Abstract
Use of environmentally friendly, decomposable natural products for effective vector control has gained considerable momentum in modern society. In this study, essential oil of Sphaeranthus indicus (Si-EO) was extracted and further phytochemical screening revealed fourteen compounds with prominent peak area percentage of 24.9 and 22.54% in 3,5-di-tert-butyl-4-hydroxybenzaldehyde and benzene,2-(1,1-dimethylethyl)-1,4-dimethoxy, respectively. The Si-EO was further evaluated for their larvicidal response against Culex quinquefasciatus and Aedes aegypti at different dosages (62.5, 125, 250 and 500 ppm). The Si-EO displayed prominent larvicidal activity at higher concentration (500 ppm) against both species of mosquitoes. The LC50 and LC90 values of oils were observed at 130 and 350 ppm against C. quinquefasciatus larvae and at 140 and 350 ppm against A. aegypti larvae, respectively. Repellent bioassay established higher protection rate at 200 ppm up to 120 min against both the mosquitoes. However, adulticidal response displayed higher mortality rate only at 700 and 800 ppm against C. quinquefasciatus and A. aegypti, respectively. Toxicological screening against mosquito predator Toxorhynchites splendens revealed that the Si-EO was harmless even at the concentration of 1500 ppm. Overall, these results suggest that the Si-EO plays a significant role as a new bio-rational product against ecological burden mosquito vectors which provides an eco-friendly alternative to synthetic pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insect-transmitted illness plays a chief source of disease, and principally, mosquito-borne disease alone spreads over 2.5 billion population throughout the world (World Health Organization 2014). Mosquitoes play a significant role in transmitting several communicable diseases such as dengue, filariasis, malaria and yellow fever that are dangerous to humans and other organisms (Thanigaivel et al. 2012). Aedes aegypti Linn. (Diptera: Culicidae) is a most important vector in India and some of the West Asian countries which spreads deadly pathogens that cause diseases like dengue (Hemingway and Ranson 2000; Kalaivani et al. 2012; Thanigaivel et al. 2012; Benelli 2015a, b). Culex quinquefasciatus (Diptera: Culicidae) is a house-resting mosquito (i.e., endophilic mosquito that rests inside a human dwelling) principal in several tropical nations. Lymphatic filariasis is possibly the most rapidly spreading vector-borne disease of humans in the tropical region, infecting more than 146 million population due to transmission by the mosquito vector C. quinquefasciatus (World Health Organization 1992).
There is an urgent need for control of mosquito vectors to check transmission of pathogenic disease-causing agents, and this has been the principal topic of various novel researches over the past decades. The methods used in different management programmes usually depend on the different lifecycle phases (egg, larvae, pupae, or adult) that are most vulnerable in the habitat where control is needed (Gutierrez et al. 2014). Currently, several commercially formulated biological and chemical repellents are available in the market. The insects in general and mosquito species may have resistant individuals which can be selected by use of chemicals (World Health Organization 1992; Ranson et al. 2011). These issues have created a hunt for eco-friendly insecticides for the mosquito vectors (Murugan et al. 2007).
Nature provides several bioactive products against vector mosquitoes in the form of plant products, marine products, microbial products, and other biological derivatives. Use of natural products of plant origin is recommended because they are effective, biodegradable, environmentally friendly, inexpensive, readily available and popular and generally have a low mammalian toxicity (Senthil-Nathan et al. 2006a, b; Govindarajan 2011; Gahukar 2012; Senthil-Nathan 2013, 2015; Selin-Rani et al. 2016a, b). Plant-derived essential oils, after millions of years of plant evolution, have become a natural source of insecticides, pesticides, larvicides, fragrant odorants, and repellents (Oyedele et al. 2002; Rajkumar and Jebanesan 2010). Adverse effects of commercially available repellents can be reduced by incorporating components of plant-derived essential oils (EOs) into pest-management treatments (Senthil-Nathan 2007).
The herb Sphaeranthus indicus Linn. that belongs to the family Asteraceae has been used in certain regions in India for diverse systems of traditional medicines for human disease treatment and ailments of human beings such as asthma, bronchitis, rheumatism, and dermatitis and extensively studied for its various biological activities (Nemade et al. 2012). S. indicus plant is distributed all over the plains and wetlands in India and other sub-tropical nations (Gogate 2000). Several reports are available for isolation of medically important essential oil from aromatic herbs and spices by steam distillation for numerous studies (Kumar et al. 2014). Due to their potential biological activities, they were given keen interest by investigators worldwide (Mahajan et al. 2015). Mosquito larvicidal activity of various solvent extracts of S. indicus (Si-EO) has been reported (Kovendan et al. 2012); however, to our knowledge, there is lack of research on the larvicidal, repellent and adulticidal activities of Si-EO. Thus, the objectives of the present investigation comprise the following: (a) isolation and characterization of major chemical compounds from essential oils derived from fresh leaves of S. indicus (Si-EO); (b) toxicological screening of Si-EO against two medically important mosquitoes C. quinquefasciatus and A. aegypti; and (c) evaluation of non-target impacts of Si-EO against the natural mosquito predator Toxorhynchites splendens (Wiedemann) compared with commercial pesticides. This may provide a novel and promising vector control agent that can be used in the development of cost-effective and practical means to protect humans from the environmental burden mosquitoes.
Materials and methods
Plant harvesting and essential oil extraction
Leaves of S. indicus Linn. (Fig. 1a) were freshly collected from Virudhunagar district (11°00 and 12°00 N; 77°28 and 78°50 E) which is situated in the south-western part of Tamil Nadu, India. The collected plants were washed and air-dried between filter paper sheets. The leaves were cut into pieces, and steam distillation extraction was performed using a Clevenger apparatus, as described by Craveiro et al. (1976). The Si-EO was extracted and collected in sanitized glass vials. Anhydrous sodium sulphate was used to remove water traces, and the Si-EO samples were preserved at 4 °C for further experiments.
GC-MS analysis
The isolated Si-EO samples were dissolved in (1:1) ratio with ethyl alcohol. From this, 2 μl of crude and fractions were dissolved in HPLC-grade methanol and subjected to GC and MS JEOL GC mate equipped with secondary electron multiplier. Further, procedure of chemical characterization was carried out by our previous methodology (Thanigaivel et al. 2017). The molecular weight, molecular formula and structure of the compounds of test materials were ascertained by interpretation on mass spectrum of GC-MS using the database of the National Institute Standard and Technology (NIST).
Mosquito rearing
The A. aegypti and C. quinquefasciatus culture has been maintained at the Biopesticides and Environmental Toxicology Laboratory (BET Lab) with our previous methodology (Kalaivani et al. 2012; Thanigaivel et al. 2012).
Larvicidal bioassay
Larvicidal activity was evaluated by following the methods of the World Health Organization (1981) with slight modifications (Thanigaivel et al. 2017). Twenty early fourth instars of C. quinquefasciatus and A. aegypti were introduced into a 250-ml glass beaker containing four different dilutions (62.5, 125, 250 and 500 ppm) of Si-EO. Control was maintained separately treated with acetone for EO. Throughout this experiment, no food was provided to the larvae and the rate of mortality was recorded after 24 h. The treatments were replicated five times, and each replicated set contained one control. The follow-up assays were carried out according to Finney’s (1971) probit method. Percentage mortality (Eq. (1)) in the treatments was corrected when necessary for mortality in the controls using Abbott’s (1925) equation (Eq. (2)).
where T is the test treatment and C is the control treatment in the treated group.
Repellent assay
The Si-EO were tested for its repellent activity against C. quinquefasciatus and A. aegypti mosquitoes at different concentrations of 75, 100, 150 and 200 ppm carried out using method cited in the World Health Organization (2009). One hundred gravid female mosquitoes (mated, 5–7 days post emergence) starved for 24 h without a blood meal but previously fed on 10% sucrose solution were kept in net cages (45 cm × 35 cm × 45 cm). An area of 3 × 10 cm on each forearm of three human volunteers was marked out with a permanent marker and the remaining area was covered with a paper sleeve with a hole corresponding to the marked area. Further, on the day of the assay, the volunteers had no contact with lotions and perfumed soaps. As a blank control, ethanol was placed on one forearm of the same volunteer with the same process as the test repellents, whereas the other forearm was untreated. A. aegypti was tested from 06.00 h to 14.00 h. Whereas, C. quinquefasciatus was treated from 18.00 h to 02.00 h. The control and treated arms were introduced simultaneously into the mosquito cage, and by gently tapping the sides of the experimental cages, the mosquitoes were activated. Each test concentration was repeated three times. The volunteers conducted their test of each concentration by inserting the treated and control arms into the cages at the same time for one full minute every 5 min. The mosquitoes that land on the hand were recorded and then shaken off before they imbibe any blood. The repellency was calculated by the following Eq. (3):
where Ta is the number of mosquitoes in the control group and Tb is the number of mosquitoes in the treated group.
Adulticidal activity
Sugar-fed adult female mosquitoes (4 to 7 days old) were used for adult toxicity studies. The mosquitoes were treated with impregnated filter paper (140 × 120 mm) diluted with different concentrations of Si-EO (400, 500, 600, 700 and 800 ppm). A blank paper consisting of only ethanol was used as control. The papers were left to dry at room temperature to evaporate off the ethanol overnight. Impregnated papers were prepared fresh prior to testing. The bioassay was conducted in an experimental kit consisting of two cylindrical plastic tubes both measuring 120 × 45 mm following with our previous procedure (Thanigaivel et al. 2017). One tube served to expose the mosquitoes to the seed extracts, and another tube was used to hold the mosquitoes before and after the exposure periods. The impregnated papers were rolled and placed in the exposure tube. Each tube was closed at one end with a 15-mesh size wire screen. Sucrose-fed and blood-starved mosquitoes (25) were released into the tube, and the mortality effects of the extracts were observed every 10 min for 3-h exposure.
Effects on non-target organisms
The toxic effect of Si-EO against beneficial aquatic predator was performed with adapted methodology of Sivagnaname and Kalyanasundaram (2004) with slight modifications (Thanigaivel et al. 2017). T. splendens were collected in the field at the same aquatic habitat of A. aegypti and maintained separately in containers containing water (45 cm in diameter and 15 cm in depth) maintained with 26 ± 2 °C and 85% RH (relative humidity). The non-target T. splendens were tested against the EO in comparison with the commercially used pesticides Temephos (Sigma-Aldrich, PESTANAL®, analytical standard). The different concentrations of Si-EO (900, 1000 and 1500 ppm) and Temephos (0.2, 0.4 and 0.5 ppm) were tested for its non-target effect. For each tested concentration, ten replicates were performed along with the five replicates of the untreated controls. Mortality rate was recorded after 24 h.
Data analysis
Mortality data from the treatments were exposed to variance analysis (ANOVA of arcsine-, logarithmic- and square root-transformed percentages), and mean of five replicates was expressed in the data. Tukey’s multiple-range test was used to determine the significant differences between the different treatments (significance at P < 0.05) using Minitab®17. Further, MicroCal software (SigmaPlot 12) was used to determine the enzyme activity. The lethal concentrations required to kill 50% (LC50) of the larvae in 24 h were calculated by probit analysis with a dependability interval of 95% using the Minitab®17 programme.
Results
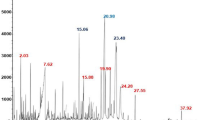
The yield percentage of the Si-EO samples was 1.5 ml/kg of leaves. The GC-MS chromatogram of the Si-EO was displayed in Fig. 1b. The essential oil volatile compounds, expressed as percentage from the total area, were shown in Table 1. It can be seen that fourteen volatile compounds up to 99.1% total area were recorded. The peak area was prominent for 3,5-di-tert-butyl-4-hydroxybenzaldehyde and benzene,2-(1,1-dimethylethyl)-1,4-dimethoxy with 24.94 and 22.54%, respectively.
The Si-EO showed prominent larval mortality with 96.80 and 96.31% at the highest concentration (500 ppm) against C. quinquefasciatus and A. aegypti, respectively. Dose-dependent mortality was observed in both the vectors (Fig. 2). The prominent mortality at 500 ppm concentration (F 3,16 = 80.87, P ≤ 0.0001) was different significantly from the 250 (F 3,16 = 80.87, P ≤ 0.035), 125 (F 3,16 = 80.87, P ≤ 0.0001), and 62.5 ppm treatments (F3,16 = 80.87, P ≤ 0.0001) and control (F 3,16 = 80.87, P ≤ 0.0001) with 82.00, 43.40, 21.00 and 5.00% mortality rate, respectively, against C. quinquefasciatus. Correspondingly, the mortality rate for the fourth instar larvae of A. aegypti at 500 ppm (F 3,16 = 68.19, P ≤ 0.0001) was significantly different from the 250 (F 3,16 = 68.19, P ≤ 0.0049), 125 (F 3,16 = 68.19, P ≤ 0.0001), and 62.5 ppm treatments (F 3,16 = 68.19, P ≤ 0.0001) and control (F 3,16 = 68.19, P ≤ 0.0001) with 80.42, 46.73, 25.31 and 5.32% mortality rate, respectively. The lethal concentrations (LC50 value) were recorded at 130 and 140 ppm against fourth instar larvae of C. quinquefasciatus (Fig. 3a) and A. aegypti (Fig. 3b), respectively.
The Si-EO gave a significant protection time against C. quinquefasciatus and A. aegypti at different dosages (Fig. 4a). Among the different concentrations tested, the prominent repellent activity was observed at 200 ppm with protection up to 210 min for both the mosquitoes. The higher concentration of 200 ppm (F 4,20 = 14.11, P < 0.004) displayed significant difference in the repellent activity to 150 (F 4,20 = 17.23, P < 0.001), 100 (F 4,20 = 21.31, P < 0.002) and 75 ppm (F 4,20 = 14.11, P < 0.004) against C. quinquefasciatus. Likewise, repellent activity of 200 ppm (F 4,20 = 15.55, P < 0.004) was found to be statistically different against other treatment concentrations of Si-EO against A. aegypti (Fig. 4b). Our results indicate that protection time was dose-dependent in both the vectors. The major phytoconstituents of the Si-EO showed complete protection against both the vectors. The Si-EO displayed 100% adulticidal mortality rate at 700 and 800 ppm against C. quinquefasciatus (Fig. 5a) and A. aegypti (Fig. 5b), respectively, within 30 min. However, other treatments did not produce >90% rates of mortality even at 90 min.
The non-target effects of the Si-EO on non-target T. splendens were low even at concentrations of 1500 ppm with mortality rate of 15.4% (F 3,16 = 23.37, P ≤ 0.001). However, Temephos at 0.5 ppm showed 97.34% mortality rate against T. splendens (Fig. 6). Besides, the development and behaviour of the mosquito predator were altered significantly after treatment with Temephos.
Discussion
Plant products have been used worldwide as vector control agents due to the diversity of biologically active compounds and reduced human toxicity compared to many synthetics (Murugan et al. 2007; Senthil-Nathan et al. 2007; Lija-Escaline et al. 2015). Screening of plants which were locally available for its mosquitocidal properties would generate eco-friendly and cost-effective means and stimulate natural efforts to improve public health against the disease-spreading vectors (Senthil-Nathan et al. 2008a, b; Benelli and Mehlhorn 2016).
Large number of plant-derived essential oils (EOs) have been reported as promising larvicidal agents against diverse mosquito vectors (Kamsuk et al. 2006; Senthil-Nathan 2007; Kalaivani et al. 2012; Benelli 2015a, b). Besides, phytochemicals derived from the plant source also displayed significant toxic responses against different mosquito vectors (Nemade et al. 2012; Pradeepa et al. 2014, 2015, 2016; Edwin et al. 2016; Govindarajan and Benelli 2016). In support to the above statement, phytochemicals derived from the Si-EO displayed promising mosquitocidal response against both the vectors. Chemical screening revealed that the peak area was prominent for 3,5-di-tert-butyl-4-hydroxybenzaldehyde and benzene,2-(1,1-dimethylethyl)-1,4-dimethoxy. Kaila et al. (2007) evaluated that 3,5-di-tert-butyl-4-hydroxybenzaldehyde showed an anti-inflammatory action and could exert actions of multiple benefits in atherosclerosis. Similarly, bicyclo[4.4.0]dec-1-ene (8.2%) compound is listed as having an antimicrobial activity (Naidoo et al. 2014). Further, previous research also proved caryophyllene as potent anti-inflammatory (Fernandes et al. 2007; Chavan et al. 2010), anti-cancer (Legault and Pichette 2007) and anti-fungal agent (Yang et al. 2000).
Controlling of vectors at the larval stage is more preferable compared to other life stages which reduces pesticide usage (Tennyson et al. 2012). The hexane fractions of S. indicus showed 100% mortality against three mosquito vectors at 10 mg/l (Vidhya and Mathew 2014). Similar to the above findings, 500 ppm concentration of Si-EO delivers approximately 97 and 95% larval mortality against C. quinquefasciatus and A. aegypti mosquitoes, respectively. It is generally accepted that botanical extracts with LC90 > 10 ppm are not effective for management of field populations (Pavela 2015). Consequently, it would be beneficial to identify and increase the fractions of compounds with the greatest effectiveness in future Si-EO studies. It should also be noted that the dosage needed to attain larval mortality depends on several factors, including larval stage, suitable temperature, and capability of the phytochemicals to enter inside the cuticle, as well as the mode and site of action (Rattan 2010; Pavela 2015).
Generally, EOs possess strong repellent properties against the mosquito vectors (Sritabutra and Soonwera 2013). However, it is important to consider that EOs used in repellent activity are not constantly harmless comparable to commercial repellents (Nerio et al. 2010; Champakaew et al. 2015). In contrast, our results did not reveal any harmful effects to the treated volunteers in the complete study period. The presence of diverse phytoconstituents increases the repellence activity of EO against diverse mosquito vectors (Trongtokit et al. 2005). Repellent activity of several EOs was linked with the presence of sesquiterpenes and monoterpenoids (Jaenson et al. 2006). Our results clearly illustrate that major compounds present in the Si-EO influenced the repellence of both the mosquitoes strongly up to 210 min. Similar to our results, Choochote et al. (2007) reported that EO derived from Zanthoxylum piperitum (L.) DC showed maximum protection period up to 1 h against A. aegypti. Likewise, Govindarajan et al. (2016a) proved Zingiber nimmonii (J. Graham) Dalzell EO showed protection up to 150 min against different mosquito vectors.
The adult mosquitoes of both the species were found to be susceptible against Si-EO treatment. The adulticidal activity of the Si-EO was more prominent at 700 and 800 ppm concentrations against both the species. Similar to our results, EO derived from Lantana camara L. established adulticidal response at 0.06 mg/cm2 against A. aegypti (Dua et al. 2010). However, Si-EO requires higher concentrations (800 ppm) to produce adulticidal actions on mosquitoes than that required for larval mortality (500 ppm). Ajaegbu et al. (2016) stated that most of the botanical EOs proved as a good larvicidal agent but failed to display their adulticidal potential against the mosquito vectors. However, in our results, Si-EO displayed decent larvicidal and adulticidal responses against both the vectors.
EO and their compounds were found to be toxic against non-targets only at higher concentrations or exposure for a longer time (Pavela 2015; Seo et al. 2012). However, our results did not exhibit significant mortality against T. splendens even at 1500 ppm concentration. This might be due to the fact that Toxorhynchites species are generally larger in size than our target mosquitoes (Benelli et al. 2016). Similarly, Pinus kesiya EO was safer towards Anisops bouvieri, Diplonychus indicus and Gambusia affinis aquatic organisms with LC50 readings ranging from 4135 to 8390 mg/ml (Govindarajan et al. 2016b). Synthetic pesticides exhibit direct harmful effects towards beneficial insects and display toxic effects on their development and behaviour (Vasantha-Srinivasan et al. 2016, 2017; Ponsankar et al. 2016a, b). Correspondingly, Temephos produce severe toxic response against T. splendens at 0.5 ppm. Therefore, the current results suggest that the Si-EO can be considered completely safe against the mosquito predator.
Conclusion
The application of botanical pesticide against mosquito vectors will not only diminish our reliance on chemical pesticides but also encourage eco-friendly and sustainable means of vector control. In conclusion, the EO extracted from the bio-rational plant S. indicus was analysed by GC-MS and evaluated for its larvicidal, repellent and adulticidal activities against C. quinquefasciatus and A. aegypti. Both mosquito larvae are susceptible to Si-EO at the higher dosage of treatments. Further, the repellent and adulticidal actions were promising. Moreover, the toxicological screening against mosquito predator also confirms that the Si-EO can be considered completely safer on the non-target insects. Overall, the present investigation encourages other scientific circles to move forward in the EO research efforts against medically challenging mosquito vectors.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18: 265–267
Ajaegbu EE, Danga SPY, Chijoke IU, Okoye FBC (2016) Mosquito adulticidal activity of the leaf extracts of Spondias mombin L. against Aedes aegypti L. and isolation of active principles. J Vector Borne Dis 53:17–22
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114:2801–2805
Benelli G (2015b) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114(9):3201–3212
Benelli G, Mehlhorn H (2016) Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–1754
Benelli G, Jeffries CL, Walker T (2016) Biological control of mosquito vectors: past, present, and future. Insects 7(52):1–18
Champakaew D, Junkum A, Chaithong U, Jitpakdi A, Riyong D, Sanghong R, Intirach J, Muangmoon R, Chansang A, Tuetun B, Pitasawat B (2015) Angelica sinensis (Umbelliferae) with proven repellent properties against Aedes aegypti, the primary dengue fever vector in Thailand. Parasitol Res 114(6):2187–2198
Chavan MJ, Wakte PS, Shinde DB (2010) Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. bark. Phytomed 17(2):149–151
Choochote W, Chaithong U, Kamsuk K, Jitpakdi A, Tippawangkosol P, Tuetun B, Champakaew D, Pitasawat B (2007) Repellent activity of selected essential oils against Aedes aegypti. Fitoterapia 78:359–364
Craveiro AA, Matos FJA, Alencar JW (1976) A simple and inexpensive steam generator for essential oils extraction. J Chem Edu 53:652
Dua VK, Pandey AC, Dash AP (2010) Adulticidal activity of essential oil of Lantana camara leaves against mosquitoes. Indian J Med Res 131:434–439
Edwin E, Vasantha-Srinivasan P, Senthil-Nathan S, Thanigaivel A, Ponsankar A, Pradeepa V, Selin-Rani S, Kalaivani K, Hunter WB, Abdel-Megeed A, Duraipandiyan V, Al-Dhabi NA (2016) Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae). Acta Trop 163:167–178
Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, Pianowski LF, Calixto JB (2007) Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. European J Pharmacol 569(3):228–236
Finney DJ (1971) Probit Analysis. 3rd ed., Cambridge University Press, Cambridge
Gahukar RT (2012) Evaluation of plant-derived products against pests and diseases of medicinal plants: a review. Crop Prot 42:202–209
Gogate VM (2000) Ayurvedic pharmacology and therapeutic uses of medicinal plants (Dravyaganvigyan). Bhartiya Vidya Bhavan, Mumbai, pp 112–114
Govindarajan M (2011) Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med 4(2):106–111
Govindarajan M, Benelli G (2016) α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol Res. doi:10.1007/s00436-016-5025-2
Govindarajan M, Rajeswary M, Arivoli S, Tennyson S, Benelli G (2016a) Larvicidal and repellent potential of Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae) essential oil: an eco-friendly tool against malaria, dengue, and lymphatic filariasis mosquito vectors? Parasitol Res 115(5):1807–1816
Govindarajan M, Rajeswary M, Benelli G (2016b) Chemical composition, toxicity and non-target effects of Pinus kesiya essential oil: an eco-friendly and novel larvicide against malaria, dengue and lymphatic filariasis mosquito vectors. Ecotoxicol Environ Saf 129:85–90
Gutierrez PM, Antepuesto AN, Eugenio BAL, Santos MFL (2014) Larvicidal activity of selected plant extracts against the dengue vector Aedes aegypti mosquito. Int Res J Biol Sci 3(4):23–32
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Jaenson TGT, Garbouli S, Palsson K (2006) Repellency of oils of lemon eucalyptus, geranium, and lavender and the mosquito repellent MyggA natural to Ixodes ricinus (Acari: Ixodidae) in the laboratory and field. J Med Entomol 43(4):731–736
Kaila R, Rao CM, Kutty NG (2007) Synthesis and evaluation of the anti-inflammatory activity of N-[2-(3,5-ditert-butyl-4-hydroxyphenyl)-4-oxothiazolidin-3-yl]-nicotinamide. ArzneimittelForsch 57(9):616–622
Kalaivani K, Senthil-Nathan S, Murugesan AG (2012) Biological activity of selected Lamiaceae and Zingiberaceae plant essential oils against the dengue vector Aedes aegypti L. (Diptera: Culicidae). Parasitol Res 110:1261–1268
Kamsuk K, Choochote W, Chaithong U, Jitpakdi A, Tippawangkosol P, Riyong D, Pitasawat B (2006) Effectiveness of Zanthoxylum piperitum-derived essential oil as an alternative repellent under laboratory and field applications. Parasitol Res 100:339–345
Kovendan K, Murugan K, Vincent S (2012) Evaluation of larvicidal activity of Acalypha alnifolia Klein ex Wild. (Euphorbiaceae) leaf extract against the malaria vector, Anopheles stephensi, dengue vector, Aedes aegypti and bancroftian filariasis vector, Culex quinquefasciatus. Parasitol Res 110:571–581
Kumar S, Mishra M, Wahab N, Warikoo R (2014) Larvicidal, repellent, and irritant potential of the seed-derived essential oil of Apium graveolens against dengue vector, Aedes aegypti L. (Diptera: Culicidae). Fronte in public health 2:147
Legault J, Pichette A (2007) Potentiating effect of b-caryophyllene on anticancer activity of a-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol 59:1643–1647
Lija-Escaline J, Senthil-Nathan S, Thanigaivel A, Pradeepa V (2015) Physiological and biochemical effects of chemical constituents from Piper nigrum Linn (Piperaceae) against the dengue vector Aedes aegypti Liston (Diptera: Culicidae). Parasitol Res 114(11):4239–4249
Mahajan NG, Chopda MZ, Mahajan RT (2015) A review on Sphaeranthus indicus Linn: multipotential medicinal plant. Int J Pharma Res Allied Sci 4(3):48–74
Murugan K, Murugan P, Noortheen A (2007) Larvicidal and repellent potential of Albizzia amara Boivin and Ocimum basilicum Linn against dengue vector, Aedes aegypti (Insecta: Diptera: Culicidae). Bioresour Technol 98:198–201
Naidoo Y, Sadashiva CT, Kasim N, Nicholas A, Naidoo G (2014) Chemical composition and antimicrobial activity of the essential oil of Ocimum obovatum E. Mey. ex Benth. (Lamiaceae). J Essent Oil Bear Pl 17(1):132–147
Nemade NV, Chopda MZ, Mahajan RT (2012) No evidence of antiprotozoal property of asteracid weeds Sphaeranthus indicus Linn. J Pharm Negative Results 3(1):46–48
Nerio LS, Olivero-Verbel J, Stashenko E (2010) Repellent activity of essential oils: a review. Bioresour Technol 101:372–378
Oyedele AO, Gbolade AA, Sosan MB, Adewoyin FB, Soyelu OL (2002) Formulation of an effective mosquito-repellent topical product from lemongrass oil. Phytomedicine 9:259–262
Pavela R (2015) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crop Prod 76:174–187
Ponsankar A, Vasantha-Srinivasan P, Senthil-Nathan S, Thanigaivel A, Edwin E, Selin-Rani S, Kalaivani K, Hunter WB, Alessandro RT, Abel-Megeed A, Paik C, Duraipandiyan V, Al-Dhabi NA (2016a) Target and non-target toxicity of botanical insecticide derived from Couroptia guianensis L. flower against generalist herbivore, Spodoptera litura Fab. and an earthworm, Eisenia foetida Savigny. Ecotoxicol Environ Saf 133:260–270
Ponsankar A, Vasantha-Srinivasan P, Thanigaivel A, Edwin E, Selin-Rani S, Chellappandian M, Kalaivani K, Annamalai M, Hunter WB, Alessandro RT, Duraipandiyan V, Al-Dhabi NA (2016b) Response of Spodoptera litura Fab. (Lepidopteran: Noctuidae) larvae to Citrullus colocynthis L. (Cucurbitales: Cucurbitales) chemical constituents: larval tolerance, food utilization and detoxifying enzyme activities. Physiol Mol Plant Pathol. doi:10.1016/j.pmpp.2016.12.006
Pradeepa V, Sathish-Narayanan S, Kirubakaran SA, Senthil-Nathan S (2014) Antimalarial efficacy of dynamic compound of plumbagin chemical constituent from Plumbago zeylanica Lin (Plumbaginaceae) against the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res 113(8):3105–3109
Pradeepa V, Sathish-Narayanan S, Kirubakaran SA, Thanigaivel A, Senthil-Nathan S (2015) Toxicity of aristolochic acids isolated from Aristolochia indica Linn (Aristolochiaceae) against the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Exp Parasitol 153:8–16
Pradeepa V, Senthil-Nathan S, Sathish-Narayanan S, Selin-Rani S, Vasantha-Srinivasan P, Thanigaivel A, Ponsankar A, Edwin E, Sakthi-Bagavathy M, Kalaivani K, Murugan K, Duraipandiyan V, Al-Dhabi NA (2016) Potential mode of action of a novel plumbagin as a mosquito repellent against the malarial vector Anopheles stephensi, (Culicidae: Diptera). Pestic Biochem Physiol 134:84–93
Rajkumar S, Jebanesan A (2010) Chemical composition and larvicidal activity of leaf essential oil from Clausena dentata (Willd) M. Roam. (Rutaceae) against the chikungunya vector, Aedes aegypti Linn. (Diptera: Culicidae). J Asia Pac Entomol 13:107–109
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V (2011) Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27(2):91–98
Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 29:913–920
Selin-Rani S, Senthil-Nathan S, Revathi K, Chandrasekaran R, Thanigaivel A, Vasantha-Srinivasan P, Ponsankar A, Edwin E, Pradeepa V (2016a) Toxicity of Alangium salvifolium Wang chemical constituents against the tobacco cutworm Spodoptera litura Fab. Pest Biochem Physiol 126:92–101
Selin-Rani S, Senthil-Nathan S, Thanigaivel A, Vasantha-Srinivasan P, Edwin E, Ponsankar A, Lija-Escaline J, Kalaivani K, Abdel-Megeed A, Hunter WB, Alessandro RT (2016b) Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere 165:257–267
Senthil-Nathan S (2007) The use of Eucalyptus tereticornis Sm. (Myrtaceae) oil (leaf extract) as a natural larvicidal agent against the malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour Technol 98:1856–1860
Senthil-Nathan S (2013) Physiological and biochemical effect of Neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front Physiol 4(359):1–17
Senthil-Nathan S (2015) A review of bio pesticides and their mode of action against insect pests. In: Environmental Sustainability-Role of Green Technologies. Springer-Verlag, pp. 49–63
Senthil-Nathan S, Kalaivani K, Sehoon K (2006a) Effects of Dysoxylum malabaricum Bedd. (Meliaceae) extract on the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour Technol 97:2077–2083
Senthil-Nathan S, Savitha G, George DK, Narmadha A, Suganya L, Chung PG (2006b) Efficacy of Melia azedarach L. extract on the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour Technol 97:1316–1323
Senthil-Nathan S, Choi MY, Paik CH, Seo HY (2007) Food consumption, utilization, and detoxification enzyme activity of the rice leaffolder larvae after treatment with Dysoxylum triterpenes. Pest Biochem Physiol 88(3):260–267
Senthil-Nathan S, Hisham A, Jayakumar G (2008a) Larvicidal and growth inhibition of the malaria vector Anopheles stephensi by triterpenes from Dysoxylum malabaricum and Dysoxylum beddomei. Fitoterapia 79:106–111
Senthil-Nathan S, Choi MY, Seo HY, Paik CH, Kalaivani K, Kim JD (2008b) Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown plant hopper Nilaparvata lugens (Stal). Ecotoxicol Environ Saf 70:244–250
Seo S, Park H, Park I (2012) Larvicidal activity of ajowan (Trachyspermum ammi) and Peru balsam (Myroxylon pereira) oils and blends of their constituents against mosquito, Aedes aegypti, acute toxicity on water flea, Daphnia magna, and aqueous residue. J Agric Food Chem 60:5909–5914
Sivagnaname N, Kalyanasundaram M (2004) Laboratory evaluation of methanolic extract of Atlantia monophylla (Family: Rutaceae) against immature stages of mosquitoes and non-target organisms. Mem Inst Oswaldo Cruz, Rio de Janeiro 99(1):115–118
Sritabutra D, Soonwera M (2013) Repellent activity of herbal essential oils against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) Asian Pac J Trop Dis 3(4):271–276
Tennyson S, Ravindran KJ, Arivoli S (2012) Screening of twenty five plant extracts for larvicidal activity against Culex quinquefasciatus Say (Diptera: Culicidae). Asian Pac J Trop Biomed 2(2):S1130–S1134
Thanigaivel A, Chandrasekaran R, Revathi K, Nisha S, Sathish-Narayanan S, Kirubakaran SA, Senthil-Nathan S (2012) Larvicidal efficacy of Adhatoda vasica (L.) Nees against the bancroftian filariasis vector Culex quinquefasciatus Say and dengue vector Aedes aegypti L. in in vitro condition. Parasitol Res 110:1993–1999
Thanigaivel A, Vasantha-Srinivasan P, Senthil-Nathan S, Edwin E, Ponsankar A, Chellappandian M, Selin-Rani S, Lija-Escaline J, Kalaivani K (2017) Impact of Terminalia chebula Retz. against Aedes aegypti L. and non-target aquatic predatory insects. Ecotoxicol Environ Saf 137:210–217
Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C (2005) Comparative repellency of 38 essential oils against mosquito bites. Phytother Res 19:303–309
Vasantha-Srinivasan P, Senthil-Nathan S, Thanigaivel A, Edwin E, Ponsankar A, Selin-Rani S, Pradeepa V, Sakthi-Bhagavathy M, Kalaivani K, Hunter WB, Duraipandiyan V, Al-Dhabi NA (2016) Developmental response of Spodoptera litura Fab. to treatments of crude volatile oil from Piper betle L. and evaluation of toxicity to earthworm, Eudrilus eugeniae Kinb. Chemosphere 155:336–347
Vasantha-Srinivasan P, Senthil-Nathan S, Ponsankar A, Thanigaivel A, Edwin E, Selin-Rani S, Chellappandian M, Pradeepa V, Lija-Escaline J, Kalaivani K (2017) Comparative analysis of mosquito (Diptera: Culicidae: Aedes aegypti Liston) responses to the insecticide Temephos and plant derived essential oil derived from Piper betle L. Ecotoxicol Environ Saf 139:439–446
Vidhya PT, Mathew N (2014) Bioassay guided fractionation of Sphaeranthus indicus extract against mosquito vectors. Int J Pharma Sci Res 5(9):3965–3971
World Health Organization (1981) Instruction for determining the susceptibility or resistance of mosquito larvae to insecticides. WHOVBC 81(807):1–6
World Health Organization (1992) Vector resistance to pesticides. Fifteenth report of the WHO expert committee on vector biology and control. WHO technical report, Geneva
World Health Organization (2009) Guidelines for efficacy testing of mosquito repellents for human skins. WHO, Geneva, pp 4–18 WHO/CDS/NTD/WHOPES/2009.4
World Health Organization (2014) A global brief on vector-borne diseases. WHO, Geneva
Yang D, Michel L, Chaumont J, Millet-Clerc J (2000) Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia 148:79–82
Acknowledgements
Author MC was supported by the Department of Science and Technology, Science and Engineering Research Board (SERB), Government of India (File No. PDF/2016/001185).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Chellappandian, M., Thanigaivel, A., Vasantha-Srinivasan, P. et al. Toxicological effects of Sphaeranthus indicus Linn. (Asteraceae) leaf essential oil against human disease vectors, Culex quinquefasciatus Say and Aedes aegypti Linn., and impacts on a beneficial mosquito predator. Environ Sci Pollut Res 25, 10294–10306 (2018). https://doi.org/10.1007/s11356-017-8952-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8952-2