Abstract
Mercury (Hg) exposure represents a significant public health concern at a global level. This study aims at assessing Hg exposure and risk among Lebanese young adults based on Hg biomonitoring and seafood intake. A group of 166 young adults were administered a questionnaire to assess Hg exposure and were asked to provide a hair sample. Risk assessment was performed: (1) using the US Environmental Protection Agency Hazard Quotient (HQ) model based on fish intake and previously studied local fish Hg concentrations, and (2) by determining the total hair Hg concentration (THHg) using continuous flow-chemical vapor generation atomic absorption spectrometry. Differences in THHg across demographic and exposure subgroups were tested using t test or ANOVA. Correlations between THHg concentrations, fish consumption, and HQ were determined by computing Pearson’s r. Higher THHg correlated with higher consumption of Mediterranean rabbitfish/spinefoots (r = 0.27; p = 0.001) and geographical location (p < 0.001) in the bivariate analysis, and remained significant in the adjusted multivariable linear regression model (geographical location: ß = 0.255, 95%CI 0.121–0.388; rabbitfish/spinefoots consumption: ß = 0.016, 95%CI 0.004–0.027). No significant correlations were found between HQ and THHg. In conclusion, this is the first study examining hair Hg levels and fish consumption in a young adult Lebanese population. Our findings constitute valuable baseline data for a local fish advisory and Hg monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a global environmental contaminant introduced to the environment from anthropogenic sources, particularly medical wastes, cement, power plants, and various industrial applications (Clarkson 1997). Hg pollution reaches aquatic systems where it undergoes biomethylation to methylmercury (MeHg). MeHg is readily taken up by fish through trophic webs, consequently bioaccumulating and biomagnifying into the top predating fish, in addition to fish-eating birds, and wild mammals (Gochfeld 2003). This makes fish consumption the primary source of Hg exposure in the general population (Nyland et al. 2011; Schaefer et al. 2014). In New Jersey, USA, one third of the sampled saltwater fish species were found to have concentrations of Hg above 0.5 ppm, which pose a human health risk for consumers (Burger and Gochfeld 2011). In addition, according to a study conducted by the US Environmental Protection Agency (EPA), around 50% of the human population living in the vicinity of US lakes had Hg tissue concentrations above 0.3 ppm (Olsen et al. 2009).
Through consumption of Hg in fish and food, populations in many countries, such as Japan, Iraq, Ghana, Seychelles, and the Faroe Islands, have been confronted with major outbreaks of Hg-induced diseases and mortality (Tchounwou et al. 2003). Hg was responsible for much toxic exposure events in history, such as the pink disease in infants (1900s), the Minimata disease in Japan (1932–1956), and the Iraq poison grain outbreak (1971) (Bakir et al. 1973; Clarkson et al. 1976; Harada 1995). These outbreaks allowed the identification of Hg primary target organs in humans, particularly the nervous system (Clarkson et al. 2003; Eto et al. 1992). MeHg, in particular, has lipophilic characteristics allowing it to penetrate the blood–brain barrier to the central nervous system (Aschner et al. 1992). Adverse health effects from Hg exposure may include tremor, impaired vision and hearing, paralysis, insomnia, emotional instability, developmental deficits during fetal development, attention deficit, and cognitive impairment during childhood (Clarkson et al. 2003).
Maternal exposure to MeHg during pregnancy was associated with psychomotor retardation, defects in attention, and motor dysfunction in multiple ethnic groups (Halbach et al. 2008; Sandborgh-Englund et al. 1998). In addition, both higher blood Hg concentrations and increased maternal fish consumption in the second trimester were associated with lower scores in cognitive and visual abilities among children in a US prospective cohort study (Oken et al. 2008). While fetal brain seems to be more susceptible to Hg toxicity, a major concern for adults involves evidence of Hg antagonizing the positive cardio-protective effects of omega-3 fatty acids, which is also normally acquired through fish consumption (Yoshizawa et al. 2002).
The Mediterranean Sea is known to be a hot spot for Hg, harboring about half of the world’s natural Hg deposits (Covelli et al. 2012). Total Hg concentrations were reported to be the highest in the liver of aquatic mammals (Squadrone et al. 2015), ranging between 0.246 and 1.74 mg/kg-DW in tuna and other types of fish caught along the Italian shore and in deep sea (Di Bella et al. 2015; Spada et al. 2012).
In addition, a strong correlation was found between frequencies of fish consumption in the Mediterranean area and total Hg levels in cord blood (Miklavcic et al. 2013). At the same time, populations living in the coastal zones on the Mediterranean Basin—about 150 million individuals—are known to have food habits revolving around local seafood consumption (Brambilla et al. 2013).
Total Hg and MeHg concentrations in the hair of fish consumers are linearly related (Pellizzari et al. 1999), with total hair Hg concentration (THHg) estimated to be 150- to 200-fold higher than Hg concentrations in blood (Gill et al. 2002). MeHg from fish consumption usually constitutes at least 80% of the total Hg analyzed in hair among fish consumers (Cernichiari et al. 1995). Therefore, THHg is considered a reliable biomarker for MeHg exposure and is often used to characterize Hg exposures as it provides a simple, integrative, and noninvasive matrix for estimating long-term average exposure (WHO, UNEP 2008). According to the US National Health and Nutrition Examination Survey (NHANES), geometric mean hair Hg concentrations were 3-fold higher among frequent fish consuming women of childbearing age compared to nonconsumers (McDowell et al. 2004). In addition, THHg increased with fish consumption and age in women of childbearing age in the state of Florida (Traynor et al. 2013). Similarly, THHg in groups that consume fish were found to be significantly higher than groups that never consumed fish in Northern Taiwan (Chien et al. 2010).
In Lebanon, a recent survey conducted by the Ministry of Environment in partnership with the United Nations Development Program (UNDP) revealed that 84% of hospitals still use Hg-based medical devices and products (UNDP 2012). The healthcare sector in Lebanon is considered as the highest contributor to environmental Hg pollution, with an estimated burden of 31 kg of Hg released annually (UNDP 2012). Healthcare facilities introduce Hg to the environment by incineration of medical wastes and by release of untreated medical wastewater, especially in the absence of a clear national waste management strategy, a problem further exacerbated by the recent solid waste crisis. So far, Lebanon has not ratified the Minimata Convention on global Hg banning. A previously conducted study demonstrates high total Hg accumulation in local fish caught along the Lebanese shore, with concentrations reaching up to 0.57 μg/g (Obeid et al. 2011). The present study aims at assessing Hg health risk in a young adult Mediterranean subpopulation in Lebanon, based on Hg biomonitoring and seafood intake.
Materials and methods
Study design and population
The study used a cross-sectional design. Participants attended an institution of higher education—with English as the main language of instruction—located in Kurah in the Northern Governorate and in the capital city Beirut (Fig. 1). To garner interest in the study, a description of the project and its goals was announced to all students by group email, displaying contact information, and encouraging students to visit recruitment booths set up on both campuses for purposes of recruitment and data collection. Recruitment ran from 9:00 am to 3:00 pm during weekdays between the months of April and July 2015. No compensation, reward, or incentive was offered in exchange for participation in the study. All volunteering students aged 18–25 years were recruited in the study. Accordingly, a sample of 166 male and female participants was identified.
Young adults were targeted to allow assessment of cumulative Hg exposure early on in life. Targeting university students has the additional benefit of controlling for potential occupational exposure to Hg. The sample size was guided by power analysis to obtain a mean hair Hg concentration with a margin of error of 0.03 μg/g, assuming a population standard deviation for hair Hg concentrations of 0.1 μg/g and a 95% confidence interval (Traynor et al. 2013).
Data collection
Institutional Review Board approval was obtained prior to conduction of the study. All participants signed an informed consent form before the interview. Trained research assistants interviewed participants in English, measured their body weight, and obtained a hair sample. Information sought included age, sociodemographics, area of education and residence, and the following potential sources of Hg: (1) fish consumption, (2) occupation if any, (3) presence of dental amalgam, (4) use of hair dyes, (5) flu vaccination, and (6) use of skin lightening creams (Al-Saleh and Al-Doush 1997). A recall period of 2 months was used for self-reported data in order to approximate the exposure period captured by the hair biomarker.
Fish consumption
A matrix was built to assess consumption of the different types of fish based on species normally caught along the Lebanese shore and constituting the majority of the species consumed by the population. The fresh fish by their English name/scientific name (local common name) included the following: shrimp/Penaeus species (Kraydiss), yellowstripe barracuda/Sphyraena chrysotaenia (Mallifa), white seabream/Diplodus sargus (Sargous), bogue/Boops boops (Ghobbos), gilt-head seabream/Sparus aurata (Ajaj), sand smelt/Atherina boyeri (Bezri), rabbitfish/spinefoots/Siganus luridus (Jarbidi/Abou Shawki), European pilchard/Sardina pilchardus (Zellek), parrot-fish/Sparisoma cretense (Sardine), and yellow goatfish/Upeneus moluccensis (Sultan Ibrahim). The frozen and canned fish, whether locally processed or imported, consisted of tuna, European pilchard, salmon, fish fillet and fish fingers, and others. Fish consumption was determined as the number of servings of each type of fish per week, and daily intake amounts were computed assuming a serving is equivalent to 120 g of fish (Chien et al. 2010). Interviewers provided participants with visual aids to help them assess the serving size and to recognize accurately the different types of fish.
Hair sampling
Hair collection was performed with stainless steel scissors cutting as close as possible to the scalp. Samples were collected from the occipital region of the head at one or more areas targeting 100–150 strands (equivalent to about 1 g of hair). Collected hair was trimmed in a way to preserve only about 2–3 cm of hair closest to the scalp and discarding the rest. Assuming hair grows at a rate of 1–1.5 cm per month, Hg concentrations measured in preserved hair samples are considered temporally consistent with the recall period of 2 months used for data collected via the administered questionnaire. Collected samples were stored on site in labeled 50-mL capped conical sterile polypropylene tubes and stored at 4 °C until lab analysis was performed.
Determination of THHg
All equipment and glassware used throughout the laboratory analysis were soaked in 10% ultrapure HNO3 solution overnight and rinsed three times with distilled deionized water (ddH2O) prior to their use. In preparation for total Hg analysis, each collected hair sample was removed from the stored tube using acid-pretreated sterilized stainless steel tweezers and placed in precleaned acid-treated 50-mL conical tubes. Each sample was then rinsed several times with acetone followed by rinsing five times with ddH2O to remove contaminants. The samples were then oven-dried at 100 °C for 2 h. Once dried, each hair sample was subdivided into three samples using precleaned stainless steel scissors in preparation for the microwave-acid-assisted digestion. Obtained samples were transferred to a high pressure and temperature Teflon-microwave reaction vessel followed by the addition of 5 mL of 65% HNO3. The vessels were then left open under a fume hood for 30 min prior to digestion. Vessels were then sealed and placed in the microwave oven where samples were digested using a three-step program: (1) room temperature (RT) to 200 °C, (2) holding at 200 °C for 30 min (pressure ≤ 10 bars), and (3) bringing to RT. The digests were then placed into precleaned 50-mL volumetric flasks where 30 mL of K2Cr2O7 (6% m/v) were added and left open under a fume hood for 2 h to ensure complete Hg reduction. Hydroxylamine chloride solution (7.5 mL) (20% m/v) were then added slowly into each volumetric flask so as to minimize any foaming and loss of sample. Finally, the volume in each flask was adjusted to the 50 mL mark using ddH2O. To obtain matrix-matched calibration curve, a five-point calibration curve with standards ranging from 0 to 8 μg Hg/L was prepared using a 1000 μg Hg/L stock solution (Hach Lange GmbH, Germany) as described (Obeid et al. 2011). Each standard solution was subjected to the same procedure as described above for the collected samples. Quantitation of total Hg in each sample was conducted in a graphite furnace atomic absorption spectrometer equipped with a vapor generation accessory (M-series Atomic Absorption Spectrometry with VP100, Thermo Scientific, USA).
Quality assurance and control
All specimens were run in batches that included subsamples, digestion blanks, matrix-matched standard calibration curves, as well as Certified Reference Material (CRM IAEA-086 methylmercury, total Hg in human hair, obtained from the International Atomic Energy Agency, Austria) to test the validity of all protocols used. All samples were analyzed in triplicate, and the mean THHg was reported for each sample. Digestion blanks were used randomly between samples to check for any cross-contamination that might occur from sample to sample. In addition, software programming of periodic analysis of standards from the curve was used to test for any instrumental variations during the analysis. The minimum percent CRM recovery from all batches was 90.4%, and the relative standard deviation (Rsd) was 1.27%. The detection limit for total mercury in hair was 14 ng/g. Whenever the values for hair Hg were below the limit of detection (LOD), a fill value equal to the batch-specific LOD divided by the square root of 2 was used (Taylor 1987).
Health risk assessment
Health risk assessment was performed according to the EPA model (USEPA 1989). A health risk, expressed as the hazard quotient (HQ), was calculated based on the comparison of estimated Hg exposure to the reference dose (RfD). The RfD is a reliable risk-characterization tool derived from animal testing and broadly used as a measure of the acceptability of population exposure levels. RfD is also used to guide risk managers and policy makers on related decisions ranging from fish consumption advisories to air emission regulations. For noncarcinogenic effects, an HQ exceeding 1.0 usually indicates a potential health risk. Exposure to Hg was estimated based on collected data on fish dietary intake and based on fish Hg concentrations previously determined (Obeid et al. 2011). The total Hg daily exposure dose (HgD) from ingestion of the different types of fish was calculated according to the US Agency for Toxic Substances and Disease Registry (ATSDR) guidelines (ATSDR 2005) as follows:
where
- C :
-
Arithmetic mean concentration of mercury in each type of fish (μg/g)
- IR :
-
Ingestion rate for each type of fish (g/day)
- EF :
-
Exposure factor (unitless); since the fish intake rate is a daily average, the exposure factor is equal to 1
- CF:
-
Conversion factor; since both C and IR are expressed in grams of fish, CF is equal to 1
- BW :
-
Collected individual body weight (kg)
- RfD :
-
EPA’s mercury reference dose (0.1 μg/kg BW/day)
Exposure to Hg was also estimated based on THHg as compared to the EPA hair Hg reference value of 1 μg/g (USEPA 1997).
Statistical analysis
Descriptive statistics for age, gender, education, and socioeconomic status were calculated to describe the study population. Both the arithmetic mean and the geometric mean (GM), and standard deviations, were used for normally distributed continuous variables, and percentages were used for categorical variables. The distribution of THHg was described by calculating the mean, 95% confidence interval, and range. The percentage of participants who had a THHg above the EPA reference dose (1 μg/g) was also determined. ANOVA was conducted to test for differences in mean hair Hg concentrations across demographic and exposure subgroups, and Pearson’s r was computed for correlation between THHg, fish intake, and the HQ. A multivariable linear regression was used to evaluate the impact of fish consumption rates on hair Hg concentrations adjusting for potential confounders, including socioeconomic status, occupational Hg exposure, dental amalgam, hair dyes, flu vaccination, and use of skin lightening creams. All variables with a p value <0.1 at the bivariate level were entered in the multivariable linear regression model. The normality assumption of the residuals was assessed by the Kolmogorov-Smirnov normality test. All analyses were conducted using the Statistical Package for Social Sciences (SPSS version 23.0 for Windows). All tests were two-tailed, and a p value <0.05 was considered statistically significant.
Results and discussion
Descriptive characteristics of the study sample and THHg
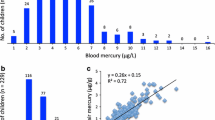
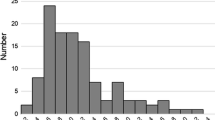
Descriptive characteristics of the study sample and bivariate analysis for THHg with covariates are summarized in Table 1. The arithmetic mean THHg for the entire study population was 0.68 μg/g (95% CI 0.61–0.75; range 0.01–2.33 μg/g), and the geometric mean (GM) was 0.43 μg/g (95% CI 0.35–0.53). THHg exceeded the EPA reference dose of 1 μg/g in 19.3% of study participants. The average body weight was 63 kg. Thirty-nine percent of the participants were from the Beirut campus. Twenty participants (12%) dyed their hair in the last 60 days prior to the study, 10 (6%) had a flu vaccine in the last 6 months, 23 (14%) had dental fillings with amalgam, and 12 (7%) reported using a skin lightning cream. In addition, 24 (15%) reported awareness of a recent Hg exposure.
Results showed a significant association between THHg and campus location. Participants attending the campus in the northern part of the country showed significantly higher hair Hg concentrations compared to participants from the capital city Beirut (0.80 vs. 0.50 μg/g, p value < 0.001).
Fish consumption, HQ, and THHg
Results showed that 85% of the total sample consists of fish consumers. When exploring the association between amounts of fish consumption for the different species and THHg, we found that participants who consumed greater quantities of rabbitfish/spinefoots (Jarbidi/Abou Shawki) show higher THHg (r = 0.27; p = 0.001). Associations with all other types of fish were not statistically significant (Table 2).
We also computed the HQ based on fish consumption amounts and previously obtained local fish Hg concentrations. The mean HQ was 1.62 (SD = 1.79; range 0–13.69), and 60.8% of participants had an HQ >1. No significant correlation was found between HQ and THHg (r = 0.038; p = 0.644).
Multivariable analysis
Family’s income, campus location, and rabbitfish/spinefoots (Jarbidi/Abou Shouki) consumption had a p value <0.1 at the bivariate level and were therefore adjusted for in the multivariable linear regression model predicting hair Hg concentrations. Interactions between fish consumption and campus location were found to be not statistically significant. In the adjusted model, campus location (North vs. Beirut: ß = 0.255, 95%CI 0.121–0.388) and rabbitfish/spinefoots consumption (ß = 0.016, 95%CI 0.004–0.027) remained significant predictors of THHg (adjusted r 2 = 0.135). The p value of the Kolmogorov-Smirnov normality test of the residual was 0.2, indicating that the normality assumption of the residuals for the linear regression holds.
Discussion
The World Health Organization considers Hg among the top ten chemicals of major public health concern (WHO, UNEP 2008). Fish and seafood consumption pose a major health risk due to its Hg content. This study assessed health risk in a population of Lebanese young adults based on THHg and fish consumption habits. Consumption of the Mediterranean rabbitfish/spinefoots, and geographical location, were the most significant predictors of THHg in the studied population. Our results support findings from earlier studies, indicating that hair Hg is influenced by fish intake (Bjornberg et al. 2005; Morrissette et al. 2004).
Several aspects of our findings need to be emphasized. First, both the arithmetic mean and the GM THHg in the study population (0.68 and 0.43 μg/g, respectively) were generally higher than those reported in North American populations; the mean hair Hg concentration was 0.37–0.59 μg/g in a group of 211 men and women from lakeside communities in Quebec, Canada (Abdelouahab et al. 2008), and 0.25 μg/g in a subgroup of 159 young adults aged 18–24 years in the State of Florida (Traynor et al. 2013), while the GM hair Hg concentrations was 0.2 μg/g in a US nationwide study of 1726 women of childbearing age (McDowell et al. 2004). Our reported mean THHg was also higher than that reported for a community in North East India (mean = 0.28 μg/g) (Masih et al. 2016), and for women in Southern Iran (mean = 0.37 μg/g) (Barghi et al. 2012). In contrast, the mean THHg found in our studied group was lower than that found in various Asian populations, including Asians living in Chicago (GM = 0.58 μg/g) (Buchanan et al. 2015), Asian-Taiwanese women (mean = 1.73 μg/g) (Chien et al. 2010), and a Japanese population (mean = 2.02 μg/g) (Nakagawa 1995). This may be explained by the fact that these populations are characterized by higher dietary fish consumption. At the same time, compared to communities living in close proximity to the Mediterranean coast, Lebanese young adults had much lower mean THHg compared to that of Tunisian young adults living in Sfax (mean = 6.2–6.7 μg/g) (Mezghani-Chaari et al. 2011), Moroccans (GM = 1.79 μg/g) (Elhamri et al. 2007), and Greek women (mean = 1.36 μg/g) (Gibicar et al. 2006). In addition, THHg in Lebanese was higher than that in young adults living in Northern Egypt (mean = 0.23 μg/g) (Mortada et al. 2002), but almost equal to young and middle-aged adults living in Naples, Italy (mean THHg = 0.63 μg/g) (Diez et al. 2008). Interestingly, at the level of dietary habits, our studied group shows levels of fish consumption similar to that of studied Italian groups (71 g/day vs. 86 g/day) (Brambilla et al. 2013), which may explain the similarity in hair Hg levels.
Second, our results identified a positive association between consumption of Mediterranean rabbitfish/spinefoots and THHg. This association mirrors previous findings which reported, in the same geographical locations, rabbitfish/spinefoots to have significant Hg concentrations (Obeid et al. 2011). Previous Mediterranean studies have identified other edible species, such as the annular seabream (D. annularis) and the European flounder (P. flesus) as contributors to Hg health risk (Maulvault et al. 2015; Mezghani-Chaari et al. 2011; Spada et al. 2012). In addition, studies on trace elements contamination did not identify a risk contributory role for Mediterranean tuna consumption (Di Bella et al. 2015), which is supported by our findings in this study.
Third, our results do not show gender differences in THHg or risk indicators as opposed to previous reports that considered gender as one of the important variables influencing Hg content of hair (Mortada et al. 2002; Nakagawa 1995; Olivero et al. 2002). This may be explained by the fact that our sample was not gender balanced since the majority of participants were women.
Fourth, the computed HQ did not show the same risk assessment profile as compared to THHg. While the percentage of participants having a THHg above 1 μg/g was 19%, the percentage of those having an HQ >1 was 61%. By the same token, HQ did not correlate with THHg. This suggests that comprehensive fish sampling in conjunction with risk index estimation and human hair Hg biomonitoring are needed using a standardized and sustainable Hg surveillance program. The absence of correlation between HQ and hair Hg concentrations may be explained by a recall bias in reporting fish consumption, which may have potentially influenced the quality of the computed hazard quotient. At the same time, we have no reasons to suspect a confounding effect since data on potential confounders was collected and these were adjusted for in the multivariable analysis.
Biomonitoring of Hg exposure in the studied population using hair showed a number of advantages as a noninvasive, fast, and credible direct exposure assessment approach that can achieve a good response rate and can serve as the basis for an environmental public health preventive action. However, it is important to note that according to the US ATSDR, hair analysis is limited by the lack of a standard sampling procedure and potential surface contamination, which may alter the internal validity of exposure results (ATSDR 2003). Further, ATSDR considers that hair analysis provides little conclusive evidence linking hair concentration of contaminants to specific health outcomes, except in the case of Hg exposure and fish consumption rates in women with childbearing age. In this study, we tried to overcome this limitation by following a hair sampling procedure well described in the literature and consistent with the recall period for collected data, and by accounting for other potential sources of Hg in the final analysis. Besides hair analysis limitations and a potential recall bias stated above, our results are not representative of the general population or the different age groups. Subgroups of the population with higher fish consumption habits in other geographical locations of the country may have higher hair Hg concentrations. This is supported by our results showing statistically significant higher THHg in participants from the Northern Governorate compared to Beirut, a more central coastal city. The observed geographical differences in hair Hg concentrations may be explained by the presence of the Chakka cement plant, a potential source of Hg emission north of the country. This requires further investigation. Another limitation of the study is the utilization of THHg instead of MeHg, the most toxic form of the element. However, it is generally embraced that the total mercury analyzed in hair among fish consumers consists mainly of MeHg (Cernichiari et al. 1995).
Conclusions
In conclusion, this is the first study examining hair Hg levels in relation to fish consumption in a young adult Lebanese population. Globally, there is a call for Hg surveillance particularly in low- and middle-income countries (Sheehan et al. 2014). The challenge of accurately estimating Hg exposure, particularly from fish consumption, lies in the ability to account for variations in fish consumption, Hg concentrations between fish species, and in geographical locations (Smith and Sahyoun 2005). Our findings highlight the need for a local fish advisory for Lebanon and constitute valuable baseline data for monitoring Hg exposure in the population. Biomonitoring programs in particular will provide an additional dimension to national surveillance and can assist in tailoring mitigation and adaptation strategies such as dietary advice and Hg risk communication (Knobeloch et al. 2011). However, the challenge for public health practitioners remains in the ability to identify a balance between benefits and risks from fish consumption.
References
Abdelouahab N et al (2008) Gender differences in the effects of organochlorines, mercury, and lead on thyroid hormone levels in lakeside communities of Quebec (Canada). Environ Res 107:380–392. doi:10.1016/j.envres.2008.01.006
Al-Saleh I, Al-Doush I (1997) Mercury content in skin-lightening creams and potential hazards to the health of Saudi women. J Toxicol Environ Health 51:123–130. doi:10.1080/00984109708984016
Aschner M, Gannon M, Kimelberg HK (1992) Interactions of trimethyl tin (TMT) with rat primary astrocyte cultures: altered uptake and efflux of rubidium, L-glutamate and D-aspartate. Brain Res 582:181–185
ATSDR (2003) Analysis of hair samples: how do hair sampling results relate to environmental Exposure?
ATSDR (2005) public health assessment guidance manual (Update). Atlanta Georgia
Bakir F et al (1973) Methylmercury poisoning in Iraq. Science 181:230–241
Barghi M, Behrooz RD, Esmaili-Sari A, Ghasempouri SM (2012) Mercury exposure assessment in Iranian pregnant women’s hair with respect to diet, amalgam filling, and lactation. Biol Trace Elem Res 148:292–301. doi:10.1007/s12011-012-9384-y
Bjornberg KA, Vahtera M, Grawe KP, Berglund M (2005) Methyl mercury exposure in Swedish women with high fish consumption. Sci Total Environ 341:45–52. doi:10.1016/j.scitotenv.2004.09.033
Brambilla G et al (2013) Mercury occurrence in Italian seafood from the Mediterranean Sea and possible intake scenarios of the Italian coastal population. Regul Toxicol Pharmacol 65:269–277. doi:10.1016/j.yrtph.2012.12.009
Buchanan S, Targos L, Nagy KL, Kearney KE, Turyk M (2015) Fish consumption and hair mercury among Asians in Chicago. J Occup Environ Med 57:1325–1330. doi:10.1097/JOM.0000000000000560
Burger J, Gochfeld M (2011) Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci Total Environ 409:1418–1429. doi:10.1016/j.scitotenv.2010.12.034
Cernichiari E et al (1995) Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. Neurotoxicology 16:705–710
Chien LC, Gao CS, Lin HH (2010) Hair mercury concentration and fish consumption: risk and perceptions of risk among women of childbearing age. Environ Res 110:123–129. doi:10.1016/j.envres.2009.10.001
Clarkson TW (1997) The toxicology of mercury. Crit Rev Clin Lab Sci 34:369–403. doi:10.3109/10408369708998098
Clarkson TW, Amin-Zaki L, Al-Tikriti SK (1976) An outbreak of methylmercury poisoning due to consumption of contaminated grain. Fed Proc 35:2395–2399
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 349:1731–1737. doi:10.1056/NEJMra022471
Covelli S, Langone L, Acquavita A, Piani R, Emili A (2012) Historical flux of mercury associated with mining and industrial sources in the Marano and Grado Lagoon (northern Adriatic Sea). Estuarine Coastal and Shelf Science 113:7–19
Di Bella G, Potorti AG, Lo Turco V, Bua D, Licata P, Cicero N, Dugo G (2015) Trace elements in Thunnus thynnus from Mediterranean Sea and benefit-risk assessment for consumers. Food Addit Contam Part B Surveill 8:175–181. doi:10.1080/19393210.2015.1030347
Diez S, Montuori P, Pagano A, Sarnacchiaro P, Bavona JM, Triassi M (2008) Hair mercury levels in an urban population from southern Italy: fish consumption as a determinant of exposure. Environ Int 34:162–167. doi:10.1016/j.envint.2007.07.015
Elhamri H et al (2007) Hair mercury levels in relation to fish consumption in a community of the Moroccan Mediterranean coast. Food Addit Contam 24:1236–1246. doi:10.1080/02652030701329611
Eto K, Oyanagi S, Itai Y, Tokunaga H, Takizawa Y, Suda I (1992) A fetal type of Minamata disease. An autopsy case report with special reference to the nervous system. Mol Chem Neuropathol 16:171–186
Gibicar D, Horvat M, Nakou S, Sarafidou J, Yager J (2006) Pilot study of intrauterine exposure to methylmercury in Eastern Aegean Islands, Greece. Sci Total Environ 367:586–595. doi:10.1016/j.scitotenv.2006.01.017
Gill US, Schwartz HM, Bigras L (2002) Results of multiyear international interlaboratory comparison program for mercury in human hair. Arch Environ Contam Toxicol 43:466–472. doi:10.1007/s00244-002-1257-5
Gochfeld M (2003) Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf 56:174–179
Halbach S et al (2008) Blood and urine mercury levels in adult amalgam patients of a randomized controlled trial: interaction of Hg species in erythrocytes. Environ Res 107:69–78. doi:10.1016/j.envres.2007.07.005
Harada M (1995) Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 25:1–24. doi:10.3109/10408449509089885
Knobeloch L, Tomasallo C, Anderson H (2011) Biomonitoring as an intervention against methylmercury exposure. Public Health Rep 126:568–574
Masih A, Taneja A, Singhvi R (2016) Exposure profiles of mercury in human hair at a terai belt of North India. Environ Geochem Health 38:145–156. doi:10.1007/s10653-015-9698-8
Maulvault AL et al (2015) Toxic elements and speciation in seafood samples from different contaminated sites in Europe. Environ Res 143:72–81. doi:10.1016/j.envres.2015.09.016
McDowell MA et al (2004) Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999-2000. Environ Health Perspect 112:1165–1171
Mezghani-Chaari S, Hamza A, Hamza-Chaffai A (2011) Mercury contamination in human hair and some marine species from Sfax coasts of Tunisia: levels and risk assessment. Environ Monit Assess 180:477–487. doi:10.1007/s10661-010-1800-1
Miklavcic A et al (2013) Mercury, arsenic and selenium exposure levels in relation to fish consumption in the Mediterranean area. Environ Res 120:7–17. doi:10.1016/j.envres.2012.08.010
Morrissette J, Takser L, St-Amour G, Smargiassi A, Lafond J, Mergler D (2004) Temporal variation of blood and hair mercury levels in pregnancy in relation to fish consumption history in a population living along the St. Lawrence River Environ Res 95:363–374. doi:10.1016/j.envres.2003.12.007
Mortada WI, Sobh MA, El-Defrawy MM, Farahat SE (2002) Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura city, Nile delta, Egypt. Environ Res 90:104–110. doi:10.1006/enrs.2002.4396
Nakagawa R (1995) Concentration of mercury in hair of Japanese people. Chemosphere 30:127–133
Nyland JF et al (2011) Biomarkers of methylmercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environ Health Perspect 119:1733–1738. doi:10.1289/ehp.1103741
Obeid PJ, El-Khoury B, Burger J, Aouad S, Younis M, Aoun A, El-Nakat JH (2011) Determination and assessment of total mercury levels in local, frozen and canned fish in Lebanon. J Environ Sci (China) 23:1564–1569
Oken E et al (2008) Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol 167:1171–1181. doi:10.1093/aje/kwn034
Olivero J, Johnson B, Arguello E (2002) Human exposure to mercury in San Jorge river basin, Colombia (South America). Sci Total Environ 289:41–47. doi:10.1016/S0048-9697(01)01018-X
Olsen AR, Snyder BD, Stahl LL, Pitt JL (2009) Survey design for lakes and reservoirs in the United States to assess contaminants in fish tissue. Environ Monit Assess 150:91–100. doi:10.1007/s10661-008-0685-8
Pellizzari ED, Fernando R, Cramer GM, Meaburn GM, Bangerter K (1999) Analysis of mercury in hair of EPA region V population. J Expo Anal Environ Epidemiol 9:393–401
Sandborgh-Englund G, Elinder CG, Langworth S, Schutz A, Ekstrand J (1998) Mercury in biological fluids after amalgam removal. J Dent Res 77:615–624
Schaefer AM, Jensen EL, Bossart GD, Reif JS (2014) Hair mercury concentrations and fish consumption patterns in Florida residents. Int J Environ Res Public Health 11:6709–6726. doi:10.3390/ijerph110706709
Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA (2014) Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull World Health Organ 92:254–269F. doi:10.2471/BLT.12.116152
Smith KM, Sahyoun NR (2005) Fish consumption: recommendations versus advisories, can they be reconciled? Nutr Rev 63:39–46
Spada L, Annicchiarico C, Cardellicchio N, Giandomenico S, Di Leo A (2012) Mercury and methylmercury concentrations in Mediterranean seafood and surface sediments, intake evaluation and risk for consumers. Int J Hyg Environ Health 215:418–426. doi:10.1016/j.ijheh.2011.09.003
Squadrone S, Chiaravalle E, Gavinelli S, Monaco G, Rizzi M, Abete MC (2015) Analysis of mercury and methylmercury concentrations, and selenium:mercury molar ratios for a toxicological assessment of sperm whales (Physeter macrocephalus) in the most recent stranding event along the Adriatic coast (Southern Italy, Mediterranean Sea). Chemosphere 138:633–641. doi:10.1016/j.chemosphere.2015.07.047
Taylor J (1987) Quality Assurance of Chemical Measurements. Lewis Publishing, Chelsea
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175. doi:10.1002/tox.10116
Traynor S, Kearney G, Olson D, Hilliard A, Palcic J, Pawlowicz M (2013) Fish consumption patterns and mercury exposure levels among women of childbearing age in Duval County. Florida J Environ Health 75:8–15
UNDP (2012) Demonstrating and promoting best techniques and practices for reducing health-care waste to avoid environmental releases of dioxins and mercury project. Retrieved from URL: http://www.undp.org.lb/ProjectFactsheet/projectDetail.cfm?projectId=135. Accessed 5 July 2016
USEPA (1989) Risk assessment guidance for superfund, volume I: human health evaluation manual (Part A), Interim Final. EPA/540/1–89/002. Washington DC
USEPA (1997) Mercury study report to congress volume IV: an assessment of exposure to mercury in the United States. EPA-452/R-97-006
WHO, UNEP (2008) Guidance for identifying populations at risk from mercury exposure. Switzerland, Geneva
Yoshizawa K et al (2002) Mercury and the risk of coronary heart disease in men. N Engl J Med 347:1755–1760. doi:10.1056/NEJMoa021437
Acknowledgements
The authors thank Dr. Gregory Kearney at the Florida Department of Health for sharing the questionnaire that was adapted for purposes of our study. This work was supported by a research grant from the University of Balamand (BIRG 20/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was reviewed and approved by the Institutional Review Board of the University of Balamand. Informed consent was obtained from all participants in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Obeid, P.J., Fares, S.A., Farhat, G.N. et al. Mercury health risk assessment among a young adult Lebanese population. Environ Sci Pollut Res 24, 9370–9378 (2017). https://doi.org/10.1007/s11356-017-8621-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8621-5