Abstract
In this study, we focused on the rate of adsorption of phosphate on to the surface of magnetite in the presence of coexisting anions, organic matters and heavy metals. Magnetite particles were prepared using a co-precipitation method. Iron (II) sulfate heptahydrate and iron (III) chloride hexahydrate were mixed and then a sodium hydroxide solution was added drop-wise in the mixed iron solution. Coexisting anions were found to have no effect on the decrease in phosphate adsorption. However, phosphate adsorbed on to magnetite surface decreased with increasing total organic carbon (TOC) concentration of natural organic matter (NOM) such as citric, oxalic, and humic acid. The amount of phosphate adsorbed rapidly decreased with the increase of NOM concentration; therefore, it can be noted that NOM concentration considerably affects the adsorption of phosphate due to the negative charge exiting on the surface of NOMs. Glucose and ethanol, meanwhile, were found to have no effect on the phosphate adsorption. The amount of phosphate adsorbed did not change in the presence of heavy metals, namely, Pb and Cd, under acidic conditions. However, under alkaline conditions, the amount of phosphate adsorbed decreased with increasing concentrations of Pb and Cd. In the case of coexisting As(III), the amount of phosphate adsorbed decreased at all pH levels with increasing As(III) concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent industrial development has led to increase in the quantity of phosphate found in the industrial effluents (Nur et al. 2016). Discharged phosphorus causes eutrophication in fresh-water bodies, such as rivers and lakes, and the anoxic zones generated by these algal blooms result in the deaths of local fish species (Hong et al. 2015; Kim et al. 2015; Lee et al. 2015; Xie et al. 2013). Phosphorus is an essential element used in various day-to-day applications, such as semiconductors, fertilizers, and food (Tang et al. 2015; Yang et al. 2016). However, earth’s reserves of phosphorus are limited and will be depleted within 50–100 years, indicating the importance of not only removal of phosphorus but also its recovery (Cordell 2010; Cordell et al. 2009). Many researchers have recently started focusing on methods to recover phosphorus from the adsorbed phosphate wastewater. Table 1 shows the phosphate adsorption capacity of different adsorbents. As shown in Table 1, phosphate adsorption capacity of magnetite was performed 15.2 mg/g at pH 4.0 in our previous study (Choi et al. 2016).
In this study, we have used magnetite for phosphate adsorption. The phosphate adsorption capacity of tantalum and zirconium was greater than that of magnetite. Neither of the materials, tantalum and zirconium, are economical, and both exhibit difficult desorption of phosphate (Thomas et al. 2016), so suitable phosphate recovery is difficult. Ce–Zr binary oxides and iron-doped activated carbon have smaller adsorption capacities than magnetite. Magnetite also has other benefits such as being comparatively inexpensive, and being highly convenient in terms of recycling as the recovery ratio of magnetite particles is greater than 99%. Magnetite has a strong magnetic field, resulting in easy recovery of particles using magnets and as a result. Therefore, magnetite is considered as a suitable material for phosphate recovery (Prasad and Satyanarayana 2012).
Phosphorus is contained in different effluents such as anaerobic digestion leachate, livestock-generated wastewater, and wastewater discharged from semiconductors (Huang et al. 2017; Kawai et al. 2016; Li et al. 2016). Recovery of phosphates from such sources of wastewater is essential for protecting the environment and reducing the depletion of phosphates.
In this study, we synthesized magnetite particles and performed their characterization. We also evaluated the effects three different types of coexisting components, i.e., anions, organic matter, and heavy metals, on phosphate adsorption on magnetite surface.
Materials and methods
Preparation of magnetite particles
Magnetite particles were prepared using a co-precipitation method. The first step in this preparation was to mix 250 mL of 0.5 M iron(II) sulfate heptahydrate (FeSO4•7H2O) solution with 1.0 M iron chloride hexahydrate (FeCl3•8H2O) solution. A 6 M sodium hydroxide (NaOH) solution was then added drop-wise into this mixture using an overhead stirrer (GT-SS20D, Green tech, Korea). The resulting precipitates were aged at 70 °C for 12 h, washed two times using deionized (DI) water and ethanol, and subsequently dried at 50 °C. Finally, the dried particles were crushed using a mortar and pestle, and a sieve was used to separate out particles with size less than 20 μm.
Characterization of magnetite particles
The particle-size distribution was measured using a particle-size analyzer (Dandong Bettersize Instruments Ltd., Bettersize2000, China). Specific surface area and pore distribution of magnetite was measured using a surface area analyzer (BET, BEL Japan Inc., Japan). The surface formation and components of magnetite were examined using a scanning electron microscopy (SEM, Carl Zeiss, Germany) and an energy dispersive X-ray spectroscopy (EDS, Carl Zeiss, Germany) operating at 15 kV.
Effect of coexisting components on phosphate adsorption
Solutions containing phosphate mixed with coexisting anions were prepared by mixing 200 mg/L of potassium dihydrogen phosphate with 1.0:0.5, 1.0:1.0, 1.0:1.5, and 1.0:2.0 M ratios of sodium nitrate, sodium bicarbonate, sodium sulfate, and sodium chloride, respectively, with DI water. The coexisting anions used in this study were nitrate, bicarbonate, sulfate, and chloride.
We examined the effects of coexisting organic matter on phosphate adsorption by analyzing the TOC concentration of the organic matter. A calibrated curve-fitting was done for each type of organic matter to determine the TOC concentration. Ethanol, glucose, citric acid (CA), oxalic acid (OA), and humic acid (HA) were all used as organic matter sources. Organic matter concentrations of 125, 250, 500, and 1000 mg/L were used on the basis of the TOC concentration findings, with 200 mg/L of phosphate prepared using potassium dihydrogen phosphate.
To determine the effects that coexisting heavy metals have on phosphate adsorption, we conducted experiments using different molar ratios of phosphate and heavy metals (P:heavy metal = 1.0:0.5, 1.0:1.0, 1.0:1.5, and 1.0:2.0) of Cu, Pb, and As(III) standard solution at pH 2–9. Furthermore, 1 g of magnetite particles and 30 mL of a solution containing phosphate mixed with coexisting anions were added drop-wise into a 50-mL conical tube that was continuously stirred using a multi-rotator (GTR-100, Green Tech Co., Korea) at room temperature.
Sampling and analysis
Each sample after the phosphate adsorption reaction was collected as a solution using a 0.45-μm syringe filter for removing magnetite particles. The phosphate concentration of each sample was then measured using the standard method 8048 with a high-range limit of 100 mg/L PO43− using an UV-spectrophotometer (Hach, DR3900, USA).
Results and discussion
Characterization
EDS is semi-quantitative analysis technique used for analysis of elements and their chemical composition. Figure 1 shows an EDS analysis of the magnetite used for examining magnetite surface components; carbon, oxygen, and iron were found as the components. Table 2 shows the magnetite composition obtained through the EDS analysis. Carbon impurity was found because the carbon tape used to set the sample for the EDS analysis. The atomic ratio of oxygen and iron was approximately 3:4, which is roughly the correct composition of magnetite.
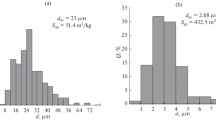
The particle-size distribution of magnetite is shown in Fig. 2. Overall, the range of the particle-size distribution was approximately 1–100 μm, and the average particle size was 24.77 μm. Figure 3 depicts BET analysis of magnetite particles. The specific surface area of magnetite was 85.6 m2/g and the pore size and pore volume were 14.7 nm and 0.32 cm3, respectively. The BET isotherm followed a type II isotherm, which means the magnetite particles exhibited a non-porous surface. SEM images of the magnetite are shown in Fig. 4. The particle size, as observed from the SEM images, was less than 100 μm, and the magnetite had an angular shape on the surface, and there are no existing pores on the magnetite surface.
Effect of coexisting components on phosphate adsorption
Anions
The effect of coexisting anions on phosphate adsorption is shown in Fig. 5a–d. Four anions, NO3−, SO42−, HCO3−, and Cl−, were used to investigate the effects of the coexisting anions. Overall, we found that the coexisting anions had no effect on phosphate adsorption. Thus, the magnetite particles were considered to be the strongest when competing to bond with the magnetite surface as compared with other material like anions.
Organic matter
Figure 6a, b shows the effect of general organic matter on phosphate adsorption for various TOC concentrations. Glucose and ethanol showed no effect on phosphate adsorption on the magnetite surface. However, the adsorbed quantity of phosphate on the magnetite surface was lower than that for the coexisting anions during the first 15 min of the experiment. Therefore, we can determine that glucose and ethanol only have an effect on phosphate adsorption only during lower adsorption kinetics.
The effect of coexisting NOM on phosphate adsorption is shown in Fig. 7a–c. The phosphate adsorbed on the magnetite surface decreased with increasing TOC concentration of the NOMs, i.e., CA, OA, and HA. A comparison between phosphate adsorption using magnetite in a phosphate solution and that with magnetite in a phosphate solution mixed with 125 mg/L TOC of NOM indicates that the NOMs significantly decreased the amount of phosphate adsorbed. This is because a negative charge existed on the surface of the NOMs; therefore, both phosphate and NOMs competed for being adsorbed on to the magnetite surface (Guan et al. 2006; Liu et al. 2008b).
Heavy metals
Figure 8 show the effect of coexisting heavy metals, i.e., Pb and Cd, on phosphate adsorption. Under acidic conditions, the amount of phosphate adsorbed on to the magnetite surface in the presence of Pb and Cd did not change; however, under alkaline conditions, the amount of phosphate adsorbed decreased as with increasing concentration of Pb and Cd. This phenomenon was caused by the distribution of Pb and Cd at different pH conditions. The existing Pb and Cd cations were below the pH of 9–10 in water (Choi et al. 2013; Jin et al. 2015). However, for As(III), the amount of phosphate adsorbed decreased at all pH levels with increasing As(III) concentrations. This is because As(III) existed as an anion in water at almost all pH levels (Mähler et al. 2013).
Conclusions
This study was conducted to examine the effects of coexisting components on phosphate adsorption when magnetite was used. The oxygen and iron on the surface of the magnetite were measured to have an atomic ratio of 3:4 and the particle-size range was 1–100 μm with an average particle size of approximately 24.77 μm. Coexisting anions were found to have a negligible effect on phosphate adsorption, and phosphate is fully adsorbed on to magnetite in this scenario. General organic matter also showed no effect on phosphate adsorption. In contrast, NOMs proved to be highly effective in decreasing the amount of phosphate adsorption. This phenomenon occurred because of the NOMs competing with phosphate for being adsorbed on to the magnetite surface as NOMs have a negative charge on the surface. The reaction of NOM adsorption on to the magnetite surface was mainly electrostatic. In the case of heavy metals, Pb and Cd showed no effect under acidic conditions but the amount of phosphate adsorbed was reduced in the presence of these metals near pH 10. This was because these metals are mostly present as cations at all pH levels in water. As(III), however, was effective in lowering the amount of phosphate adsorbed at all pH levels because As(III) exists in an anionic form at all pH levels in water. These anions competed with phosphate for being adsorbed on to the magnetite surface.
References
Choi J, Ide A, Truong YB, Kyratzis IL, Caruso RA (2013) High surface area mesoporous titanium–zirconium oxide nanofibrous web: a heavy metal ion adsorbent. J Mater Chem A 1:5847–5853
Choi J, Chung J, Lee W, Kim J-O (2016) Phosphorous adsorption on synthesized magnetite in wastewater. J Ind Eng Chem 34:198–203
Cordell D (2010) The story of phosphorus: sustainability implications of global phosphorus scarcity for food security
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305
Guan X-H, Shang C, Chen G-H (2006) Competitive adsorption of organic matter with phosphate on aluminum hydroxide. J Colloid Interface Sci 296:51–58
Hong K-H, Yoo I-S, Kim S-H, Chang D, Sunwoo Y, Kim D-G (2015) Application of brass scrubber filter with copper hydroxide nanocomposite structure for phosphate removal. Environ Eng Res 20:199–204
Huang H, Liu J, Zhang P, Zhang D, Gao F (2017) Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem Eng J 307:696–706
Jin Z, Gao H, Hu L (2015) Removal of Pb (ii) by nano-titanium oxide investigated by batch. XPS and model techniques Rsc Advances 5:88520–88528
Kawai M, Nagao N, Kawasaki N, Imai A, Toda T (2016) Improvement of COD removal by controlling the substrate degradability during the anaerobic digestion of recalcitrant wastewater. J Environ Manag 181:838–846
Kim J-H, Park J-A, Kang J-K, Kim S-B, Lee C-G, Lee S-H, Choi J-W (2015) Phosphate sorption to quintinite in aqueous solutions: kinetic, thermodynamic and equilibrium analyses. Environ Eng Res 20:73–78
Lee W-H, Chung J, Kim J-O (2015) Characteristics of phosphate adsorption using prepared magnetic iron oxide (MIO) by co-precipitation method in water. Journal of Korean Society of Water and Wastewater 29:609–615
Li W, Loyola-Licea C, Crowley DE, Ahmad Z (2016) Performance of a two-phase biotrickling filter packed with biochar chips for treatment of wastewater containing high nitrogen and phosphorus concentrations. Process Saf Environ Prot 102:150–158
Liu H, Sun X, Yin C, Hu C (2008a) Removal of phosphate by mesoporous ZrO2. J Hazard Mater 151:616–622
Liu G, Zhang X, Talley JW, Neal CR, Wang H (2008b) Effect of NOM on arsenic adsorption by TiO 2 in simulated As (III)-contaminated raw waters. Water Res 42:2309–2319
Mähler J, Persson I, Herbert RB (2013) Hydration of arsenic oxyacid species. Dalton Trans 42:1364–1377
Nur T, Loganathan P, Kandasamy J, Vigneswaran S (2016) Phosphate adsorption from membrane bioreactor effluent using Dowex 21K XLT and recovery as struvite and hydroxyapatite. Int J Environ Res Public Health 13:277
Prasad AS, Satyanarayana B (2012) Synthesis of aryl 2-oxazolines from aromatic nitriles and aminoalcohols using magnetically recoverable Pd/Fe3O4. Der Pharma Chemica 4:93–99
Su Y, Cui H, Li Q, Gao S, Shang JK (2013) Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res 47:5018–5026
Su Y, Yang W, Sun W, Li Q, Shang JK (2015) Synthesis of mesoporous cerium–zirconium binary oxide nanoadsorbents by a solvothermal process and their effective adsorption of phosphate from water. Chem Eng J 268:270–279
Tang G et al (2015) Phosphate glass-clad tellurium semiconductor core optical fibers. J Alloys Compd 633:1–4
Thomas A, Sridhar S, Aghyarian S, Watkins-Curry P, Chan JY, Pozzi A, Rodrigues DC (2016) Corrosion behavior of zirconia in acidulated phosphate fluoride. J Appl Oral Sci 24:52–60
Wang Z, Nie E, Li J, Yang M, Zhao Y, Luo X, Zheng Z (2012) Equilibrium and kinetics of adsorption of phosphate onto iron-doped activated carbon. Environ Sci Pollut Res 19:2908–2917
Xie F, Wu F, Liu G, Mu Y, Feng C, Wang H, Giesy JP (2013) Removal of phosphate from eutrophic lakes through adsorption by in situ formation of magnesium hydroxide from diatomite. Environ Sci Technol 48:582–590
Yang S, Jin P, Wang X, Zhang Q, Chen X (2016) Phosphate recovery through adsorption assisted precipitation using novel precipitation material developed from building waste: behavior and mechanism. Chem Eng J 292:246–254
Yu S-H, Dong X-L, Gong H, Jiang H, Liu Z-G (2012) Adsorption kinetic and thermodynamic studies of phosphate onto tantalum hydroxide. Water Environ Res 84:2115–2122
Acknowledgments
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean Government (NRF-2016R1A2A1A05005388). Partial support was provided by the Korea Ministry of Environment (MOE) as the Advanced Technology Program for Environmental Industry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Lee, WH., Kim, JO. Effect of coexisting components on phosphate adsorption using magnetite particles in water. Environ Sci Pollut Res 26, 1054–1060 (2019). https://doi.org/10.1007/s11356-017-8528-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8528-1