Abstract
Nitrate pollution in water is a common environmental problem worldwide. The Qinhe Basin (QHB) faces with the risk of eutrophication. To clarify nitrate pollution of river water, water chemical data, water isotope values (δD and δ18O–H2O), and dual nitrate isotope values (δ15N–NO3− and δ18O–NO3−) were used to discern sources and transformation mechanisms of nitrogen in the QHB. The nitrate concentrations of river water ranged from 0.71 to 20.81 mg L−1. The δD and δ18O–H2O values of river water varied from − 74 to −52‰ and from − 10.8 to − 7.2‰, with an average value of − 60‰ and − 8.2‰, respectively. The δ15N–NO3− and δ18O–NO3− values of nitrate ranged from − 6.7 to + 14.8‰ and from − 6.0 to + 5.6‰, with a mean value of + 4.6‰ and − 0.6‰, respectively. Assimilation by algae and the mixing of soil nitrogen, chemical fertilizer, sewage, and industrial wastewater could account for increasing δ15N–NO3− values. There was neither significantly positive nor negative correlation between δ15N–NO3− and δ18O–NO3− in river water, indicating that no obvious denitrification shifted isotopic values of nitrate in the QHB. Based on the dual isotopic values of nitrate and land use change in the watershed, it could be concluded that intensive nitrification dominated in the QHB, and dissolved nitrate was mainly derived from nitrification of ammonium in fertilizer, soil nitrogen, and domestic sewage. As the primary nitrate sources identified in the QHB, effective fertilization and afforestation can be taken to protect water resource from nitrate pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nitrate contamination of water, which is associated with anthropogenic activities including fossil fuel burning, agricultural fertilization, and discharge of manure and sewage (Barnes and Raymond 2010; Xue et al. 2012), is a pervasive environmental problem in the world (Fukada et al. 2004; Zhang et al. 2014). Excess nitrate in water can cause myriad environmental and ecological problems, such as eutrophication of lakes and reservoirs, toxic algal blooms, and water acidification (Curtis et al. 2011; Li et al. 2019; Li et al. 2013). Furthermore, nitrate with a high concentration in drinking water is also harmful to human health and can cause methemoglobinemia in infants (Fan and Steinberg 1996; Fewtrell 2004). Therefore, to identify nitrate sources and elucidate the processes affecting local nitrate concentrations are imperative to effectively control nitrate pollution in freshwater ecosystems. Traditional methods of identifying nitrate sources can be accomplished by a combination of nitrate concentration, flux data, land use, and the hydrological characteristic of the study areas (Chang et al. 2002; Li et al. 2015b; Xing et al. 2013). However, the nitrate concentration is influenced by assimilation, mixing, ammonification (reaction 1), nitrification (reactions 2–4 (Kool et al. 2007)), and denitrification (reaction 5 (Tesoriero et al. 2000)) processes. As a result, traditional methods cannot well access sources and transformations of nitrogen.
The nitrogen and oxygen isotopic compositions of nitrate have been proved to be an effective tool to elucidate nitrate sources and decipher main nitrogen cycle processes (Deutsch et al. 2009; Kelley et al. 2013; Liu et al. 2006; Vrzel et al. 2016; Xing and Liu 2016). The dual-isotope approach is based on the fact that different sources of nitrate have distinct isotopic compositions. Nitrate in the fresh water system is mainly derived from chemical fertilizer, soil organic nitrogen, atmospheric precipitation, livestock waste, and sewage effluent (Chen et al. 2014; Kendall et al. 2007; Ohte 2013). The typical δ15N values of chemical fertilizer, which is produced by fixation of atmospheric N2, range from − 6 to + 6‰ (Xue et al. 2009). The δ18O–NO3− values of synthetic nitrate fertilizer vary between + 17 and + 25‰ (Kendall et al. 2007). The δ15N values of soil nitrogen affected by the rate of ammonification and nitrification range from 0 to + 8‰ (Ding et al. 2014; Xue et al. 2009). The δ18O values of nitrate derived from nitrification originating from ammonia fertilizer and mineralized soil organic nitrogen vary from − 10 to + 10‰ (Kelley et al. 2013). The δ15N and δ18O signatures of nitrate from atmospheric precipitation range from − 13 to + 13‰ and from + 25 to + 94‰, respectively (Kendall et al. 2007; Xue et al. 2009; Yue et al. 2017). Compared with other nitrogen sources, nitrate from manure and sewage has high δ15N values from + 7 to more than + 20‰ and low δ18O values from − 5 to + 10‰ (Ding et al. 2014; El Gaouzi et al. 2013; Lee et al. 2008). Meanwhile, denitrification results in δ15N and δ18O values of the remaining nitrate increasing with a relationship of 1.3:1 to 2.1:1 (Wexler et al. 2014; Xu et al. 2016; Xue et al. 2009). The δ18O values of water combining with δ18O values of atmospheric O2 can determine whether nitrification happened (Hollocher 1984; Kelley et al. 2013).
Qinhe is the second largest river of Shanxi province, China, which provides water resources for industrial, agricultural, and domestic uses along the river. However, the water quality of Qinhe Basin (QHB) deteriorated owing to the increasing discharge of industrial wastewater, agricultural, and domestic sewage (Fu et al. 2013). Recently, the water pollution problem of QHB has attracted concerns of numerous researchers (Feng et al. 2010; Fu 2012; Qin et al. 2016; Wang et al. 2013a; Zhang et al. 2012). The river water in QHB faces with the risk of eutrophication (Fu 2012). The nitrate of the river water in this area ranged from 4.93 to 43.24 mg/L (Qin et al. 2016), and the water bodies were polluted by urban point sources, agricultural, and rural non-point sources (Wang et al. 2012). In order to prevent nitrate contamination, it is necessary to evaluate the nitrate sources of river water in the QHB. The purpose of this study was to identify the sources and transformations of nitrate in the QHB using isotopic compositions of nitrate and river water. The results of this study should be useful to improve the management measures of water quality and control nitrate pollution in the QHB.
Materials and methods
Study area description
The QHB, located in the north China between 35° 03′ to 36° 48′ N latitude and 112° 03′ to 113° 30′ E longitude, is the main tributary of the Yellow River, with a drainage area of 1.35 × 104 km2 and length of 485 km. It, originating from Erlangshen ditch south of Taiyue Mountain in Shanxi province, flows through Shanxi and Henan provinces, and finally empties into the Yellow River at Nanjia Village in Wuzhi County. The climate of QHB is mainly dominated by the temperate continental monsoon, and the annual mean temperature ranges from 10 to 14.4 °C. The annual average precipitation is about 550–700 mm, mainly occurring from June to September when approximately 70% of the total annual precipitation falls (Bai et al. 2014). The terrain of the study area is high in the south and low in the north. The landforms of the region are divided into two large units, that is, Taiyue Mountain and Southeast Plateau of Shanxi province (Hu et al. 2012). The study area consists of four landscape types, including stony Mountain area, earth-rock hilly region, valley area, and alluvial plain region. The main strata of the headstream of QHB in Taiyue Mountain and the downstream of QHB in Taihang Mountain are Ordovician limestone, Cambro-Ordovician limestone, Lime sandstone, and Permian sandstone (Zhang et al. 2015). The sedimentary rocks widely exposed in the upper and middle stream of QHB are Triassic sandstone and sandy mudstone (Qin et al. 2016). There are mainly mountain areas and few human activities in the upstream. The midstream of the study area is characterized by high population density, developed industry, and frequent anthropogenic activities. The downstream of QHB is mainly agricultural area, intensive residential, and industrial areas. The Dan River is the biggest tributary of Qinhe Basin, originating from Danzhuling at Zhao village in Shanxi province. It loads wastewater of industrial enterprises in Gaoping and Jincheng cities and urban domestic sewage (Ma et al. 2010; Wang et al. 2012), which causes water quality to deteriorate severely.
Sampling and analysis
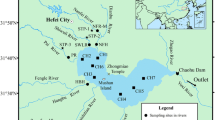
The sampling sites of surface water are shown in Fig. 1. A total of 24 surface water samples from Qinhe Basin were collected in August (wet season) 2015. Water parameters, such as temperature, pH, electrical conductivity (EC), and dissolved oxygen (DO), were measured using field meters (SX716 and SX731, Sanxin, China). Then, the water samples were collected in 5-L pre-cleaned plastic bags and filtered through 0.22 μm cellulose acetate membrane within 24 h and then stored in 4 °C environment before analysis.
Sampling sites of QHB (modified from Zhang et al. 2015)
The concentrations of anions (NO3− and Cl−) were determined by ionic chromatography (ICS90, Dionex) at the State Key Laboratory of Environmental Geochemistry in Guiyang City, China. The hydrogen and oxygen isotopic compositions of water were analyzed with an elemental analyzer (Flash 2000 HT) isotope ratio mass spectrometer (IRMS, MAT-253, Thermo Fisher Scientific) at the Provincial Key Laboratory of Henan Polytechnic University in Jiaozuo City, China. The analytical precisions of δD and δ18O–H2O were better than 2‰ and 0.2‰, respectively. The international standard VSMOW was used to calibrate δD and δ18O–H2O values.
The isotopic compositions of nitrate in river water were determined at the State Key Laboratory of Subtropical Mountain Ecology in Fuzhou City, China, with an IRMS (MAT-253, Thermo Fisher Scientific) attached Precon using the cadmium reduction method (McIlvin and Altabet 2005; Xue et al. 2009). Analytical precision for δ15N–NO3− and δ18O–NO3− were 0.31‰ and 0.55‰, respectively. The international standard USGS34, USGS35, and IAEA-N3 were used to calibrate the ratios of δ15N–NO3− and δ18O–NO3−.
The stable isotope ratios are reported using the delta (δ) per thousand (‰) notation relative to an international standard.
Where R represents D/H, 15N/14N, or 18O/16O. The value of 15N/14N reference standard is N2 in atmospheric air; the value of D/H and 18O/16O reference standard is the international Vienna Standard Mean Ocean Water (VSMOW).
Results
Chemical compositions of river water
The hydrochemical compositions of water samples collected in August 2015 were summarized in Table 1. The pH of river water ranged from 7.19 to 9.28 with an average value of 8.27, and DO varied from 0 to 24.92 mg L−1 with an average value of 9.95 mg·L−1. The EC value of river water in upstream, midstream, and downstream of QHB varied from 361 to 1002 μS cm−1, from 425 to 781 μS cm−1and from 719 to 1305 μS cm−1, with an average value of 564 μS cm−1, 526 μS cm−1, and 928 μS cm−1, respectively. The EC value of river water in the mainstream and tributaries varied from 382 to 846 μS cm−1 and from 361 to 1305 μS cm−1, with an average value of 572 μS cm−1 and 683 μS cm−1, respectively. The concentrations of NO3− in the upstream, midstream, and downstream of QHB ranged from 3.21 to 20.81 mg L−1, from 2.32 to 16.99 mg L−1 and from 0.71 to 16.32 mg L−1, with a mean value of 7.37 mg L−1, 7.88 mg L−1, and 8.33 mg L−1, respectively. The range of nitrate concentrations of river water in the mainstream was from 2.19 to 16.99 mg L−1, with a mean value of 7.16 mg L−1, while the nitrate concentrations of river water in the tributaries varied from 0.71 to 20.81 mg L−1, with an average of 8.32 mg L−1. The average value of NO3− (7.16 mg L−1) in the mainstream of QHB was lower than that of the Yellow River (11.07 mg L−1) in the high flow season (Yue et al. 2017). The Cl− of river water in the upstream, midstream, and downstream of QHB ranged from 2.33 to 43.21 mg L−1, from 8.32 to 33.79 mg L−1, and from 27.08 to 112.29 mg L−1, with an average value of 17.83 mg L−1, 19.51 mg L−1, and 55.58 mg L−1, respectively. The Cl− contents of river water in the mainstream varied from 2.33 to 50.98 mg L−1 with an average value of 21.23 mg L−1, while the Cl− contents in the tributaries ranged from 6.97 to 112.29 mg L−1 with average values of 31.28 mg L−1.
Isotopic compositions of river water
As shown in Table 1, the δD and δ18O–H2O values of river water varied from − 74 to − 52‰ and from − 10.8 to − 7.2‰, with a mean value of − 60‰ and − 8.2‰, respectively. As indicated in Fig. 2a, the water sample with the most negative δD and δ18O–H2O values was located in the headstream (M1), and the water sample with positive values exhibited in the downstream (T11).
The δ15N–NO3− and δ18O–NO3− values of river water ranged from − 6.7 to + 14.8‰ and from − 6.0 to + 5.6‰, with an average of + 4.6 and − 0.6‰, respectively (Table 1). The δ15N–NO3− value of river water in the upstream, midstream, and downstream of QHB varied from − 6.7 to + 9.6‰, from + 1.9 to + 10.9‰, and from + 6.0 to + 14.8‰, with a mean value of + 2.9‰, + 5.4‰, and + 9.0‰, respectively. The average of δ15N–NO3− value in the upstream was lower than those in the midstream and downstream. The δ18O–NO3− value of river water in the upstream, midstream, and downstream of QHB ranged from − 5.2 to + 5.5‰, from − 6.0 to + 5.6‰, and from − 5.8 to − 1.7‰, with a mean value of + 0.1‰, − 0.6‰, and − 3.1‰, respectively. As shown in Fig. 3a, the δ15N–NO3− values increased slowly with abruptly high values in M3, M8, and M12; however, the oxygen isotope values increased slowly with high values in M3 and M8 and decreased in M9 and then increase slowly to the downstream.
Discussion
The source of river water and controlling factors of water isotopes
The local meteoric water line (LMWL), which was reported as δD = 6.42δ18O – 4.66 (Jia et al. 2015). In addition, the global meteoric water line (GMWL) was defined as δD = 8δ18O + 10 (Craig 1961). As indicated in Fig. 2a, the hydrogen and oxygen isotopic compositions of river water in the QHB distributed below GMWL and LMWL, suggesting that atmospheric precipitation was the major source of river water.
The δD and δ18O–H2O values of samples (M5, M6, M8, and T11) located below the lower right of LMWL, which was caused by evaporation of river water enriching hydrogen and oxygen isotope values of the residual water. The M5 was sampled in the downstream of the Maliangeda reservoir with relatively slow velocity where evaporation caused the enrichment of δD and δ18O–H2O values. M6 and M8 were also sampled in reaches with slow water speed where evaporation dominated the water isotopes of river water. T11 and T12 were sampled in tributary Danhe River where the river received much wastewater from sewage, agriculture, and industrial enterprises (Ma et al. 2010; Wang et al. 2012). Industrial and agriculture water induced runoff reduction, and water recycling caused further evaporation (Gao et al. 2011) with increasing hydrogen and oxygen values of river water in these sites.
Groundwater recharge occurred in the headwater (M1) and downstream (M9 and M10) where river water has relatively negative water isotope values (Fig. 2c, d). The most negative water isotope values of M1 was due to the direct recharge from spring water undergoing no obvious evaporation and keeping water isotope values of local wet precipitation. The reach from M9 to M10 was located in the Taihang Mountain where river flew through the mountain in deep valley receiving much spring water. Tributary water had more negative water isotope values than those in most mainstream water indicating groundwater was dominated water source in these tributaries due to tributaries sourced from mountain area (Li et al. 2015a). The residual samples did not experience obvious groundwater recharge (Fig. 2c, d).
The relationship between d excess (d = δD − 8δ18O) and total dissolved solid (TDS) value was often used to identify the controlling factors of dissolved components in groundwater due to evaporation process resulted in increasing of TDS values together with decreasing of d excess values; however, leaching process of minerals only resulted in increasing of TDS values but not change of d excess values (Huang and Pang 2012; Qin et al. 2016). The EC values of river water were significantly associated with TDS; therefore, we use the plot between d excess and EC to discern the controlling processes of dissolved components in the river water. As indicated in Fig. 2b, the increase of EC values and the decrease of d excess values of river water were mainly found in M5, M6, and M8 where evaporation was responsible for the increase of EC values in these water samples. Water leaching process was responsible for the increase of EC values of most river water. It was worth to note that dissolved components in groundwater mostly derived from the water-rock reaction along the flow path. Groundwater infiltration and groundwater direct input into river water as spring were important factors to control the riverine components in the QHB. This conclusion was supported by the fact that the water exchange between surface water and groundwater was 1.03 × 109 m3 in 1988 in the QHB (Wang et al. 2006). The average EC value of river water in the midstream (526 μS cm−1) was lower than that of river water in the upstream (564 μS cm−1) was due to groundwater input from sandstone aquifer with low EC value (Zhang et al. 2015). The increase of EC values in the downstream (M10, M11, M12, T11, and T12) was not only due to groundwater input from karst aquifer with high dissolved components but also due to direct wastewater input from sewage, agriculture, and industrial enterprises.
Sources of dissolved nitrate in the river water
Spatial variations of nitrate contents in river water were due to different nitrate sources and variable nitrogen transforming paths. As shown in Fig. 4a, the nitrate concentrations of the river water were variable in the different reaches of the QHB with nitrate contents less than 8 mg/L from M1 to M8, but larger than 10 mg/L from M9 to the mouth except M12. The enhancement of NO3− and Cl− concentrations from M9 to M11 was related to the input of sewage water, industrial wastewater, and agricultural fertilizer (Han 2008). The decreasing NO3− concentrations (Fig. 4a) but increasing Cl− contents (Fig. 4b) in M3, M8, and M12 were possibly due to input of groundwater and/or assimilation by algae at these sampling sites with DO contents of 10.28 mg/L, 24.92 mg/L, and 9.97 mg/L (Table 1). However, positive oxygen isotope values of M3, M8, and M12 indicated no obvious groundwater input (Fig. 2c, d); internal algae assimilation could be responsible for the decreasing of nitrate concentrations in these sites due to relatively steady conditions. The high NO3− and Cl− concentration in T6 (Fig. 4) was probably related to the input of industrial wastewater from coking plant and coal preparation plant. The nitrate concentration of T9 was larger than 16 mg/L possibly owing to coal mining activities and wastewater from chemical enterprises (Fu et al. 2013). The DO concentration of T12 was 0 mg/L (Table 1), which was suitable for denitrification (Rivett et al. 2008).
The δ15N–NO3− and δ18O–NO3− values of river water in the source (M1) of the QHB were − 6.7‰ and − 3.9‰, which suggested that nitrate was mainly sourced from NH4+ in the rainwater and subsequent nitration. The δ15N–NO3− values of sites (M3, M8, and M12) were more than + 7‰ (Fig. 3a), as mentioned above, indicating that nitrate assimilation by algae was responsible for the nitrogen and oxygen isotope enrichment of residual nitrate in water. Wang et al. (2006) proved that the percentage of exchange between surface water and groundwater in the area located from the source of QHB to Wuzhi hydrological station was more than 80% of total groundwater resources in 1 year. As shown in Fig. 3b, the δ18O–NO3− values of sites (M9 and M10) were close to that of the headstream (M1), which was probably due to groundwater input corresponding to negative hydrogen and oxygen isotope values in M9 and M10 (Fig. 2c, d). However, the δ15N–NO3− values of these sites (+ 5.8‰ and + 6.0‰) were significantly different from the source of QHB (− 6.7‰), which indicated that except groundwater nitrate input, there must be another important nitrate sources. Negative oxygen isotope values of nitrate could be formed from nitration of ammonium, and nitrate in M1 was mainly controlled by nitration of ammonium from rainwater with small Cl− content (Table 1). However, nitrate in M9 and M10 was mainly affected by nitration from sewage and industrial wastewater with high Cl− contents (Table 1). As indicated in Fig. 3a, the δ15N–NO3− values of tributaries (T1 and T2) were larger than + 7‰, indicating that domestic sewage from town residential area had an important effect on nitrate isotopic compositions. The nitrogen isotopic value of T6 was close to − 5‰ (Fig. 3a), which was mainly attributed to the input of ammonium in the fertilizer and industrial wastewater with high Cl− content (Table 1) from coal mining company (Wang et al. 2013b). The δ15N–NO3− value of T9 increased also due to wastewater from coal mining and chemical enterprises (Fu et al. 2013).
Transformations of nitrogen in the QHB
Mixing of different sources and assimilation by algae
The isotopic compositions of nitrate are usually modified by physical and biogeochemical processes. The nitrate derived from various potential sources seldom kept constant isotopic values, which was probably changed by diverse isotope fractionation in different periods of mixing (Kendall et al. 2007). The cross plot between δ15N–NO3− and the inverse of nitrate was often used to elucidate the mixing processes. If two different nitrate sources mixed, the δ15N–NO3− is linearly positively correlated with 1/ [NO3−] (Kendall et al. 2007; Xu et al. 2016). There was a significantly positive relationship between δ15N–NO3− and 1/ [NO3−] (Fig. 5a), which indicated that the mixing processes could account for the shifting of δ15N–NO3− values. As shown in Fig. 5a, the nitrate of river water was originated from at least two different sources: one with δ15N–NO3− values below + 5‰, another with δ15N–NO3− values above + 7‰. The δ15N–NO3− values of river water in the QHB less than + 5‰ indicated that nitrate was primarily derived from soil nitrogen and chemical fertilizer. It was worth to note that nitrate from rain ammonium was one of the important components in soil nitrogen. Although δ15N–NO3− values of nitrate from rainwater and fertilizer and industrial wastewater were within a similar range, NO3− and Cl− concentrations were quite different (Fig. 5a, b). Some river water with δ15N–NO3− values larger than + 7‰ had potential nitrate from sewage and manure, and increasing of δ15N–NO3− values with a decreasing of NO3− concentration but relatively constant or increased Cl− contents may be due to occurrence of assimilation of nitrate by algae in water samples with DO contents more than 3 mg/L, e.g., M3, M8, and M12 (Fig. 5a).
Therefore, the nitrate in headwater was mainly derived from nitrification of ammonium from rainwater and soil organic nitrogen oxidation. Sewage and industrial wastewater were important nitrate sources in the domestic area, e.g., M3, M8, M12, T6, and T9. Algae assimilation was dominated in M3, M8, and M12 due to the relatively steady station and rich organic nutrition from sewage water.
Denitrification
Denitrification was an important process to remove nitrogen by the reduction of nitrate to gaseous nitrogen (Kendall et al. 2007; Wang et al. 2017; Xu et al. 2016). The denitrification causes nitrate concentrations to decrease and an enrichment of isotopic values of the remaining nitrate (Xue et al. 2009). In the denitrification process, nitrogen isotopic enrichment factors vary from − 40 to − 5‰ (Panno et al. 2006; Xue et al. 2009), and oxygen isotopic enrichment factors vary from − 18 to − 8‰ (Xue et al. 2009). The linear relationship between δ15N–NO3− and δ18O–NO3− (1.3:1 to 2.1:1) can provide evidence for denitrification (Xu et al. 2016; Xue et al. 2009). As indicated in Fig. 6, there was no significant relationship between δ15N–NO3− and δ18O–NO3− in river water (P > 0.05). Meanwhile, most water samples in the QHB had DO concentrations more than 2 mg/L (Table 1), which was not beneficial for denitrification (Rivett et al. 2008). Therefore, the relationship between δ15N–NO3− and δ18O–NO3− indicated that no obvious denitrification occurred in the QHB.
Nitrification
Nitrification was a process that ammonium was oxidized to nitrate mediated by a few different sorts of autotrophic bacteria or archaea (Kendall et al. 2007; Xu et al. 2016). Nitrogen enrichment factors in the nitrification process range from − 38 to − 14‰ (Casciotti et al. 2003; Xu et al. 2016). It is reported that the oxygen atoms derived from nitrification gain two thirds of oxygen from water and one third from atmospheric oxygen (Andersson and Hooper 1983). The δ18O–H2O values of river water in QHB ranged from − 10.8 to − 7.2‰ (Table 1), and the δ18O value of atmospheric O2 is 23.5‰ (Durka et al. 1994; Hollocher 1984). If there had been no isotope fractionation, the theoretical values of δ18O–NO3− originated from nitrification would have ranged from + 0.6 ‰ to + 3.0‰. Several δ18O–NO3− values of river water from upstream and midstream were higher than theoretical values (Fig. 7), which probably caused by the variable proportion of oxygen from water and O2, oxygen isotope fractionation, and different biological processes (Kendall et al. 2007; Mayer et al. 2001). Kool et al. (2011) found that oxygen changes in water affect δ18O values of nitrate in soil ecosystems. It may be the primary reason that some δ18O–NO3− values were lower than theoretical values (Fig. 7), which possibly suggested that more oxygen from the water was transformed into nitrate in the nitrification process. As shown in Fig. 7, the δ18O–NO3− values of river water were distributed around theoretical nitrification line and were in the range of − 10 to + 10‰ in which δ18O–NO3− signatures derived from nitrification (Kendall et al. 2007). Thus, the oxygen isotopic compositions of nitrate indicated that the nitrate in river water of QHB would be affected by nitrification.
Identification of nitrate sources based on dual isotope approach
In the riverine ecosystem, the nitrate possibly originates from atmospheric deposition, agricultural fertilizer input, soil nitrogen, manure, urban domestic sewage, and industrial wastewater (Xu et al. 2016). The dual-isotope approach is used to access the nitrate sources of the QHB due to different nitrate sources having distinct isotopic compositions. As shown in Fig. 8, the δ15N–NO3− and δ18O–NO3− values fell into three sections, suggesting that NH4+ in fertilizer and rain, soil nitrogen, manure, and urban domestic sewage might be the nitrate sources of river water in the QHB. The δ18O–NO3− values of nitrate in precipitation (+ 25 to + 94‰) and nitrate fertilizer (+ 17‰ and + 25‰) are higher than that of nitrate from manure and sewage, and higher than that of nitrate from nitrification of soil nitrogen and ammonium in rain and fertilizer (Kendall et al. 2007; Xue et al. 2009; Yue et al. 2017). The δ18O–NO3− signatures of nitrate indicated that nitrate in atmospheric deposition and nitrate fertilizer contributed a minor proportion of nitrate to the river water.
Some researchers reported that the concentrations and isotopic compositions of nitrate in river water probably influenced by land use and human activities in the watershed (Ohte et al. 2010; Xing and Liu 2016; Yue et al. 2014). As shown in Fig. 8, the nitrate isotopic values (δ15N–NO3−, − 6.7 to + 9.6‰; δ18O–NO3−, − 5.2 to + 5.5‰) in the upstream indicated that nitrate was mainly derived from NH4+ in the rain and soil nitrogen, which was corresponding to the river flowing through dense forest and regions with few human activities. There were many coal mining plants, coking plants, and power plants distributed along the riverside of midstream in the QHB, and large amounts of industrial wastewater and urban domestic sewage discharged into the river. Meanwhile, the δ15N–NO3− of river water receiving industrial wastewater were smaller than + 5‰ (Xu et al. 2016). Therefore, the isotopic compositions of nitrate (δ15N–NO3−, + 1.9 to + 10.9‰; δ18O–NO3−, − 6.0 to + 5.6‰) in the midstream suggested that nitrate was primarily derived from industrial wastewater, soil nitrogen, ammonium fertilizer, and domestic sewage, which suggested that the main nitrate sources of river water agreed with pollution sources distribution in the midstream of QHB. The higher δ15N–NO3− values of nitrate were observed in the downstream of QHB where it is characterized as dense agricultural and industrial activities and high population density. The δ15N–NO3− values in the downstream ranged from + 6.0 to + 14.8‰, which was close to the δ15N–NO3− values of nitrate (from + 5.5 to + 11.6‰) reported by Zhang et al. (2012). The isotopic values of nitrate in the downstream indicated that nitrate mainly originated from soil nitrogen, manure, and sewage. In summary, the nitrate isotopic compositions of river water in the QHB indicated that the nitrate mainly derived from soil nitrogen, ammonium in fertilizer, and domestic sewage.
Conclusions
This study evaluated sources and transformations of nitrogen in the QHB by combining isotopic compositions of nitrate and H2O with water chemistry data. The hydrogen and oxygen isotopic compositions of water indicated that atmospheric precipitation was the main source of river water. The nitrogen and oxygen isotopic values were mainly affected by the mixing process and nitrification process. No obvious denitrification shifted nitrate isotopic values of the QHB. The mixing process and assimilation by algae would be responsible for δ15N–NO3− values increasing. The δ15N and δ18O values of nitrate in river water of the QHB demonstrated that nitrate sources were mainly derived from nitrification of ammonia fertilizer, soil nitrogen, and domestic sewage. Some measures should be taken to alleviate nitrate pollution, such as effective fertilization and planting trees to prevent soil erosion. In the future study, different end members of isotopic compositions of nitrate should be determined to evaluate the contribution proportions of potential nitrate sources.
References
Andersson KK, Hooper AB (1983) O2 and H2O are each the source of one O in NO2 − produced from NH3 by Nitrosomonas: 15N-NMR evidence. FEBS Lett 164:236–240
Bai P, Liu WH, Guo MJ (2014) Impacts of climate variability and human activities on decrease in streamflow in the Qinhe River, China. Theor Appl Climatol 117:293–301
Barnes RT, Raymond PA (2010) Land-use controls on sources and processing of nitrate in small watersheds: insights from dual isotopic analysis. Ecol Appl 20:1961–1978
Casciotti KL, Sigman DM, Ward BB (2003) Linking diversity and stable isotope fractionation in ammonia-oxidizing bacteria. Geomicrobiol J 20:335–353
Chang CCY, Kendall C, Silva SR, Battaglin WA, Campbell DH (2002) Nitrate stable isotopes: tools for determining nitrate sources among different land uses in the Mississippi River Basin. Can J Fish Aquat Sci 59:1874–1885
Chen ZX, Yu L, Liu WG, Lam MHW, Liu GJ, Yin XB (2014) Nitrogen and oxygen isotopic compositions of water-soluble nitrate in Taihu Lake water system, China: implication for nitrate sources and biogeochemical process. Environ Earth Sci 71:217–223
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Curtis CJ, Heaton THE, Simpson GL et al (2011) Dominance of biologically produced nitrate in upland waters of Great Britain indicated by stable isotopes. Biogeochemistry 111:535–554
Deutsch B, Voss M, Fischer H (2009) Nitrogen transformation processes in the Elbe River: distinguishing between assimilation and denitrification by means of stable isotope ratios in nitrate. Aquat Sci 71:228–237
Ding JT, Xi BD, Gao RT, He L, Liu H, Dai X, Yu Y (2014) Identifying diffused nitrate sources in a stream in an agricultural field using a dual isotopic approach. Sci Total Environ 484:10–18
Durka W, Schulze ED, Gebauer G, Voerkeliust S (1994) Effects of forest decline on uptake and leaching of deposited nitrate determined from 15N and 18O measurements. Nature 372:765–767
El Gaouzi FZJ, Sebilo M, Ribstein P et al (2013) Using δ15N and δ18O values to identify sources of nitrate in karstic springs in the Paris basin (France). Appl Geochem 35:230–243
Fan AM, Steinberg VE (1996) Health implications of nitrate and nitrite in drinking water: an update on methemoglobinemia occurrence and reproductive and developmental toxicity. Regul Toxicol Pharmacol 23:35–43
Feng JL, Wang GL, Sun JH, Sun SP, Liu XF (2010) Metals in water and surface sediments from Henan reaches of the Yellow River, China. Sci China Chem 53:1217–1224
Fewtrell L (2004) Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect 112:1371–1374
Fu P (2012) Heavy metal pollution in the Qin River. Dissertation, Shanxi University (In Chinese)
Fu P, Wang F, Ma XP et al (2013) Vertical distribution of heavy metals in sediments of Qinhe River and its risk assessment. Chin J Appl Environ Biol 19:305–312 (In Chinese)
Fukada T, Hiscock KM, Dennis PF (2004) A dual-isotope approach to the nitrogen hydrochemistry of an urban aquifer. Appl Geochem 19:709–719
Gao JF, Ding TP, Luo XR et al (2011) δD and δ18O variations of water in the Yellow River and its significance in environmental studies. Acta Geol Sin 85:596–602 (In Chinese)
Han J (2008) Evaluation on the present situation of water quality and pollution analysis of Qinhe River’ s Shanxi section. Sci-tech Information Development and Economy 18:215–216 (In Chinese)
Hollocher TC (1984) Source of the oxygen atoms of nitrate in the oxidation of nitrite by Nitrobacter agilis and evidence against a P-O-N anhydride mechanism in oxidative phosphorylation. Arch Biochem Biophys 233:721–727
Hu CH, Wang JJ, Wang H et al (2012) Quantitative response of observed runoff in Qinhe River watershed to environmental change. Climate Change Research 8:213–219 (In Chinese)
Huang TM, Pang ZH (2012) The role of deuterium excess in determining the water salinisation mechanism: a case study of the arid Tarim River basin, NW China. Appl Geochem 27:2382–2388
Jia ZX, Zang HF, Zheng XQ et al (2015) Research on characteristics of hydrogen and oxygen isotopes of precipitation in Taiyuan area. Jounral of Water Resources and Water Engineering 26:22–25 (In Chinese)
Kelley CJ, Keller CK, Evans RD, Orr CH, Smith JL, Harlow BA (2013) Nitrate-nitrogen and oxygen isotope ratios for identification of nitrate sources and dominant nitrogen cycle processes in a tile-drained dryland agricultural field. Soil Biol Biochem 57:731–738
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener RH, Lajtha K (eds) Stable isotopes in ecology and environmental science, 2nd edn. Blackwell Publishing, Hoboken, pp 375–449
Kool DM, Wrage N, Oenema O, Dolfing J, van Groenigen JW (2007) Oxygen exchange between (de)nitrification intermediates and H2O and its implications for source determination of NO3 − and N2O: a review. Rapid Commun Mass Spectrom 21:3569–3578
Kool DM, Wrage N, Oenema O, van Kessel C, van Groenigen JW (2011) Oxygen exchange with water alters the oxygen isotopic signature of nitrate in soil ecosystems. Soil Biol Biochem 43:1180–1185
Lee KS, Bong YS, Lee D, Kim Y, Kim K (2008) Tracing the sources of nitrate in the Han River watershed in Korea, using delta15N-NO3 − and delta18O-NO3 − values. Sci Total Environ 395:117–124
Li C, Li SL, Yue FJ, Liu J, Zhong J, Yan ZF, Zhang RC, Wang ZJ, Xu S (2019) Identification of sources and transformations of nitrate in the Xijiang River using nitrate isotopes and Bayesian model. Sci Total Environ 646:801–810
Li SL, Liu CQ, Li J, Xue Z, Guan J, Lang Y, Ding H, Li L (2013) Evaluation of nitrate source in surface water of southwestern China based on stable isotopes. Environ Earth Sci 68:219–228
Li SL, Yue FJ, Liu CQ et al (2015a) The O and H isotope characteristics of water from major rivers in China. Chin J Geochem 34:28–37
Li WB, Song YB, Xu HK, Chen LY, Dai WH, Dong M (2015b) Ion-exchange method in the collection of nitrate from freshwater ecosystems for nitrogen and oxygen isotope analysis: a review. Environ Sci Pollut Res 22:9575–9588
Liu CQ, Li SL, Lang Y et al (2006) Using δ15N- and δ18O-values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ Sci Technol 40:6928–6933
Ma XP, Jing WX, Wang Q et al (2010) Heavy metal pollution in the surface sediment of Dan River and its ecological risk assessment. Journal of Agro-Environment Science 29:1180–1186 (In Chinese)
Mayer B, Bollwerk SM, Mansfeldt T, Hütter B, Veizer J (2001) The oxygen isotope composition of nitrate generated by nitrification in acid forest floors. Geochim Cosmochim Acta 65:2743–2756
McIlvin MR, Altabet MA (2005) Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal Chem 77:5589–5595
Nestler A, Berglund M, Accoe F, Duta S, Xue D, Boeckx P, Taylor P (2011) Isotopes for improved management of nitrate pollution in aqueous resources: review of surface water field studies. Environ Sci Pollut Res 18:519–533
Ohte N (2013) Tracing sources and pathways of dissolved nitrate in forest and river ecosystems using high-resolution isotopic techniques: a review. Ecol Res 28:749–757
Ohte N, Tayasu I, Kohzu A, Yoshimizu C, Osaka K', Makabe A, Koba K, Yoshida N, Nagata T (2010) Spatial distribution of nitrate sources of rivers in the Lake Biwa watershed, Japan: controlling factors revealed by nitrogen and oxygen isotope values. Water Resour Res 46:W07505
Panno SV, Hackley KC, Kelly WR, Hwang HH (2006) Isotopic evidence of nitrate sources and denitrification in the Mississippi River, Illinois. J Environ Qual 35:495–504
Qin Y, Zhang D, Zhao ZQ (2016) Spatial and temporal variations of hydrochemical compositions of river water in Qinhe Basin. Chin J Ecol 35:1516–1524 (In Chinese)
Rivett MO, Buss SR, Morgan P, Smith JWN, Bemment CD (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res 42:4215–4232
Tesoriero AJ, Liebscher H, Cox SE (2000) Mechanism and rate of denitrification in an agricultural watershed: electron and mass balance along groundwater flow paths. Water Resour Res 36:1545–1559
Vrzel J, Vukovic-Gacic B, Kolarevic S et al (2016) Determination of the sources of nitrate and the microbiological sources of pollution in the Sava River Basin. Sci Total Environ 573:1460–1471
Wang CY, Yang YH, Zhou F et al (2012) Spatio-temporal characteristics and source identification of water pollutants in river Qinhe Basin. Acta Sci Circum 32:2267–2278 (In Chinese)
Wang F, Fu P, Ma XP et al (2013a) Distribution of organochlorine pesticides along the Qinhe River and its risk evaluation. Chin J Appl Environ Biol 19:670–676 (In Chinese)
Wang L, Ni GH, Hu HP (2006) Simulation of interactions between surface water and groundwater in Qin River basin. J Tsinghua Univ (Sci & Tech) 46:1978–1981 1986 (In Chinese)
Wang SQ, Tang CY, Song XF, Yuan R, Wang Q, Zhang Y (2013b) Using major ions and δ15N-NO3 − to identify nitrate sources and fate in an alluvial aquifer of the Baiyangdian lake watershed, North China Plain. Environ Sci Process Impacts 15:1430–1443
Wang ZJ, Yue FJ, Zeng J, Li SL (2017) The influence of urbanization on karst rivers based on nutrient concentration and nitrate dual isotopes: an example from southwestern China. Acta Geochim 36:446–451
Wexler SK, Goodale CL, McGuire KJ et al (2014) Isotopic signals of summer denitrification in a northern hardwood forested catchment. Proc Natl Acad Sci U S A 111:16413–16418
Xing M, Liu WG (2016) Using dual isotopes to identify sources and transformations of nitrogen in water catchments with different land uses, Loess Plateau of China. Environ Sci Pollut Res 23:388–401
Xing M, Liu WG, Wang ZF, Hu J (2013) Relationship of nitrate isotopic character to population density in the Loess Plateau of Northwest China. Appl Geochem 35:110–119
Xu SG, Kang PP, Sun Y (2016) A stable isotope approach and its application for identifying nitrate source and transformation process in water. Environ Sci Pollut Res 23:1133–1148
Xue DM, Botte J, De Baets B et al (2009) Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Res 43:1159–1170
Xue DM, De Baets B, Van Cleemput O et al (2012) Use of a Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water. Environ Pollut 161:43–49
Yue FJ, Li SL, Liu CQ et al (2017) Tracing nitrate sources with dual isotopes and long term monitoring of nitrogen species in the Yellow River, China. Sci Rep 7:506–515
Yue FJ, Liu CQ, Li SL, Zhao ZQ, Liu XL, Ding H, Liu BJ, Zhong J (2014) Analysis of δ15N and δ18O to identify nitrate sources and transformations in Songhua River, Northeast China. J Hydrol 519:329–339
Zhang D, Qin Y, Zhao ZQ (2015) Chemical weathering of carbonate rocks by sulfuric acid on small basin in North China. Acta Sci Circum 35:3568–3678 (In Chinese)
Zhang D, Yang W, Zhao JL (2012) Tracing nitrate sources of the Yellow River and its tributaries with nitrogen isotope. J Ecol Rural Environ 28:622–627 (In Chinese)
Zhang Y, Li F, Zhang Q, Li J, Liu Q (2014) Tracing nitrate pollution sources and transformation in surface- and ground-waters using environmental isotopes. Sci Total Environ 490:213–222
Acknowledgments
We would like to thank Mr. Li Xiao-Dong and Mr. Lei Guo-Liang for their help in determining isotopic compositions of nitrate. We would like to thank Wang Sai for his help during field sampling. We also would like to thank two anonymous referees whose comments and suggestions on an earlier version have greatly improved our manuscript.
Funding
The work was supported by the National Natural Science Foundation of China (Grant Nos. 41573095 and 41573064).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Qin, Y., Zhang, D. & Wang, F. Using nitrogen and oxygen isotopes to access sources and transformations of nitrogen in the Qinhe Basin, North China. Environ Sci Pollut Res 26, 738–748 (2019). https://doi.org/10.1007/s11356-018-3660-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3660-0