC had a positive correlation with soil pH at 40-cm depths (P < 0.01), and had a negative correlation with the concentrations of soil SO42− (P < 0.01) at 40-cm depths and NO3− at 20-cm depths (P < 0.01) (Table 1). Atmospheric inorganic N deposition was negatively correlated with soil pH values at 40-cm depths (at 0–10 cm and 10–20 cm, P = 0.07; at 20–40 cm, P < 0.05).
The concentrations of soil SO42− were positively correlated with the concentrations of soil Mg2+ (at 0–10 cm and 20–40 cm, P < 0.05; at 10–20 cm, P < 0.001) and Ca2+ (P < 0.001), and with the concentrations of soil K+ at 10–20 cm (P < 0.05) and 20–40 cm (P < 0.01). Soil NO3− concentrations also had positive correlations with soil Mg2+ concentrations at 0–10 cm (P < 0.001) and 20–40 cm (P < 0.05), and with soil K+ concentrations at 10–20 cm (P < 0.05). However, no significant correlations were observed between soil SO42− or NO3− concentrations and soil Na+ concentrations (Table 2).
Discussion
Subsoil acidification in evergreen broadleaf forests
Urbanization in the PRD region was reported to drive soil acidification in pine plantations (Huang et al. 2015c). Significant decline in soil pH from rural to urban sites found here (Fig. 3) also evidenced that urbanization in the PRD led to soil acidification in evergreen broadleaf forests, and to a more serious extent than in pine plantations. Firstly, pH values were generally 3.5–4.5, which is a narrower range than that registered in pine plantations (3.5–5.3). Secondly, the pH along the rural–urban gradient declined more than 0.60 U on average at 40-cm depths, which was larger than that observed in pine plantations (0.44 U). Thirdly, no significant differences in soil pH and ANC were found among different soil depths (40-cm) in evergreen broadleaf forests, while a significant difference in pH according to depth was observed in pine plantations. Hence, subsoil acidification (40-cm depths) occurs in the evergreen broadleaf forests of the PRD region in South China.
Subsoil acidification is a more severe environmental issue than topsoil acidification, because subsoil acidification further decreases root elongation via restraining the absorption of nutrient and water, which results in poorer root systems and restricted plant growth, worse, reduces even loses the ability of plants to resist to disease and insect pests because of nutrient deficiency. Furthermore, limited use of nutrients and water due to poor root systems may accelerate nutrient loss and groundwater eutrophication and contamination, which can be intensified by heavy rainfall in this region. Soil microorganisms also change their community composition and diversity to adapt the acidic environment (Cruz-Paredes et al. 2017), which is also detrimental to plant growth and development. Consequently, the whole ecosystem structure and function are rapidly destroyed. To make it worse, subsoil acidification is considered irreversible, owing to the difficulty of its amelioration (Tang et al. 2011). Therefore, soil acidification in the PRD region is very serious and should receive special attention.
Characteristics of forest soil acidification in the PRD region
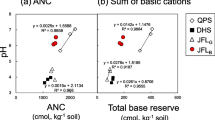
As a crucial indicator of ecological vulnerability to acidification (Lu et al. 2015; Vogt et al. 2006), ANC is calculated based on the concentrations of soil water-soluble cations and anions. Overall, ANC had a consistent positive relationship with soil pH, significantly decreasing from rural to urban sites (Fig. 4; r > 0.60, P < 0.001), agreeing with that reported for the temperate region (Driscoll et al. 2001). Negative ANC was always observed in urban and urban/suburban sites at the whole 40-cm depths, indicating that evergreen broadleaf forest ecosystems have poor soil buffering capacity (Lu et al. 2014) and cannot neutralize and hold excess acid input (Lu et al. 2015). This finding was consistent with the values of ANC observed at 40-cm depths in DHS (Lu et al. 2015). Moreover, ANC was dominated by soil SO42− and NO3−; it was greatly affected by the concentrations of soil SO42− and NO3−, not by base cations (Figs. 4 and 5), suggesting that N and S deposition strongly contribute to soil acidification, which supported our hypothesis.
The BS obtained here was all lower than 20% and even below 10% in urban/suburban, suburban/rural, and rural sites (Fig. 6). Because 20% is the BS threshold for predicting damage caused by acidification (Hicks et al. 2008), the values found on the present study indicate that base cations were very depleted for the four types sites (Fig. 7), which was consistent with that reported for the temperate region (Driscoll et al. 2001). The low BS found in strongly acidic soils in the humid tropics is attributable to the rapid dissolution and leaching of weatherable minerals driven by high precipitation (Lu et al. 2014). Despite depletion, the concentrations of base cations showed a significant decline from urban to urban/suburban and to suburban/rural sites, in this order (Fig. 7), suggesting the presence of important urban sources of these cations (Huang et al. 2015b). For example, Ca2+, which mainly originates from dust transported from local and anthropogenic sources such as industrial and construction activities (Larssen and Carmichael 2006). Urban sources of base cations could effectively reduce the production of H+ and the accumulation of Al3+ in urban sites with lower concentrations than suburban sites (Fig. 7). The concentrations of soil SO42− and NO3− also positively affected base cations concentrations, especially for Mg2+ and Ca2+, based on their significant positive correlations (Table 2), also indicating their common urban sources. Moreover, Mg2+ and Ca2+ leaching and mobilization were also correlated with NO3− (Lu et al. 2014) and SO42− (Sokolova and Alekseeva 2008).
Soil exchangeable Al3+, as the dominant component of soil cation pools, was present at much higher levels than any other cation, exceeding the sum of base cations in base equivalents (Fig. 7), thereby indicating Al3+ release, which is an important acid buffering process in acidic soils (Mulder et al. 1989) experiencing S control (Driscoll et al. 2001) and N deposition (Huang et al. 2015a; Lu et al. 2015). However, Al3+ concentrations in the urban sites were always lower than that in the urban/suburban sites, especially at 10-20-cm and 20-40 cm soil depths, while Fe3+ concentrations were always higher in the urban sites than in the urban/suburban sites (Fig. 7), suggesting Fe3+ release. A significant negative correlation was also observed between soil pH and Fe3+ concentrations (Table 1; at 0–10 cm, P < 0.05; at 10–20 cm, P < 0.01), indicating a potential Fe buffer. Both Al buffer and potential Fe releases were also observed in the pine plantations (Huang et al. 2015c).
The absence of any effect of soil depth on soil pH and ANC up to 40 cm was recognized as an important feature of subsoil acidification in this region, as evidenced by the non-significant differences for these two parameters across the three soil depths (i.e., 0–10, 10–20, and 20–40 cm) in all sites. CEC was not greatly impacted by soil depth, which also supported the loss of soil depth effects. Depth effects are an important feature of soil that is reflected in all soil properties, including soil pH, organic carbon content, and soil metal concentration (Minasny et al. 2016). Strong leaching is frequent in the tropics due to heavy precipitation (Table S1) (Adhikari et al. 2014), and it strongly affects soil pH at various depths (Adhikari et al. 2014). Eventually, soil depth effects on pH and ANC at 40-cm diminish and even disappear, and the impact of acidification reaches the subsoil (below 40-cm depths).
Sulfur deposition is still contributing to soil acidification
Urbanization induces subsoil acidification in the PRD region, as evidenced by the significant effects (P < 0.01) of urbanization on soil pH and by the significant correlations of soil pH and ANC with soil NO3− and SO42− concentrations (P < 0.001; Table 1) at 40-cm depths. Soil SO42− and NO3− concentrations have common urban sources, namely the SO2 and NOx produced in highly urbanized areas, which are the precursors of S and N deposition, respectively, as shown by the significant positive correlations between their concentrations (Table 1). Hence, besides N deposition, S deposition also contributes to soil acidification in this region.
In China, S emission has been controlled since 2001 (Fang et al. 2013), and has declined by 75% since 2007 (Li et al. 2017), but S deposition was still high (Fang et al. 2013). S deposition is an important source of SO42−, which can be retained and accumulated in soils via adsorption (Sokolova and Alekseeva 2008), negatively affecting soil pH (McBride 1994). Sulfur deposition has been reported to continuously contribute to soil acidification in croplands (Guo et al. 2010) and subtropical forests (Huang et al. 2015a) in China. In this study, S deposition contributed to high soil SO42− concentrations up to 40-cm depths and to its dominance in ANC, especially in urban sites (Figs. 3 and 4), suggesting its continuing contribution to soil acidification. Significant positive correlations (Table 2) between the concentrations of soil SO42− and Mg2+ (P < 0.05) or Ca2+ (P < 0.01) also suggested that the deposition is their common source and affects their concentrations. The result was supported by Du et al. (2015) that S deposition still greatly contributes to the formation of urban acid islands in the cities of southern China.
The contribution of S deposition to soil acidification is enhanced by the tropical climate and soil properties of the PRD region. In this region, high precipitation and temperature, the annual averages of which ranged from 1566 to 2133 mm and 19.65 to 22.22 °C, respectively (Table S1), can positively influence the amount of SO42− adsorbed (Sokolova and Alekseeva 2008). As all forest soils were lateritic red soil, which is highly weathered and with low pH (mostly in the range 3.6–4.5), their SO42− adsorption capacity is high (Gobran et al. 1998). Previous studies have shown that when soil pH is 4, SO42− adsorption can reach a maximum (Nodvin et al. 1986;) via an irreversible process (Gobran et al. 1998). In the urban sites, soil pH values up to 40-cm depths were around 4.0, and the highest soil SO42− concentration was observed in these sites (Fig. 5), suggesting that their SO42− adsorption capacity is higher than that of the other three types of sites. Moreover, SO42− might be retained for longer periods (> 30–50 years) in the tropics than in the temperate region, as strong leaching via rainfall helps the effect of S deposition to reach the subsoil.
High N deposition also strengthens the effects of S deposition on soil acidification. In the PRD region, N deposition was more than 30 kg N ha−1 year−1 and was dominated by NO3− (Huang et al. 2015b), which results in N-rich and even N-saturated conditions, as that observed in DHS (Gurmesa et al. 2016), enhancing SO42− adsorption (Huang et al. 2015a), and NO3− leaching (Gurmesa et al. 2016). Consequently, ANC is more influenced by SO42− and NO3− than by base cations (Dentener et al. 2006), although they have important urban sources. Therefore, there might be an interaction involving N deposition and S deposition on soil acidification, but it needs further investigation to be validated. Because of China, among the countries with highest N deposition (Gu et al. 2015), the effect of N deposition on S deposition driving soil acidification cannot be ignored, and S deposition’s contribution to soil acidification should receive much and continued attention in the future.
Conclusions
Significant subsoil acidification (at 40-cm depths) was observed in the evergreen broadleaf forests of the PRD region, South China, indicating reduced soil pH values (about 0.60 U lower in urban sites than in rural sites), depletion of base cations, Al and Fe release, negative ANC dominated by SO42− and NO3−, and no depth effects on pH and ANC at 40-cm depths. The contribution of S deposition to this acidification was identified based on high soil SO42− concentrations and significant positive correlations between soil SO42− and ANC, pH, and base cations. Our findings indicated that soil acidification in the PRD region is very serious, and S deposition is still an important driver of this acidification, albeit the control of S emission for more than a decade. Therefore, recovering acidified soils is very difficult, and abating both S and N emission, instead of controlling only S or N emission, seems to be a more effective measure for the sustainable management of these tropical forest ecosystems.
References
Adhikari K, Kheir RB, Greve M, Greve MH, Malone MB, Minasny B, McBratney A (2014) Mapping soil pH and bulk density at multiple soil depths in Denmark. In: Arrouays D, McKenzie N, Hempel J, de Forges AR, McBratney AB (eds) Global soil map: basis of the global spatial soil information system. Taylor & Francis, London, pp 155–160
Alewell C, Manderscheid B, Gerstberger P, Matzner E (2000) Effects of reduced atmospheric deposition on soil solution chemistry and elemental contents of spruce needles in NE-Bavaria, Germany. J Plant Nutr Soil Sci 163:509–516
Chen H, Zhang W, Gilliam F, Liu L, Huang J, Zhang T, Wang W, Mo J (2013) Changes in soil carbon sequestration in Pinus massoniana forests along an urban-to-rural gradient of southern China. Biogeosciences 10:6609–6616
Cruz-Paredes C, Wallander H, Kjøller R, Rousk J (2017) Using community trait-distributions to assign microbial responses to pH changes and Cd in forest soils treated with wood ash. Soil Biol Biochem 112:153–164
Dentener F, Drevet J, Lamarque JF, Bey I, Eickhout B, Fiore AM, Hauglustaine D, Worowitz LW, Krol M, Kulshrestha UC, Lawrence M, Galy-Lacaux C, Rast S, Shindell D, Stevenson D, Van Noije T, Atherton C, Bell N, Bergman D, Butler T, Cofala J, Collins B, Doherty R, Ellingsen K, Galloway J, Gauss M, Montanaro V, Müller JF, Pitari G, Rodriguez J, Sanderson M, Solmon F, Strahan S, Schultz M, Sudo K, Szopa S, Wild O (2006) Nitrogen and sulfur deposition on regional and global scales: multimodel evaluation. Glob Biogeochem Cycles 20:GB4003. https://doi.org/10.1029/2005GB002672
Ding S, Qiao G, Guo Y, Wu Y, Wang B (2015) Study on the urban heat island and meteorological elements over the Pearl River Delta. J Trop Meteorol 31(5):681–690 (in Chinese with English abstract)
Driscoll CT, Lawrence GB, Bulger AJ, Butler TJ, Cronan CS, Eagar C, Lambert KF, Likens GE, Stoddard JL, Weathers KC (2001) Acidic deposition in the northeastern United States: sources and inputs, ecosystems effects, and management strategies. BioScience 51:180–198
Du E, de Vries W, Liu X, Fang J, Galloway JN, Jiang Y (2015) Spatial boundary of urban ‘acid islands’ in southern China. Sci Rep 5:12625
Fang Y, Wang X, Zhu F, Wu Z, Li J, Zhong L, Chen DH, Yoh M (2013) Three-decade changes in chemical composition of precipitation in Guangzhou city, southern China: has precipitation recovered from acidification following sulphur dioxide emission control? Tellus Ser B Chem Phys Meteorol 65:20213
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vöosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70(2):153–226
Environmental Protection Department of Guangdong Province (GEPGP) (2001-2015) Annual bulletin of environmental statistics of Guangdong Province. (in Chinese) http://www.gdep.gov.cn/zlkz/tjxx/?sTop=0
Gimeno L, Marín E, del Teso T, Bourhim S (2001) How effective has been the reduction of SO2 emissions on the effect of acid rain on ecosystems? Sci Total Environ 275:63–70
Gobran GR, Selim HM, Hultberg H, Anderson I (1998) Sulfate adsorption-deposition is a Swedish forest soil. Water Air Soil Pollut 108:411–424
Gu B, Ju X, Chang J, Ge Y, Vitousek PM (2015) Integrated reactive nitrogen budgets and future trends in China. PNAS 112(28):8792–8797
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KW, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010
Gurmesa AG, Lu X, Gundersen P, Mao Q, Zhou K, Fang Y, Mo J (2016) High retention of 15N-labeled nitrogen deposition in a nitrogen saturated old-growth tropical forest. Glob Chang Biol 22:3608–3620
Hedin LO, Granat L, Likens GE, Buishand TA, Galloway JN, Butler TJ, Rodhe H (1994) Steep declines in atmospheric base cations in regions of Europe and North America. Nature 367:351–354
Hicks WK, Kuylenstierna JC, Owen Z, Dentener F, Seip HM, Rodhe H (2008) Soil sensitivity to acidification in Asia: status and prospects. Ambio 37:295–303
Hizal A, Gökbulak F, Zengin M, Erean M, Karakaş A, Tuğrul D (2013) Effect of vegetation change from native broadleaf forest to coniferous plantation on selected soil properties. Environ Monit Assess 185:10249–10256
Huang Y, Kang R, Mulder J, Zhang T, Duan L (2015a) Nitrogen saturation, soil acidification, and ecological effects in a subtropical pine forest on acid soil in Southwest China. J Geophys Res 120:2457–2472
Huang J, Zhang W, Zhu X, Gilliam FS, Chen H, Lu X (2015b) Urbanization in China changes the composition and main sources of wet inorganic nitrogen deposition. Environ Sci Pollut Res 22:6526–6534
Huang J, Zhang W, Mo J, Wang S, Liu J, Chen H (2015c) Urbanization in China significantly drives soil acidification of Pinus massoniana forests. Sci Rep 5:13512
Kirk GJD, Bellamy PH, Lark RM (2010) Changes in soil pH across England and Wales in response to decreased acid deposition. Glob Chang Biol 16:3111–3119
Larssen T, Carmichael GR (2006) Acid rain and acidification in China: the importance of base cation deposition. Environ Pollut 110:89–102
Lawrence GB, Hazlett PW, Fernandez IJ, Ouimet R, Bailey SW, Shortle WC (2015) Declining acidic deposition begins reversal of forest-soil acidification in the Northeastern U.S. and Eastern Canada. Environ Sci Technol 49:13103–13111
Li C, McLinden C, Fioletov V, Krotkov N, Carn S, Joiner J, Streets D, He H, Ren X, Li Z, Dickerson RR (2017) India is overtaking China as the world’s largest emitter of anthropogenic sulfur dioxide. Sci Rep 7:14303
Liu GS, Jiang NH, Zhang LD, Liu ZL (1996) Soil physical and chemical analysis and description of soil profiles. China Standards Press, Beijing (in Chinese)
Lu X, Mao Q, Gilliam F, Luo Y, Mo J (2014) Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob Chang Biol 20:3790–3801
Lu X, Mao Q, Mo J, Gilliam F, Zhou G, Luo Y, Zhang W, Huang J (2015) Divergent responses of soil buffering capacity to long-term N deposition in three tropical forests with different land-use history. Environ Sci Technol 49:4072–4080
Mao B, Deng X, An X, Liu X, Li F, Liu X (2014) Spatial and temporal distributions of tropospheric CO2 concentrations over Guangdong province based on satellite observations. China Environ Sci 34(5):1098–1106 (in Chinese with English abstract)
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York
Minasny B, Stockmann U, Hartemink AE, McBratney AB (2016) Measuring and modeling soil depth functions. In: Hartemink AE, Minasny B (eds) Digital soil morphometrics. Springer, Dordrecht, pp 225–240
Mulder J, Van Breeman N, Eijck HC (1989) Depletion of soil aluminum by acid deposition and implications for acid neutralization. Nature 337:247–249
Mulder J, Stein A (1994) The solubility of aluminum in acidic forest soils: long-term changes due to acid deposition. Geochim Cosmochim Acta 58:85–94
Nodvin SC, Driscoll CT, Likens GE (1986) The effect of pH on sulfate adsorption by a forest soil. Soil Sci 142:69–75
Sokolova TA, Alekseeva SA (2008) Adsorption of sulfate ions by soils (a review). Eurasian Soil Sci 41:158–167
Tang C, Conyers MK, Nuruzzaman M, Poile GJ, Liu DL (2011) Biological amelioration of subsoil acidity through managing nitrate uptake by wheat crops. Plant Soil 338:383–397
Tian D, Niu S (2015) A global analysis of soil acidification caused by nitrogen deposition. Environ Res Lett 10:024019
Vestreng V, Myhre G, Fagerli H, Reis S, Tarrasón L (2007) Twenty-five years of continuous sulphur dioxide emission reduction in Europe. Atmos Chem Phys 7:3663–3681
Vogt RD, Seip HM, Larssen T, Zhao D, Xiang R, Xiao J, Luo J, Zhao Y (2006) Potential acidifying capacity of deposition experiences from regions with high NH4 + and dry deposition in China. Sci Total Environ 367:394–404
Warby RAF, Johnson CE, Driscoll CT (2009) Continuing acidification of organic soils across the Northeastern USA: 1984–2001. Soil Sci Soc Am J 73:274–284
Yang Y, Ji C, Ma W, Wang S, Wang S, Han W, Mohammat A, Robinson D, Smith P (2012) Significant soil acidification across northern China’s grasslands during 1980s-2000s. Glob Chang Biol 18:2292–2300
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant Nos. 41573073 and 41731176), Natural Science Foundation of Guangdong Province (Grant No. 2016A030313154), and Open Fund of State Key Laboratory of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences (No. OGL-201409).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Huang, J., Zhou, K., Zhang, W. et al. Sulfur deposition still contributes to forest soil acidification in the Pearl River Delta, South China, despite the control of sulfur dioxide emission since 2001. Environ Sci Pollut Res 26, 12928–12939 (2019). https://doi.org/10.1007/s11356-019-04831-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04831-w