Abstract
A gas standard mixture containing 22 chlorinated hydrocarbons in high purity nitrogen was prepared using a two-step weighing method and a gasifying apparatus developed in-house. The concentration of each component was determined using a gas chromatograph with flame ionization detection (GC/FID). Linear regression analysis of every component was performed using the gas standard mixture with concentrations ranging from 1 to 10 μmol/mol, showing the complete gasification of volatile organic compound (VOCs) species in a selected cylinder. Repeatability was also examined to ensure the reliability of the preparation method. In addition, no significant difference was observed between domestic treated and imported treated cylinders, which were conducive to reduction of the cost of raw materials. Moreover, the results of stability testing at different pressures and long-term stability tests indicated that the gas standard at 1 μmol/mol level with relative expanded uncertainties of 5% was stable above 2 MPa for a minimum of 12 months. Finally, a quantity comparison was conducted between the gas standard and a commercial gas standard from Scott Specialty Gases (now Air Liquide America Specialty Gases). The excellent agreement of every species suggested the favorable accuracy of our gas standard. Therefore, this reference material can be applied to routine observation of VOCs and for other purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) have raised wide concerns during the past decades due to the fact that it may cause severe air pollution (Gao et al. 2016; He et al. 2015; Lyu et al. 2016; Wang et al. 2008). It is well-known that VOCs are ozone precursors and play an important role in the formation of secondary organic aerosol (Sun et al. 2016). Moreover, epidemiologic studies indicate that chronic exposure to VOCs and ozone have an adverse effect on human health and increased mortality (D'Andrea and Reddy 2016; Gong et al. 2017; Wang et al. 2008). Thus, volatile halogenated hydrocarbon compounds have been identified as highly carcinogenic and toxic by a number of major environment safety agencies. With the rapid development of industries, high concentrations of VOCs have been detected in China in recent years (Lei et al. 2013; Li et al. 2015; Ling et al. 2011; Shao et al. 2016; Wei et al. 2014; Wu et al. 2016; Xie et al. 2016). The Department of Environmental Protection in China has issued “Ambient air—Determination of volatile halogenated hydrocarbons—Activated charcoal adsorption and carbon disulfide desorption/gas chromatographic method” (HJ 645-2013) as the standard method for the determination of volatile halogenated hydrocarbon compounds.
Gas reference materials with accurate concentrations and long-term stability at different pressure ranges are urgently needed for proficiency testing and routine observation of chlorinated hydrocarbons at ambient concentration levels. A single-step microgravimetric procedure for the preparation of VOCs was previously developed at the National Institute of Standards and Technology (NIST) in 1983 (Rhoderick 1997; Rhoderick et al. 1993). Then in the past 30 years, the NIST has developed several different kinds of gas standard mixture containing two to as many as 30 hydrocarbons (Rhoderick and Yen 2006; Rhoderick et al. 2010) which were reliable but have a long development and delivery time, and can be expensive.
However, no corresponding gas standard was available in China. In this research, a standard reference material containing 22 chlorinated hydrocarbons at 1 μmol/mol was developed using a two-step microgravimetric method. Linear regression analyses of every component and repeatability of the preparation method were performed. In addition, domestically and imported treated cylinders were all suitable in the study. Moreover, the results of this research indicated that the gas standard for 22 chlorinated hydrocarbons at 1 μmol/mol level with relative expanded uncertainties of 5% was stable above 2 MPa in cylinders for a minimum of 12 months.
Experimental
Reagents
The chlorinated hydrocarbons with purities from 99 to 99.99% were obtained from commercial suppliers, and nitrogen at a specified purity of 99.9995% was obtained from a commercial supplier. All the components were identified using gas chromatography-mass spectrometry (GC-MS), and their purity analyses were performed using gas chromatography with flame ionization detection (GC-FID). The purity determined by the chemical suppliers’ and the authors’ laboratory, along with the commercial suppliers, are all shown in Table 1.
Gas cylinders
New aluminum gas cylinders were obtained from commercial suppliers. The imported cylinders were from Air Liquide America Specialty Gases where they had treated the interior cylinders walls using a proprietary technique called Aculife IV to render the active sites of the inner cylinder walls inert. The domestic treated cylinders were from the Institute of Metal Research, Chinese Academy of Science, which were treated with electroless Ni-P alloy plating. Domestic none-treated cylinders were from Shenyang Gas Cylinder Safety Technology Co., Ltd. The cylinders with an internal volume of 2 L were equipped with a YQF-1 stainless steel valve.
Apparatus
An 81-V-HCE-30G series two-pan balance (HNU Systems, Inc., USA) with 30 kg capacity and 0.001 g sensitivity was used for weighing cylinders. The sealed chlorinated hydrocarbon liquids were weighed using AE240 series electrical balance (Mettler Toledo, Switzerland) with a capacity of 200 g and a readability of 0.01 mg.
Preparation of gas standard
Chloromethane, chloroethane, and chloroethylene are gaseous, and other chlorinated hydrocarbons are liquids at room temperature, especially for o-dichlorobenzene which gasifies at a high temperature (180 °C). Thus, it is difficult to develop the standard reference material containing 22 hydrochloric hydrocarbons through a normal method. A two-step weighing method and a home-made VOC gasifying apparatus, which insures all compounds are completely gasified, were adopted.
The first step is to achieve “premix 1.” Chloromethane at 5.013 mmol/mol, chloroethane at 7.453 mmol/mol, and chloroethylene at 14.60 mmol/mol in nitrogen at a final pressure of 12.0 MPa were prepared. A new 2L cylinder was evacuated and weighed using the two-pan balance. This was followed by adding to the cylinder pre-calculated amounts of chloromethane, chloroethane, and chloroethylene. This was followed by the addition of nitrogen into the cylinder through a manifold system in a calculated amount to yield premix 1 resulting in a concentration of about 150 μmol/mol at a final pressure of 12.0 MPa.
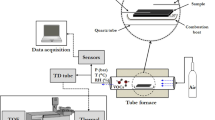
The next step was to prepare the standard reference material containing the other 19 liquid chlorinated hydrocarbons and premix 1 at 1 μmol/mol. The other 19 liquid chlorinated hydrocarbon (19 VOC) mixture was prepared by weighting about 1 g of each compound (which is shown in Table 2) into a volumetric flask. The injection syringe with a valve was used in the weighting process to prevent the loss of liquid chlorinated hydrocarbons with a low boiling point. A sample of the 19 VOC mixture was taken by injection syringe and weighed using the electrical balance in a calculated amount to yield a concentration of about 1 μmol/mol in 12.0 MPa nitrogen. The specific amount of 19 VOC was added into a new, evacuated, and weighed cylinder with the help of the home-made VOC gasifying apparatus for ensuring complete gasification. The same procedure was done to the premix 1 in the same cylinder. The flow diagram of the preparation of the gas standard is described in Fig. 1.
Analysis method
The standard reference material containing the 22 chlorinated hydrocarbons was analyzed using GC-FID. A 60 m by 0.53 mm i.d. capillary column coated with a 3-μm-thick film of DB-624 was used to separate the 22 chlorinated hydrocarbon compounds. The column temperature was started at 37 °C and kept for 3 min and then heated to 180 °C at 10 °C/min and held for 5 min. ChemStation software was used to integrate the peaks and then transfer the data to an Excel spreadsheet. Figure 2 shows a chromatogram of the 22 chlorinated hydrocarbon gas standard.
Representative chromatogram of the 22 chlorinated hydrocarbon gas standard. Note:1 chloromethane; 2 chloroethylene; 3 chloroethane; 4 1,1-dichloroethene; 5 dichloromethane; 6 trans-1,2-dichloroethene; 7 1,1-dichloroehtane; 8 cis-1,2-dichloroethene; 9 chloroform; 10 1,1,1-trichloroethane; 11 carbon tetrachloride; 12 1,2-dichloroehtane; 13 trichloroethylene; 14 1,2-dichloropropane; 15 1,1,2-trichloroethane; 16 tetrachloroethylene; 17 chlorobenzene; 18 1,1,1,2-tetrachlorobenzene; 19 1,1,2,2-tetrachlorobenzene; 20 1,3-dichlorobenzene; 21 1,4-dichlorobenzene; 22 1,2-dichlorobenzene
Results and discussion
Reproducibility of preparation procedure
Reproducibility of the preparation technique is an important factor in the accuracy and reliability of the reference material. Two approaches were performed to test the reproducibility. The first is to prepare five gas standards, using the described method, with concentrations ranging from 1 to 10 μmol/mol at intervals of about 2 or 3 μmol/mol. These standards are then analyzed using GC-FID. In order to minimize the error, the standards were measured at least six times. The signal response models as a function of their concentrations were set up by data fitting, and the models with their standard deviations are all listed in Table 3 which showed good reproducibility. The other approach is to determine the five gas standards at 1 μmol/mol at the pressure of 12 MPa using GC-FID. The reproducibility of the preparation method can also be evaluated by the relative standard deviation of the relative signal response (R) which was calculated by Formula (1). The results which are displayed in Table 4 showed that the relative standard deviations of R of each component were less than 0.7% and confirmed that the preparation method was reliable.
where A = peak area and C = concentration.
Cylinder selection
The cylinder selection is necessary because the various components of the gas standard may adsorb or react with each other in the active site of the inner cylinder wall. The cylinder containing the 22 chlorinated hydrocarbon gas mixture was named the mother cylinder. A dedicated pipeline which should be as short as possible was used to transfer the chlorinated hydrocarbons from the mother cylinder to another cylinder which was called sub-cylinder until the pressure equilibrated between the two cylinders. The mother cylinder and the sub-cylinder were both tested using GC-FID, and the relative deviations (E) of the signal response between these two were used to estimate cylinder quality. Imported treated cylinders (A), domestic treated cylinders (B), and domestic none-treated cylinders(C) were included. Table 5 shows the relative deviations of A, B, and C (only one cylinder data was listed in this study considering that others have shown the same trend), and the relative deviations of A and B were less than 1%, whereas, while the lower molecular mass compounds are less than 1%, the heavier molecular mass compounds appear to be a problem in the C cylinders with deviations more than 1.5%. So A and B can be used to prepare the 22 chlorinated hydrocarbon gas standard.
Stability testing at different pressures
Stability testing at different pressures was carried out in view of some chlorinated hydrocarbon gases with high molecular which may stratify in the gas cylinder and verify with the pressure. The concentration differences of all components at different pressures were used to measure the stability. Six standard reference materials were prepared to a pressure above 10 MPa, and then each cylinder was bled down to 10, 8, 6, 4, 2, and 1 MPa and the signal response of GC-FID was tested at each pressure to assay the stability. Uncertainty of instability was calculated using Formulas (2–5).
where m is the number of repeat measurements, n is the number of different pressures, N is the total number of measurements, \( \overline{Xj} \) is the average value at each pressure, and \( \overline{\overline{X}} \) is the average value of all the measured data.
The uncertainties of instability at different pressures are all shown in Table 6 (only one cylinder data was listed considering that others have the same trend) from which it can be seen that the u bb of 1,1,2,2-tetrachlorobenzene, m-dichlorobenzene, p-dichlorobenzene, and o-dichlorobenzene were significantly increased as the pressure went down and uncertainties of instability were higher than 2% when the sample pressure was lower than 2 MPa. Thus, the minimum pressure used was determined as 2 MPa.
Long-term stability testing
The long-term stability of the reference material was measured and analyzed according to the ISO Guide 35. Six cylinders of the gas standard were prepared and measured using GC-FID every 3 months over a period of 12 months. New primary gas standards were prepared before analyzing gas standard samples for long-term stability.
The concentration of each component is shown in Fig. 3 (only one cylinder data was listed, considering that others have the same trend), and no meaningful trend was observed; thus, a concentration value as a function of time set up by data fitting was used as an empirical model which is shown in Formula (7). The slopes (b 0) with its standard deviations (s(b 1)) and intercept (b 1) were computed using Formulas (8–10) and are all displayed in Table 7. The absolute value of b 1 was tested for significance by comparing with an appropriate t-factor (t 0.95,n-2 × s(b 1)), and b 1 values were all less than the control value of the t test. As a consequence, all slopes were insignificant and no instability was observed. Twenty-two chlorinated hydrocarbons in nitrogen can maintain stability for 12 months. The uncertainty contribution due to long-term stability ranged from 0.42 to 0.84%.
Certified concentration value
The comparison method was recommended by ISO 6143, and ISO 35 was used to calculate the uncertainty value of the 22 chlorinated hydrocarbon standard reference material.
The uncertainty of 22 chlorinated hydrocarbons in the gas standards included uncertainty of the certified value (u char ), uncertainty of stability at different pressures (u bb ), and uncertainty of long-term stability (u lts ). The uncertainty of the certified value (u char ) was calculated using Formula 11, and the uncertainty contribution due to long-term stability was computed using Formula 12. The related combined uncertainty (u CRM ) was calculated using Formula 13. Uncertainty of the certified value, uncertainty of stability at different pressures, uncertainty of long-term stability, and expanded uncertainty (U) where k = 2 are all displayed in Table 8.
where x i (m) is the mass fraction in the standard reference material of component i; x i (mol) is the molar fraction in the standard reference material of component i; n is the number of gas species; m i is the weight amount of component i; m p is the weight amount of diluent gas; M i is the molar weight of component i; Mp is the molar weight of diluent gas; x p is the purity of diluent gas; and x i,A is the purity of raw component i.
where t is the shelf life
Quantity comparison
To verify the accuracy of concentration of the gas standard, 15 chlorinated hydrocarbons both in TO14 and in this gas standard prepared in our lab were checked. The gas standard prepared in our lab was used as reference gas to compare with Scott Specialty Gases. The standard value (C CRM ), the uncertainty of Scott Specialty Gases which was given by suppliers (u CRM ), the measured value (C meas ), the standard deviation of measurement (SD), and the uncertainty of preparation and measurement (u meas ) are all shown in Table 9. The differences between standard value and measured value were all less than the expanded uncertainty which proved that they have a good consistency.
Conclusions
Using the described gravimetric technique, gas standard mixtures containing 22 chlorinated hydrocarbons were prepared in nitrogen at 1 μmol/mol with expanded uncertainty of 5%. The repeatability of the preparation method has been demonstrated, as well as the performance of the domestic and imported treated cylinders. Meanwhile, all the components remain homogeneous above 2 MPa in the cylinder and maintain stability no less than 12 months to keep concentration values accurate and reliable. This 22 chlorinated hydrocarbon gas standard material can be used in quality control and quality assurance for the detection of chlorinated hydrocarbon, analytical method of chlorinated hydrocarbon, and certification value for unknown concentration gas samples in the future.
References
D'Andrea MA, Reddy GK (2016) Illness symptoms experienced by children exposed to benzene after a flaring incident at the BP refinery facility in Texas City. Clin Pediatr 55:1143–1151. doi:10.1177/0009922816641463
Gao F, Calatayud V, García-Breijo F, Reig-Armiñana J, Feng Z (2016) Effects of elevated ozone on physiological, anatomical and ultrastructural characteristics of four common urban tree species in China. Ecol Indic 67:367–379. doi:10.1016/j.ecolind.2016.03.012
Gong Y, Wei Y, Cheng J, Jiang T, Chen L, Xu B (2017) Health risk assessment and personal exposure to volatile organic compounds (VOCs) in metro carriages—a case study in Shanghai, China. Sci Total Environ 574:1432–1438. doi:10.1016/j.scitotenv.2016.08.072
He Q, Yan Y, Li H, Zhang Y, Chen L, Wang Y (2015) Characteristics and reactivity of volatile organic compounds from non-coal emission sources in China. Atmos Environ 115:153–162. doi:10.1016/j.atmosenv.2015.05.066
Lei T et al (2013) Pollution characteristics of ambient volatile organic compounds (VOCs) in the southeast coastal cities of China. Environmental of Science and Pollution Research 20:2603–2615
Li L, Xie S, Zeng L, Wu R, Li J (2015) Characteristics of volatile organic compounds and their role in ground-level ozone formation in the Beijing-Tianjin-Hebei region, China. Atmos Environ 113:247–254. doi:10.1016/j.atmosenv.2015.05.021
Ling ZH, Guo H, Cheng HR, Yu YF (2011) Sources of ambient volatile organic compounds and their contributions to photochemical ozone formation at a site in the Pearl River Delta, southern China. Environ Pollut 159:2310–2319. doi:10.1016/j.envpol.2011.05.001
Lyu XP, Chen N, Guo H, Zhang WH, Wang N, Wang Y, Liu M (2016) Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci Total Environ 541:200–209. doi:10.1016/j.scitotenv.2015.09.093
Rhoderick GC (1997) Development of a fifteen component hydrocarbon gas standard reference material at 5 nmol/mol in nitrogen Fresenius. J Anal Chem 359:477–483
Rhoderick GC, Yen JH (2006) Development of a NIST standard reference material containing thirty volatile organic compounds at 5 nmol/mol in nitrogen. Anal Chem 78(9):3125–3132
Rhoderick GC, Zlellnski WL, Jr R, Miller W (1993) Gas standards containing halogenated compounds for atmospheric measurements. Environmental science & Technology 27:2849–2854
Rhoderick GC, Duewer DL, Ning L, Desirant K (2010) Hydrocarbon gas standards at the pmol/mol level to support ambient atmospheric measurements. Analytical Chmistry 82:859–867
Shao P, An J, Xin J, Wu F, Wang J, Ji D, Wang Y (2016) Source apportionment of VOCs and the contribution to photochemical ozone formation during summer in the typical industrial area in the Yangtze River Delta, China. Atmos Res 176-177:64–74. doi:10.1016/j.atmosres.2016.02.015
Sun J, Wu F, Hu B, Tang G, Zhang J, Wang Y (2016) VOC characteristics, emissions and contributions to SOA formation during hazy episodes. Atmos Environ 141:560–570. doi:10.1016/j.atmosenv.2016.06.060
Wang Q, Han Z, Wang T, Zhang R (2008) Impacts of biogenic emissions of VOC and NOx on tropospheric ozone during summertime in eastern China. Sci Total Environ 395:41–49. doi:10.1016/j.scitotenv.2008.01.059
Wei W, Cheng S, Li G, Wang G, Wang H (2014) Characteristics of ozone and ozone precursors (VOCs and NOx) around a petroleum refinery in Beijing, China. J Environ Sci 26:332–342. doi:10.1016/s1001-0742(13)60412-x
Wu R, Li J, Hao Y, Li Y, Zeng L, Xie S (2016) Evolution process and sources of ambient volatile organic compounds during a severe haze event in Beijing, China. Sci Total Environ 560-561:62–72. doi:10.1016/j.scitotenv.2016.04.030
Xie M et al (2016) Temporal characterization and regional contribution to O3 and NOx at an urban and a suburban site in Nanjing, China. Sci Total Environ 551-552:533–545. doi:10.1016/j.scitotenv.2016.02.047
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Li, N., Du, J., Yang, J. et al. Development of a standard reference material containing 22 chlorinated hydrocarbon gases at 1 μmol/mol in nitrogen. Environ Sci Pollut Res 24, 24177–24186 (2017). https://doi.org/10.1007/s11356-017-9774-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9774-y