Abstract
São Domingos belongs among the most important historic Iberian Pyrite Belt Cu mines. The anthrosoil is contaminated by a very high content of heavy metals and metalloids. The study was focused on evaluating the interaction of some chemical elements (Ca, Mg, Fe, Mn, Cu, Pb, Zn, Ag, Cd, Ni, Co, As, Sb) in the system soil vs. five autochthonous dominant plant species: Pinus pinaster Aiton, Quercus rotundifolia Lam., Agrostis sp., Juncus conglomeratus L. and Juncus effusus L. The plants are heavily contaminated by Cu, Pb, As and Zn. The bioconcentration factor proved that they exhibit features of metal tolerant excluders. The trees are accumulators of Ag, whereas the graminoids are hyper-accumulators of Ag and Juncus effusus of Co. The translocation factor confirmed that the selected elements are immobilised in the roots except for Mn and Zn in Pinus pinaster and Mn in Quercus rotundifolia and Juncus conglomeratus. The bioaccumulation of Mn, Zn and Cu at low pH increases. The increased content of Ca and Mg in the soil inhibits, in the case of some metals and metalloids, their intake to plants. Although the studied plants, despite their fitness and vitality at the contaminated sites, are not suitable for phytoextraction (except Co and Ag), they can be used for phytostabilisation at the mining habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The São Domingos mine was exploited since the Roman Age and later, between 1857 and 1966, by the Mason and Barry Company. The ore body is formed by a sub-vertical E-W direction body, associated with black shales and volcanic felsic and basic rocks of the IPB volcano–sedimentary complex (Strunian to Upper Visean age; Matos et al. 2006, 2008; Fig. 1). These rocks are surrounded by thrust faults, SW-vergent, by shales and quartzites of the Phyllite–Quartzite Formation of the Famennian epoch. The mineralisation is represented by massive sulphide and stockwork ore (pyrite, chalcopyrite, sphalerite, galena, tetrahedrite, arsenopyrite and sulphosalts). Intense oxidation and supergene enrichment are related to deposit erosion and represented by haematite and limonite gossan and a covellite/chalcocite zone. The mining products consisted of pyrite, roasted pyrite, sulphur and copper. Native copper was obtained by cementation from ore leaching at the Moitinha plateaus (Matos et al. 2006). Related to the mining process, intense acid mine drainage occurred and large areas are occupied by different mine wastes (Quental et al. 2003; Matos 2004). The extractive activities affected a total area of 3,076,900 m2, from the São Domingos to the Chumbeiro dam, located 11 km downstream. A total of 14.7 Mm3 of mining waste has been estimated. The São Domingos mining waste related with the exploitation of the gossan open pit is dispersed in the northern mine area, near the old open pit and close to the village of São Domingos. Evaluated by the Conasa company, the total inferred mineral resource is estimated at 2.38 Mt of non-conditioned volumes, with an average grade of 0.77 g t−1 gold and 8.26 g t−1 silver, totalling a metal content of 59.489 oz t gold and 633.488 oz t silver (Matos 2004; Álvarez-Valero et al. 2008, Vieira 2015, Vieira et al. 2015). According to these authors, considering non-conditioned mine waste (including tailings located in urban areas), a total of 4.0 Mt of waste is present in the mine’s northern area, with an average grade of 0.64 g t−1 Au and 7.30 g t−1 Ag.
Surrounding the São Domingos Cu deposit is one of the most contaminated localities by mining activity in all of Europe, helping to enable the study on the impact of heavy metals on country components.
The soil profile is poorly developed, showing commonly < 1 m profiles as is usual in the south Portugal Alentejo region. The parental rocks of the soils are as follows: shales, quartzites and volcanic rocks in the northern area and shales and greywackes in the southern area. The anthrosoil composition is intimately also connected with the spoil material, strongly affected by primary and secondary ore minerals, whose decomposition saturates the country by high heavy metal content and produces great acidity levels.
In the mining area, acid mine drainage is the most important element mobility factor (Abreu et al. 2010; Bálintová et al. 2012; Batista et al. 2012b; Holub et al. 2015). Seepage waters were formed by surface rain water run-off in and under the tailings and slags in the limit between the consolidated bedrock material and unconsolidated weathered material run into the main São Domingos stream with the lowest pH values and the highest Fe and sulphur ion concentrations (Quental et al. 2003; Matos et al. 2006; Álvarez-Valero et al. 2008; Abreu et al. 2010; Mateus et al. 2011; Batista et al. 2012a, b). Microbial analysis indicates the presence of microorganisms, capable of surviving at pH < 1, Fe and S oxidates (Acidithiobacillus ferrooxidans), Fe oxidates (Leptospirillum ferrooxidans) and S oxidates (Acidithiobacillus thiooxidans) and also moderate acidophiles (pH 3 to 6; Bryan et al. 2006). The oxidation processes, combined with the inability of carbonates to neutralise, results in acid production that lowers the pH of the water and raises the conductivity and metal concentration in the solution (Batista et al. 2012a, b). Groundwater concentrations are low in metals, except for Mn, and at some locations, the Zn and redox potential and high pH also indicate that the contaminated water is confined to the main São Domingos stream (Martins et al. 2007). Several studies (e.g. Batista et al. 2007, 2012b, 2013; Abreu et al. 2008, 2012; Gonzalez-Fernandez et al. 2011; Alvarenga et al. 2012, 2014; Santos et al. 2014; Andráš et al. 2016) show significant impacts on plants, especially in areas that are proximal to mine waste with sulphide mineralisation, where soils present high grades of As, Cu, Pb and Zn.
The aim of the research was to identify dominant autochthonous conifers, broadleaves and graminoids at the São Domingos mining area and reference site and study the input of selected metals/metalloids on these plant species, their bioaccumulation and translocation processes with respect to pH and to the ability of Ca/Mg to inhibit their transfer in the system soil–plants. Consequently, the obtained results were used to identify the suitable type of phytoremediation application.
Material and methods
Sampling of soil and plants
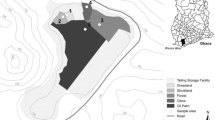
The data sampling was planned considering the local waste disposal and mine site characteristics according to the existing geological and mining mapping (Matos 2004). The key selected sectors were the mine open pit and São Domingos Valley where the tailings, landfills and acid water lagoons are located. The reference area was situated to a locality which is not affected by ore mineralisation (Fig. 2), situated about 1.5 km from the northern border of the São Domingos mining area in the western direction.
Thirty soil (from the root ball of individual sampling plants) and 30 individual plant samples from 5 taxa (roots, shoots or leaves/needles from 8 individuals of Agrostis sp., 4 Juncus conglomeratus, 4 J. effusus, 5 Pinus pinaster, 9 Quercus rotundifolia) were collected from the mining area (Fig. 2). The field work was done in August 2013 at the time when the above-ground organs of individuals were fully developed. From each locality, one clump of graminoids and samples from one adult tree was collected. The averaged root sample was prepared from five individual drilling holes to the lateral roots. The analysed branch samples represent averaged samples from five 3-year-old branches and from the terminal parts of branches were collected the leaves/needles. The plant species were collected from sites which represented the variability of the habitats at the São Domingos mining area (varying from dry scratchy groves with a dominion of Pinus pinaster and Quercus rotundifolia, xerophilous and mesophilous open grassland habitats with Agrostis sp. up to wetlands with Juncus conglomeratus and J. effusus). We also took into account the minimum distance of 10 m between the trees and the 5 m between the herbs. The reference area was selected to reflect the same rock type as the studied mining area. From the reference area, only three plant species were collected, because the area is dry, without wetlands with Juncus spp.
The nomenclature of vascular plants is according to Euro+Med PlantBase database (Domina 2011).
Handling of soil and plant samples and analytical methods
The soil from the roots was carefully removed by means of a soft brush and then washed three times in distilled water. The plants were cut into little pieces and dried for 2 weeks at room temperature. They were consequently dried for 6 h in drying plant, model ED, APT Line II, Binder at a temperature of 40 °C.
The soil samples marked as SDO-1 to SDO-35 were dried at the laboratory temperature. The rinse pH of the soil was determined in water suspension and the paste pH in 1 M KCl (64 g KCl/1000 ml H2O) lixivium. Fifteen millilitres of distilled water or 1 M solution of KCl was added to 5 g of the sample in a glass bake, and this suspension was mixed by electromagnetic stirrers for 2 h, then both pH and Eh were measured in the laboratories of the Geological Institute of the Slovak Academy of Science in Banská Bystrica using pH metre EUTECH instruments according to Sobek et al. (1978). The determined pH and Eh values were re-counted for standard hydrogen electrode count according to Pitter (2009).
The pH measured in the water suspension is known as rinse pH, whereas the pH in the KCl-soil (NaCl-soil) suspension is known as the paste pH. The paste pH not only represents the balance between the concentration of hydrogen (H+) and hydroxyl (OH−) ions in the solution but it also reflects the function of the adsorbed Al3+ ions in the colloidal complexes of the soil. Al3+ ions could be released into the soil solution through the activity of hydrolytically neutral salts (NaCl, KCl, CaCl2). As Eh is also dependant on the pH of the soil solution, we can use the rH2 factor for a better comparison between Eh values at different pH:
In well-aerated soils, rH2 ranges between 28 and 34, whereas in non-altered soils, the rH2 value is < 20 (Richter and Hlůšek 2003).
The paste pH(KCl) of anthropogenic soil enables the determination of the ionic composition of the soil sorption complex and the cation exchange capacity (McNeill and Khakee 1992; Čurlík and Šefčík 1999; Čurlík et al. 2003). The pHH2O – pHKCl difference is expressed by the DpH value. A positive value is equal to the occurrence of soil colloids with a negative charge, and a negative value reflects the occurrence of colloids with a positive charge.
The ICP-MS analyses of soil/technosoil samples were realised in the ACME Laboratory (Vancouver, Canada) from samples of 2 g in weight. The samples were homogenised and dried at the laboratory temperature. The grinding in an agate mill was realised in the laboratory of the Geological Institute of the Slovak Academy of Science in Banská Bystrica.
Two grammes of rock powder was wetted with a few drops of water and then digested into dry vapour in a H2O–HF–HclO4–HNO3 mixture with a rate of 2:2:1:1. After adding 10 ml of 50% HCl, the samples were slowly heated on a water bath under continual mixing. The cooled solution was refilled with HCl to an exact volume and ICP-MS was analysed.
Contamination indices and indices of environmental impact soil–plant
The environmental impact of heavy metals or metalloids and the degree of the pollution of the soil (sediments) can be described with the help of several parameters: the contamination factor (CF) or enrichment factor, pollution load index (PLI) and geoaccumulation index, Igeo.
The CF is a ratio of the element content in the soil of the contaminated area divided by the content of the element in the background soil (Kalender and Çiçek Uçar 2013). Some authors designate this value as the enrichment factor (Kisku et al. 2000; Singh et al. 2010). It is calculated according to the following formula:
According to Hakanson (1980) and Varol (2011), the CF< 1 means low contamination, 1 < CF < 3 is a moderate contamination, CF > 3 to 6 is a considerable contamination and CF > 6 means a very high contamination.
For the whole studied area, the PLI can be calculated as follows:
According to Tomlinson et al. (1980) and Varol (2011), pollution exists when PLI > 1.
The geoaccumulation index (Igeo) is possible calculate according to the following equation:
where Cn is the metal content in soil/sediment from the contaminated area and is the metal content in the background soil (Nowrouzi and Pourkhabbaz 2014). The 1.5 factor is the background matrix correction factor. According to the Igeo index value, Varol (2011) distinguishes the following classes:
-
Igeo ≤ 0—unpolluted
-
0 < Igeo < 1—unpolluted to moderately polluted
-
1 < Igeo < 2—moderately polluted
-
2 < Igeo < 3—moderately to heavily polluted
-
3 < Igeo < 4—heavily polluted
-
4 < Igeo < 5—heavily to extremely polluted
-
5 > Igeo—extremely polluted
For the bioaccumulation calculation, two parameters are important: the bioconcentration factor (BCF) and the translocation factor (TF). BCF, separately for every species, was calculated three ways: (i) as the ratio of average element concentration in an entire plant (shoot plus roots in herbaceous species or leaves/needles plus branches plus root in trees) vs. average element concentration in soil samples from the root ball of this species, (ii) as the ratio of average element concentration in the roots vs. the element content in the soil and (iii) as the ratio of average element concentration only in the aerial parts of plants to the average element concentration in the soil (Brooks 1998; Boussen et al. 2013; Boim et al. 2016). If BCF < 1, the plant is an excluder; if BCF = 1, the plant is an indicator; and if BCF > 1, the plant is an accumulator to hyper-accumulator (Baker 1981). The first and second ways define the plants that are generally suitable for phytostabilisation, while the third way defines plants suitable for phytoextraction.
The translocation factor (TF) reflects the rate of the chemical concentration of the contaminant in the leaves or shoots vs. the concentration of the contaminant in the roots (Singh et al. 2010). This parameter shows in which part of the plant the contaminant (in the case of this study, the individual heavy metals or metalloids) is preferentially accumulated.
Statistical analysis
The correlation analyses were done in IBM SPSS Statistics, version 19.0 (IBM Corp. Released 2010).
Results
Soil contamination assessment
Soil samples collected in the surrounding of the open pit mining area (SDO-1 to SDO-30) show a rinse pH(H2O) ranging between 2.74 and 7.10 (on average 4.29). The paste pH(KCl) varies from 2.69 to 7.02, on average 4.19 (Tab. 1); thus, the soil has an acidic character.
The Eh(H2O) ranges from − 10.7 to 243.4 (on average 153.3) and Eh(KCl) values from − 5.6 to 225.7 (on average 172.9). Eh values indicate mainly suboxic conditions.
The values of the rH2 factor (13.43–15.39) confirm badly aerated conditions. The DpH values, with the exception of two negative values in samples SDO-21 and SDO-25, are as follows: 0.05 to 0.85 (on average 0.34). Such values indicate the presence of soil colloids with a negative surface charge. Two soil samples (SDO-13 and SDO-25) seem to be extraordinary in comparison with the rest of the samples. Not only are the DpH values negative or low, but the pH in these samples is also not strongly acid but close to a neutral value, and the Eh is relatively low. These samples represent not contaminated “isles”, e.g. in the surrounding of the Achada do Gamo old sulphur factory area (Fig. 2).
Analytical data in Table 1 presents the content of selected chemical elements in the soil from São Domingos. The content of heavy metals and metalloids decreases in the range Fe > Pb > As > Zn > Cu > Mn > Sb > Bi > Ni > Co > Ag > Cd. The top soil shows the highest values except for Fe (3.29–25.24%) and Pb. The variability for Pb ranges from 80.1 mg kg−1 (sample SDO-26) to >10,000 mg kg−1 (samples SDO-4 and SDO-5). Accordingly, the top highest concentration of Cu (6204.7 mg kg−1) was evaluated in the same sample SDO-13.
The soil at the reference area has close to a neutral pH (pH(H2O) 6.91 and pH(KCl) 6.61 on average) and contains a substantially lower content of heavy metals and metalloids (Table 2), mainly Pb (82 times lower), Sb (36 times), As (29 times), Bi (25 times), Cu (18 times) and Zn (7 times). Here, the content of heavy metals and metalloids decreases in the range Fe > Mn > Zn > As > Pb and Ni > Cu > Co > Sb > Bi > Cd > Ag. It points to a higher content of Mn (four times) and Ni (two times) on this site. The rH2 index not only is higher—varying from 17.61 to 20.84 (on average 19.13)—but it also detected that the soil is not well aerated. The DpH index value is very similar (on average 0.30) to the value from the contaminated area.
The high correlation degree was described between couples Cu/Co (r = 0.817), Pb/Sb (r = 0.788), Pb/Bi (r = 0.707 and Fe/Zn (r = 0.679) at São Domingos. The highest negative correlation was found between couples Ni/As (r = − 0.567) and Mn/As (r = − 0.518).
The average of the Ca content at São Domingos is 0.51% and of Mg 0.31%. The Ca/Mg ratio is 1.645, but the degree of Ca/Mg correlation (Fig. 3) is only low (r = 0.07). At reference area, the Ca content is lower (0.13%) and Mg a little higher (0.37%), that is Ca/Mg ratio is also higher (r = 0.351).
Among Ca and Cu, Pb, Ag and As, negative correlations were described, because Ca inhibits their input to plant organs, especially in Pinus pinaster. The moderate degree of negative correlation between acidity (pH) and bioconcentration factor, therefore a higher bioavailability at a lower pH, was determined in the case of Mn (r = − 0.411), Zn (r = − 0.307) and Cd (r = − 0.219). The other metals did not show this dependence (e.g. Cu; Fig. 4).
Contamination indices
The values of the calculated CF show the high contamination of the mining area mainly by Pb, Sb, As, Bi, Cu, Zn and Ag; moderate contamination by Fe, Cd and Ni; and low contamination by Mn (Table 3). The Co content in the soil of the reference area is low (20 mg kg−1 in average), but a little bit higher as compared to the mining area (23.1 mg kg−1); the CF = 0.62 (low contamination). Ca and Mg are, from the viewpoint of the contamination present in the country, not relevant.
In addition, the pollution load index PLI = 1.56 confirms that the studied area is strongly contaminated.
The geoaccumulation index Igeo proved that the soil in the mining area is extremely polluted by Pb, As, Sb and Bi, heavily polluted by Cu, moderately to heavily polluted by Zn and Ag as well as moderately polluted by Fe and Cd (Table 3).
Chemical elements in plant organs and their interaction in the system soil–plant
The content of selected elements in individual parts of the studied plants is presented in Table 4.
According to Table 4, the highest Fe, Cu, Pb, Zn and Ni contents were determined in the roots of Agrostis sp., Co in the needles of Pinus, Mn in the leaves of Quercus, As in the roots of Juncus effusus and Sb in the roots of Juncus conglomeratus. The lowest heavy metal and metalloid content were found in Pinus and Quercus (except for Mn).
The sequence of the element content in plants differs more according to the individual species than according to the studied sites, among which this sequence is almost identical (Table 5). Fe, Ca and Mg generally show the highest degree of bioavailability as well as the ability to transport these elements to the shoots. In descending order, Mn follows as bioaccumulation in woody plants and Agrostis sp. (no in roots Juncus spp.), whereas Pb is the following one as far as herbs are concerned. Pb remains more or less immobile in the roots, and Mn is efficiently transported to the shoots (with the exception of Juncus effusus), just like Zn (with the exception of Agrostis sp.). Zn and Mn in Quercus are mainly accumulated in the leaves.
The heavy metal and metalloid accumulation in the system soil–plants is, with the exception of several few cases, low (Table 6). The trees are Ag accumulators, whereas the graminoids are the hyper-accumulators (the Ag content in roots is 400–700 times higher as in soil). The translocation of Ag from the roots to the shoots was not confirmed. Mn is also relatively mobile and its accumulation decreases in the following order: Pinus pinaster, Juncus conglomeratus and Quercus rotundifolia. This element is also well translocated into the aerial parts of this species. Juncus effusus accumulates Co very well in all organs (BCF > 9). Thus, it may serve as the potential plant species for the phytoremediation of cobalt-contaminated areas. Pinus pinaster is an accumulator of Cd and an indicator for Co in São Domingos (Table 4).
All of the studied heavy metals and metalloids are preferentially accumulated in the roots, except for Mn, which is mainly accumulated in the leaves or shoots (not in Agrostis sp. and Juncus effusus). In the trees, Zn and Co (in Pinus) are also mainly transported to assimilation organs.
Discussion
For growth and development, each plant needs the appropriate amounts of mineral salts, i.e. macronutrients and micronutrients. The mining processes exposed thousands of tons of nutrient-poor waste rock and subsoil to weathering.
The transfer and accumulation of heavy metals and metalloids to the plant organs influence numerous factors, for example the heavy metal content in the soil and soil properties (e.g. the mould content, the content of the organic matter of clay minerals, the cation exchange capacity and Ca and Mg presence). The input of heavy metal and metalloids to plants is usually and substantially influenced by Ca and Mg and respectively by the Ca/Mg ratio in the sorption complex (Čurlík et al. 2015).
Heavy metals and metalloids are mainly represented in the soil in the form of salts, which are usually unavailable for plant uptake. Only their limited portion is present in the form of an aqueous phase. The uptake of heavy metals and metalloids is controlled both by biological–chemical processes in the root system and by the influence of the presence of some heavy metals or metalloids in soils or in plants. The lack of some micronutrients in the soil can cause the excessive accumulation of individual metals in organs of plants (Fijalkowski et al. 2012).
On the other hand, at the contaminated sites (such as the São Domingos mining area), the excess of numerous heavy metals and metalloids caused disturbances in the metabolism of plants and influenced both the transport and assimilation of heavy metals and metalloids in the organs of plants (Padmavathiamma and Li 2007). The correlation among metallic elements and macroelements (e.g. Ca, Mg) is variable.
The main factor which controls the distribution and mobility of heavy metals and metalloids is pH. Soil type, soil moisture (at São Domingos, the soil is very dry), the presence of individual minerals as well as their content, the intensity of the weathering process, organic matter in the soil, characteristics of edaphone, its composition and the uptake of metals by plants substantially affect the distribution of heavy metals and metalloids. The increase of H+ ion concentration causes an increase in heavy metals and metalloid mobility (Alkorta et al. 2004; Vamerali et al. 2010). The highly contaminated, strongly acidic soils at São Domingos are relatively poor in organic matter content, so the mobility of metallic elements is substantially higher than under neutral or alkaline conditions. On the other hand, organic matter can cause both the release and immobilisation of heavy metals (Kelly 2008).
Another factor which controls the mobility of elements is the oxidation–reduction potential of the soil. According to Kavamura and Esposito (2010), the lack of oxygen in the soil causes the start up and affects an increase in the mobility of most of the heavy metals and metalloids.
The occurrence of individual heavy metals in various forms (various chemical compounds and various oxidation states) significantly affects their mobility. Typically, the most mobile metals are Cd, Zn and Mo, whereas Cr, Ni and Pb are substantially less mobile (Prasad and Freitas 2003; Batista et al. 2012a). The current study also proved the high Mn input into the organs of the plants.
The accumulation of selected metals in different plant species is very variable. It depends not only on the plant species but also on the soil characteristics (Chunilall et al. 2005), which we also confirmed. Plants have several mechanisms by which they can control heavy metals and metalloid tolerance, mainly chelation and sequestration, which are able to remove toxic metals from sensitive sites or incorporate essential metals to their specific cellular destination (Viehweger 2014).
The highest Fe content at São Domingos was recorded in the graminoids. Fe is one of the major constituents of the soil (0.5 to 5%). It is usually present in Fe3+ form (Greenwood and Earnshaw 1990). The bioavailability of such an Fe oxidation status at pH values suitable for plant growth is severely limited by the low solubility of Fe hydroxides. Dicotyledons and non-graminaceous monocotyledons are characterised by an Fe3+ solubilisation which is usually mediated by the acidification of the rhizosphere, by completion with chelating compounds and by reduction to Fe2+, taken up by the roots by means of a transporter for Fe2+. Phytosiderophores (organic acids) are synthesised by the roots of plants and then released into the rhizosphere: there, they form complexes with Fe. The phytosiderophore-plus Fe complex moves into the root across the membrane. Movement of Fe occurs from one part of the shoot (in particularly, from senescent leaves) to other shoot parts via phloem; however, most scientists believe that Fe is not easily re-translocated into plant shoots (Hochmuth 2011). On the contrary, grasses (in our case Agrostis sp.) are characterised by a different mechanism for Fe acquisition, with Fe mobilising mainly by means of the roots (Pinton et al. 2007).
As far as further heavy metals are concerned, Zn is mainly acquired from the soil solution as Zn2+. However, zinc toxicity is rare and occurs in post-mining and smelting sites, especially with acidic soils. Here the graminaceous species are generally less sensitive to higher concentrations of Zn than most vascular plants, especially dicotyledons. Many plant metallophytes tolerate zinc, but for most cultivated species, it is toxic. Initial research has suggested that the most tolerant species are plants of the family Poaceae, as well as Lamiaceae and Caryophyllaceae, but subsequent studies did not confirm this phylogenetic predisposition to the evolution of Zn hyper-tolerance. Nowadays, zinc hyper-tolerant plants from Poaceae are also available as cultural species and they are successfully used in the remediation of soils contaminated with Zn and also Pb and other heavy metals on post-mining sites (Broadley et al. 2007).
Singh et al. (2010) and Lehout et al. (2018) confirmed a significant correlation in the processes of metal translocation from root to shoot between Cd and Cr, Cu, Mn, Fe; Cr/Pb, Mn and between Pb and Fe; between Cd and Cu, Pb, Zn; and Zn and Cu. This study considered other relationships: a positive correlation in the couples Cu vs. Co, Pb vs. Sb and Bi and Fe vs. Zn and a negative correlation in the couple Ni/As and Mn/As.
A negative correlation between acidity (pH) and bioconcentration factor usually exists, and it means a higher degree of the bioavailability at lower pH values (Sobek et al. 1978; Singh et al. 2010). At the São Domingos site, it was described only in case of Mn, Zn and Cd.
The Ca/Mg > 1 ratio is characteristic of basic rocks (McCarten 1992), so it corresponds with the composition of the wall rocks at the São Domingos mining area.
Calcium is usually better soluble than magnesium, and therefore, it is also more bioavailable. Mg shows a tendency to migrate to the deeper soil horizons (Bowen 1979). This tendency is typical mainly for more acidic soils, as it also is at São Domingos. Ca is usually accumulated in the upper soil horizons. This feature is also influenced by its content in the paced plant tissues (Verbruggen and Hermans 2013).
The high Mg content can cause the strong toxicity of plants (Proctor 1971, 2003; Brooks 1987; Brady et al. 2005; Alexander et al. 2007); therefore, the relatively low Mg content in the soil at São Domingos (0.31% Mg on average) could have a positive influence on the vegetation. Actually, Mg is not harmful to plants but it inhibits Ca and K bioavailability; therefore, the symptoms of Mg toxicity are actually caused by Ca and K lack (Gunes et al. 1998; Merhaut 2007).
Arsenic is (unlike Pb, Cd and Hg) relatively mobile even in neutral or slightly alkaline soils (Yu 2005), but its mobility increases in the acidic conditions typical of São Domingos. Its bioavailability and toxicity is controlled not only by the character of soil solutions (by pH and Eh), the presence of oxyhydroxides and sorption on clay minerals (Davranchea et al. 2003; Förster and Salomons 2004), but it can be increased also by the presence of Ca2+, Mg2+ and Fe2+ (Dixit and Hering 2006). The relatively low bioavailability of As at the deposit is also influenced by the ability to form solid phases (Sadiq 1997; Matera and Le Hécho 2001)—secondary minerals, only slightly soluble oxides of an identical chemical formula (As2O3), arsenolite and claudetite, were described at the deposit by Álvarez-Valero et al. (2008).
Sb shows in acid conditions at low pH values a strong sorption ability on clay minerals as well as on Fe oxides and Fe hydroxides (Kabata-Pendias and Mukherjee 2007). It is the reason why Sb at the reference area, where the pH is higher as in the mining area, has a better bioavailability.
The studied plants show the highest metal concentrations in Agrostis sp., common across the entire mining area (Turisová et al. 2014). The phytoremediation features of the relatively widespread plants of the taxons Cistus (4 species) and Erica (2 species) were studied by Abreu et al. (2008, 2012). The authors conditioned the selection by finding that they did not display visual symptoms of toxicity, in spite of the fact that they grew on very contaminated sites. Both the presently studied plant species and species studied by the above-mentioned authors did not translocate hazardous elements to the shoots to a degree, which would enable the indication of them as accumulators. Higher concentrations of the metals and metalloids were noted in Erica andevalensis and Erica australis by Anawar et al. (2010) and Anawar (2012), but these species, together with Juncus sp., Lavandula stoechas subsp. luisieri, Daphne gnidium, Rumex induratus, Ulex parviflorus subsp. eriocladus and Genista hirsuta, are not suitable for phytoremediation but may have a major importance for the rehabilitation and recovery of the contaminated mining area.
Freitas et al. (2004a) have published the most detailed study dealing with 135 plant species from 39 families exhibiting resistance to trace metals from north–east of Portugal—a serpentinised area in which tissues were detected with a considerable content of Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn. Both the present study of Quercus rotundifolia as well as the study of the above-mentioned authors of Quercus pyrenaica and Quercus ilex proved the high Mn content input to their organs. The highest metal content was described in Alyssum serpyllifolium. According to Fecenko and Ložek (2000), manganese input is influenced by the redox potential, content of organic matters, activity of microorganisms, temperature and mainly by pH. The low pH values typical of São Domingos are very favourable for the high mobility and bioaccumulation of Mn2+. The highest Mn content was usually detected in young needles/leaves (Richter and Hlůšek 2003).
In 24 plant species, also including the presently studied Juncus conglomeratus, Juncus effusus, Pinus pinaster and representatives from the genera Agrostis and Quercus growing in the São Domingos area, also studied by Freitas et al. (2004b), show the highest Pb and As content in the shoots of Juncus conglomeratus (84.8 and 23.5 mg kg−1). According to our results, the Pb content in this species is very changeable (it vary from 4 to 154 mg kg−1) and does not correlate with the Pb content in the soil, where it is present predominantly in the form of anglesite—PbSO4, cerussite—PbSO3 or leadhillite—Pb4(CO3)2(SO4)(OH)2 (Álvarez-Valero et al. 2008). Low solubility is typical for all of these minerals. Moreover, clay minerals, Mn oxides as well as Fe and Al hydroxides are very good sorbents for Pb salts. The formation of insoluble humin complexes causes its fixation in the upper horizons of the soil (Huang et al. 2011). These features substantially influence the very limited Pb bioavailability (Rapant et al. 1996). However, according to the findings of the present study, grass Agrostis sp. has on average a much higher Pb and Ag content (Pb 217 mg kg−1, As 43 mg kg−1).
Our present study proved the relatively higher Ag, As, Ni and Zn contents in the leaves and branches of Quercus rotundifolia and higher Ag, As, Pb and Zn contents in Pinus pinaster as described by Freitas et al. (2004a) in Quercus ilex. Most minerals release during the weathering of Zn2+, which is partly sorbed on the surface of natural sorbents but in acidic conditions is very mobile and bioavailable (Tölgyessy 1989; McLean and Bledsoe 1992). The studied tree species, due to their great biomass, can be very effective for metal phytoextraction and phytostabilisation.
Conclusion
The studied dominant plant species in the polygon of the mining area accumulate high concentrations of heavy metals and metalloids (Cu, Pb, Zn, Co, As, Sb). The concentrations of these metals are accumulated predominantly in the roots. The strategy of metal accumulation in the plant organs and tissues is dependent on specific species, regardless of their life forms and preferences of habitats with different ecological conditions, as well as on the metal/metalloid geochemical behaviour. The mobility of elements (metals/metalloids) in the soil–plant system can also be controlled by pH and by the relationship between them and the Ca/Mg content in the soil. Despite the good plant fitness and vitality of the studied metal-tolerant species, these are not suitable for phytoextraction both because of the heavy contamination of the mining and post-mining country components and due to their prevailing strategy as excluders. They may be applied only for phytostabilisation purposes.
References
Abreu MM, Tavares MT, Batista MJ (2008) Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos, Portugal. J Geochem Explor 96:210–222. https://doi.org/10.1016/j.gexplo.2007.04.007
Abreu MM, Batista MJ, Magalhães MCF, Matos JX (2010) Acid mine drainage in the Portuguese Iberian Pyrite Belt. In: Brock CR (ed) Mine drainage and related problems. Nova Science Publishers, New York, pp 71–118
Abreu MM, Santos ES, Magalhães MCF, Batista MJ (2012) São Domingos mine wastes phytostabilization using spontaneous plant species. In: 9th International Symposium on Environmental Geochemistry, 15th–21th July 2012: Aveiro, field guidebook: multidisciplinary contribution for environmental characterization and improvement at the São Domingos mining site, pp 42–49
Alexander EB, Coleman RG, Keeler-Wolf T, Harrison S (2007) Serpentine geoecology of Western North America: geology, soils, and vegetation. Oxford University Press, New York
Alkorta I, Hernandez-Alica J, Becerril JM, Amezaga I, Albizu I, Garbisu C (2004) Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead and arsenic. Rev Environ Sci Technol 3:71–90. https://doi.org/10.1023/B:RESB.0000040059.70899.3d
Alvarenga P, Palma P, Varennes A, Cunha-Queda A (2012) A contribution towards the risk assessment of soils from the São Domingos mine (Portugal): chemical, microbial and ecotoxicological indicators. Environ Pollut 161:50–56. https://doi.org/10.1016/j.envpol.2011.09.044
Alvarenga P, Simões I, Palma P, Amaral O, Matos JX (2014) Field study on the accumulation of trace elements by vegetables produced in the vicinity of abandoned pyrite mines. Sci Total Environ 470–471:1233–1242. https://doi.org/10.1016/j.scitotenv.2013.10.087
Álvarez-Valero AM, Pérez-López R, Matos J, Capitán MA, Nieto JM, Sáez R, Delgado J, Caraballo M (2008) Potential environmental impact at São Domingos mining district (Iberian Pyrite Belt, SW Iberian Peninsula): evidence from a chemical and mineralogical characterization. Environ Geol 55:1797–1809. https://doi.org/10.1007/s00254-007-1131-x
Anawar HM (2012) Role of private sector and national policy for rehabilitation of abandoned mines in Portugal. In: McCullough CD, Lund MA, Wyse L (eds) International Mine Water Association Symposium, Bunbury, Australia, pp 295–300
Anawar HM, Freitas MC, Canha N (2010) Contamination and phytoremediation of arsenic and other heavy metals and evaluation of different drying process to determine metals in plant species grown in a mining impacted area. In: Proceedings of 15th International Conference on Heavy Metals in the Environment, September 19–23, Gdańsk, pp 483–485
Andráš P, Turisová I, Buccheri G, Matos JMX, Dirner V (2016) Comparison of heavy-metal bioaccumulation properties in Pinus sp. and Quercus sp. in selected European Cu deposits. Web Ecol 16:81–87. https://doi.org/10.5194/we-16-81-2016
Baker AJM (1981) Accumulators and excluders—strategies in the response of plant to heavy metals. J Plant Nutr 3:643–654. https://doi.org/10.1080/01904168109362867
Bálintová M, Petriláková A, Singovszka E (2012) Study of metals distribution between water and sediment in the Smolnik Creek (Slovakia) contaminated by acid mine drainage. Chem Eng Trans 28:73–78. https://doi.org/10.3303/CET1228013
Batista MJ, Abreu MM, Serrano Pinto M (2007) Biogeochemistry in Neves Corvo mining region, Iberian Pyrite Belt, Portugal. J Geochem Explor 92:159–176. https://doi.org/10.1016/j.gexplo.2006.08.004
Batista MJ, Abreu MM, Locutura J, Oliveira DPS, Matos JX, Silva C, Bel-Ann A, Martins LP (2012a) Evaluation of trace elements mobility from soils to sediments between the Iberian Pyrite Belt and the Atlantic Ocean. J Geochem Explor 123:61–68. https://doi.org/10.1016/j.gexplo.2012.06.011
Batista MJ, Matos JX, Abreu MM (2012b) Acid drainage potential at S. Domingos mine. In: Silva EF, Reis AP, Patinha C, Pereira E, Rodrigues S (Eds) Multidisciplinary contribution for environmental characterization and improvement at the S. Domingos mining site. Field Trip Guidebook of the 9th Int. Symposium on Environmental Geochemistry, PLM-Plural S. A., pp 13–17
Batista MJ, Oliveira DPS, Abreu MM, Locutura J, Shepherd T, Matos JX, Bel-Ann A, Martins LP (2013) Sources, background and enrichment of lead and other elements: lower Guadiana River. Geoderma 193–194:265–274. https://doi.org/10.1016/j.geoderma.2012.08.033
Boim AG, Melo LC, Moreno FN, Alleoni LR (2016) Bioconcentration factors and the risk concentrations of potentially toxic elements in garden soils. J Environ Manag 170:21–27. https://doi.org/10.1016/j.jenvman.2016.01.006
Boussen S, Soubranda M, Bril H, Ouerfelli K, Abdeljaouad S (2013) Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 192:227–236. https://doi.org/10.1016/j.geoderma.2012.08.029
Bowen HJM (1979) Hodnocení těžkých kovů v odpadech a průmyslově vyráběných kompostech. In: Proceeding of Conference Kompostování odpadů a životné prostředí, ČSVTS, Praha, pp 83–94 (in Czech)
Brady KU, Kruckeberg AR, Bradshaw HD Jr (2005) Evolutionary ecology of plant adaptation to serpentine soils. Annu Rev Ecol Evol Syst 36:243–266. https://doi.org/10.1146/annurev.ecolsys.35.021103.105730
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702. https://doi.org/10.1111/j.1469-8137.2007.01996.x
Brooks R (1987) Serpentine and its vegetation: a multidisciplinary approach. Dioscorides Press, Portland
Brooks R (1998) Plants that hyperaccumulate heavy metals: their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. CAB International, Wallingford
Bryan C, Hallberg KB, Johnson DB (2006) Mobilisation of metals in mineral tailings at the abandoned São Domingos copper mine (Portugal) by indigenous acidophilic bacteria. Hydometallurgy 83:184–194. https://doi.org/10.1016/j.hydromet.2006.03.023
Chunilall V, Kindness A, Jonnalagadda SB (2005) Heavy metal uptake by two edible Amaranthus herbs grown on soils contaminated with lead, mercury, cadmium and nickel. J Environ Sci Health 40:375–384. https://doi.org/10.1081/PFC-200045573
Čurlík J, Šefčík P (1999) Geochemical atlas of Slovak Republic, part V: soils. Ministry of the Environment of the Slovak Republic, Geological Survey of Slovak Republic, Bratislava (in Slovak)
Čurlík J, Bedrna Z, Hanes J, Holobradý K, Hrtánek B, Kotvas F, Masaryk Š, Paulen J (2003) Pôdna reakcia a jej úprava. Suma Print, Bratislava (in Slovak)
Čurlík J, Kolesár M, Ďurža O, Hiller E (2015) Dandelion (Taraxacum officinale) and agrimony (Agrimonia eupatoria) as indicators of geogenic contamination of flysch soils in eastern Slovakia. Arch Environ Contam Toxicol 70:475–486. https://doi.org/10.1007/s00244-015-0206-z
Davranchea M, Bollingera JC, Bril H (2003) Effect of reductive conditions on metal mobility from wasteland solids: an example from the Mortagne-du-Nord site (France). Appl Geochem 18:383–394. https://doi.org/10.1016/S0883-2927(02)00075-6
Dixit S, Hering JG (2006) Sorption of Fe(II) and As(III) on goethite in single- and dual-sorbate systems. Chem Geol 228:6–15. https://doi.org/10.1016/j.chemgeo.2005.11.015
Domina G (2011) The Euro+Med PlantBase. In The information resource for Euro-Mediterranean plant diversity. http://ww2.bgbm.org/EuroPlusMed/query.asp. Accessed 23 Mar 2017
Fecenko J, Ložek O (2000) Výživa a hnojenie poľných plodín. Slovak Agricultural University, Nitra (in Slovak)
Fijalkowski K, Kacprzak M, Grobelak A, Placek A (2012) The influence of selected soil parameters on the mobility of heavy metals in soils. Inžynieria i Ochrana Środowiska 15:81–92
Förster U, Salomons W (2004) Elements and compounds in sediments. In: Merian E, Anke M, Ihnat M, Stoeppler M (eds) Elements and their compounds in the environment: occurrence, analysis and biological relevance. Vol. 1, part I.8. Wiley, Weinheim, pp 149–162
Freitas H, Prasad MNV, Pratas J (2004a) Analysis of serpentinophytes from north–east of Portugal for trace metal accumulation–relevance to the management of mine environment. Chemosphere 54:1625–1642. https://doi.org/10.1016/j.chemosphere.2003.09.045
Freitas H, Prasad MNV, Pratas J (2004b) Plant community tolerant to trace elements growing on the degraded soils of São Domingos mine in the south east of Portugal: environmental implications. Environ Int 30:65–72. https://doi.org/10.1016/S01604120(03)00149-1
Gonzalez-Fernandez O, Batista MJ, Abreu MM, Queralt I, Carvalho ML (2011) Elemental characterization of edible plants and soils in an abandoned mining region: assessment of environmental risk. X-Ray Spectrom 40:353–363. https://doi.org/10.1002/xrs.1348
Greenwood NN, Earnshaw A (1990) Chemie der Elemente. Wiley-VCH, Würzburg
Gunes A, Alpaslan M, Inal A (1998) Critical nutrient concentrations and antagonistic and synergistic relationships among the nutrients of NFT-grown young tomato plants. J Plant Nutr 21:2035–3047. https://doi.org/10.1080/01904169809365542
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Hochmuth G (2011) Iron (Fe) nutrition of plants. Soil and Water Department, UF/IFAS Extension 7 pp
Holub M, Bálintová M, Singovszka E (2015) Quality of the bottom sediments in the area affected by mining activities. Pollack Periodica 10:109–116. https://doi.org/10.1556/606.2015.10.3.11
Huang MP, Li Y, Sumner ME (2011) Handbook of soil sciences, properties and processes, 2nd edn. CRC Press, Boca Raton
IBM Corp. Released (2010) IBM SPSS Statistics for Windows, Version 19.0. Armonk, IBM Corp., New York
Kabata-Pendias A, Mukherjee AB (2007) Trace elements form soil to human. Springer-Verlag, Berlin
Kalender L, Çiçek Uçar S (2013) Assessment of metal contamination in sediments in the tributaries of the Euphrates River, using pollution indices and the determination of the pollution source. Turkey. J Geochem Explor 134:73–84. https://doi.org/10.1016/j.gexplo.2013.08.005
Kavamura VN, Esposito E (2010) Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol Adv 28:61–69. https://doi.org/10.1016/j.biotechadv.2009.09.002
Kelly G (2008) Application of recycled organics in mine site rehabilitation. Department of Environment and Climate Change NSW, Sydney 37 pp
Kisku GC, Barman SC, Bhargava SK (2000) Contamination of soil and plants with potentially toxic elements irrigated with mixed industrial effluent and its impact on the environment. Water Air Soil Poll 120:121–137. https://doi.org/10.1023/A:1005202304584
Lehout A, Charchar N, Nourine H, Bouyahmed H (2018) The effect of heavy metals on plant communities distribution in an abandoned mining area (Northeast-Algeria). Carpath J Earth Environ 13:3745. https://doi.org/10.26471/cjees/2018/013/004
Martins L, Batista MJ, Matos JX et al (2007) As origens do chumbo na parte inferior da bacia do Guadiana. In: VI Cong. Geoquímica, UTAD Vila Real, pp 278–281
Matera V, Le Hécho L (2001) Arsenic behavior in contaminated soils: mobility and speciation. In: Selim HM, Spark DL (eds) Heavy metals release in soil. Lewis Publisher, Boca Raton, pp 207–235
Mateus A, Pinto A, Alves LC, Matos JX, Figueiras J, Neng N (2011) Roman and modern slag at S. Domingos mine (IPB, Portugal): compositional features and implications for their long-term stability and potential re-use. Int J Environ Waste Manag 8:133–158. https://doi.org/10.1504/IJEWM.2011.040971
Matos JX (2004) Carta geológico-mineira de São Domingos, 1 : 5000. INETI
Matos JX, Pereira Z, Oliveira V, Oliveira JT (2006) The geological setting of the São Domingos pyrite orebody, Iberian Pyrite Belt. In: VII Cong. Nac. Geologia, Estremoz, Évora, pp 283–286
Matos JX, Martins LP, Oliveira JT, Pereira Z, Batista MJ, Quental L (2008) Rota da pirite no sector português da Faixa Piritosa Ibérica, desafios para um desenvolvimento sustentado do turismo geológico e mineiro. Projecto RUMYS, programa CYTED. In: Carrion P (ed) Livro Rutas Minerales en Iberoamérica. Sup. Politécnica del Litoral, Guayaquil, Equador, pp 136–155 (in Portuguese)
McCarten N (1992) Community structure and habitat relations in a serpentine grassland in California. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic serpentine soils. Proceeding of the 1st international conference on serpentine ecology. University of California, Davis California, pp 207–211
McLean JE, Bledsoe BE (1992) Behaviour of metals in soils. Ground Water Issue. United States Environmental Protection Agency. Office of Water and Office of Solid Waste, EPA-540/S-92/018, Washington DC
McNeil PL, Khakee R (1992) Disruptions of muscle fiber plasma membranes. Role in exerciseinduced damage. Am J Path 140:1097–1109
Merhaut DJ (2007) Magnesium. In: Barker AV, Pilbeam DJ (eds) Handbook of plant nutrition. Taylor and Francis, Boca Raton, pp 145–181
Nowrouzi M, Pourkhabbaz A (2014) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of hara biosphere reserve, Iran. Chem Spec Bioavailab 26:99–105. https://doi.org/10.3184/095422914X13951584546986
Padmavathiamma PK, Li LY (2007) Phytoremediation technology: hyper-accumulation metals in plants. Water Air Soil Poll 184:105–126. https://doi.org/10.1007/s11270-007-9401-5
Pinton R, Varanini Z, Nannipieri P (eds) (2007) The rhizosphere: biochemistry and organic substances at the soil-plant interface, 2nd edn. CRP Press Taylor and Francis Group, New York
Pitter P (1990) Hydrochemie. 2nd edn. SNTL, Praha (in Czech)
Prasad MNV, Freitas H (2003) Metal hyperaccumulation in plants—biodiversity prospecting for phytoremediation technology. Electron J Biotechnol 6:275–321. https://doi.org/10.2225/vol6-issue3-fulltext-6
Proctor J (1971) The plant ecology of serpentine: III. The influence of a high magnesium/calcium ratio and high nickel and chromium levels in some British and Swedish serpentine soils. J Ecol 59:827–842 http://www.jstor.org/stable/2258143
Proctor J (2003) Vegetation and soil and plant chemistry on ultramafic rocks in the tropical Far East. Perspectives in Plant Ecology Evolution and Systematics 6:105–124. https://doi.org/10.1078/1433-8319-00045
Quental L, Brito MG, Sousa AJ et al (2003) Utilização de imagens hiperespectrais na avaliação da contaminação mineira em S. Domingos, Faixa Piritosa, Alentejo [CD-ROM]. Ciências da Terra (UNL), Lisboa, M33-M36 (in Portuguese)
Rapant S, Vrana K, Bodiš D (1996) Geochemical atlas of Slovakia 1—groundwater. State Geological Survey of Dionýz Štúr, Bratislava (in Slovak)
Richter R, Hlůšek J (2003) Soil fertility. Ústav zemědělských a potravinářských informací. Ministerstvo zemědelství, Praha (in Slovak)
Sadiq M (1997) Arsenic chemistry in soils: an overview of thermodynamic predictions and field observations. Water Air Soil Poll 3:117–136. https://doi.org/10.1007/BF02404751
Santos ES, Abreu MM, Batista MJ, Magalhães MC, Fernandes E (2014) Inter-population variation on the accumulation and translocation of potentially harmful chemical elements in Cistus ladanifer L. from Brancanes, Caveira, Chança, Lousal, Neves Corvo and São Domingos mines in the Portuguese Iberian Pyrite Belt. J Soils Sediments 14:758–772. https://doi.org/10.1007/s11368-014-0852-1
Singh R, Singh DP, Kumar N, Bhargava SK, Barman SC (2010) Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J Environ Biol 31:421–430
Sobek AA, Schuller WA, Freeman J R, Smith RM (1978) Field and laboratory methods applicable to overburden and minesoils. U. S. Environmental Protection Agency, Environmental Protection Technology, EPA 600/2-78-054
Tölgyessy J (1989) Chémia, biológia a toxikológia vody a ovzdušia. VEDA, vydavateľstvo SAV, Bratislava (in Slovak)
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgolander Meeresun 33:566–575. https://doi.org/10.1007/BF02414780
Turisová I, Štrba T, Andráš P, Aschenbrenner Š (2014) Floristic composition on the abandoned copper heaps in Central Slovakia. Romanian J Miner Deposits 87:61–64
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8:1–17. https://doi.org/10.1007/s10311-009-0268-0
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364. https://doi.org/10.1016/j.jhazmat.2011.08.051
Verbruggen N, Hermans C (2013) Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 368:87–99. https://doi.org/10.1007/s11104-013-1589-0
Viehweger K (2014) How plants cope with heavy metals. Bot Stud 55:35. https://doi.org/10.1186/1999-3110-55-35
Vieira A (2015) Avaliação do Potencial Mineiro das Escombreiras da Mina de Sao Domingos. MSc Thesis, Évora University
Vieira A, Matos JX, Lopes L, Martins R (2015) Evaluation of the mining potential of the São Domingos mine wastes, Iberian Pyrite Belt. In: Cong. Ibérico Geoquímica/XVIII Semana Geoquímica, LNEG, pp 208–211
Yu MH (2005) Environmental toxicology: biological and health effects of pollutants. CRC Press, Boca Raton
Funding
The article was financially supported by the Slovak Research and Development Agency (projects VEGA 2/0040/17 and VEGA 1/0291/19).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Andráš, P., Matos, J.X., Turisová, I. et al. The interaction of heavy metals and metalloids in the soil–plant system in the São Domingos mining area (Iberian Pyrite Belt, Portugal). Environ Sci Pollut Res 25, 20615–20630 (2018). https://doi.org/10.1007/s11356-018-2205-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2205-x