Abstract
The present work studies the removal of nutrients (nitrate and phosphate) from a synthetic wastewater simulating a secondary treatment effluent using the microalgae Chlorella vulgaris in autotrophic photobioreactors, together with an analysis of the critical points affecting the scaling-up process from laboratory to pilot scale. Laboratory experiments were done in open agitated 1-L photobioreactors under batch operation mode, while pilot-scale experiments were done using a 150-L closed tubular photobioreactor under continuous operation mode. In both scales, nitrate was the limiting substrate and the effect of its concentration on microalgae performance was studied. From laboratory experiments, an average microalgae productivity of 85 mgVSS L−1 day−1 and approximate maximum N-NO3− and P-PO43− removal rates of 8 mg N gVSS−1 day−1, and 2.6 mg P gVSS−1 day−1 were found. Regarding pilot scale, the average microalgae productivity slightly decreased (76 mgVSS L−1 day−1) while the approximate maximum N-NO3− and P-PO43− removal rates slightly were increased (11.7 mg N gVSS−1 day−1 and 3.04 mg P gVSS−1 day−1) with respect to the laboratory-scale results. The pilot-scale operation worked under lower levels of turbulence and higher dissolved oxygen concentration and light intensity than laboratory experiments; those parameters were difficult to control and they can be identified as the critical points in the differences found on both nutrient removal and microalgae production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient removal in modern domestic wastewater treatment plants is usually included in the secondary biological step, and it is achieved by means of well-known processes such as nitrification, denitrification, and biological phosphorus removal (Ekama 2015); the whole of them is called as Biological Nutrient Removal (BNR). Nitrification is practically and successfully implemented in the aerobic step most of the classical biological treatments, but denitrification and P removal may not be considered in the plant design (old plants) or may not work properly in modern plants because of the operation costs or the lack of pre-hydrolized easily biodegradable organic matter in the wastewater, which is an important requisite (Zeng et al. 2015). Thus, it is usual that nitrogen removal is not satisfactory and it is present mainly as N-NO3− in the effluent, and also the P concentration may exceed the discharge limit, which caused adverse environmental impacts.
Because of this problem, there are currently different tertiary systems for the removal of nitrate and phosphate in secondary effluents. Some of them are based on physicochemical fundamentals but they are associated with significant costs due to the consumption of chemical reagents. An alternative to such physicochemical methods may be the biological nutrient removal using microalgae and photobioreactors. There is currently a lot of information in the scientific literature about the use of microalgae for N and P removal in wastewater (Cai et al. 2013). It can be achieved through an autotrophic photosynthetic process capable to treat a secondary effluent with no biodegradable organic matter. Microalgae may be also used in mixotropic processes, a combination of heterotrophic and autotrophic biological treatment in which bacteria and microalgae simultaneously remove organic carbon, carbon dioxide from heterotrophic respiration, nitrogen, and phosphorus (Zeng et al. 2015). Since microalgae prefer ammonium rather than oxidized forms of nitrogen, the number of scientific papers focused on ammonium removal is higher than those about nitrate removal, despite the fact that nitrate is the main nitrogen form in secondary effluents from wastewater treatment plants (Cai et al. 2013). Moreover, the potential of nitrate-accumulating microalgae for nutrient recovery has not been adequately investigated to date (Coppens et al. 2014).
In addition to nutrients capture, photosynthetic autotrophic processes using microalgae have been widely used for carbon dioxide removal in exhaust combustion gas and for microalgae biomass production because of its multiple uses. Unlike sewage sludge from wastewater treatment plants, microalgae biomass is considered as a valuable raw material instead of a biowaste. Many applications of microalgae biomass have been proposed and investigated (Odjadjare et al. 2017): (i) transformation into valuable bioproducts such as lipids, oil, fatty acids, pigments, vitamins, and proteins; (ii) transformation into energy sources, e.g., biofuels, biogas, or biohydrogen; and (iii) animal food manufacture. The cost of such biomass production could be significantly reduced by using treated sewage as inorganic nutrients source (Cabanelas et al. 2013).
However, most of the research work regarding microalgae nutrient removal has been made under laboratory scale, while the research focused on scale-up to pilot- or full-scale photobioreactors did not receive so much attention. It is very important to study the differences that could appear in the scale-up step because some parameters such as temperature, light intensity, or turbulence are easily controlled under lab scale but not under pilot scale (Acién Fernández et al. 2013). Several authors reported scale-up investigations where both nutrient removal efficiency (Van den Hende et al. 2014) and microalgae productivity (Lam et al. 2016) decreased in pilot-scale photobioreactors.
In this context, the present work shows the results of an experimental study in which Chlorella vulgaris microalgae was used to remove nitrate and phosphate from a synthetic wastewater simulating a secondary treatment effluent. The study was performed both at laboratory and pilot scales. Laboratory experiments were done in open agitated photobioreactors under batch operation mode and using different nitrate concentrations, while pilot-scale experiments were carried out in a closed tubular photobioreactor (150 L reaction volume) under continuous operation mode. One of the novelty points is that most of the nitrogen removal works using microalgae are focused on ammonium removal, while the use of nitrate is not so common. Moreover, the second novelty point is focused on the scaling-up process, and the critical operating parameters to perform scale-up have been identified. Thus, the objectives of the present work were: (i) to assess the N-NO3− and P-PO43− removal rates and biomass yields both under laboratory and pilot scale, (ii) to study the relationship between the differences found in those parameters and the main operating variables for both scales, and (iii) to identify the most critical operating parameters in the scale-up process.
Materials and methods
Microalgae and growth medium
C. vulgaris was obtained from the Culture Collection of Algae in the University of Las Palmas de Gran Canaria (Spain). The microalgae culture was incubated in closed flasks using Bold’s Basal Medium, BBM, as synthetic culture medium (Frumento et al. 2016), weekly growth cycles at ambient temperature (approximately 21 °C) and day/night alternation. Air with 10% CO2 simulating flue gas was bubbled during light periods (Judd et al. 2015; Duarte et al. 2016).
BBM was chosen as a synthetic medium to simulate the secondary effluent as it contains nitrate and phosphate as the main inorganic nutrients, although P concentrations used in BBM were higher than in the usual secondary effluent levels. BBM also presents buffer capacity for pH control, and it was the liquid medium used both in the laboratory and pilot-scale experiments. Its composition was the following (mg L−1): NaNO3, 250.0; CaCl2·2H2O, 25.0; MgSO4·7H2O, 75.0; K2HPO4, 175.0; KH2PO4, 75.0; NaCl, 25.0; EDTA, 50.0; KOH, 31.0; H3BO3, 11.5; FeSO4·7H2O, 5.0; ZnSO4·7H2O, 8.8; MnCl2·4H2O, 1.8; and CuSO4·5H2O, 1.6. pH was between 6.6 and 6.8.
Experimental installations
Figure 1 shows a scheme of the experimental installation used for the lab-scale experiments. It consisted of a photobioreactor with the following parts: (1) a system for atmospheric air feeding enriched in CO2 (2), in order to simulate a combustion exhaust gas; (3) a thermostated closed chamber with adjustable temperature; (4) UEETK (USA) 28 cm LED lamps for artificial lighting and a timer (5) to adjust the duration of light/dark cycles; (6) a multiple magnetic stirring system and several 1-L glass bottles (7) which acted as completely mixed batch reactors that contained the microalgae suspensions and the liquid growth medium, receiving the air/CO2 mixture flow through bubbling (8).
Figure 2 shows a scheme (a) and a photograph (b) of the pilot-scale installation. It consisted of a tubular photobioreactor that contained the following parts: (1) a system for atmospheric air feeding enriched in CO2 similar to the one used in laboratory, (2) a CO2 absorption tank (100 L) with a mechanical stirring system that contained the liquid growth medium, (3) the feeding system of the liquid growth medium saturated in CO2 consisting of a peristaltic pump, (4) a 150-L tubular photobioreactor composed by consecutive tubes of 1.5 m long and 9 cm diameter, (5) a degasification unit, (6) a peristaltic pump for liquid flow recirculation, (7) an effluent outlet, and (8) an atmospheric air compressor to improve the turbulence. It was also equipped with temperature and lighting sensors. The system worked under continuous operation mode as a completely mixed biological reactor without biomass recirculation. The whole pilot-scale installation was located into a greenhouse (Fig. 2b) which allowed temperature control by an air-conditioning device, and maximum light intensity control by a manual adjustable solar radiation mitigation system. The greenhouse was located next to the Institute of Chemical and Environmental Technology of the University of Castilla-La Mancha, Ciudad Real (Spain).
Experimental procedure
Lab-scale nutrient (nitrate and phosphate) removal experiments were performed under batch operation mode. Glass bottles were filled with the growth liquid medium. Depending on the experiments, the growth medium contained different nitrate concentrations. The same initial amount of microalgae was inoculated in all bottles and then magnetic mixing and light/darkness cycles were connected during 10 days. Air containing 10% (v/v) CO2 was bubbled only during light cycles. Temperature was 21 °C. Mixing rate was 10 s−1 (Approximate Reynolds number of 25·103). Light/darkness cycles were 12 h/12 h and light intensity was 100 μmol m−2 s−1. Three experiments were performed: N1, N2, and N3 that contained initial concentrations of 14.6, 28.2, and 40.8 mg N-NO3− L−1, respectively. Experiments were made by triplicate. The liquid samples were taken every 10 h from start to day 3, and then every 24 h until the end.
Pilot-scale nutrient removal experiments were made under continuous operation mode during 7 months. The system worked as a tubular completely mixed biological reactor without biomass recirculation; that is, the hydraulic retention time was the same as the cell (microalgae) retention time. The photobioreactor was filled with the BBM liquid growth medium and inoculated with an initial amount of microalgae culture (approximately 0.15 gVSS L−1) from the laboratory. A batch operation was applied during the first week, as acclimation step in order to reach enough microalgae concentration, and then a continuous flow of CO2-saturated liquid growth medium containing 40.8 mg N-NO3− L−1 was used (as in the N3 laboratory experiment). According to the results previously found in the laboratory experiments, hydraulic retention time were varied throughout the experiment, 5.5 days in the first month, 6.0 days in the second month, and 6.5 days in months 3 to 7 (which was the main stationary period to compare with the laboratory results), corresponding to mean flow rates of 27.3, 25.0, and 23.1 L day−1, respectively. Atmospheric air was supplied by a compressor to favor liquid flow and mixing and degasification of excess dissolved oxygen (Acién Fernández et al. 2013).
Analytical methods
All analytical methods followed Standard Methods (A.P.H.A. 1998). Microalgae concentration in the liquid samples was measured as volatile suspended solids (VSS) by weight loss after ignition at 550 °C. Nitrogen (N-NO3−) and phosphorus (P-PO43−) concentrations were measured by colorimetric methods using a DR2700 Hach portable spectrophotometer (Colorado, USA). Dissolved inorganic carbon (IC) was measured by a TOC analyzer (Shimadzu TOC-VCSH, Columbia, USA). The pH was measured by a pH-meter (PCE-228M). Dissolved oxygen was measured using a YSI 5000 dissolved oxygen probe. Light intensity was measured by a Collihigh illuminometer.
Results and discussion
Evolution and control of experimental conditions
Experimental conditions were easily controlled in the laboratory experiments. Temperature was maintained at 21.0 ± 2 °C, pH was 6.5 ± 0.5, and saturation dissolved oxygen concentration was 7.0 ± 1.2 mg L−1. Light intensity was kept constant at 100 μmol m−2 s−1.
Pilot-scale conditions were more difficult to control than in the laboratory. Temperature, pH, dissolved oxygen concentration, and lighting values monitored throughout the experimental period have been included as supplementary material (online resource, Fig. S1). Temperature values varied between 15 and 28 °C during the first 100 days, although it were better controlled and maintained (around 24 °C) during the rest of the experiment; pH varied between 6.5 and 8.0 by means of the buffer capacity of the BBM growth medium; dissolved oxygen concentration varied between 6 and 12 mg L−1 approximately, and, finally, light intensity reached higher values than those of lab-scale tests (in the range 100–300 μmol m−2 s−1) during the first 100 days, although it was better controlled (in values around 90 μmol m−2 s−1) throughout the rest of the experimental period. Observed variations in temperature and lighting were mainly due to changes in external climatic conditions, while pH changes may be attributed to variations in carbon availability throughout the experiment (Acién Fernández et al. 2013). Therefore, it seems clear that, in spite of the control systems implemented in the pilot plant used, the achievement of precise control of the operating conditions at the pilot plant scale is a complex issue that needs to be adequately addressed in future experiments.
Nutrient removal and microalgae production in laboratory
Figure 3a shows the growth of microalgae during the laboratory batch experiments using different values of the initial nitrate concentration (mean values from three replicates). As previously indicated, batch experiments were performed under excess concentrations of N and P, being N-NO3− the limiting nutrient at the end of the experiments (as discussed in Fig. 4). Microalgae growth profiles were straight lines indicative of first-order growth kinetics, without a clear influence of the initial nitrate concentration. The maximum growth rate began to decline from day 8. An average microalgae productivity value of 85 mgVSS L−1 day−1 was calculated during this period. Taking into account also the average biomass concentration, an approximate maximum specific growth rate of 0.18 day−1 may be calculated, which corresponds to an approximate hydraulic retention time of 5.5 days in a hypothetical continuous operation mode.
Figure 3b shows the dissolved inorganic carbon (IC) consumption measured in the closed batch tests carried out in the laboratory (average values from three replicates). The gas mixture air/CO2 (10% v/v) was bubbled into the microalgae suspension (0.25 gVSS L−1) in BBM medium until it was saturated with CO2. Then, the air/CO2 flow was stopped and the dissolved IC concentration consumption was measured during several hours. A constant maximum carbon consumption rate can be observed during the first 9 h approximately. It means that an excess of inorganic carbon, as well as nitrogen and phosphorus, was kept during the first 9 h and therefore it was not the limiting substrate for microalgae growth. Under such conditions, the maximum microalgae IC consumption rate in the laboratory photobioreactor may be calculated, obtaining a value of 118 mg C gVSS−1 day−1. Taking into account the microalgae productivity value previously calculated, an approximate biomass yield of 1.2 gVSS gC−1 was obtained.
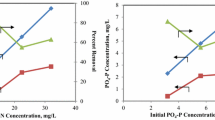
Figure 4a shows the N-NO3− concentration profiles in the three laboratory batch experiments performed with different initial nitrate concentrations. Figure 4b shows the values of the biomass specific N-NO3− removal rates (mg N gVSS−1 day−1). Assuming the typical variability of the experimental results in this type of biological processes (see dashed lines in Fig. 4b), it is possible to observe a Monod-type trend in the removal rates and, thus, it may be approximately established the range in which the specific N removal rate is maximum. Maximum N removal rate was approximately 8 mg N gVSS−1 day−1, and it began to decrease from N concentrations of approximately 18 mg L−1 (that is, approximately from day 5 in the N3 experiment) and, from that concentration, it can be assumed that N became the limiting nutrient as the liquid medium was always saturated in CO2 and P concentration was quite high (Fig. 5). N removal rates during the last days were very low and some additional days would be necessary to the complete N depletion.
Figure 5a shows the P-PO43− concentration profiles in the laboratory batch experiments while Fig. 5b shows the specific P removal rates (mg P gVSS−1 day−1). The average biomass specific P removal rate observed during the first days, that is, in the period where the maximum N removal rate was kept (no N limitations), was approximately 2.6 mg P gVSS−1 day−1. As it was previously indicated, P was not a limiting nutrient in the present work. Moreover, the buffer capacity of the BBM growth medium kept pH between 6.0 and 7.0, avoiding P precipitation (Cai et al. 2013).
From the approximate values of the nutrient removal rates previously calculated, a mass stoichiometric removal ratio IC/N/P of 100/6.8/2.2 was found. It would indicate a mass removal N/P ratio of approximately 3.1, which can be considered as a low value compared to previously reported values (N/P = 7) regarding N and P removal by microalgae (Acién Fernández et al. 2013; Ruiz et al. 2013); nevertheless, these works usually refer to ammonium nitrogen capture instead of nitrate.
Nutrient removal and microalgae production in the pilot-scale photobioreactor
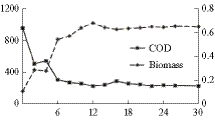
Figure 6 shows (a) the microalgae and (b) the dissolved IC concentrations in the effluent of the pilot-scale photobioreactor during the 7 months of continuous operation. According to the results obtained in the laboratory experiments, the hydraulic retention time (HRT) used in the first month of operation was 5.5 days. However, as the removal of the limiting nutrient (nitrate) was not completed (see later discussion, Fig. 7), HRT was increased to 6.0 days in the second month and, finally, to 6.5 days during the rest of the continuous operation period. It can be observed in Fig. 6a that the effluent microalgae concentration was approximately stabilized in 0.5 gVSS L−1 (horizontal line), which means an average microalgae productivity of 76 mgVSS L−1 day−1, that is approximately 12% lower than the productivity value found in laboratory (“Nutrient removal and microalgae production in laboratory” section).
The IC effluent concentration (Fig. 6b) showed significant fluctuations but an approximate average value of 85 mg L−1 (horizontal line) may be considered; it corresponded to an approximate removal efficiency of 82% with respect to the IC in the saturated liquid stream flowing into the photobioreactor from the absorption tank. Thus, an IC removal rate value of 121 mg C gVSS−1 day−1 and a biomass yield of 1.26 gVSS gC−1 were calculated, which are similar values to those found in the laboratory experiments.
The microalgae productivity values obtained in the present work could be compared to other previously reported values. We have selected previous works regarding nitrogen (mainly ammonium) and phosphate removal in secondary effluents using C. vulgaris. For instance, Ruiz et al. (2013) reported values between 40 and 170 g L−1 day−1, Gao et al. (2014) reported 10.3 g L−1 day−1, and Marbelia et al. (2014) reported 33 g L−1 day−1 using 5 days as HRT. Honda et al. (2012) reviewed microalgae productivity values between 48 and 1500 mg L−1 day−1 under different experimental conditions. Finally, Arbib et al. (2013) reported values between 600 and 800 g L−1 day−1 using again 5 days as HRT. All these reported results show great variability depending on the specific experimental conditions.
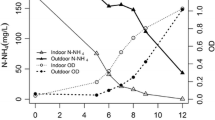
Figure 7 shows the effluent nutrient concentrations (N-NO3− and P-PO43−) during the operation of the pilot-scale photobioreactor (data points) together with the inlet N and P concentrations (horizontal lines). As previously indicated, phosphorus was always in excess, therefore nitrate being the limiting nutrient and the parameter used to decide about the HRT conditions. Effluent N-NO3− concentrations were in the range 0–25 mg L−1 throughout the first month of operation (HRT 5.5 days), which indicated that this nutrient was not completely used. On the contrary, after increasing HRT to 6.5 days, N was almost completely consumed and thus N became as limiting nutrient. According to the high P inlet concentration and the lower P capture capacity of microalgae, P removal efficiency was quite lower than N removal efficiency.
From Fig. 7, average effluent N and P concentrations were estimated, being 2.1 and 38.3 mg L−1, respectively. They corresponded to nutrient removal rates of 5.9 mg N L−1 day−1 and 1.5 mg P L−1 day−1, respectively, and biomass specific removal rates of 11.7 mg N gVSS−1 day−1 and 3.04 mg P gVSS−1 day−1. According to the IC, N, and P removal rates at the final stationary period, the mass stoichiometric removal ratio IC/N/P was calculated as 100/9.8/2.5. It means a N/P ratio of 3.9, which is higher than that obtained in the laboratory experiments (“Evolution and control of experimental conditions” section) but still lower than those formerly reported in the literature (Acién Fernández et al. 2013; Ruiz et al. 2013).
As previously stated, nitrate removal studies by microalgae are not as common as ammonium removal studies. Honda et al. (2012) reported lower growth rate values for C. vulgaris using nitrate compared to those using ammonium. Gao et al. (2014) reported that C. vulgaris did not seem to use nitrate as substrate while, in contrast, Aslan and Kapdan (2006) reported that C. vulgaris removed 510 mg N-NO3− and 29 mg P-PO43− in a 6-day batch period. Finally, Coppens et al. (2014) showed some results for different nitrate-accumulating microalgae (but not for C. vulgaris).
Scaling up implications and comparison with classical BNR techniques
The scaling up has been carried out in the present work using as main criteria to use a similar HRT in lab and pilot plant experiments. Additionally, air was fed in the same conditions, an excess of IC and P concentrations was kept, and we tried to maintain similar values of T, pH, and lighting. However, different results were obtained under both scales. The main differences observed in the pilot-scale test with respect to laboratory were a slight decrease in microalgae productivity (which caused that higher HRT were necessary) and a slight increase in N and P removal rates. So, in general, it cannot be said that scaling up of our process caused a clear efficiency decrease as reported in previous works (Ruiz et al. 2013; Van den Hende et al. 2014).
The main differences observed in the operating conditions between the two processes (laboratory and pilot scales) were the liquid flow mode and turbulence levels, the dissolved oxygen concentrations, and the lighting level. High dissolved oxygen concentrations in the pilot-scale process could have influenced negatively the microalgae performance as inhibition could appear at oxygen concentrations from 7 mg L−1 (Acién Fernández et al. 2013). An air compressor was necessary to avoid dead flow zones in the horizontal tubes which would cause even higher oxygen accumulation. Moreover, the movement induced to microalgae is quite different in both systems, reaching much lower turbulence levels in the pilot scale; in fact, Reynolds number was 25·103 in laboratory test while it was lower than 10 in the pilot plant. Arbib et al. (2013) reported that high turbulence is necessary to avoid biofouling and excessive dissolved oxygen levels. Biofouling (microalgae accumulation in the internal reactor surface) was detected only in the pilot-scale operation in the present work, which was related to the low turbulence and the high reactor specific surface (m2 m−3). Biofouling in the pilot scale caused organic waste accumulation that could eventually produce problems such as reported by Grobbelaar (2012), i.e., predators, pathogens, and alien microalgae invasion. Regarding the light intensity, since the higher specific surface of the pilot-scale photobioreactor, it could receive more light than the lab-scale reactor; in fact, light intensity was only correctly controlled in the pilot plant during the last stationary 100 days. In general, it can be said that all abovementioned factors were difficult to control and would cause the differences found between the results from laboratory and pilot scales.
Regarding the nutrient removal applications, on one hand, the current microalgae technology is clearly slower and less effective than classical BNR secondary processes but, on the other hand, it could be a more sustainable technology. Judd et al. (2015) reported the advantages and disadvantages of using microalgae instead of the classical BNR processes. According to these authors, because of the lower nutrient removal rates and the high surface (low depth) necessary for microalgae, PBR systems may be 15 times slower than classical activated sludge BNR systems, and overall there is two order of magnitude difference in footprint between them. Against this, the biomass yield is quite lower in PBR and microalgae is considered a valuable product, and they also conclude that microalgae nutrient removal is less effective but involves lower operation costs and, additionally, allows CO2 capture. Additionally, Marbelia et al. (2014) described different scenarios to combine classical wastewater treatment plants with microalgae nutrient removal and they propose approximate values of power consumption and operation costs, and they reported that conventional BNR are energy-intensive and involve extra equipment instruments which may cover 60–80% of the total energy consumption in the treatment process.
Conclusions
The results showed in this work showed that the microalgae C. vulgaris is capable to effectively remove nitrate and phosphate from a synthetic secondary effluent, with no need of organic carbon and rendering a valuable waste material. Although N and P removal rates were low compared to classical biological nutrient removal secondary processes, the use of microalgae could be considered a more sustainable technology for wastewater treatment.
Microalgae productivity values found here were similar to previous reported works. The work in a 150-L pilot plant showed the difficulties to keep an adequate control of the process variables. Nevertheless, although microalgae production was lower than those of the laboratory tests, N and P removal rates were slightly increased in the pilot plant. On summary, it can be said that scaling up of the process caused some differences with respect to the laboratory results being them mainly attributed to the differences in turbulence, dissolved oxygen concentrations, and lighting levels. These points have been identified as to be critical, and so it is considered that future efforts should be made to improve control of such factors and thus to allow studies that compare laboratory and pilot-scale systems.
References
A.P.H.A.-A.W.W.A.-W.P.C.F (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Acién Fernández FG, Fernández Sevilla JM, Molina Grima E (2013) Photobioreactors for the production of microalgae. Rev Environ Sci Biotechnol 12:131–151
Arbib Z, Ruiz J, Álvarez-Díaz P, Garrido-Pérez C, Barragán J, Perales JA (2013) Long term outdoor operation of a tubular airlift pilot photobioreactor and a high rate algal pond as tertiary treatment of urban wastewater. Ecol Eng 52:143–153
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70
Cabanelas ITD, Ruiz J, Arbib Z, Chinalia FA, Pérez CG, Rogalla F, Nascimento IA, Perales JA (2013) Comparing the use of different domestic wastewaters for coupling microalgae production and nutrient removal. Bioresour Technol 131:429–436
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev 19:360–369
Coppens J, Decostere B, Van Hulle S, Nopens I, Vlaeminck SE, De Gelder L, Boon N (2014) Kinetic exploration of nitrate-accumulating microalgae for nutrient recovery. Appl Microbiol Biotechnol 98:8377–8387
Duarte JH, Fanka LS, Costa JAV (2016) Utilization of simulated flue gas containing CO2, SO2, NO and ash for Chlorella fusca cultivation. Bioresour Technol 214:159–165
Ekama GA (2015) Recent developments in biological nutrient removal. Water SA 41:515–524
Frumento D, Aliakbarian B, Casazza AA, Converti A, Al Arni S, da Silva MF (2016) Chlorella vulgaris as a lipid source: cultivation on air and seawater-simulating medium in a helicoidal photobioreactor. Biotechnol Prog 32:279–284
Gao F, Yang ZH, Li C, Wang YJ, Jin WH, Deng YB (2014) Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresour Technol 167:441–446
Grobbelaar JU (2012) Microalgae mass culture: the constraints of scaling-up. J Appl Phycol 24:315–318
Honda R, Boonnorat J, Chiemchaisri C, Chiemchaisri W, Yamamoto K (2012) Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Bioresour Technol 125:59–64
Judd S, van den Broeke LJP, Shurair M, Kuti Y, Znad H (2015) Algal remediation of CO2 and nutrient discharges: a review. Water Res 87:356–366
Lam MK, Lee KT, Khoo CG, Uemura Y, Lim JW (2016) Growth kinetic study of Chlorella vulgaris using lab-scale and pilot-scale photobioreactor: effect of CO2 concentration. J Eng Sci Tech 7:73–87
Marbelia L, Bilad MR, Passaris I, Discart V, Vandamme D, Beuckels A, Muylaert K, Vankelecom IFJ (2014) Membrane photobioreactors for integrated microalgae cultivation and nutrient remediation of membrane bioreactors effluent. Bioresour Technol 163:228–235
Odjadjare EC, Mutanda T, Olaniran AO (2017) Potential biotechnological application of microalgae: a critical review. Crit Rev Biotechnol 37:37–52
Ruiz J, Arbib Z, Álvarez-Díaz PD, Garrido-Pérez C, Barragán J, Perales JA (2013) Photobiotreatment model (PhBT): a kinetic model for microalgae biomass growth and nutrient removal in wastewater. Environ Technol 34:979–991
Van Den Hende S, Beelen V, Bore G, Boon N, Vervaeren H (2014) Up-scaling aquaculture wastewater treatment by microalgal bacterial flocs: from lab reactors to an outdoor raceway pond. Bioresour Technol 159:342–354
Zeng X, Guo X, Su G, Danquah MK, Zhang S, Lu Y, Sun Y, Lin L (2015) Bioprocess considerations for microalgal-based wastewater treatment and biomass production. Renew Sust Energ Rev 42:1385–1392
Acknowledgments
The author would like to thank the company C.T. Ingenieros for funding this research and the students Rosa M. Sánchez, Pedro Capilla, Antonio J. Expósito, and Juan L. Lillo for their assistance in carrying out the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Bingcai Pan
Electronic supplementary material
ESM 1
(DOCX 137 kb)
Rights and permissions
About this article
Cite this article
Villaseñor Camacho, J., Fernández Marchante, C.M. & Rodríguez Romero, L. Analysis of a photobioreactor scaling up for tertiary wastewater treatment: denitrification, phosphorus removal, and microalgae production. Environ Sci Pollut Res 25, 29279–29286 (2018). https://doi.org/10.1007/s11356-018-2890-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2890-5