Abstract
Rivers may receive pharmaceuticals, personal care products, and environment estrogens, which are emerging concerns, from various sources. Understanding the fate of these emerging contaminants (ECs) from the sources to their receiving river is important for assessing their ecosystem risk. Here, the occurrence, seasonal variation, spatial distribution, and ecological risk of 22 ECs in water and sediments from the Jilin Songhua River, as well as in the effluents from the riverside Jilin wastewater treatment plant (WWTP) were investigated. Results indicated that estriol with the highest median concentration of 21.5 ng L−1 in the river water and with the highest median concentration of 481.5 ng g−1 in the sediments, and methylparaben with the highest concentration of 29.6 ± 2.9 ng L−1 in the WWTP effluents were the predominant contaminants. The total concentration of ECs in the river water in the dry season was about 1.5 times higher than that in the wet season. The concentrations of these ECs close to the contaminated tributary and the WWTP were relatively high. Risk assessment showed that the maximum risk quotient value of estrone was 1.07 in the river water and estriol was 2.10 in the effluents. In addition, erythromycin posed generally medium risk in the river water and WWTP effluents. It should be paid attention to the prior control of the three contaminants in the river region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Regarding emerging contaminants (ECs), the use of pharmaceuticals is for both human and veterinary treatment. Personal care products (PCPs) are increasingly used for human applications. Environment estrogens (EEs) are secreted by livestock and poultry. Their discharge has gradually increased due to the global economic development. In recent years, the ECs have been widely detected in different aquatic environmental compartments with concentrations ranging from ng L−1 to μg L−1 in aqueous matrices and ng g−1 to μg g−1 in solid matrices (Moreno-Gonzalez et al. 2015; Gong et al. 2016; Peng et al. 2017). ECs access the river water by various ways, but their main input is through treated and untreated wastewater discharges, which the result of that conventional wastewater treatment plants (WWTPs) are unable to completely remove these contaminants (Wang and Wang 2016). In addition, these ECs could also enter into the natural waters through the agricultural runoff (Ali et al. 2018). The continuous discharge could cause potential resistance genes to develop, endocrine disruption, and result in long-term potential risk to the aquatic ecosystems (Arlos et al. 2018; Yi et al. 2017; Archer et al. 2017).
The occurrence and ecological risk of ECs including pharmaceuticals, PCPs, and EEs in major river systems in China have been widely investigated in recent years (Zhang et al. 2015; Pan and Chu 2018; Li et al. 2018). However, the investigation of ECs in Songhua River, the third major river in China, is scarce. The Songhua River is located in northeastern China which is the earliest industrial base of China. The pollution incidents of toxic pollutants including heavy metal mercury and nitrobenzene in the river once attracted the most public attention. In recent decades, with the production process transformation of the riverside chemical industrial companies and the implementation of pollution control measures since 2006, the ecological risk of these toxic pollutants in the Songhua River is decreasing year by year (Wang et al. 2012; Dong et al. 2016a,b; Meng et al. 2016). However, the ecological risk presented by the ECs is still unknown.
The goals of this study were to (1) investigate the occurrence, seasonal variation, and spatial distribution of 22 ECs including 17 pharmaceuticals, 3 EEs, and 2 PCPs in both river water and surface sediments of the 500-km Songhua River in Jilin Province; (2) evaluate the relationship between the ECs in riverside WWTP effluents and the receiving river water; and (3) assess the ecological risk of these ECs in the river water and WWTP effluents.
Materials and methods
Chemicals and reagents
Standards of 22 ECs were purchased from Dr. Ehrenstorfer GmBH (Augsburg, Germany). They include 17 pharmaceuticals, 3 EEs, and 2 PCPs. Seventeen pharmaceuticals including three categories: 12 antibiotics including sulfamethoxazole (SMX), sulfamonomethoxine (SMM), sulfaquinoxaline (SQX), norfloxacin (NOR), ciprofloxacin (CIP), enrofloxacin (ENR), ofloxacin (OFL), erythromycin (ETM), amoxicillin (AMX), doxycycline (DC), florfenicol (FFC), lincomycin (LIN), ibuprofen (IBP), naproxen (NPX), acetaminophen (AP), diclofenac (DLF), caffeine (CAF); 3 EEs include estrone (E1), estradiol (E2), and estriol (E3), and 2 PCPs including triclosan (TCS) and methylparaben (MPB). Their some physicochemical properties are listed in Table S1 in Supplementary Material. 13C3-caffeine, 13C3-ibuprofen, 13C2-estrone, and 13C12-triclosan purchased from BOC Sciences (Shirley, NY, USA) and 13C6-sulfamethazine purchased from Cambridge Isotope Labs (Andover, MA, USA) were used as surrogate standards. Oasis HLB solid-phase extraction (SPE) cartridges (200 mg and 6 mL for water sample extraction and 500 mg and 6 mL for sediment extraction) were obtained from Waters Corporation (Milford, MA, USA). Chromatography-grade methanol and formic acid were obtained from Merck (Darmstadt, Germany). Ultrapure water was prepared using a Milli-Q water purification system (Millipore Corporation, USA). Stock solutions (100 mg L−1) of individual ECs and the surrogate standards were prepared in methanol. All stock solutions were stored in the dark at − 20 °C prior to use. Working solutions were prepared daily from the stock solutions by serial dilution to prevent their instability from affecting the experiment.
Sampling sites and sample collection
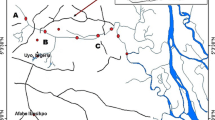
The distribution of sampling sites in the Jilin Songhua River is shown in Fig. 1. The basic information of the sampling sites is listed in Tables S2. River water and sediment samples were collected from ten sites along the Jilin Songhua River from upstream (S1) to downstream (S10), and effluent samples were collected from the riverside Jilin WWTP (W) in July (wet season) and October (dry season) 2017. Site S1 is located downstream Baishan reservoir. S2 is located in the main stream after merging by the Huifa River which is the largest tributary of Jilin Songhua River. The Huifa River flows 1.4 × 104 km2 area basin. S3 is located downstream Songhua Lake and upstream Jilin City. W represents the WWTP of Jilin City. The inhabitant quantity served by the WWTP is about 1.52 M, and its wastewater treatment capacity is 4.5 × 104 m3 day−1. S4 and S5 are located in about 3 and 45 km downstream the WWTP, respectively. S6, S7, S8, and S10 are located in the suburban region. S9 is located in downstream Songyuan City. The river flow near site S8 was 1592 and 245 m3 s−1 in the July and October sampling events, respectively (http://www.slswxx.cn/). During the wet season in the study, the monthly average amount of precipitation was 148.0 mm, 15% more than normal year. During the dry season, the monthly average amount of precipitation was 11.8 mm, 9% less than normal year (http://www.weather.com.cn/). Overlying water (0.5 m depth) and surface sediments (top 10 cm) were collected. Grab effluent samples were collected from the riverside WWTP discharge channel. All samples were collected in duplicate from each site. The collected water and sediment samples were stored in an icebox and transported to the laboratory, and were immediately pretreated for the experiment. The pH and dissolved oxygen (DO) of the water samples were measured in situ, and the total organic carbon (TOC) contents of the sediment samples were measured by a TOC analyzer (TOC-L, Shimadzu, Japan) in the laboratory. These data are listed in Table S3.
Sample pretreatment, instrumental analysis, and quality assurance
All liquid samples including the river water and WWTP effluents (500 mL) spiked with 50 ng of the surrogate standard (13C6-sulfamethazine) were filtered through 0.45-μm glass fabric filters before extraction, followed sulfuric acid (1 mol L−1) to adjust the pH to 4.0 and 0.2 g of Na2EDTA. The samples were extracted using Oasis HLB SPE cartridges. Each cartridge was sequentially conditioned with 6.0 mL of methanol and 6.0 mL of Milli-Q water prior to use, and the water samples were loaded at a flow rate of 10 mL min−1. Following this, the extraction cartridge was rinsed with 6 mL of Milli-Q water to remove excess Na2EDTA and dried under a negative-pressure nitrogen stream. After drying, the cartridge was eluted from its case with 6.0 mL of methanol. The eluates were almost dried at 30 °C in a water bath with a gentle nitrogen stream, and 50 ng of per internal standard was added (13C3-caffeine, 13C3-ibuprofen, 13C2-estrone, and 13C12-triclosan). The residues detected by positive and negative mode were dissolved with methanol/0.1% formic acid water (15:85, v/v) and methanol-water (10:90, v/v) to the volume of 1.0 mL, respectively. The final extracts were filtered through a 0.22-μm glass fabric filter into 2-mL amber vials for liquid chromatography by a tandem mass spectrometry system (LC-MS/MS) analysis. Sediment samples were pretreated by ultrasound-assisted extraction and SPE. Firstly, 5 g of the sediment was freeze-dried and passed through a 74-μm sieve. They were then weighed in a 50-mL centrifuge tube with 50 ng of 13C6-sulfamethazine as a standard substitute and 0.4 g of Na2EDTA. The sediment samples were then mixed for 2 min and then received 30 mL of methanol before undergoing ultrasonic extraction in a water bath at 30 °C for 20 min. The extracts were centrifuged at 10,000 rpm for 2 min to collect the supernatant, which was transferred to a 50-mL centrifuge tube. The ultrasonic extraction and centrifuging procedure was repeated twice. The combined extracts were evaporated on a rotary concentrator (110 Pa, 30 °C, r = 120 rpm) to concentrate the solution to 10 mL, which was then diluted to 500 mL with Milli-Q water. The following steps were the same as the SPE procedure of water samples.
The 22 ECs were identified on a LC-MS/MS (API 4000, Applied Biosystems, USA). ECs were separated on a Dionex C18 column (2.1 × 150 mm, 3 μm particle size). Ten microliters of the extract were automatically injected, and the LC oven temperature was 25 °C during sample analysis. Gradient conditions were applied at a 0.20-mL min−1 flow rate. Analyses were conducted in the negative mode for 8 of the compounds (all NSAIDs, PCPs, and FFC) and the positive mode for the other 14 compounds. The conditions of positive and negative mode phases and gradient elution are listed in Tables S4 and S5. The positive mobile phase gradient elution was as follows: the initial gradient was 15% of methanol for 1.0 min, which increased to 25% from 1.0–3.0 min and held for 1 min. It increased to 85% from 4.0 to 13.0 min, remained at 85% until 14.0 min, decreased to 15% from 14.0 to 14.1 min, and then remained at 15%, with a total runtime of 19 min. The negative mobile phase gradient elution was as follows: the initial gradient was 10% of methanol for 3.0 min, which increased to 90% from 3.0 to 8.0 min, remained at 90% until 12.0 min, decreased to 10% from 12.0 to 12.1 min, and then remained at 10%, with a total runtime of 17.0 min. The instrument parameters were as follows: ion spray voltage − 3500 V; curtain gas 45 psi (0.31 MPa); nebulizer gas 35 psi (0.24 MPa); auxiliary gas 40 psi (0.28 MPa); collision gas 6.0 psi (0.15 MPa); source temperature 550 °C. The mass spectrometer was operated in the multiple reaction monitoring (MRM) mode. The ion selection parameters are listed in Table S6.
For quality assurance and control, the recoveries of each spiked sample, method detection limit (MDL), method quantification limit (MQL), field blank, matrix spike recoveries, and duplicate samples were analyzed. MDL and MQL were calculated based on a signal to noise ratio (S/N) of 3 to 10. The MQL was 0.3–3.3 ng L−1 for the liquid samples and 0.5–6.5 ng g−1 for the sediments (Table S7). All EC compounds in the blanks fell below the MQL. Sufficient recoveries were achieved for all ECs, ranging between 66.2 and 109.2%, and all concentrations were not recovery corrected. The precision of the method represented by the relative standard deviation (RSD) percentage of the spiked measurements was 4.1–15.3%.
Data analyses
The risk quotient (RQ) was used to evaluate the ecological risk of the ECs. The equation for calculating the RQMEC conducted by the measured environment concentrations (MEC) of ECs is as follows:
where MEC is the measured environmental concentration, and PNEC is the predicted no-effect concentration. In this study, the lowest PNEC values of ECs in water were obtained from the literatures which are listed in Table S1. When the RQ exceeds 1.0, the risk is high. When the RQ ranges from 0.01 to 0.1 and 0.1 to 1, the risks are low and medium, respectively (Hernando et al. 2006).
The weighted measured environment concentration (WMEC) was calculated as follows:
where Cave and Cmax are the average and maximum concentrations, respectively; Rtot is the detection frequency (%); and R90%max is the ratio of the number of samples with concentrations exceeding the 90% maximum concentration to the total number of samples (Ben et al. 2018). Hence, RQWMEC conducted by weighted measured environment concentrations (WMEC) is calculated as follows:
Statistical analyses were all conducted using IBM SPSS Statistics (version 22.0) and OriginPro (version 8.0) to identify significant differences and relationships of the ECs (p < 0.05).
Results and discussion
Occurrence of the ECs in the river water, sediments, and WWTP effluents
As shown in Table 1, 21 ECs were detected at least once either in the samples of river water, sediments, and WWTP effluents. Only NOR was never detected in any sample. In the river water, E3 was the most frequently detected compounds in 80 and 100% of the samples in the wet and dry season, respectively. CAF showed the second highest detection frequency of 100% in the wet season and 70% in dry season, followed by MPB, with the detection frequency of 100% in the wet season and 60% in dry season. For the other detected ECs, the minimal detection frequency was 20% (TCS) in the wet season, and 20% (CIP, OFL, and TCS) in the dry season. The highest maximum concentration was 26.9 ng L−1 (E3) in the wet season and 37.3 ng L−1 (E1) in the dry season. The median concentrations ranged from quantification limits to 18.5 and 21.5 ng L−1 (E3) in the wet and dry season, respectively. Pharmaceuticals were detected at different frequencies and concentrations. In the wet season, CAF was found to be the compound with the highest maximum concentration of 26.3 ng L−1 and highest median concentration of 6.8 ng L−1, followed by DC with the maximum concentration of 15.3 ng L−1 and median concentration of 4.2 ng L−1, whereas SMX was below their quantification limits. In the dry season, comparable median concentrations were observed for CAF with 6.0 ng L−1, DC with 9.3 ng L−1, and SMX with 7.4 ng L−1, respectively. For the three EEs, the higher maximum concentration of E1 was 37.3 ng L−1 in the dry season, E2 was 16.1 ng L−1 in the dry season, and E3 was 29.4 ng L−1 in the dry season. For PCPs, MPB were detected at high median concentrations of 6.0 ng L−1 in the wet season, while low median concentrations of 1.3 ng L−1 in the dry season. TCS had the same mean concentrations of 0.2 ng L−1 in both wet and dry seasons.
In the sediments, SMX, SMM, SQX, AMX, and LIN were not detected. Among all the 22 ECs, AP and MPB were the most frequently detected compounds in 100% of the samples in the wet and dry season, respectively. CIP and IBP showed the second highest detection frequency of 100% in the wet season and 90% in dry season. For the other detected ECs, the minimal detection frequency was 30% (DC and TCS) in the wet season and 20% (TCS) in the dry season. The highest maximum concentration was 745.9 and 517.7 ng g−1 (E3) in the wet and dry season, respectively. The median concentrations ranged from quantification limits to 481.5 and 138.2 ng g−1 (E3) in the wet and dry seasons, respectively. Pharmaceuticals were also detected at different frequencies and concentrations. In the wet season, IBP was found to be the compound with the highest maximum concentration of 95.0 ng g−1 and median concentration of 38.2 ng g−1, followed by ETM with the maximum concentration of 63.8 ng g−1 and the median concentration of 13.3 ng g−1. DLF had the maximum concentration of 50.6 ng g−1 and highest median concentration of 40.2 ng g−1. In the dry season, comparable median concentrations were observed for IBP with 7.5 ng g−1, ETM with 7.8 ng g−1, and DLF with 20.0 ng g−1, respectively. For three EEs, the higher maximum concentration of E1 was 492.5 ng g−1 in the dry season, E2 was 391.1 ng g−1 in the dry season, and E3 was 745.9 ng g−1 in the wet season. For PCPs, MPB were detected at high median concentrations of 27.7 ng g−1 in the wet season, while low median concentrations of 11.5 ng g−1 in the dry season. TCS had the same maximum concentrations of 2.1 ng g−1 in both wet and dry seasons.
In the WWTP effluents, 20 out of the 22 surveyed ECs were detected, while only the concentrations of NOR and LIN were below the MQL. The highest detected concentration was 29.6 ± 2.9 ng L−1 (MPB) in the wet season and 22.4 ± 2.2 ng L−1 (SMX) in dry season. For pharmaceuticals, in the wet season, SMX was found to be the compound with the highest detected concentration of 17.0 ± 1.6 ng L−1, followed by AMX with the concentration of 14.1 ± 1.4 ng L−1, CAF with the concentration of 11.9 ± 1.2 ng L−1. In the dry season, comparable the detected concentrations were observed for SMX with 22.4 ± 2.2 ng L−1, AMX with 8.9 ± 0.9 ng L−1 and CAF with 7.3 ± 0.7 ng L−1, respectively. For the three EEs, the higher detected concentration of E1 was 5.2 ± 0.5 ng L−1 in the dry season, E2 was 18.7 ± 1.8 ng L−1 in the dry season, and E3 was 15.9 ± 1.5 ng L−1 in the dry season. For PCPs, MPB were detected at high concentrations of 29.6 ± 2.9 ng L−1 in the wet season, while low concentrations of 12.5 ± 1.2 ng L−1 in the dry season. These were much higher than the concentrations of TCS with 1.5 ± 0.1 ng L−1 in the wet season and 2.0 ± 0.2 ng L−1 in the dry season.
Summarily, E3 with the highest median concentration of 21.5 ng L−1 in the dry season were the predominant contaminant in the river water, E3 with the highest median concentration of 481.5 ng g−1 in the wet season were predominant in the sediments, and MPB with the highest concentration of 29.6 ± 2.9 ng L−1 in the wet season were predominant in the WWTP effluents. It was found that the concentration of MPB was 151.0 ng L−1 in the WWTP effluents located in the Pearl River region (Yang et al. 2017). It could indicate that the usage and discharge of MPB in the Songhua River region in the northeastern China were much lower than those in the Pearl River region in the southern China.
Seasonal variation of the ECs in the river water, sediments, and WWTP effluents
The concentrations of ECs in the river water and sediments at different sampling sites, as well as the WWTP effluents in the wet and dry seasons are shown in Fig. 2. In the river water, the total concentrations of these ECs (ΣECs) ranged from 64.3 to 157.7 ng L−1 with the mean value of 115.0 ng L−1 among the ten sampling sites in the dry season, while it ranged from 11.2 to 127.1 ng L−1 with the mean value of 78.7 ng L−1 in the wet season. The mean ΣECs in the river water in the dry season were about 1.5 times higher than that in the wet season. In the sediments, the ΣECs ranged from 146.3 to 1196.3 ng g−1 with the mean value of 626.6 ng g−1 in the dry season, while it ranged from 170.5 to 1186.6 ng g−1 with the mean value of 831.0 ng g−1 in the wet season. The mean ΣECs in the sediments in the dry season were lower than that in the wet season. In the WWTP effluents, the ΣECs were 169.1 ng L−1 in dry season and 152.5 ng L−1 in wet season. There was no obvious difference between the two seasons which indicated the treatment effectiveness for these ECs in the WWTP was relatively stable.
Calculated on the hydrology and meteorology data described in the above section, the monthly average amount of precipitation during the wet season was 12.5 times higher than that during the dry season, and the river flow in the wet season was 6.5 times higher than that during the dry season. The seasonal variation of the most ECs concentrations in the river water could be attributed to the rainfall and river flow (Fang et al. 2017; Proia et al. 2016).
Spatial distribution of the ECs in the river water and sediments
The ΣECs exhibited obvious spatial differences among the ten sampling sites in the river water and sediments (Fig. 2). In the river water, the highest ΣECs were 157.7 ng L−1 at S4 in dry season and 122.9 ng L−1 at S2 in wet season. In the sediments, the highest ΣECs were1196.3 ng g−1 at S4 in dry season, and 1186.6 ng g−1 at S2 in wet season. Sites S2 and S4 showed relatively high concentrations of the ECs. The difference of pollutant concentrations among different sampling sites in rivers could be influenced by various factors, such as river flow, distance with point and non-point sources (Chen et al. 2018; Li et al. 2017a,b; Wu et al. 2016). Site S2 is located downstream Huifa River, which is the largest tributary of the Jilin Songhua River. The Huifa River runs through 13 small- and medium-sized towns with high-intensive agricultural activities. The agricultural runoff, industrial and domestic wastewater from riverside towns could lead to higher concentration of the ECs in it. The Huifa River could be the main contributor of the ECs in site S2. Site S4 is located downstream the WWTP of Jilin City. The higher concentrations of the ECs in S4 should be related to the discharge of the WWTP effluents. It was also found that the higher concentration level of ECs in the mainstream of Haihe River was also affected by the merging of several polluted tributaries and the discharge of riverside WWTP effluents (Chen et al. 2018; Dai et al. 2016). So, it could be proposed that the investigation of ECs in contaminated tributaries and the riverside WWTP effluents in the major rivers in China need to pay attention.
In addition, in order to understand the relationship of ECs between the WWTP effluents and the receiving water, the Spearman correlation analysis was conducted among individual EC concentrations in the WWTP effluents and the river water downstream sampling sites S4 and S5. The calculated correlation coefficients of individual EC concentrations in the WWTP effluents and the river water in S4 were 0.48 and 0.63 (p < 0.01) in wet and dry seasons, respectively. However, the calculated values between the effluents and S5 showed no correlation (p > 0.01). It indicated that the discharge of WWTP effluents were one of the main sources of these ECs for the receiving river, but its effects could be limited. The similar results were also reported in the study on the Huangpu River (Wu et al. 2016).
Ecological risk assessment of the ECs in the river water and WWTP effluents
The RQ values of the 22 ECs in the Jilin Songhua River water and the WWTP effluents were calculated (Figs. 3 and 4). The PNECs for ECs were obtained from reported literature using the most sensitive aquatic species based on their chronic toxicity data (Table S1). In the river water in the wet season, the RQMEC value of E1, E3, OFL, ETM, LIN, and MPB were 0.24, 0.25, 0.12, 0.18, 0.11, and 0.19, respectively. It indicated that 27.3% of these ECs in the river water in the wet season showed medium risk (0.1 < RQMEC-wet < 1). The RQWMEC values of E1, E3, and MPB were 0.13, 0.20, and 0.19, respectively. It indicated that 13.6% of these ECs in the river water in the wet season showed medium risk (0.1 < RQWMEC-wet < 1). In the dry season, the RQMEC value of E1, E3, ETM, and DC were 0.38, 0.49, 0.18, and 0.15, respectively. It indicated that 18.2% of the ECs in the river water in the dry season showed medium risk (0.1 < RQMEC-dry < 1). The RQWMEC value of ETM, E1, and E3 were 0.15, 0.24, and 0.49, respectively. It indicated that 13.6% of these ECs in the river water in the dry season showed medium risk (0.1 < RQWMEC-dry < 1).
Among the ten sampling sites, more types of these ECs showed medium or high risk at S2 and S4. E1 posed high risk at S2 with the RQ value of 1.07 in the dry season. Seven out of 22 ECs posed medium risk at S2 and S4 in the wet season and 6 out of 22 ECs posed medium risk at S2 and S4 in the dry season. Among them, ETM, E1, and E3 in the river water at S2 and S4 in the two seasons posed medium risk. In the WWTP effluents, E3 posed high risk with the maximum RQ value of 2.10 in the dry season. AMX, ETM, DC, DLF, E1, and MPB posed medium risk in the dry season and AMX, SMX, ETM, E1, E3, and MPB posed medium risk in the wet season. Summarily, ETM, E1, and E3 posed medium or high risk in the river water and WWTP effluents. It was reported that ETM also posed medium risk in the heavily-polluted Haihe River, and E1 and E3 posed medium risk in Yangtze River (Chen et al. 2018; Nie et al. 2015). Compared with the two major rivers in China, the ecological risk of ETM, E1, and E3 in the Jilin Songhua River were at the same or higher level. Therefore, the potential ecological risk and associated adverse effects of the three ECs in this river may need to pay attention.
Conclusions
The occurrence, seasonal variation, spatial distribution, and ecological risk of 22 ECs in water and sediments from the Jilin Songhua River, as well as in Jilin WWTP effluents were investigated for the first time. Results indicated that E3 were the dominant contaminants in the river water and sediments, and MPB was the dominant contaminants in the WWTP effluents of Jilin City. The total concentration of ECs in the river water in the dry season was about 1.5 times higher than that in the wet season, but the total concentration of ECs in sediments in the dry season was lower than that in the wet season. The concentrations of these ECs close to the contaminated tributary and the WWTP were relatively high. Risk assessment showed that ETM, E1, and E3 posed medium or high risk in the river water and Jilin WWTP effluents. So, the three ECs should be preferentially controlled.
References
Ali JM, D'Souza DL, Schwarz K, Allmon LG, Singh RP, Snow DD, Bartelt-Hunt SL, Kolok AS (2018) Response and recovery of fathead minnows (Pimephales promelas) following early life exposure to water and sediment found within agricultural runoff from the Elkhorn River, Nebraska, USA. Sci Total Environ 618:1371–1381
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTP and environmental waters. Chemosphere 174:437–446
Arlos MJ, Parker WJ, Bicudo JR, Law P, Marjan P, Andrews SA, Servos MR (2018) Multi-year prediction of estrogenicity in municipal wastewater effluents. Sci Total Environ 610:1103–1112
Ben WW, Zhu B, Yuan XJ, Zhang Y, Yang M, Qiang ZM (2018) Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: comparison of wastewater treatment processes. Water Res 130:38–46
Chen HY, Jing LJ, Teng YJ, Wang JS (2018) Characterization of antibiotics in a large-scale river system of China: occurrence pattern, spatiotemporal distribution and environmental risks. Sci Total Environ 618:409–418
Dai GH, Wang B, Fu CC, Dong R, Huang J, Deng SB, Wang YJ, Yu G (2016) Pharmaceuticals and personal care products (PPCPs) in urban and suburban rivers of Beijing, China: occurrence, source apportionment and potential ecological risk. Environ Sci Process Impacts 18:445–455
Dong DM, Liu XX, Hua XY, Guo ZY, Li LF, Zhang LW, Xie YJ (2016a) Sedimentary record of polycyclic aromatic hydrocarbons in Songhua River, China. Environ Earth Sci 75:508
Dong DM, Zhang LW, Liu S, Guo ZY, Hua XY (2016b) Antibiotics in water and sediments from Liao River in Jilin Province, China: occurrence, distribution, and risk assessment. Environ Earth Sci 75:1202
Fang HS, Zhang Q, Nie XP, Chen BW, Xiao YD, Zhou QB, Liao W, Liang XM (2017) Occurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 173:99–106
Gong J, Duan DD, Yang Y, Ran Y, Chen DY (2016) Seasonal variation and partitioning of endocrine disrupting chemicals in waters and sediments of the Pearl River system, South China. Environ Pollut 219:735–741
Hernando M, Mezcua M, Fernandez-Alba AR, Barcelo D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Li N, Tian Y, Zhang J, Zuo W, Zhan W, Zhang J (2017a) Heavy metal contamination status and source apportionment in sediments of Songhua River Harbin region, Northeast China. Environ Sci Pollut Res 24:3214–3225
Li RL, Liang J, Gong ZB, Zhang NN, Duan HL (2017b) Occurrence, spatial distribution, historical trend and ecological risk of phthalate esters in the Jiulong River, Southeast China. Sci Total Environ 580:388–397
Li S, Shi WZ, Liu W, Li HM, Zhang W, Hu JR, Ke YC, Sun WL, Ni JR (2018) A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci Total Environ 615:906–917
Meng FS, Wang YY, Zhang LS, Cheng PX, Xue H, Meng DB (2016) Organic pollutant types and concentration changes of the water from Songhua River, China, in 1975-2013. Water Air Soil Pollut 227:214
Moreno-Gonzalez R, Rodriguez-Mozaz S, Gros M, Barcelo D, Leon VM (2015) Seasonal distribution of pharmaceuticals in marine water and sediment from a mediterranean coastal lagoon (SE Spain). Environ Res 138:326–344
Nie MH, Yan CX, Dong WB, Liu M, Zhou JL, Yang Y (2015) Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter, and sediments of the Yangtze estuary. Chemosphere 127:109–116
Pan M, Chu LM (2018) Occurrence of antibiotics and antibiotic resistance genes in soils from wastewater irrigation areas in the Pearl River Delta region, southern China. Sci Total Environ 624:145–152
Peng FJ, Pan CG, Zhang M, Zhang NS, Windfeld R, Salvito D, Selck H, Van den Brink PJ, Ying GG (2017) Occurrence and ecological risk assessment of emerging organic chemicals in urban rivers: Guangzhou as a case study in China. Sci Total Environ 589:46–55
Proia L, von Schiller D, Sanchez-Melsio A, Sabater S, Borrego CM, Rodriguez-Mozaz S, Balcazar JL (2016) Occurrence and persistence of antibiotic resistance genes in river biofilms after wastewater inputs in small rivers. Environ Pollut 210:121–128
Wang C, Feng YJ, Zhao SS, Li BL (2012) A dynamic contaminant fate model of organic compound: a case study of nitrobenzene pollution in Songhua River, China. Chemosphere 88:69–76
Wang JL, Wang SZ (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J Environ Manag 182:620–640
Wu MH, Que CJ, Xu G, Sun YF, Ma J, Xu H, Tang L (2016) Occurrence, fate and interrelation of selected antibiotics in sewage treatment plants and their receiving surface water. Ecotoxicol Environ Saf 132:132–139
Yang YY, Liu WR, Liu YS, Zhao JL, Zhang QQ, Zhang M, Ying GG (2017) Suitability of pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) as wastewater indicators in the Pearl River Delta, South China. Sci Total Environ 590:611–619
Yi XZ, Tran NH, Yin TR, He YL, Gin KYH (2017) Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res 121:46–60
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 21577047 and 21307041), Scientific Research Project of Environmental Protection Bureau of Jilin Province (No. 2017-15), and the 111 Project (No. B16020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Ester Heath
Electronic supplementary material
ESM 1
(DOC 264 kb)
Rights and permissions
About this article
Cite this article
He, S., Dong, D., Zhang, X. et al. Occurrence and ecological risk assessment of 22 emerging contaminants in the Jilin Songhua River (Northeast China). Environ Sci Pollut Res 25, 24003–24012 (2018). https://doi.org/10.1007/s11356-018-2459-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2459-3