Abstract
Polycyclic aromatic hydrocarbons (PAHs), some of which are classified as possible carcinogens (WHO), have been detected in cooking fumes in considerable amounts. Distribution of 24 PAHs on varying particle sizes was analyzed in cooking emission. Analysis of cooking fumes from vegetarian and non-vegetarian food was carried out separately in the kitchen of a hostel mess in IIT Kanpur during November 2012 and February 2013. Respirable suspended particulate matter (RSPM) and particle-bound polycyclic aromatic hydrocarbons (PPAHs) showed a similar sequence regarding concentration observed in vegetarian and non-vegetarian food. PAHs with carcinogenic potential was detected and quantified mostly in the fine particles. Total PAH concentrations in the fine and ultrafine ranges together accounted for > 90% of the total carcinogenic PAHs, highlighting them as primary carriers of PAHs rather than coarser particles. Benzo [a] pyrene (B [a]P) levels contribute > 70% to total carcinogenic potential and > 60%, to mutagenic potential, respectively. The total toxicity impact on the workers due to the PAHs emitted from cooking fumes was 3.374 × 10−10 DALYs, with B [a] P contributing the most (> 70%) despite its low concentration. Exposure to cooking fumes especially for people involved in this activity on a daily basis (chefs, hostel mess workers, among others) raises health concerns. An extensive examination of impacts due to exposure to emissions in both particle and gas phase on a long-term basis is required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the Global burden of disease (IHME 2016), risk due to air pollution, incorporating ambient particulate matter pollution and household air pollution due to solid fuel, was a major cause of premature mortality and morbidity not only in India but globally. In India, it resulted into causing approximately 1.6 million of deaths and 46 million of disability-adjusted life years (DALYs) lost per year, accounting for 16% of total deaths and 9.8% of total deaths, respectively.
Progressive exposure to harmful compounds present indoor can be highly significant because of people spending approximately 80% of their time indoor (Gupta and Bhandari 2011; Lu et al. 2008; Stabile et al. 2015). Besides, poor ventilation conditions in the modern building to minimize the energy uses compromising the indoor air quality, despite its environmental benefit, raising health concerns (Balakrishnan et al. 2004). A significant source of indoor air pollution worldwide includes furnishing, construction materials, combustion of fuel, coal, and tobacco, ventilation system and cooking. Cooking and fuel/tobacco combustion is the main source of respirable suspended particulate matter (RSPM) in the indoor environment (Zhang and Smith 2018). Among RSPM, fine (aerodynamic diameter, dp ≤ 1 μm) and ultrafine (dp ≤ 0.1 μm) particles are of significant concern since they can penetrate deep into the respiratory tract and thus into the blood circulation system (Schwartz et al. 1996). RSPM is an excellent carrier of absorbed inorganic and organic compounds such as PAHs (polycyclic aromatic hydrocarbons). Polycyclic aromatic hydrocarbons have been reported as carcinogenic and mutagenic (WHO 1998), found ubiquitously in the environment, and to have developmental, reproductive, cardio-, immune-, and neuro-toxicities in laboratory animals and human (ASTDR 1995; IARC 1983).
Various studies on indoor air quality have identified cooking as one of the most significant particle generating activities indoors (Zhao et al. 2007; Buonanno et al. 2009). A recent study on health risk faced by Indian population through dietary PAHs (Singh and Agarwal 2018) has examined levels of these compounds on different everyday food types, including cereals, bread, biscuit, oils, etc. The study reported total concentrations of PAHs in these products ranging from 0.18 to 61,967 μg/kg. Furthermore, it was examined (Wei and Balasubramanian 2008) that cooking method involving the use of oil at high temperatures, such as frying, pan-frying, and deep-frying, released a higher amount of PAHs compared with those that include the use of water, such as boiling and steaming. Also, high-temperature frying leads to the production of higher molecular weight PAHs, while low-temperature cooking results in the formation of more amount of low molecular weight PAHs. PAHs with high molecular weight, mainly present in the particulate phase, are more carcinogenic than those with low molecular weight (Oanh et al. 2000; Zhu et al. 2009). Buonanno et al. (2009) reported higher aerosol mass emission when cooking fatty food (i.e., food containing a higher percentage of fat) resulting in higher indoor concentration than while cooking vegetables. Emission of PAHs in cooking fumes is not only related to the cooking method, but also to the cooking ingredients. It was found that cooking meat produced far greater PAH concentrations than frying vegetables (Schauer et al. 1999). Higher quantities of oil are generally used in stir-frying, commonly used in Malay and Chinese cooking than simmering which is the most common technique used for the preparation of Indian dishes.
Research on PAH emission in indoor air from cooking is limited and has mainly focused on rural India and cooking fuels like biomass, coal, and kerosene oil (Pandit et al. 2001; Bhargava et al. 2004). PAH concentrations are seen to increase substantially during biomass burning. Exposure to carcinogenic PAHs in indoor-air during cooking over ‘chulha’ in rural India is highly likely in the breathing zone and surrounding areas while cooking (Bhargava et al. 2004). The chronic exposure to carcinogenic PAHs along with multiple chemicals or biological agents in indoor air in the form of RSPM could be a risk factor for acute pulmonary illness, asthma, pulmonary tuberculosis, and lung cancer in people involved in the cooking activity, mainly women in India. Knowledge of variation in emission from different food types and cooking methods used in urban India, the occurrence of particle-bound polycyclic aromatic hydrocarbons (PPAHs) on finer PM leading to health risk is limited.
Objective
The primary aim of this study is an assessment of health risk to people involved in cooking activity through exposure to PPAHs in fumes emitted during cooking at a mess in IIT Kanpur campus. This was done by measurement of RSPM and determination of the size-segregated distribution of PPAHs in fumes emitted from different types of food cooked. Data collected have been used to estimate the combined cancer and non-cancer burden of disease, in terms of disability-adjusted life years (DALYs), attributable to daily exposure to PPAHs in cooking fumes.
Methodology
Sampling sites and experimental setup
Active air samples were collected from a Hostel mess (kitchen) in fully residential IIT Kanpur campus, with around 6000 population. Hostel messes on campus were surveyed to ascertain the infrastructure and nature and amount of food cooked. The selection was based on the cooking area, ventilation conditions (exhaust and hood), and the amount of food cooked. The samples were collected in a hostel mess which caters to around 600 students. Other selection criteria which were considered include the amount of food cooked per day and the frequency of cooking (number of times a week) of non-vegetarian food like chicken and fish, to include all types of food cooked and increase the sampling efficiency. Sampling was conducted three times over a 4-month period, November 2012 to February 2013, during the regular semester and semester break (Table 1). Each set includes all three types of food cooked—vegetarian (veg), chicken, and fish.
In this study, the chimneys over cooking areas were kept on, and hence, the level of RSPM measured is the actual emission to which the workers were exposed. By managing the flow direction of fans in the kitchen, intermixing of fumes from a different type of food, vegetarian, chicken, and fish was avoided.
Sampling schedule
Samples were collected during the main cooking period three times a day (breakfast, lunch, and dinner) over a 4-month period (Nov 2012 to Feb 2013). The chicken was cooked for dinner while fish for lunch. Sampling duration and instrument used are described for each of the three sets in Table 1. Sampling in set 1 and set 2 (using OPC) has been conducted on the basis of food type cooked—vegetarian, chicken, and fish. This means that separate filters in OPC were used for different types of food cooked (vegetarian, fish, or chicken) and resulted in three filters, one for each food type, in set 1 and set 2 separately. To ascertain size-segregated particle-bound PAH emission from food cooked during a day, sampling was conducted using MOUDI on a single day in February (set 3). Only vegetarian food was cooked in the mess on this day. In summary, set 1 and set 2 provide average levels of pollutants over one mealtime while set 3 provides information on levels over one complete day. Combining the number of sampling days for set 1 and set 2 reveals that data for vegetarian food has been collected over 17 days followed by 20, and 17 days for chicken and fish, respectively.
Instrumentation and sampler location inside the mess
Air samples conducted to determine particle size distribution using an Optical Particle Counter (OPC) (GRIMM Aerosol 1.108). Size-segregated distribution of particles (and particle-bound PAHs) was ascertained using a Micro-Orifice Uniform Deposit Impactor (MOUDI) during November 2012–February 2013. The OPC had a flow rate of 1.2 L/min, and its gravimetric filters had a pore size of 0.12 μm and diameter of 47 mm. The MOUDI had a flow rate of 30 L/min such that sampling duration could be shortened considerably to obtain quantifiable results. The nominal cut sizes of the 10 MOUDI stage filters are 0.056, 0.1, 0.18, 0.32, 0.56, 1.0, 1.8, 3.2, 5.6, and 10 μm. The collected samples were then analyzed in a gas chromatograph-mass spectrometer (GC-MS) for PAHs.
The two instruments (OPC and MOUDI) were placed close to the stove where food was being cooked as seen in Fig. 1, which shows the location of samplers in the hostel kitchen. Presence of a semi-partition wall between the cooking stoves minimized the possibility of cross-contamination of air samples during cooking. Samplers were placed close to the stove on which the food was being cooked. Separate stoves were used for cooking vegetarian and non-vegetarian food. This wall separates the areas where non-vegetarian food was cooked from vegetarian food. The fans were not used during the cooking period, thereby ensuring reduced cross-contamination of particulate matter from each stove.
Sample analysis
Collected samples were analyzed for PAHs using gas chromatography-mass spectrometer (GC-MS) (Model Clarus 600, Perkin Elmer) using an elite silica capillary column, DB5-MS (30 m, 0.25 mm I. D, 0.25 μm film thickness; Perkin Elmer, Santa Clara, USA). Helium (XL grade) was used as the carrier gas. The mass spectrometer was operated in the electron impact mode (70 eV) with the ion source at 200 °C. The samples were injected in 1/19 splits at an injector temperature of 260 °C. The oven was programmed as the initial temperature of 70 °C for 2 min, heated to 180 °C at 10 °C min−1, then to 230 °C at 6 °C min−1 and finally heated to 280 °C at 2 °C min−1 and held at the highest temperature for 5 min.
Analytes
The PAH standards for 24 compounds were used as external standards. These included namely isophorone (Ip), hexachlorocyclopentadiene (C-56), acenaphthylene (AcPy), dimethyl phthalate (DMP), diethyl phthalate (DEP), fluorene (Flu), hexachlorobenzene (HCB), anthracene (Ant), phenanthrene (PA), di n butyl phthalate (DBP), pyrene (Pyr), chlorobenzilate (CB), bis-2-ethyl hexyl adipate (BEHA), butyl benzyl phthalate (BBP), bis-2-ethyl hexyl phthalate (BEHP), benz [a] anthracene (B [a]A), chrysene (Chr), benzo [k] fluoranthene (B [k]F), benzo [b] fluoranthene (B [b]F), benzo [a] pyrene (B [a]P), dibenz [a,h] anthracene (D [a,h]A), and indeno(1,2,3-c,d) pyrene (IP). 2-Methylnaphthalene (Sigma-Aldrich Labrochemikalein, GmbH) was used as internal standards.
Health risk assessment
B [a]P-equivalent concentration for PAHs
Benzo (a) pyrene (B [a]P) is a PAH which is highly toxic to humans and animals. It has five aromatic rings fused in a honeycomb structure. The main sources of B [a] P are diesel exhaust particles, industrial emissions, wood burning smoke, etc. In this study, carcinogenicity and mutagenic potential of cooking fumes have been estimated through calculations of B [a]P-TEQ (toxicity equivalent) and B [a]P-MEQ (mutagenic equivalent). Out of 24 PAHs analyzed, the ME factor is available for 7 of the PAHs detected (all being carcinogenic except B [ghi]). To permit comparison between toxicity and mutagenicity potential of fumes, B [a]P-TEQ and B [a]P-MEQ are calculated (see equations below) for these seven PAHs only.
-
B [a]P-TEQ (carcinogenic equivalents (ng/m3)) and B [a]P-MEQ were calculated by multiplying the concentrations of each PAH compound with its TEF (toxic equivalency factor) for cancer potency relative to B [a] P (Nisbet and LaGoy 1992). B [a]P-TEQ levels for the sum of nonvolatile PAHs (∑7PAH; MW ≥ 228) were calculated as follows:
$$ {\displaystyle \begin{array}{l}{\left(\mathrm{BaP}-\mathrm{TEQ}\right)}_{\sum 7\mathrm{PAH}}=\left[\mathrm{BaA}\right]\times 0.1+\left[\mathrm{Chry}\right]\times 0.01+\left[\mathrm{BbFA}\right]\times 0.1+\left[\mathrm{BkFA}\right]\times 0.1+\kern0.5em \\ {}\left[\mathrm{BaP}\right]\times 1+\left[\mathrm{IP}\right]\times 0.1+\left[\mathrm{BghiP}\right]\times 0.01\kern4.079998em \end{array}} $$(1) -
B [a]P-MEQ (mutagenic equivalents, ng/m3) were calculated by multiplying the concentrations of each PAH compound with its MEF relative to B [a] P (Durant et al. 1999). B [a]P-MEQ levels for the sum of nonvolatile PAHs (∑ 7PAH; MW ≥ 228) were calculated as follows:
$$ {\displaystyle \begin{array}{l}{\left(\mathrm{BaP}-\mathrm{MEQ}\right)}_{\sum 7\mathrm{PAH}}=\left[\mathrm{BaA}\right]\times 0.082+\left[\mathrm{Chry}\right]\times 0.017+\left[\mathrm{BbFA}\right]\times 0.25+\left[\mathrm{BkFA}\right]\times 0.11+\\ {}\left[\mathrm{BaP}\right]\times 1+\kern0.36em \left[\mathrm{IP}\right]\times 0.31+\left[\mathrm{BghiP}\right]\times 0.19\ \end{array}} $$(2)
Disability adjusted life years
Health impact on a human is calculated due to exposure from cooking fumes emission in terms of DALYs. The term DALYs, disability adjusted life years, for a disease is the summation of the life years lost due to premature mortality and because of some disability, so that a person is unable to live productively. Human health impact for the PAH emission from cooking was calculated using the approach proposed by Hellweg et al. (2009) and Chaudhary and Hellweg (2014) (Eq. (3)).
where impacti is the impact on human health [cases per functional unit], mi is the mass of individual PAH and metals, EFi is the human health effect factor [cases per kilogram intake], and iF is the intake fraction, the fraction of emitted mass inhaled by the people present, calculated by Eq. (4).
where IR is the inhalation rate (m3/h) (United States Environmental Protection Agency (US EPA 2011)), h is the exposure time (h/day), V is the room volume (m3), 24 is the hours per day (hour/day), N is the air changes per hour (1/h), and P is the number of people exposed. Air change per hour is calculated as follows:
Rp (cfm per person) is the people outdoor air rate, Pz is the number of people present in the zone, Ra (cfm per sq. ft.) is the outdoor airflow rate required per unit area, and Az (sq. ft.) is the net occupiable floor area of the zone. The values used in the calculation were obtained American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE (2015)) and are mentioned in Table 1 (Supplementary material).
The intake fraction (mass of emitted pollutants inhaled per unit emitted mass) calculated by using Eq. (4) for the workers present while cooking in the mess kitchen was 0.00705447.
The effect factor (EFi) is taken from the USEtox model, developed by UNEP/SETAC which is an environmental fate, exposure, and effect model, based on scientific consensus. The health impact is calculated in terms of “disability adjusted life years” (DALYs) instead of “cases per kilogram intake” (Rosenbaum et al. 2008). The concept of DALYs was originally developed by WHO (World Health Organization) and was proposed as a powerful idea to address human health damages. It represents the sum of the years of life lost (potential life) due to premature mortality and years of productive life lost due to the disability caused by the exposure to some harmful element for humans or potential handicap. The units of the effect factor available in the USEtox data are in “cases per kilogram intake”, and in this study, they are converted to DALYs by multiplying with the conversion factor (known as damage factor (DALYs per cases)) of 11.5 for carcinogenic and 2.7 for non-carcinogenic chemicals as suggested by Huijbregts et al. (2005) (Eq. (7)).
While calculating the impact on human health, it was assumed that the most significant exposure pathway for effect on health was ingestion, excluding ingestion and dermal contact. The human health effect factor (EF), used in Eq. (3) for impact calculation, was not available for all the chemicals (PAHs) in the USEtox database. To getting an approximate estimation of the human health impact, the effect factor is estimated using close isomers of the compound whose effect factor is known. Various parameters used in Eqs. (4), (5), and (6) are listed in Table 1 (Supplementary material).
Results and discussion
Mass concentration of respirable suspended particulate matter in cooking fumes
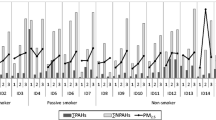
Mass concentration of respirable particles (dp ≤ 2.0 μm) in cooking fumes for different types of food being cooked in the hostel (Fig. 1, Supplementary material), calculated from the particle count data obtained from OPC used during set 1 and set 2 (total of 8 sampling days), shows lower levels in case of vegetarian food compared to non-vegetarian food. In vegetarian food, the level of RSPM calculated was 73.52 ± 57.45 μg/m3 followed by ~ 1.3 times that of vegetarian food for chicken (93.29 ± 49.93 μg/m3) and the highest for fish ~ 1.5 (106.89 ± 62.72 μg/m3) times of vegetarian food. In summary, the mass concentration of RSPM that were recorded from OPC is higher for non-vegetarian food types (fish > chicken) that were greater than for vegetarian food. This observation is similar to that reported by Buonanno et al. (2009). In their study, peak values for mass concentration were seen to be highest in case of bacon (352 μg/m3), and lowest was for eggplant (78 μg/m3).
Variation in particle-bound PAH concentrations emitted from different food types
A total of 24 PAHs were analyzed in this study (listed in Methodology). Half of the analytes, i.e., 12 PAHs were detected on OPC filter samples (total 6) and the PAHs detected in each sample, can be seen in Table 2 (Supplementary material). The variations in individual particle-bound PAH and total PAH concentrations detected in the three food types (vegetarian, chicken, and fish) during a regular semester (set 1 in November) and winter recess (set 2 in January) are presented in Table 3 (Supplementary material). Total PPAH concentrations observed (10–35 ng/m3) in this study are comparable to the indoor PAH concentrations reported for emissions from different gas cooking methods (See and Balasubramanian 2006) and at public places in Hong Kong (Lu et al. 2008).
Maximum number of PAHs was detected in vegetarian food (n = 9 and 11 for set 1 and set 2, respectively) followed by chicken (n = 10 and 11) and then fish (n = 4 and 5). Lower molecular weight PAHs were detected in fish and anthracene contributed significantly to PAH mass. For set 1, anthracene and bis-2-ethylhexyl-phthalate accounted for 95% of the total mass of PAHs for fish and 88% for chicken. In set 2 for fish, 80% of total mass of PAHs was contributed by anthracene and benzo [b] fluoranthene while anthracene and benzo [ghi] perylene added to 46% of total mass of PAHs for chicken. The contribution of individual entities to a total load of PAH mass is more evenly distributed for emissions captured from vegetarian food. More number of carcinogenic PAHs were detected in emissions from vegetarian and chicken food cooking (set 2).
For set 1, the trend among the food types for PPAHs (non-vegetarian > vegetarian) (Fig. 2) is the same as that observed for particle emissions. This is in agreement with the hypothesis that the emission of PPAHs in cooking fumes not only is related to the cooking method but also to the cooking ingredients (chicken > vegetarian) (Buonanno et al. 2009).
Impact of cooking oil on PAH emissions in cooking fumes
Several studies from China have examined emissions solely from cooking oils including soybean, lard, and sunflower. Both carcinogenic and cytotoxic PAHs have been detected (Chiang et al. 1997; Lu et al. 2000). The cooking oil used for cooking of food in hostel mess is refined soybean oil purchased from a local vendor. The study on soybean oil by Chen and Chen (2001) reported a large number of PAHs, which includes 10 out of 16 PAHs detected (MOUDI) in our study. This suggests that cooking fuel influences the PAHs observed in cooking fumes. However, the difference in the amount used in laboratory studies and real cooking can be very high and warrants further research on type and amount of cooking fuels used in India.
Influence of the amount of food cooked on pollutant levels
Amount of PAHs generated in set 2 is almost the same for all food types (Fig. 2) and is much less than in set 1 for both chicken and fish samples. This is surprising since both the samples have been collected during winter time (Nov and Jan) and have similar sample volume (~ 45 h for all samples). The most likely reason for this observation is the difference between the amount of food cooked in November (semester in session) and January (semester recess). Hostels are fully occupied during November while the number of hostel occupants is much lesser during the recess time in January. This results in the lower amount of food being cooked as a whole. Moreover, hostel authorities allow only a minimum quantity of non-vegetarian food to be cooked during recess time which is quite less as compared to November. Reduction in the amount of non-vegetarian food being cooked results in lesser amount of emissions and hence the lower levels observed in set 2.
Size segregated distribution of PPAHs
Data from MOUDI was used to determine the concentration of various PAHs in different size bins. Briefly, impactor stages S1 to S3 account for the coarse range of deposition, while S4 to S9 and S10 denote the fine and ultrafine ranges, respectively. Details of PAHs detected in different stages of MOUDI, including carcinogens, are provided in Table 4 (Supplementary material).
Constituents of the total PAHs in samples obtained from MOUDI
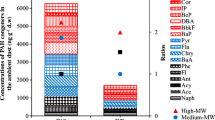
In total, 16 different PAHs were detected in all the samples collected by MOUDI. Out of these, seven PAHs are probable human carcinogens (group B2), as listed by US EPA. Detection of PAHs was highest in the fine range (n = 13 in S6) followed by ultrafine (n = 11 in S10). In contrast, lesser PAHs (9) were detected in the coarse range (S1–S3).
Carcinogenic PAHs
The abundance of carcinogenic PAHs is higher generally in the finer and ultrafine stages (S5–S10) (Fig. 3) while the wealth of non-carcinogenic PAHs is more in stages S1–S5 (coarse to fine) (Fig. 4). Less than 10% (approximately 7%) of carcinogenic PAHs were detected in coarser range, while the rest were in fine and ultrafine range. The total concentration of carcinogenic PAHs detected was 15.97 ng/m3. The maximum concentration of carcinogenic PAHs was found on stage 7 (cutoff diameter = 0.32 μm) contributing 48.5%. Higher incidence of PAHs on fines is again a confirmation of the fact that the concentration of PAHs increases with the decrease in the size of the particulate matter emitted while cooking. This goes on to show that PAHs are present in fines and ultra-fines in larger concentrations because of availability of a larger surface area for adsorption (Chiang et al. 1999). Smaller particles have a higher specific area and a higher attachment rate for organic pollutants and hence contain a considerable amount of organic carbon, which allows for more PAH adsorption (Kawanaka et al. 2009).
Cause of concern
B [a] P and chrysene made up to 53.95% of the total carcinogenic PAHs. B [a] P is the major contributor (almost 32%) for the ultrafine range. Presence of carcinogenic PAHs in the fine and ultrafine particles in cooking fumes is a cause for health concern. Given the stable conditions of the indoor environments, this can be a cause for a large number of respiratory and pulmonary diseases as well as lung cancer (Gurbani et al. 2013). Greater research that sheds light on the chemical properties of smaller particles and their interaction inside the human respiratory tract is warranted.
Toxic and mutagenic potential of PAHs
An attempt has been made to investigate possible risk to people exposed to cooking fumes containing the seven PAHs detected in the ten MOUDI impactor stages (Table 4, Supplementary material). The placement of samplers and continuous operation chimneys over cooking areas during cooking time ensures that the level of particle-bound PAHs measured is the actual emission to which the workers were exposed.
As mentioned earlier, B [a]P-TEQ represents the carcinogenic potential and B [a]P-MEQ represents the mutagenic potential of a particular PAHs. Carcinogenic and mutagenic potential of individual PAHs, B [a]P-equivalent (B [a]P-TEQ and B [a]P-MEQ) concentrations, were calculated using Eq. (1) and Eq. (2), respectively. Results are presented in Fig. 2 (Supplementary material) and the percentage contributions of individual PAHs to the total mutagenicity and carcinogenicity listed in Table 2. Levels of (B [a]P-TEQ)∑7PAH and (B [a]P-MEQ)∑7PAH were 3.78 ng/m3 and 4.04 ng/m3, respectively. For all samples studied, the most substantial contribution of individual PAHs to (B [a]P-TEQ)∑7PAH and (B [a]P-MEQ) ∑7PAH was made by B [a] P, followed by B [a] A for (B [a]P-TEQ) ∑7PAH and B [b] F for (B [a]P-MEQ) ∑7PAH.
Results obtained in this study for (B [a]P-TEQ) ∑7PAH were comparable to those obtained from a study in residential units in two Japanese cities (Ohura et al. 2004). This study in Japan concluded that contribution of B [a] P to the total carcinogenic potential of PPAHs was dominant in the range of 51% to 64%. In our study, the total carcinogenic potential is > 70%. There was a similar study conducted in New York City (Jung et al. 2010) which suggested that residential exposure may pose an increased risk of cancer and mutation to PAHs, at levels encountered in New York City air, especially during the heating season. Therefore, high levels of B [a]P-TEQ in this study suggest that from long-term exposure to PAHs present in cooking fumes, the mess workers are subjected to higher carcinogenic risks. It should be noted that the current research provides with the preliminary calculations and results of risk assessment, which are suggestive and not conclusive. Further intense research and data collection are required for evaluation of lifetime cancer risks involved.
DALYs as an indicator of damage to human health
Cancer and non-cancer burden of disease attributable to PPAHs detected on RSPM, in disability-adjusted life years (DALYs), were calculated. Around 25 workers were present in the kitchen while the cooking was in progress. The total human toxicity impact, combined for all 25 workers present, due to the emission of PAHs from cooking was evaluated as 3.374 × 10−10 DALYs. Toxicity effect on every individual present there was calculated as 1.3497 × 10−11 DALYs/person. Among the 16 compounds examined, B [a] P has the most extreme effect on health. The concentration of individual PAHs and effect factor (EF) are provided in Table 5 (Supplementary material) and details for the impact of individual PAHs on the health of workers present in the cooking area can be seen in Table 6 (Supplementary material).
Pollutant mass vs. human health effect factor
Figure 5a, b demonstrates the % contribution of individual PAHs to mass in the non-carcinogenic and carcinogenic group, and to health (in terms of DALYs). Equation (3) clearly shows that there is direct inter-dependence between the mass of PAHs present on particles and its impact factor. It would seem that higher mass would lead to higher DALYs. However, in the case of carcinogenic PAHs, lowest DALY value appears for a compound with the most significant contribution to overall mass. The concentration of bis-2-ethyl hexyl phthalate is maximum (445.387 ng/m3; mass 6413.57 ng) among all the PAHs, but the impact seems to be quite less on health (< 2% of total DALYs). Maximum effect on the health of workers is because of B [a] P with mass contribution less than 0.4%. This indicates that mass term is overpowered by the human health effect factor. The values obtained for DALY are likely to be underestimated since EFs for all PAHs emitted have not been documented in the USEtox database. So, EF values were specified according to their respective structures, with an intention to provide an approximate estimate of impact.
In this study, the estimation of life years lost due to inhalation is evaluated from the emission from the cooking of vegetarian food only. It implies number of years lost due to inhalation of cooking fumes throughout the day just once and cannot be generalized to DALY value based on exposure to cooking fumes in hostel mess for a whole year. The PAH concentrations obtained in set 1 and set 2 are very different for the food types (non-veg > veg). With PAH concentrations being lower in vegetarian food, DALYs calculated present an underestimation of overall impact.
Health risks in outdoor and indoor environments
A recent study conducted in Nagpur district, India, reported that global burden of disease in terms of DALYs, caused by ambient PM2.5-bound PAHs, results in 0.011 DALYs/person/year (Etchie et al. 2018). In the current research, an attempt was made to determine cancer and non-cancer burden of disease, due to RSPM-bound PAHs emitted from cooking, which was calculated to be 0.049 × 10−07 DALYs/person/year. Our study is source specific in that it determines DALYs due to one of many sources of indoor pollution while this is not the case for research in Nagpur. It examines particles that arise from multiple sources, including vehicular exhaust. A comparison between observed DALY values highlights the need for more intensive data collection from known sources of indoor air pollution to enable assessment of overall DALYs which includes the influence of processes impacting air quality of the indoor microenvironment.
Conclusion
Emissions from the cooking of different food types showed a variation in number and type of PPAHs emitted. Maximum number of particle-bound PAHs occurred in fine and ultrafine range which also accounts for greater than 90% of carcinogenic PAH concentrations. Less than 10% of carcinogenic fraction occurred in the coarser particles. B [a] P, the major contributor to ultrafine particle size bin, accounts for > 65% to toxic and mutagenic potential and > 70% of the total disability-adjusted life years. This indicates that inhalation of cooking fumes poses a significant hazard to the health of mess workers and prolonged exposure could lead to carcinogenic risk. During calculation of DALYs, it was found that mass was overpowered by the effect factor, mainly in case of carcinogenic PAHs, resulting in chemicals with lower mass having higher DALY values. Parameters like toxicity equivalent or incremental hazard risk provide us with the extent of toxicity of chemicals and incremental risk of cancer. However, calculations based on data from this pilot study suggest effective risk assessment through quantification of the impact on life years due to exposure to a particular chemical can be accomplished by DALYs.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (1995) Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs). https://www.atsdr.cdc.gov/ToxProfiles/tp69.pdf. Accessed April 2018
American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) (2015) Ventilation for acceptable indoor air quality. ANSI/ASHRAE Standard 62.1–2013. https://ashrae.iwrapper.com/ViewOnline/Standard_62.1-2016. Accessed March 2018
Balakrishnan K, Mehta S, Kumar P, Ramaswamy P (2004) Indoor air pollution associated with household fuel use in India, an exposure assessment and modeling exercise in rural districts of Andhra Pradesh, India. pp 1-114. https://www.esmap.org/sites/default/files/esmap-files/Rpt_India_IndiaFULL.pdf. Accessed April 2018
Bhargava A, Khanna RN, Bhargava SK, Kumar S (2004) Exposure risk to carcinogenic PAHs in indoor-air during biomass combustion whilst cooking in rural India. Atmos Environ 38(28):4761–4767
Buonanno G, Morawska L, Stabile L (2009) Particle emission factors during cooking activities. Atmos Environ 43(20):4761–4767
Chaudhary A, Hellweg S (2014) Including indoor Offgassed emissions in the life cycle inventories of wood products. Environ Sci Technol 48(24):14607–146014
Chen BH, Chen YC (2001) Formation of polycyclic aromatic hydrocarbons in the smoke from heated model lipids and food lipids. J Agric Food Chem 49:5238–5243
Chiang TA, Wu PF, Wang LF, Lee H, Lee CH, Ko YC (1997) Mutagenicity and polycyclic aromatic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Mutat Res 381:157–161
Chiang TA, Wu P, Ko YC (1999) Identification of carcinogens in cooking oil fumes. Environ Res 81(1):18–22
Durant JL, Lafleur AL, William FB, Donhoffner LL, Penman BW, Crespi CL (1999) Mutagenicity of C24H14 PAH in human cells expressing CYP1A1. Mutat Res 446:1–14
Etchie TO, Sivanesan S, Etchie AT, Adewuyi GO, Krishnamurthi K, George KV, Rao PS (2018) The burden of disease attributable to ambient PM2.5-bound PAHs exposure in Nagpur, India. Chemosphere 204:277–289
Gupta A, Bhandari M (2011) Monitoring and control of particulate matter in indoor air : a review. J Appl Nat Sci 3(1):139–150
Gurbani D, Kumar S, Kumar A, Pandey AK, Ana GR, Verma A, Husain A (2013) International journal of hygiene and polycyclic aromatic hydrocarbons and their Quinones modulate the metabolic profile and induce DNA damage in human alveolar and bronchiolar cells. Int J Hyg Environ Health 216(5):553–565
Hellweg S, Demou E, Bruzzi R, Meijer A, Rosenbaum RK, Huijbregts MAJ, McKone TE (2009) Integrating human indoor air pollutant exposure within life cycle impact assessment. Environ Sci Technol 43(6):1670–1679
Huijbregts MAJ, Rombouts LJA, Ragas AMJ, Meent DVD (2005) Human-toxicological effect and damage factors of carcinogenic and noncarcinogenic Chemicals for Life Cycle Impact Assessment. Integr Environ Assess Manag 1(3):1–181
IARC (1983) IARC monographs on the evaluation of carcinogenic risk of chemicals to man: polynuclear aromatic compounds, part 1, chemical and environmental data. International Agency for Research on Cancer. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono32.pdf. Accessed May 2018
Institute for Health Metrics and Evaluation (IHME) (2016) GBDCompareDataVisualization. Seattle, WA: IHME, University of Washington. http://vizhub.healthdata.org/gbd-compare. Accessed May 2018
International Programme on Chemical Safety (IPCS) (1998) Environmental Health Criterion: Selected non-heterocyclic polycyclic aromatic hydrocarbons, World Health Organisation (WHO). http://www.inchem.org/documents/ehc/ehc/ehc202.htm
Jung KH, Yan B, Chillrud SN, Perera FP, Whyatt R (2010) Assessment of benzo ( a ) pyrene-equivalent carcinogenicity and mutagenicity of residential indoor versus outdoor polycyclic aromatic hydrocarbons exposing young children in new York City. Int J Environ Res Public Health 7(5):1889–1900
Kawanaka Y, Tsuchiya Y, Yun SJ, Sakamoto K (2009) Size distributions of polycyclic aromatic hydrocarbons in the atmosphere and estimation of the contribution of ultrafine particles to their lung deposition. Environ Sci Technol 43:6851–6856
Kim NT, Reutergadh LB, Dung NT, Yu M, Yao W, Co HX (2000) Polycyclic aromatic hydrocarbons in the airborne particulate matter at a location 40 km north of Bangkok, Thailand. Atmos Environ 34:4557–4563
Lu YF, Lin CM, Fang YC (2000) The relationship between arachidonic acid content and tissue lipids in rats fed cholesterol-containing diets. Nutr Sci J 25:108–114
Lu H, Zhu L, Chen S (2008) Pollution level, phase distribution and health risk of polycyclic aromatic hydrocarbons in indoor air at public places of Hangzhou, China. Environ Pollut 152(3):569–575
Nisbet ICT, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16(3):290–300
Oanh NTK, Reutergadh LB, Dung NT, Yu M, Yao W, Co HX (2000) Polycyclic aromatic hydrocarbons in the airborne particulate matter at a location 40 km north of Bangkok, Thailand. Atmos Environ 34:4557–4563
Ohura T, Amagai T, Sugiyama T, Fusaya M (2004) Characteristics of particle matter and associated polycyclic aromatic hydrocarbons in indoor and outdoor air in two cities in Shizuoka, Japan. Atmos Eniron 38:2045–2054
Pandit GGU, Srivastava PK, Rao AMM (2001) Monitoring of indoor volatile organic compounds and polycyclic aromatic hydrocarbons arising from kerosene cooking fuel. Sci Total Environ 279(1):159–165
Rosenbaum RK, Bachmann TM, Gold LS, Huijbregts MAJ, Jolliet O, Juraske R, Koehler A (2008) USEtox - the UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater Ecotoxicity in life cycle impact assessment. Int J Life Cycle Assess 13(7):532–546
Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT (1999) Measurement of emissions from air pollution sources . 1 . C 1 through C 29 organic compounds from meat charbroiling. Environ Sci Technol 33(10):1566–1577
Schwartz J, Dockery DW, Neas LM (1996) Is daily mortality associated specifically with fine particles ? Journal of the air & Waste Management Association is Daily Mortality Associated Specifically with fine particles? J Air Water Manag Assoc 46(10):927–939
See SW, Balasubramanian R (2006) Health risk assessment of occupational exposure to particulate-phase polycyclic aromatic hydrocarbons associated with Chinese, Malay and Indian cooking. J Environ Monit 8(3):369–376
Singh L, Agarwal T (2018) Chemosphere PAHs in Indian diet : assessing the Cancer risk. Chemosphere 202:366–376
Stabile L, Fuoco FC, Marini S, Buonanno G (2015) Effects of the exposure to indoor cooking-generated particles on nitric oxide exhaled by women. Atmos Environ 103:238–246
U.S. EPA (2011) Exposure Factors Handbook 2011 Edition (Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F. https://www.nrc.gov/docs/ML1400/ML14007A666.pdf. Accessed March 2018
Wei S, Balasubramanian R (2008) Chemical characteristics of fine particles emitted from different gas cooking methods. Atmos Environ 42:8852–8862
World Health Organization & International Programme on Chemical Safety (1998) . Selected non-heterocyclic polycyclic aromatic hydrocarbons. Geneva : World Health Organization. http://www.inchem.org/documents/ehc/ehc/ehc202.htm. Acessed May 2018
Zhang JJ, Smith KR (2018) Indoor air pollution : a Global Health concern. Br Med Bull 68:209–225. https://doi.org/10.1093/bmb/ldg029
Zhao Y, Hu M-Ã, Slanina S, Zhang Y (2007) The molecular distribution of fine particulate organic matter emitted from Western-style fast food cooking. Atmos Environ 41:8163–8171
Zhu L, Hao L, Shuguang C, Takashi A (2009) Pollution level, phase distribution and source analysis of polycyclic aromatic hydrocarbons in residential air in Hangzhou, China. J Hazard Mater 162(2–3):1165–1170
Acknowledgments
The support and co-operation of the mess workers during sample collection are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 97 kb)
Rights and permissions
About this article
Cite this article
Goel, A., Ola, D. & Veetil, A.V. Burden of disease for workers attributable to exposure through inhalation of PPAHs in RSPM from cooking fumes. Environ Sci Pollut Res 26, 8885–8894 (2019). https://doi.org/10.1007/s11356-019-04242-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04242-x