Abstract
Lead (Pb) stress adversely affects in planta nutrient homeostasis and metabolism when present at elevated concentration in the surrounding media. The present study was aimed at investigation of organic acid exudations, elemental contents, growth, and lipid peroxidation in two wild plants (Amaranthus viridis L. and Portulaca oleracea L.), exhibiting differential root to shoot Pb translocation, under Pb stress. Plants were placed in soil spiked with lead chloride (PbCl2) concentrations of 0, 15, 30, 45, or 60 mg Pb/kg soil, in rhizoboxes supplied with nylon nets around the roots. The plant mucilage taken from root surfaces, mirroring the rhizospheric solution, was analyzed for various organic acids. Lead stress resulted in a release of basified root exudates from both plants. Exudates of P. oleracea roots showed a higher pH. In both plants, the pH rising effect was diminished at the highest Pb treatment level. The exudation of citric acid, glutamic acid (in both plants), and fumaric acid (in P. oleracea only) was significantly increased with applied Pb levels. In both plant species, root and shoot Pb contents increased while nutrients (Ca, Mg, and K) decreased with increasing Pb treatment levels, predominantly in A. viridis. At 60 mg Pb/kg soil, shoot Na content of A. viridis was significantly higher as compared to untreated control. Higher Pb treatment levels decreased plant fresh and dry masses as well as the quantity of photosynthetic pigments due to enhanced levels of plant H2O2 and thiobarbituric acid reactive substances in both species. Photosynthetic, growth, and oxidative stress parameters were grouped into three distinct dendrogram sections depending on their similarities under Pb stress. A positive correlation was identified between Pb contents of studied plants and secretion of different organic acids. It is concluded that Pb stress significantly impaired the growth of A. viridis and P. oleracea as a result of nutritional ion imbalance, and the response was cultivar-specific and dependent on exogenous applied Pb levels. Differential lipid oxidation, uptake of nutrients (Ca, Mg, and K) and exudation of citric acid, fumaric acid, and glutamic acid could serve as suitable physiological indicators for adaptations of P. oleracea to Pb enriched environment. The findings may help in devising strategies for Pb stabilization to soil colloids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to Environmental Protection Agency (EPA), USA, lead (Pb) is one of the five most toxic heavy metals which is harmful to biotic systems and listed as the major contaminant and great threat to the environment (Wang et al. 2013). It has received great attention of scientists due to its ability to stay in the environment for a prolonged period of time, i.e., 150–5000 years (Fahr et al. 2013). A major proportion of Pb, present in soil and water bodies, comes from erosion and weathering of rocks (Gadd 2010). In addition, Pb sources include municipality sewage sludge, Pb-supplemented paints, explosives, gasoline, mining/smelting activities, fuel combustion, chemical fertilizers, Pb containing acid batteries, and fusible alloys (Mukai et al. 2001). The soil-Pb concentration varies from 2 to 29 mg/kg of soil in various industrial zones of Pakistan (Malik et al. 2010). Presence of Pb in soil-plant system affects seed germination, seedling growth, root development, chlorophyll synthesis, transpiration rate, biomass, and yield (Pourrut et al. 2011; Bharwana et al. 2014).
High levels of Pb not only inhibit enzymatic activities and membrane permeability in plants but also initiate oxidative stress due to over production of reactive oxygen species (ROS) (Reddy et al. 2005). As the difference between ROS production and elimination increases under Pb stress, it disturbs the cell redox equilibrium and exposes the cells to oxidative stress causing deterioration of biological macromolecules, enhanced lipid peroxidation, and, hence, membrane dismantling (Pourrut et al. 2011). Srivastava et al. (2014) suggested that oxidative stress boosts Pb toxicity in rice (Oryza sativa L.).
The arable (wheat, and maize), as well as wild macrophytes (common cotton grass, alpine pennycress, and ryegrass), responds to toxic elements, i.e., Pb by initiating alterations in rhizospheric pH (Stoltz and Greger 2002; Blossfeld et al. 2010; Yang et al. 2011; Javed et al. 2013; Tanwir et al. 2015). The major components which contribute towards fluctuations in the rhizospheric pH are the activity of the proton pumps, imbalance of nutrients and metallic cations uptake, production of respiratory CO2, and secretion of organic acids and protons by plant roots.
Under heavy metal stress, plant roots secrete low molecular weight organic acids (LMWOAs) and certain other chelating substances which may modulate the bioavailability of metallic cations (Haoliange et al. 2007; Qin et al. 2007; Zeng et al. 2008). The exudation of LMWOAs can probably be interpreted as an adaptation of plants to adverse conditions, especially to toxic Pb concentrations. The dissociated carboxylic acids, holding negative charges, may interact with dissolved cations mainly through formation of metal-carboxylic acid complexes leading to metal immobilization. The composition of exuded LMWOAs may vary from species to species. Moderately high levels of toxic elements induced Eriophorum angustifolium roots to release various organic acids, but the exudation was less at higher treatment level (Javed et al. 2013). Lead generally precipitates in soil sediment together with phosphates, sulfates, and rhizospheric chemicals (Blaylock and Huang 2000). Plants control metal uptake and transport by formation of metal-organic acid (in their anionic forms) complexes (Kochian et al. 2005). An increase in Pb uptake was observed after addition of citric acid in Vicia faba and Typha angustifolia (Muhammad et al. 2009; Shahid et al. 2012). The Pb immobilization in root zone is mainly attributed to its ability to form a complex with histidine, while the translocation to the upper plant organs is due to the formation of its complexes with organic acids (Massaccesi et al. 2014). Furthermore, organic acids may also serve as an alternative source to EDTA in forming complexes with Pb when considering enhanced metal uptake from contaminated soil/sediment (Shakoor et al. 2014).
At normal plant cytosolic pH (7–7.5), organic acids are present as anions and upon release from roots, instead of binding with metallic cations, these anions can take up protons and cause rhizospheric basification. Metal stress suppressed the activity of H+-ATPase which in turn reduced protons extrusion and caused a rise in external pH (Janicka-Russak et al. 2012). Such basification mechanisms by plants are also likely to cause metal stabilization in the rhizosphere due to reduced metal ions mobility (Javed et al. 2013). The addition of organic acids can also cause soil acidosis depending on the number of carboxylic groups they carry, as well as the initial soil pH (Zhi-An et al. 2008).
Reports exist regarding the impact of Pb toxicity on nutritional ion (Ca2+ and K+) imbalances in various plants (Trivedi and Erdei 1992), e.g., Brassica napus grown under Pb stress (Ali et al. 2014). Moreover, Pb can also prevent cation exchange in the roots for K+, Ca2+, Mg2+, Fe2+, and Zn2+ (Sharma and Dubey 2005).
Portulaca oleracea L. and Amaranthus viridis L. are wild macrophytes distributed in various regions of Pakistan. In an earlier study, both plant species were reported to withstand Pb toxicity and exhibited significant differences in their root to shoot Pb translocation (Malik et al. 2010). However, the mechanisms of Pb tolerance which induced physio-biochemical alterations, as well as the exudation of various organic acids, that enable the adaptation of these wild plants to Pb-polluted rhizosphere, have not been investigated. In the present study, we (a) evaluated the possible role of different organic acids in Pb uptake, distribution, and tolerance in P. oleracea and A. viridis under Pb stress and (b) assessed the interference of Pb uptake with nutritious ion homeostasis and growth of these plants. For the first time, we investigated and discussed the organic acid exudations of P. oleracea and A. viridis under Pb stress with reference to their growth and photosynthetic attributes. It was hypothesized that P. oleracea and A. viridis plants will initiate changes in their rhizospheric environment by secreting different organic acids enabling these macrophytes to tolerate Pb toxicity. Exuded organic acids may modulate Pb uptake and translocation, nutrients, and plant physio-biochemical attributes. Appropriate understanding of physio-biochemical acclimatization, as well as its dependency on organic acids exudations, can assist effective utilization of P. oleracea and A. viridis for decontamination programs of Pb-polluted soil colloids/sediments.
Materials and methods
Plant acclimatization and rhizobox experiment
Wild grown plants of Amaranthus viridis L. and Portulaca oleracea L., having uniform growth, were collected from Botanical Garden, located at Government College University, Faisalabad, in flat planes of northeast Punjab, Pakistan (31°24/N, 73°04/E). After washing with distilled water, plants were mounted in Styrofoam plates which covered 1-L black containers having aerated 1% Hoagland solution (Eliasson 1978). There were five to six plants per container, which were placed in a growth chamber at temperature of 22 ± 2 °C having RH of 70% and light/dark conditions of 16 h/8 h. Concentration of Hoagland solution was gradually increased up to 100% in order to acclimatize the plants and was replenished periodically. After acclimatization for 4 weeks, rhizoboxes were used to collect and examine organic acid contents in mucilage which was dissolved from root surface of plants as described by Javed et al. (2013, 2017) and UdDin et al. (2015). Amaranthus viridis L. and P. oleracea L. plants having uniform size and fully expanded leaves were washed thoroughly and transferred to the rhizoboxes (length = 18 cm, width = 18 cm, thickness = 3 cm) (Greger and Landberg 2008). The pH of the experimental soil was 7.64 with EC of 3.06 dSm−1 having Pb concentration of 0.28 mg/kg, available N of 682 mg/kg, available P of 5.2 mg/kg, total Mg of 78.7 mg/kg, total Ca of 730.2 mg/kg, total Fe of 169.8 mg/kg, total K of 256 mg/kg and 1.19% organic matter. Soil was autoclaved and spiked with PbCl2, 8 weeks in advance, to achieve the final concentrations of Pb as 0.28 (natural soil Pb level/control), 15, 30, 45, or 60 mg/kg soil (Malik et al. 2010) before use in rhizoboxes. In each rhizobox, four plants were used where plant roots were placed in a nylon net (25 μm) which prevented direct root-soil contact. For each treatment level, four replicates were maintained.
Collection of root exudates
Two seedlings were taken from rhizoboxes after 72 h and roots were thoroughly rinsed with distilled water. The solution obtained from rinsing of roots was filtered through a filter with 0.45 μm pore size (Millex-HA, Millipore) and was subsequently collected in Eppendorf tubes (Greger and Landberg 2008). Sodium hydroxide solution (0.01 M) was mixed with all the samples in order to analyze the organic acids except the ones used for pH measurements and analysis of oxalic acid (Javed et al. 2013). The collected samples were then freeze-dried with the help of liquid nitrogen and stored at − 80 °C before further analysis of the organic acids.
Analysis of root exudates
Quantitative analysis of organic acids was carried out with high-performance liquid chromatography (HPLC) equipped with Flexer FX-10 UHPLC isocratic pump (PerkinElmer, MA, USA). For this purpose, the freeze-dried root exudate samples were mixed with ethanol (80%) and 20 μl of the solutions was eluded isocratically into a C18 column (Brownlee Analytical C-183 μm; length 150 mm × 4.6 mm2, USA). The mobile phase of the HPLC was comprised of acidified acetonitrile solution consisting of acetonitrile:H2SO4:acetic acid at 15:4:1, respectively, and at pH 4.9. The samples were analyzed for a time period of 10 min at a flow rate of 1.0 ml min−1. The inner column temperature was maintained at 45 °C, and organic acid quantification was executed with the help of a detector (UV-vis Series 200, USA) at 214 nm wavelength based upon the retention times as well as the UV spectra relative to their standards (UdDin et al. 2015).
The pH of the root exudates was recorded after dissolving the freeze-dried samples in redistilled water with the help of micro-pH glass electrode by using a pH meter (ISTEK Model 4005-08007, Seoul, South Korea).
Plant lead and nutritious ion analysis
The plants which were harvested from rhizoboxes after 72 h of Pb exposure for collection of root exudates were subsequently analyzed for Pb as well as Ca, Mg, K, and Na contents. For metal analysis, the plant roots were washed with distilled water twice, quickly dipped in EDTA (20 mM), and thereafter rinsed again with distilled water in order to remove the adsorbed metals from the root surfaces. The plants were separated into the roots and shoots and were oven dried for 24 h at 105 °C. Oven-dried ground material (0.5 g) was then shifted into digestion flasks which were incubated overnight at room temperature after addition of 5 ml conc. H2SO4 (Wolf 1982). Afterwards, 0.5 ml H2O2 (35%) was poured into the flasks which were placed on a hot plate at temperature of 350 °C until no fume was produced. The digestion flasks were removed from the hot plate and allowed to cool down. The flasks were again placed on a hot plate at temperature of 350 °C after addition of H2O2, and this step was repeated until transparent/clear digestion mixtures were obtained. Digested mixtures were diluted in volumetric flasks up to 50 ml and were stored at 4 °C till further analysis. The Pb and nutrient contents (Ca, Mg, K, and Na) of the digested plant samples were analyzed by atomic absorption spectrophotometer (Hitachi, Model 7JO-8024, Tokyo, Japan) by using flame spectrophotometry. Standard reference materials (SRM) and standard solutions were used during metal analysis in order to minimize the matrix affect.
Plant physio-biochemical analysis
Remaining two plants from each rhizobox were used (eight plants for each Pb treatment level) for the measurement of different physio-biochemical attributes.
Plant chlorophyll and carotenoid contents
Method of Arnon (1949) was used to assay plant chlorophyll and carotenoid contents. After homogenization in acetone (80%), fresh plant material was kept at 4 °C overnight. After centrifugation at 3000× g for 15 min, the solution was filtered and absorbance of the supernatant was recorded by using a spectrophotometer (Hitachi U-2001, Tokyo, Japan) at wavelengths of 663, 645, and 480 mm. Chlorophyll a, chlorophyll b, and total chlorophyll as well as total carotenoid contents were calculated by the methods described by Arnon (1949) and Davies (1976), respectively.
Measurement of lipid peroxidation
The level of lipid peroxidation products of fresh leaf tissue was measured according to method of Heath and Packer (1968) in terms of thiobarbituric acid reactive substances (TBARS) assayed by thiobarbituric acid (TBA) reaction. Plant material was homogenized with 0.1% trichloroacetic acid (TCA) by using an ultra-mixer (POLYTRON 2000, Switzerland). The homogenates were centrifuged for 5 min at 10000×g, and supernatant was mixed with TCA (20%) and TBA (0.5%) and then heated for 30 min at 100 °C. The tubes were placed on ice to quickly stop the reaction, and samples were again centrifuged at 10000× g for 5 min. The absorbance of supernatant was assayed at 532 nm and adjusted for non-specific absorbance at 600 nm by using Ultrospec 3000 (Biochrom Ltd. Cambridge, England). The extinction coefficient was 155 mM cm−1.
Plant’s hydrogen peroxide contents
The plant’s H2O2 content was measured colorimetrically as described by Jana and Choudhuri (1981). Leaf tissues (50 mg) were homogenized in 3 ml of 50 mM phosphate buffer at pH of 6.5, and the homogenate was centrifuged for 25 min at 6000×g. Extracted solution (3 ml) was then mixed with 1 ml titanium sulfate (0.1%) in H2SO4 (20% v/v), and the reaction mixture was again centrifuged for 15 min at 6000×g. In order to measure H2O2 contents, intensity of the yellow-colored supernatant was measured at 410 nm by using Ultrospec 3000 (Biochrom Ltd. Cambridge, England) where the extinction coefficient was 0.28 mmol−1 cm−1.
Statistical analysis
Simple regression and analysis of variance (ANOVA) were used for statistical data analysis by a statistical program “R” (version 2.13.0). Tukey’s honest significant differences test (HSD-test) was used for recognition of treatment differences at significance levels of p ≤ 0.05. Prior to statistical analysis, logarithmic or inverse transformations were used to normalize the data, when necessary.
Results
Organic acid contents and pH in root exudates of A. viridis and P. oleracea under lead stress
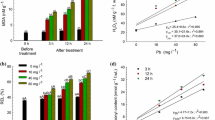
In the absence of Pb stress, the pH of 6.88 and 7.12 was observed in the mucilage of A. viridis and P. oleracea, respectively (Table 1). A consistent increase in pH in the root exudates of P. oleracea (up to 45 mg/kg) and in A. viridis (up to 30 mg/kg) was observed. However, pH increase at the highest level of Pb was less, where a significant pH decrease was recorded for A. viridis at 60 mg/kg Pb treatment.
The organic acids (citric, malic, fumaric, oxalic, and glutamic acids) were an important component of root exudates. The organic acid contents were determined in the two plant species, i.e., A. viridis and P. oleracea grown with various applied Pb levels. In both plant species, citric acid exudation was enhanced with increase in applied Pb levels. The maximum citric acid secretion was observed, at 60 mg Pb/kg soil, by the roots of P. oleracea which was 24% higher than the citric acid released by A. viridis (Table 1). The release of all other studied organic acids also followed a similar trend. The roots exudates of P. oleracea had 6.75, 21, 1.06, and 7.32% higher malic acid, fumaric acid, oxalic acid, and glutamic acids in comparison to root exudates of A. viridis when both species were grown in the presence of 60 mg Pb/kg soil.
Physiological attributes of A. viridis and P. oleracea under lead stress
The plant fresh and dry weights were decreased when seedlings were grown in the presence of Pb for 72 h. The decrease was associated with applied Pb levels, though Pb-induced effects were more pronounced in A. viridis (Table 2). The photosynthetic pigments (chlorophylls a and b and total chlorophyll) as well as accessory pigments (carotenoids) were also reduced under Pb stress. An increase in H2O2 content was observed together with increase in applied Pb stress which was more pronounced in P. oleracea. At 60 mg Pb/kg soil, A. viridis and P. oleracea showed 146 and 95% higher H2O2 content, respectively, in comparison to the one exhibited by respective control plants.
An increase in exogenous Pb in soil resulted in higher membrane lipid peroxidation, as evident from TBARS contents at various Pb levels (Table 2). At the highest applied Pb stress, i.e., 60 mg/kg soil, plants of A. viridis exhibited a higher lipid peroxidation, as evident from 71% higher TBARS contents (6.07 μmol/g FW), than exhibited by P. oleracea (3.55 μmol/g FW).
Root and shoot lead contents
The results showed that the absorption of Pb by the roots was increased in both plant species, i.e., A. viridis and P. oleracea with increasing applied Pb concentrations (Fig. 1). The two species exhibited differential response and, at 60 mg/kg soil, the former species showed three times higher Pb and the latter one showed two times higher Pb, when compared to respective control group (plants grown without Pb). The maximum shoot Pb content was observed in A. viridis (50.96 μg/g DW) followed by P. oleracea (31.73 μg/g DW) at 60 mg Pb/kg soil indicating a higher root to shoot translocation in the former plant species.
Lead (Pb) contents of Amaranthus viridis L. and Portulaca oleracea L. roots and shoots grown with different PbCl2 concentrations of 0, 15, 30, 45, or 60 mg/kg soil in rhizoboxes. The control treatment designated as 0 mg/kg soil Pb was natural soil having Pb level of 0.28 mg/kg soil with no exogenous Pb application. Significant differences in roots and shoots Pb contents of A. viridis and P. oleracia are represented by letters A–E and a–e, respectively. n = 4, Mean ± SE
Root and shoot Ca, K, Mg, and Na contents
The two plant species, A. viridis and P. oleracea, showed the same Ca uptake when no Pb was applied (control group) (Fig. 2). Exogenous Pb application resulted in a decrease in Ca uptake with the applied Pb level. The maximum decrease in Ca uptake (48% less in comparison to respective control) was exhibited by A. viridis at 60 mg Pb/kg soil while P. oleracea showed 37% decrease, with respect to its corresponding control, at the same Pb level. The root to shoot Ca translocation was also negatively affected by Pb and, at 60 mg Pb/kg soil, A. viridis showed more reduction in comparison to P. oleracea.
Calcium (Ca) contents of Amaranthus viridis L. and Portulaca oleracea L. roots and shoots grown with different PbCl2 concentrations of 0, 15, 30, 45, or 60 mg/kg soil in rhizoboxes. The control treatment designated as 0 mg/kg soil Pb was natural soil having Pb level of 0.28 mg/kg soil with no exogenous Pb application. Significant differences in roots and shoots Ca contents of A. viridis and P. oleracia are represented by letters A–D and a–c, respectively. n = 4, Mean ± SE
The results indicated that the uptake of K by the roots of A. viridis decreased with increasing Pb concentration with a maximum decrease at 60 mg Pb/kg soil (Fig. 3). P. oleracea roots showed an increase in K uptake (at 15 mg/kg soil), an insignificant change at 30 mg/kg soil, and a significant decrease at 45 and 60 mg/kg soil as compared with respective control. At 60 mg/kg soil, A. viridis showed 63.5% decrease while P. oleracea roots showed 44% decrease in K uptake, as compared with respective control. A similar trend was observed for root to shoot translocation of K by the two plant species.
Potassium (K) contents of Amaranthus viridis L. and Portulaca oleracea L. roots and shoots grown with different PbCl2 concentrations of 0, 15, 30, 45, or 60 mg/kg soil in rhizoboxes. The control treatment designated as 0 mg/kg soil Pb was natural soil having Pb level of 0.28 mg/kg soil with no exogenous Pb application. Significant differences in roots and shoots K contents of A. viridis and P. oleracia are represented by letters A–E and a–d, respectively. n = 4, Mean ± SE
The obtained data also revealed that increased Pb levels resulted in decreased Mg uptake by the roots of both plant species, though differences existed between the two macrophytes. A. viridis showed more reduction in Mg uptake in comparison to P. oleracea as shown in Fig. 4. Root to shoot translocation of Mg was also negatively influenced by Pb, and the two plant species exhibited similar decreasing trend for Mg translocation. Amaranthus viridis showed a pronounced reduction as compared to P. oleracea, particularly at 60 mg/kg soil.
Magnesium (Mg) contents of Amaranthus viridis L. and Portulaca oleracea L. roots and shoots grown with different PbCl2 concentrations of 0, 15, 30, 45, or 60 mg/kg soil in rhizoboxes. The control treatment designated as 0 mg/kg soil Pb was natural soil having Pb level of 0.28 mg/kg soil with no exogenous Pb application. Significant differences in roots and shoots Mg contents of A. viridis and P. oleracia are represented by letters A–E and a–e, respectively. n = 4, Mean ± SE
The uptake of Na by A. viridis roots decreased with increase in applied Pb level up to 45 mg/kg soil (Fig. 5). At highest Pb treatment (60 mg/kg soil), A. viridis roots showed increase in uptake of Na in comparison to lower Pb levels (15, 30, or 45 mg/kg soil), though the Na content was less (66.3 mg/g DW) than the control plants (69.5 mg/g DW). The Na uptake by the roots of P. oleracea decreased with increase in Pb treatment, and maximum reduction was observed at 60 mg/kg soil. Moreover, P. oleracea showed more reduction in Na uptake as compared to A. viridis. Root to shoot translocation was also negatively influenced as was evident from reduced shoot Na content in both the plant species, and the reduction was more prominent in P. oleracea as compared to A. viridis.
Sodium (Na) contents of Amaranthus viridis L. and Portulaca oleracea L. roots and shoots grown with different PbCl2 concentrations of 0, 15, 30, 45, or 60 mg/kg soil in rhizoboxes. The control treatment designated as 0 mg/kg soil Pb was natural soil having Pb level of 0.28 mg/kg soil with no exogenous Pb application. Significant differences in roots and shoots Na contents of A. viridis and P. oleracia are represented by letters A–C and a–e, respectively. n = 4, Mean ± SE
Correlation coefficients, principle component analysis, and dendrograms
Significantly positive correlations among Pb contents and the studied organic acids were revealed by Pearson’s correlation coefficients, as well as PCA (Table 3, Fig. 6). A significantly positive correlation was identified between root Pb contents and citric acid (0.920***), malic acid (0.906***), fumaric acid (0.901***), oxalic acid (0.946***), and glutamic acid (0.970***). The exudation of citric acid (0.984***), malic acid (0.695***), fumaric acid (0.969***), oxalic acid (0.786***), and glutamic acid (0.956***) was also positively correlated with shoot Pb contents. The organic acids exhibited significantly positive correlations with H2O2 and TBARS. However, organic acids were found to be negatively correlated with plant biomass, photosynthetic accessary pigments, and nutrient contents. Dendrogram analysis revealed the groups of plant physiological attributes of A. viridis and P. oleracea which were affected in a similar fashion under Pb stress (Fig. S1). Photosynthetic, growth, and oxidative stress parameters were grouped into three distinct sections depending on their similarities under Pb stress.
Principle component analysis (PCA) showing associations among growth, exuded organic acids, and root and shoot elemental contents of Amaranthus viridis L. and Portulaca oleracea L. CA = citric acid; MA = malic acid; FA = fumaric acid; OA = oxalic acid; GA = glutamic acid; R Pb = root lead; S Pb = shoot lead; R Ca = root calcium; S Ca = shoot calcium; R Mg = root magnesium; S Mg = shoot magnesium; R K = root potassium; S K = shoot potassium; R Na = root sodium; S Na = shoot sodium; H2O2 = hydrogen peroxide; Chl. a = chlorophyll a; Chl. b = chlorophyll b; T. Chl = total chorophyll; Car = carotenoid; TBARS = thiobarbituric acid reactive substances; DW = dry weight; FW = fresh weight.
Discussion
Organic acid contents and pH in root mucilage of A. viridis and P. oleracea under Pb stress
The highest pH in the root mucilage was recorded at moderate Pb treatments, i.e., 30 mg/kg soil (for A. viridis) and at 45 mg/kg soil (for P. oleracea). While, at higher Pb treatment (60 mg/kg soil), the basification response was diminished in both the studied plant species (Table 1). Our results corroborated the findings of Javed et al. (2013) who reported that the roots of Eriophorum angustifolium caused rhizospheric basification when stressed by toxic elements (including Pb) at lower treatment level. It can be interpreted that at the highest Pb treatment level, the plants were stressed and lost their pH modulation response. Furthermore, the organic acids are exuded as anions by anion channels (Zhu et al. 2011) and their release remain balanced under metal stress by cations/proton efflux as reported for poplar roots (Qin et al. 2007). In Cucumis sativus, metal stress impaired the pumping activities of H+-ATPase, which caused a decreased efflux of protons (Janicka-Russak et al. 2012). This could explain the rhizospheric basification in the present study under low Pb stress. The pH of root exudates decreased at higher Pb levels which is likely due to the increased contents of LMWOAs, whose carboxyl groups contributed towards the increase of H+ in the solution as reported for castor (Ricinus communis L.) under metal stress (Huang et al. 2016). An investigation with Eriophorum angustifolium revealed acidosis of the exudates at higher Pb treatments (Javed 2011). It has been suggested that the pH decrease by organic acids only happened if the initial soil pH was high, as it was in the present study where the starting soil pH was 7.64. Addition of organic acids to soil promotes ammonification which produces protons and leads to a pH decrease (Paul et al. 2001). Therefore, acidosis of root exudates might also depend on nitrogen conversion triggered by decomposition of the exuded organic acids.
Increasing Pb treatment levels influenced organic acids exudations in root mucilage of A. viridis and P. oleracea which might be of importance for plant adaptation to Pb-enriched environments (Table 1). Differential root exudation response of A. viridis and P. oleracea seems to depend on different Pb tolerance levels as proposed previously for Cynodon dactylon and Zea mays under metal stress (Xie et al. 2014; Javed et al. 2017). Exudation of different organic acids might be due to better nutrient homeostasis of P. oleracea, as an increase of K level also caused an elevated exudation in Phelipanche aegyptiaca and Orobanche cumana (Zhang et al. 2015). It is likely that the better nutrients absorption of K by P. oleracea roots and altered cell membrane permeability were related to secretion of organic acids.
The secreted organic acids may protect the plants by reducing acropetal metal transport to shoots due to metal-ion complex formation with organic acid anions in P. olerace a, compared to A. viridis (Kochian et al. 2005). Therefore, organic acid exudation is a likely mechanism by which P. oleracea tolerates Pb stress. In this regard, citric acid, malic acid, fumaric acid, oxalic acid, and glutamic acid are significantly important for P. oleracea, because a strong correlation was obtained between root Pb contents and organic acid exudations (Table 2). Despite of in planta Pb accumulation, Pb may be non-toxic if bound to organic ligands as compared to free Pb2+ ions. The dicarboxylic acids exuded by A. viridis and P. oleracea hold more negative charges, thus providing more binding sites, as well as higher binding affinity to Pb in comparison to monocarboxylic acids. Our results are in accordance with the finding that exudation of oxalic, malic, and lactic acids was significantly increased by the roots of Phyllostachys pubescens under Pb stress which enhanced Pb adsorption to soil colloids (Chen et al. 2016). Therefore, in P. oleracea, exudation of citric, glutamic, and fumaric acids could potentially limit the uptake of Pb by enhancing Pb sorption to the soil due to a rise in pH.
Metal stress stimulates organic acids exudation by acting through various mechanisms existing in plants, including both basic (Zhao et al. 2003) and stress-specific processes (Ma et al. 2000). In A. viridis and P. oleracea, the former mechanism is likely to occur, as their roots exuded various organic acids and Pb stress further activates organic acid exudations. Such an idea corroborated the findings of Javed et al. (2013), who suggested a similar mechanism for common cotton grass when exposed to a mixture of toxic elements including Pb. Reduction in shoot growth of A. viridis and P. oleracea seems to minimize by increased organic acid production, and the ameliorative effect can be stronger if organic acids are secreted in higher amount.
Effect of Pb stress on growth and photosynthetic pigments of A. viridis and P. oleracea
Plant fresh and dry weights were significantly decreased with Pb treatment levels in A. viridis and P. oleracea (Table 2). Increase in plant H2O2 contents will in turn increase TBARS levels by initiating a chain reaction in unsaturated lipids, which is likely to negatively affect the growth of A. viridis and P. oleracea. In both species, a marked reduction of plant dry weights, compared to fresh weights, points out that Pb affects photosynthetic activity of A. viridis and P. oleracea more than their growth. Earlier works also described significant decrease in dry weight of different plant parts in Plantago major, Sesbania grandiflora, Triticum aestivum, Lens culinaris, Spinacia oleracea, and Solanum lycopersicum under Pb stress (Kosobrukhov et al. 2004; Akinci et al. 2010; Lamhamdi et al. 2013; Malar et al. 2014). In addition, Pb stress in the roots of Lemna minor suppressed the cell division and thereby reduced the plant growth (Samar-dakiewicz and Wozny 2005). Dendrogram analysis showed that biomass (fresh weight and dry weight) of the studied plants was affected in a similar way and related parameters were grouped as G-2 (Fig. S1).
Lead (Pb) stress significantly reduced the plant photosynthetic pigments (chlorophylls a and b and total chlorophyll) as well as accessory pigments (carotenoids), and the effect was Pb dose-dependent and plant-specific. In A. viridis, a significant reduction in chlorophyll b contents was observed which resulted in decrease of total chlorophyll contents as compared to P. oleracea which exhibited marked reduction in chlorophyll a under Pb stress. These results point out that Pb stress differentially interferes with the photosynthetic machinery in A. viridis and P. oleracea. Our results are in line with Shahid et al. (2014) who observed a reduction in photosynthetic pigments in Vicia faba after applying different levels of Pb stress. Earlier studies show that lead-induced reduction of chlorophyll arises from diminution in the number of thylakoids/grana, chloroplast disorganization, direct inhibition of chlorophyll biosynthesis, and alterations in chlorophyll structure due to Pb replacement of nutrients like Mg and Cu (Haider et al. 2006; Akinci et al. 2010). Phaseolus mungo and L. culinaris plants were reported to undertake adaptive mechanisms to protect photosynthetic machinery by increased number of trichomes and stomata which enabled these macrophytes to sustain the efficiency of photosystem II and to minimize water evaporation losses under Pb stress (Azmat et al. 2009). In the present study, Pb-induced increase in H2O2 is likely to interfere with electron transport chain of the studied plants and, thus, affects the plant pigment contents, ultimately reducing the growth.
Lead-induced reduction in carotenoid contents might result from altered K uptake by the roots as reported by Elloumi et al. (2014). Under Pb stress, plants generate reactive oxidative species (ROS) which, in turn, significantly modulate the physiology and morphology of plants. The reduced carotenoid content is also likely due to over production of ROS which damages the photosynthetic pigments, as well as other macromolecules (Yadav 2010). Dendrogram analysis reveals that photosynthetic pigments, i.e., chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids are affected collectively and are grouped as G-1 (Fig. S1).
Lipid peroxidation of A. viridis and P. oleracea under Pb stress
The increase in exogenous Pb results in higher H2O2 (ROS) contents and membrane lipid peroxidation predominantly in A. viridis (Table 2). Excessive H2O2 production causes oxidative stress, as reported for rice under Pb treatment (Srivastava et al. 2014), and is likely to be commenced by molecular oxygen excitation (O2) to generate singlet oxygen or by electron transfer to O2 and genesis of free radicals, i.e., O2− and OH− (Shigeoka et al. 2002). Higher levels of cellular H2O2 might be attributed to their enhanced generation and/or due to reduced scavenging capacity. Reactive oxygen species (ROS), if not efficiently scavenged, reduce plant growth and development by reacting with important metabolites and macromolecules (Richards et al. 2015). Therefore, increased H2O2 levels seem to interfere with electron transport chain of the studied plants and thus affect the plant pigment contents, ultimately reducing their growth. Under lead stress, the higher levels of Kreb’s cycle intermediates (organic acids) could be reflective of elevated mitochondrial activities which ultimately produce more reducing agents and ATP. Increased metabolic activity thereby results in oxidative stress, as is seen here under lead stress by the positive correlation between organic acids and H2O2 as well as TBARS (Table 3). A Pb-induced higher level of ROS, lipid peroxidation, and reduction of plant dry mass with increasing Pb concentration in nutrient solution have previously been reported for the Vicia faba root (Pourrut et al. 2011). Oxidative stress (hydrogen peroxide and TBARS contents) parameters exhibit a similar pattern and are grouped as G-3 (Fig. S1). Our results reveal that P. oleracea plants maintain lower levels of H2O2 and TBARS under Pb stress as compared to A. viridis and thereby have a higher adaptability to Pb-polluted environment.
Pb uptake of A. viridis and P. oleracea under different Pb treatment levels
Lead compartmentalization in the roots and shoots of A. viridis and P. oleracea was significantly increased with increasing Pb treatment levels where root Pb accumulation was higher than the accumulation in shoots (Fig. 1). Our results corroborates the findings of Malik et al. (2010) who also reported that Pb mainly sequesters in the roots of A. viridis and P. oleracea and both the species exhibit differential root to shoot Pb translocation. Plant endodermis possesses casparian strips which restricts Pb transport to areal parts of plants (Verma and Dubey 2003). The observed difference in Pb uptake between A. viridis and P. oleracea might be attributed to higher capacity of P. oleracea to resist Pb-induced oxidative stress as reported earlier (Lamhamdi et al. 2013). The lower root to shoot Pb translocation in P. oleracea points out its better adaptation to Pb-polluted environment and might be dependent upon exodermis lignification/suberization which in turn restricts root Pb accumulation (Cheng et al. 2015). These authors suggested that exodermal lignification/suberization also can alter Pb availability by initiating changes in rhizospheric environment. In addition, Pb2+ binds to carboxyl groups which are possibly pectin compounds of the root cell walls (Inoue et al. 2013). Release of citric acid, glutamic acid, and fumaric acid in root exudates was increased at higher Pb treatment levels which decreased the root Pb accumulation particularly in P. oleracea. At normal plant cytosolic pH, organic acids are present as anions which when released may bind to protons resulting in rhizospheric basification. Such plant-induced basification mechanisms may result in stabilization of heavy metals due to their reduced mobility in the rhizosphere (Javed et al. 2013).
Uptake of Ca, K, Mg, and Na by A. viridis and P. oleracea under Pb stress
With increasing Pb treatments levels, calcium (Ca), magnesium (Mg), and potassium (K) contents of A. viridis and P. oleracea roots and shoots were decreased significantly, though differentially in both plant species. In all Pb treatment levels, P. oleracea appears less disturbed than A. viridis regarding studied macronutrients. The K contents in A. viridis and P. oleracea were significantly lower as compared to corresponding control plants except for the roots of P. oleracea at 15 mg Pb/kg soil where K contents increased (Fig. 3). A similar inverse relationship between Pb and K concentrations was found in Pisum sativum, Z. mays, and Solanum lycopersicum (Paivoke 2002; Malkowski et al. 2002; Akinci et al. 2010). The decrease in carotenoid contents of A. viridis and P. oleracea in the present study might result from alterations in K uptake under Pb stress (Elloumi et al. 2014) or due to enhanced level of H2O2 which can damage plant carotenoid contents (Shahid et al. 2014; Hossain et al. 2012). Parallel conclusions can also be made for Ca and Mg contents in P. oleracea which appear to be more resistant against Pb toxicity than A. viridis (Fig. 2 and Fig. 4). A similar inverse relationship between Pb and Ca ion accumulation has also been reported and might be attributed to ionic similarity of two elements that permits Pb to replace Ca during specific physiological functions (Azmat et al. 2009).
Our results corroborate the findings of Ali et al. (2014) who reported diminished uptake of nutrients in B. napus under Pb stress. Such Pb-induced nutrient ion imbalance varies from species to species (Lamhamdi et al. 2013). The uptake of nutrient ions inhibited by Pb stress blocks the entry of various ions to the absorption sites on the plant roots (Godbold and Kettner 1991). However, in the present study, very large reductions of plant ionic content can rarely arise from just an ion uptake inhibition, but is also likely due to extra ion leakage from root surface due to increased TBARS contents. This could have happened in the present study based on the fact that the release of organic acid anions from the plant root cells must be balanced by an efflux of cations (Ryan et al. 2001). Furthermore, proton pumping ability of H+-ATPase at the plasmalemma of plant cell decreases under metal stress. Therefore, nutritious cations must be co-released with organic acid anions under Pb toxicity and result in major loss of nutrient ions (Kochian et al. 2005). Potassium ions were reported to be co-released with organic acid anions in poplar roots under metal stress (Qin et al. 2007).
Lead treatment also reduced the uptake of sodium (Na) in the roots and shoots of A. viridis and P. oleracea (Fig. 5). Interestingly, root Na contents of A. viridis significantly increased at 60 mg/kg soil Pb treatment. Sodium is a micronutrient for plants, and higher Na uptake or accumulation in the roots of A. viridis could result from competition among the cations. Under metal stress, higher accumulation of monovalent cations has also been reported by Rhodes and Hanson (1993). Thus, sodium together with Pb should affect plant mineral nutrition.
Conclusions
It can be concluded that the roots of A. viridis and P. oleracea respond to moderately elevated Pb stress by release of root exudates with slightly basic pH, while the basicity of root exudates is diminished at higher Pb levels. The Pb stress triggers the exudation of various organic acids by the roots of A. viridis and P. oleracea, where the latter species produce organic acids at higher concentration. Lead stress reduces the growth and photosynthetic pigments of the studied plants irrespective of genotypic differences by inducing oxidative damage. Citric acid, malic acid, oxalic acid, fumaric acid, and glutamic acid exudations are positively correlated with root Pb contents, which may maintain optimum Ca, Mg, and K contents in P. oleracea. The efficient organic acids exudation and better uptake of nutrient ions enable the adaptations of P. oleracea to Pb-polluted environment. The present study focused on short-term Pb effects on organic acid exudations of A. viridis and P. oleracea. Therefore, investigations during long term with respect to plant root exudates, plant survival and function should be the subject of future investigations. A better understanding of root exudation patterns of A. viridis and P. oleracea under Pb stress may provide effective approaches to optimize plant-based remediation strategies of metal-contaminated environments.
References
Akinci IE, Akinci S, Yilmaz K (2010) Response of tomato (Solanum lycopersicum L.) to lead toxicity: growth, element uptake, chlorophyll and water content. Afr J Agric Res 5:416–423
Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W (2014) Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crops Prod 52:617–626
Arnon DI (1949) Copper enzyme in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24(1):1–15. https://doi.org/10.1104/pp.24.1.1
Azmat R, Haider S, Nasreen H, Aziz F, Riaz M (2009) A viable alternative mechanism in adapting the plants to heavy metal environment. Pak J Bot 41:2729–2738
Bharwana SA, Ali S, Farooq MA, Ali B, Iqbal N, Abbas F, Ahmad MSA (2014) Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ Sci Pollut Res 21(1):717–731. https://doi.org/10.1007/s11356-013-1920-6
Blaylock MJ, Huang JW (2000) Phytoextraction of metals. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. John Wiley & Sons, New York, pp 53–71
Blossfeld S, Perriguey P, Sterckeman T, Morel J, Losch R (2010) Rhizosphere pH dynamics in trace-metal-contaminated soils monitored with planar pH optodes. Plant Soil 330(1-2):173–184. https://doi.org/10.1007/s11104-009-0190-z
Chen J, Shafi M, Wang Y, Wu J, Ye Z, Liu C, Zhong B, Guo H, He L, Liu D (2016) Organic acid compounds in root exudation of moso bamboo (Phyllostachys pubescens) and its bioactivity as affected by heavy metals. Environ Sci Pollut Res 23(20):20977–20984. https://doi.org/10.1007/s11356-016-7323-8
Cheng H, Wang Y, Liu Y, Ye ZH, Wu ML, Sun CC (2015) Pb uptake and tolerance in the two selected mangroves with different root lignification and suberization. Ecotoxicology 24(7-8):1650–1658. https://doi.org/10.1007/s10646-015-1473-1
Davies BH (1976) Carotenoids. In: Goodwin TW (ed) Chemistry and biochemistry of plant pigments. Academic, London, pp 138–165
Eliasson L (1978) Effect of nutrients and light on growth and root formation in Pisum sativum cuttings. Plant Physiol 43(1):13–18. https://doi.org/10.1111/j.1399-3054.1978.tb01560.x
Elloumi N, Zouari M, Chaari L, Jomni C, Marzouk B, Elloumi FBA (2014) Effects of cadmium on lipids of almond seedlings (Prunus dulcis). Bot Stud 55(1):61. https://doi.org/10.1186/s40529-014-0061-7
Fahr M, Laplaze L, Bendaou N, Hocher V, Mzibri EM, Bogusz D, Smouni A (2013) Effect of lead on root. Front Plant Sci 4:175. https://doi.org/10.3389/fpls.2013.00175
Gadd GM (2010) Metals, minerals and microbes: geo microbiology and bioremediation. Microbiology 156(3):609–643. https://doi.org/10.1099/mic.0.037143-0
Godbold DL, Kettner C (1991) Use of root elongation studies to determine aluminium and lead toxicity in Picea abies seedlings. J Plant Physiol 138(2):231–235. https://doi.org/10.1016/S0176-1617(11)80276-2
Greger M, Landberg T (2008) Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant Soil 312(1–2):195–205
Haider S, Kanwal S, Uddin F, Azmat R (2006) Phytotoxicity of Pb II: changes in chlorophyll absorption spectrum due to toxic metal Pb stress on Phaseolus mungo and Lens culinaris. Pak J Biol Sci 9:2062–2068
Haoliange L, Chongling Y, Jingchun L (2007) Low-molecular-weight organic acids exuded by mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environ Exp Bot 61(2):159–166. https://doi.org/10.1016/j.envexpbot.2007.05.007
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hossain AM, Piyatida P, Silva TAJ, Fujita1 M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot Article ID 872875 1–7. https://doi.org/10.1155/2012/872875
Huang G, Guo G, Yao S, Zhang N, Hu H (2016) Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) under Cu stress. Int J Phytoremediat 18(1):33–40. https://doi.org/10.1080/15226514.2015.1058333
Inoue H, Fukuoka D, Tatai Y, Kamachi HY, Hayatsu M, Ono M et al (2013) Properties of lead deposits in cell walls of radish (Raphanus sativus) roots. J Plant Res 126(1):51–61. https://doi.org/10.1007/s10265-012-0494-6
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Janicka-Russak M, Kabała K, Burzyński M (2012) Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J Exp Bot 63(11):4133–4142. https://doi.org/10.1093/jxb/ers097
Javed MT (2011) Mechanisms behind pH changes by plant roots and shoots caused by elevated concentration of toxic elements. PhD thesis, Department of Botany, Stockholm University, Stockholm, pp 1–40
Javed MT, Stoltz E, Lindberg S, Greger M (2013) Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements. Environ Sci Pollut Res 20(3):1876–1880. https://doi.org/10.1007/s11356-012-1413-z
Javed MT, Akram MS, Tanwir K, Javed Chaudhary HJ, Ali Q, Stoltz E, Lindberg S (2017) Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Saf 141:216–225. https://doi.org/10.1016/j.ecoenv.2017.03.027
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274(1-2):175–195. https://doi.org/10.1007/s11104-004-1158-7
Kosobrukhov A, Knyazeva I, Mudrik V (2004) Plantago major plants responses to increase content of lead in soil: growth and photosynthesis. Plant Growth Regul 42(2):145–151. https://doi.org/10.1023/B:GROW.0000017490.59607.6b
Lamhamdi M, El Galiou O, Bakrim A, Novoa-Munoz JC, Arias-Esteves M, Aarab A, Lafont R (2013) Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedling. Saudi J Biol Sci 20(1):29–36. https://doi.org/10.1016/j.sjbs.2012.09.001
Ma JF, Taketa S, Yang ZM (2000) Aluminium tolerance genes on the short arm of chromosome 3R are linked to organic acid release in triticale. Plant Physiol 122(3):687–694. https://doi.org/10.1104/pp.122.3.687
Malar S, Manikandan R, Favas CJP, Sahi VS, Venkatachalam P (2014) Effect of lead on phytotoxicity, growth, biochemical alterations and its role on genomic templates tability in Sesbania grandiflora: a potential plant for phytoremediation. Ecotox Environ Safe 108:249–257
Malik NR, Husain ZS, Nazir A (2010) Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak J Bot 42(1):291–301
Malkowski E, Kita A, Galas W, Karcz W, Kuperberg JM (2002) Lead distribution in corn seedlings (Zea mays L.) and its effect on growth and the concentrations of potassium and calcium. Plant Growth Regul 37(1):69–76. https://doi.org/10.1023/A:1020305400324
Massaccesi L, Meneghini C, ComaCi T, D’Amato R, Onofrij A, Businelli D (2014) Ligands involved in Pb immobilization and transport in lettuce, radish, tomato and Italian ryegrass. J Plant Nutr Soil Sci 177(5):766–774. https://doi.org/10.1002/jpln.201200581
Muhammad D, Chen F, Zhao J, Zhang G, Wu F (2009) Comparison of EDTA and citricacid-enhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifolia. Int J Phytoremediat 11(6):558–574. https://doi.org/10.1080/15226510902717580
Mukai H, Tanaka A, Fujii T, Zeng Y, Hon Y, Tang J et al (2001) Regional characteristics of sulfur and lead isotope ratios in the atmosphere at several Chinese urban sites. Environ Sci Technol 35(6):1064–1071. https://doi.org/10.1021/es001399u
Paivoke AEA (2002) Soil lead alters phytase activity and mineral nutrient balance of Pisum sativum. Environ Exp Bot 48(1):61–73. https://doi.org/10.1016/S0098-8472(02)00011-4
Paul KI, Black AS, Conyers MK (2001) Effect of plant residue return on the development of surface soil pH gradients. Biol Fert Soils 33:75–82
Pourrut B, Jean S, Silvestre J, Pinelli E (2011) Lead-induced DNA damage in Vicia faba root cells: potential involvement of oxidative stress. Mutat Res 726(2):123–128. https://doi.org/10.1016/j.mrgentox.2011.09.001
Qin R, Hirano Y, Burner I (2007) Exudation of organic acid anions from poplar roots after exposure to Al, Cu, and Zn. Tree Physiol 27(2):313–320. https://doi.org/10.1093/treephys/27.2.313
Reddy AM, Kumar SG, Jyonthsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.) Chemosphere 60(1):97–104. https://doi.org/10.1016/j.chemosphere.2004.11.092
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44(1):357–384
Richards SL, Wilkins KA, Swarbreck SM, Anderson AA, Habib N, Smith AG, McAinsh M, Davies JM (2015) The hydroxyl radical in plants: from seed to seed. J Exp Bot 66(1):37–46. https://doi.org/10.1093/jxb/eru398
Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52(1):527–560. https://doi.org/10.1146/annurev.arplant.52.1.527
Samar-dakiewicz S, Wozny A (2005) Cell division in Lemna minor roots treated with lead. Aquat Bot 83(4):289–295. https://doi.org/10.1016/j.aquabot.2005.06.007
Shahid M, Pinelli E, Dumat C (2012) Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater 220:1–12
Shahid M, Austruy A, Echevarria G, Arshad M, Sanaullah M, Aslam M, Nadeem M, Nasim W, Dumat C (2014) EDTA-enhanced phytoremediation of heavy metals: a review. Soil Sediment Contam 23(4):389–416. https://doi.org/10.1080/15320383.2014.831029
Shakoor MB, Ali S, Hameed A, Farid M, Hussain S, Yasmeen T, Najeeb U, Bharwana SA, Abbasi GH (2014) Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol Environ Saf 109:38–47. https://doi.org/10.1016/j.ecoenv.2014.07.033
Sharma P, Dubey RS (2005) Pb toxicity in plants. Braz J Plant Physiol 17(1):35–52. https://doi.org/10.1590/S1677-04202005000100004
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53(372):1305–1319. https://doi.org/10.1093/jexbot/53.372.1305
Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey RS (2014) Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251(5):1047–1065. https://doi.org/10.1007/s00709-014-0614-3
Stoltz E, Greger M (2002) Cottongrass effects on trace elements in submersed mine tailings. J Environ Qual 31(5):1477–1483. https://doi.org/10.2134/jeq2002.1477
Tanwir K, Akram SA, Masood S, Chaudhary HJ, Lindberg S, Javed MT (2015) Cadmium-induced rhizospheric pH dynamics modulated nutrient acquisition and physiological attributes of maize (Zea mays L.) Environ Sci Pollut Res 22(12):9193–9203. https://doi.org/10.1007/s11356-015-4076-8
Trivedi S, Erdei L (1992) Effects of cadmium and Pb on the accumulation of Ca2+ and K+ and on the influx and translocation of K+ in wheat of low and high K+ status. Physiol Plant 84(1):94–100. https://doi.org/10.1034/j.1399-3054.1992.840115.x
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotox Environ Saf 113:271–278. https://doi.org/10.1016/j.ecoenv.2014.12.014
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wang Y, Xu L, Chen YL, Shen H, Gong YQ, Limera C et al (2013) Transcriptome profiling of radish (Raphanus sativus L.) root and identification of genes involved in response to lead (Pb) stress with next generation sequencing. PLoS One 8(6):e66539. https://doi.org/10.1371/journal.pone.0066539
Wolf B (1982) A comprehensive system of leaf analysis and its use for diagnosing top nutrient status. Commun Soil Sci Plant Anal 13:1035–1059
Xie Y, Hu L, Du Z, Sun X, Amombo E, Fan J, Fu J (2014) Effects of cadmium exposure on growth and metabolic profile of bermudagrass [Cynodon dactylon (L.) Pers.] PLoS One 9(12):e115279. https://doi.org/10.1371/journal.pone.0115279
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yang Y, Wang QL, Geng MJ, Guo ZH, Zhao Z (2011) Rhizosphere pH difference regulated by plasma membrane H+-ATPase is related to differential Al-tolerance of two wheat cultivars. Plant Soil Environ 57(5):201–206
Zeng F, Chen S, Miao Y, Wu F, Zhang G (2008) Changes of organic acid exudation and rhizosphere pH in rice plants under chromium stress. Environ Pollut 155(2):284–289. https://doi.org/10.1016/j.envpol.2007.11.019
Zhang M, Ma YQ, Zhong W, Jia XT, DR W, Yu R, Ye XX (2015) N–P–K ratio affects exudation of germination stimulants and resistance of tobacco seedlings to broomrapes. Plant Growth Regul 76(3):281–288. https://doi.org/10.1007/s10725-014-9999-4
Zhao Z, Ma JF, Sato K, Takeda K (2003) Different Al resistance and citrate secretion in barley (Hordeum vulgare L.) Planta 217(5):794–800. https://doi.org/10.1007/s00425-003-1043-2
Zhi-An L, Bi Z, Han-Ping X, Yong-Zhen D, Wan-Neng T, Sheng-Lei F (2008) Role of low-molecule-weight organic acids and their salts in regulating soil pH. Pedosphere 18(2):137–148
Zhu XF, Zheng C, Hu YT, Jiang T, Liu Y, Dong NY, Yang JL, Zheng SJ (2011) Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant Cell Environ 34:1055–1064
Acknowledgements
We are thankful to Prof. Dr. Sylvia Lindberg and Dr. Sajid Masood for critical proofreading of the manuscript.
Funding
We gratefully acknowledge the Higher Education Commission (HEC), Pakistan, for provision of funds (Grant No: PD-IPFP/HRD/HEC/2013/3021) to Dr. M. Tariq Javed for the execution of the reported work.
Author information
Authors and Affiliations
Contributions
MTJ and MSA designed the experiments. HG and KT performed the experiments and the physiological/biochemical analysis with assistance of MTJ. NH, QA, and NKN performed the statistical analysis of the data and reviewed the manuscript. NI contributed to data discussion. All the authors approved the final manuscript.
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Fig. S1
The dendrograms showing the similarity for the studied physiological attributes of Amaranthus viridis L. and Portulaca oleracea L. grown with PbCl2 concentrations of 0, 15, 30, 45, or 60 mg/kg soil in rhizoboxes. TBARS = thiobarbituric acid reactive substances, PFW = plant fresh weight, PDW = plant dry weight, T Chl = total chlorophyll, Chl. a = chlorophyll a, Car = carotenoids, Chl. b = chlorophyll b (DOCX 160 kb)
Rights and permissions
About this article
Cite this article
Javed, M.T., Akram, M.S., Habib, N. et al. Deciphering the growth, organic acid exudations, and ionic homeostasis of Amaranthus viridis L. and Portulaca oleracea L. under lead chloride stress. Environ Sci Pollut Res 25, 2958–2971 (2018). https://doi.org/10.1007/s11356-017-0735-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0735-2