Abstract
2,4,6-Trichloroanisole (TCA) is an odorous compound that is often detected in tap water and is difficult to be removed via water treatment. In this study, the transformation efficiency of TCA in the presence of persulfate (PS) activated by iron (Fe2+, Fe0, and Fe3+) was investigated for the first time. The effects of the activator dosage, oxidant dosage, pH, dosing method, chelating agents, humic acid, and temperature were evaluated. The degradation rate of TCA increased with increasing PS dosages (0.12–0.48 mM) and initial Fe2+ concentrations (0.12–0.96 mM), while it decreased with higher Fe2+ concentrations. Fe2+/PS and Fe0/PS systems achieved their best TCA removal efficiency at pH 7 and 2.5, respectively. According to the results of electron paramagnetic resonance (EPR), the contribution of SO4 −• to TCA degradation was much higher than that of •OH. Gradual addition of Fe2+ improved TCA degradation compared to single addition. Citric acid (CA) promoted TCA degradation under Fe2+/PS at the beginning of the reaction, but inhibited it after 10 min. Ethylenediaminetetraacetic acid (EDTA) improved the TCA removal rate with an EDTA/Fe2+ molar ratio of 0.5:1, while it decreased it at higher EDTA/Fe2+ molar ratios. Oxalic acid (OA) negatively affected TCA degradation with increasing OA/Fe2+ molar ratios. Among all of the chelating agents, only CA increased TCA degradation by Fe0/PS. Humic acid promoted TCA degradation by Fe2+/PS at the proper dosage (1 mg/L). Under our specific conditions and over the temperature ranging from 10 to 25 °C, no change was observed in the reaction kinetics. It was found that 2,4,6-trichlorophenol (TCP) was the only detected oxidation product. The presence of an Fe2+-Fe3+ redox cycle in iron-activated PS systems was confirmed by TCA degradation under the Fe3+/PS system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4,6-Trichloroanisole (TCA) is a typical earthy and musty compound that can cause unpleasant taste and odor in tap water (Maillet et al. 2009). In general, it is generated from the biomethylation of chlorophenol by microorganisms in the drinking water distribution system (Park et al. 2007), or from chlorination of anisole during chlorine disinfection in drinking water treatment plants (Zhang et al. 2016). Moreover, TCA can also be released into surface water by wine industrial (Marquez-Sillero et al. 2011) and wastewater discharge (Urase and Sasaki 2013). Because TCA’s odor threshold concentration is very low (0.03 ng/L) (Marquez-Sillero et al. 2011; Prat et al. 2011), consumers can still smell its off flavor even at low concentrations. In addition to its flavor, 2,4,6-TCA—as an aromatic halogenated DBP—has toxicological effects (Zhang et al. 2016). Therefore, the occurrence of TCA in source water and tap water should not be ignored by the public.
Due to its water solubility and physical properties, TCA is difficult to be removed from water by conventional water treatment technologies including coagulation, sedimentation, filtration, and disinfection (Song and O’Shea 2007). Therefore, several pretreatments and advanced treatments have been applied to remove TCA from drinking water in recent years (Bruce et al. 2002; Chestnutt et al. 2007). Lalezary et al. (1986) introduced pre-oxidation methods (e.g., potassium permanganate, chlorination, and ozonation) to conventional drinking water treatment to promote TCA degradation. However, only ozonation presented satisfactory TCA removal efficiency. Another popular method is the use of activated carbon (AC) including powdered AC (PAC) and granular AC (GAC) (Chestnutt et al. 2007; Lalezary et al. 1986). Although they can adsorb TCA effectively, PAC creates problems in the sludge removal and disposal (Chestnutt et al. 2007), and GAC causes hygienic and esthetic problems by enhancing microbial and invertebrate growth in the filters (Wang et al. 2014). Membrane processes (e.g., ultrafiltration, nanofiltration, reverse osmosis) have also been applied to remove TCA (Park et al. 2007). Both reverse osmosis and nanofiltration reduce TCA effectively (Bruchet and Laine 2005). However, the manufacturing cost and membrane fouling limit the application of membranes in water treatment plants. Recently, advanced oxidation processes (AOPs) that involve the generation of hydroxyl radical (•OH) as the predominant species for the degradation of micropollutants are attractive alternatives and have received considerable attention. Several AOPs such as photocatalytic degradation (Vlachos et al. 2008), gamma radiation (Vlachos et al. 2008), and catalytic ozonation (Qi et al. 2009a, b, c) have already been used in the degradation of TCA. Qi et al. (2009a, b, c) have performed many studies on the catalytic ozonation of TCA in the presence of aluminum oxides or bauxite. They found that the ozonation of TCA by γ-AlOOH, raw bauxite, and iron modified bauxite showed 80.3, 86.0, and > 99% conversion of TCA, respectively, at the end of the reaction time. However, the removal rate obtained by single ozonation only ranged from 34.6 to 55%.
During the past 10 years, persulfate (PS) has been considered a new advanced oxidation chemical that can generate reactive sulfate radical (SO4 −•) with oxidation-reduction potential of 2.6 V (Berlin 1986). Because of the high-redox potential of SO4 −•, PS can oxidize most of the organic pollutants in water (Matzek and Carter 2016). PS can be attractive for application in chemical oxidation due to many advantages, such as high solubility in water (2.5 M at 20 °C), high stability, and relative low cost (Rodriguez et al. 2014). PS can be activated by heat (Johnson et al. 2008), alkaline (Guan et al. 2011), ultraviolet light (Lin et al. 2011), or transition metal (Fe0, Fe2+, Cu2+, Co2+, and Ag+) (Anipsitakis and Dionysiou 2004). If Fe2+ is employed as activator, the overall reaction between iron and PS can be described by Eq. (1) (Kolthoff et al. 1951). However, it should be noted that if excess quantities of Fe2+ are added, the SO4 −• may react with Fe2+ (see Eq. (2)) (Kolthoff et al. 1951), so a limited amount of SO4 −• oxidizes the target organics.
Therefore, it is important to consider the Fe2+ concentration in the persulfate activation system, and it is necessary to take measures to avoid the rapid conversion of Fe2+ to Fe3+ and the scavenging of SO4 −• (Liang and Guo 2010). It has been reported by Liang et al. (2004a) that sequential addition of small amounts of Fe2+ and the use of chelating agents such as citric acid (CA) to control the Fe2+ concentration in solution could promote the activated persulfate oxidation of trichloroethylene. An alternative activator of PS reported could be zero-valent iron (Fe0). Fe0 is believed to be a source of Fe2+ through corrosion by PS (see Eq. (3) (Liang and Guo 2010)). In addition to this oxidation reaction, two other oxidation pathways may take place, as shown in Eqs. (4) (Liang and Guo 2010), and (5) and (6) (Rodriguez et al. 2014).
In several studies, ferric ion (Fe3+), which is transformed from Fe2+ through Eq. (2), can be reduced by organic compounds or their intermediate products (see Eq. (7) (Tan et al. 2012)). As a result, Fe2+ can be produced to activate PS again and a Fe3+-Fe2+ cycle forms. Nevertheless, the process is highly dependent on the redox potential of Fe3+, which may not have the capability to oxidize recalcitrant organics. So it is interesting to study the removal efficiency of target compounds under an Fe3+/PS system when a new pollutant is introduced.
In the past 2 years, the application of PS-activated oxidation of odor-causing compounds has been studied by several researchers. Xie et al. (2015) firstly reported that both 2-methylisoborneol (2-MIB) and geosmin could be degraded effectively using a UV/PS process. Bu et al. (2017) found that electrogenerated PS could successfully remove 2-MIB and geosmin from water. Luo et al. (2016) investigated the degradation of TCA by SO4 −• both kinetically and mechanistically. However, PS activation by different valent iron and its application in TCA oxidation have not been studied systematically. Therefore, the purpose of this paper is to investigate the oxidation of TCA by iron (Fe0, Fe2+, Fe3+)-activated PS. The first series of experiments used Fe2+ as an activator. Experiments were designed to determine the influences of Fe2+ and S2O8 2− concentrations, pH, dosing method of Fe2+, chelating agents, and humic acid. Furthermore, electron paramagnetic resonance (EPR) techniques were used to determine the free radicals of the reaction system. Additionally, the possible products were investigated by the use of gas chromatography-mass spectrometry (GC-MS). The second series of experiments used Fe0 as an activator. The influences of S2O8 2− concentration, Fe0 dosage and pH, were also evaluated. Finally, TCA oxidation under the Fe3+/PS system was studied.
Experimental
Chemicals
2,4,6-Trichloroanisloe (TCA), 2,4,6-trichlorophenol (TCP), 2-isobutyl-3-methoxypyrazine (IB) (99%), and 5,5-dimethyl-1-pyrrolidine N-oxide (DMPO) were of chromatogram grade and obtained from Sigma-Aldrich Chemical Co. Ltd. Sodium persulfate (Na2S2O8), ferrous sulfate (FeSO4·7H2O), ferric sulfate (Fe2SO4·5H2O), zerovalent iron (Fe) (particle size, 10~44 μm), sodium thiosulfate (Na2S2O3), monosodium phosphate (NaH2PO4), disodium hydrogen phosphate (Na2HPO4), sodium phosphate (Na3PO4), critic acid (C6H8O7) (CA), oxalic acid (H2C2O4) (OA), disodium ethylene-diamine-tetra-acetate (C10H14N2O8Na2) (EDTA), humic acid (HA), and n-hexane (C6H14) were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd., China. Both chemicals were used without any further purification. All solutions were prepared in de-ionized water.
Stock solutions of TCA (1 g/L in methane), TCP (100 mg/L in methane), and IB (1 g/L in methane) were prepared in 15-mL Teflon screw capped bottles and stored at 4 °C in the fridge. Sodium persulfate (0.24 M), ferrous sulfate (0.24 M, pH 3.7), and ferric sulfate (0.24 M) stock solutions were prepared fresh each time before the experiments. The concentrations of chelating agents (CA, OA, EDTA) and HA stock solutions were 0.24 M and 0.5 g/L, respectively. All of them were prepared in 20-mL amber bottles.
Degradation experiments
All experiments were carried out in 500-mL glass bottles with Teflon-faced septa placed on a magnetic stirrer. Before each experiment, 500 mL of 100 μg/L (0.47 μM) TCA was added to the reaction bottle and a certain volume of the solution was taken as the 0-min sample. Then, a certain volume (0.5~2 mL) of ferrous sulfate (or ferric sulfate, or zerovalent iron) solution was added to the solution. For the experiments with chelating agents or humic acid, appropriate volumes (0.1~2.25 mL) of these stock solutions were added to the solution. The reaction time began when a certain volume (0.5~2 mL) of sodium persulfate solution was added to the reactor. The pH of the solution was adjusted to a chosen value by using phosphate buffer (10 mM). At each designated sampling time, a certain volume of solution, which was quenched with excess sodium thiosulfate, was sampled with a 5-mL injector. The sampled solution was filtered with a glass fiber filter (25 mm in diameter) before analysis. The reaction temperature was controlled by a magnetic stirrer with a hot plate. For Fe2+ or Fe3+ experiments, reaction solutions were stirred by magnetic stirrer at 250 rpm. For Fe0 experiments, the solutions were stirred with a mechanical stirrer instead to prevent iron from absorbing on the magnet.
Electron paramagnetic resonance (EPR) studies
The SO4 −• and HO• generated by PS-activated using Fe2+ were identified by EPR using DMPO as the spin-trapping agent. The EPR spectra were obtained at room temperature using a BRUKER A300 EPR spectrometer (Switzerland) with a resonance frequency of 9.866 GHz, microwave power of 20 mW, modulation amplitude of 1.00 G, sweep width of 200 G, center field of 3480 G, time constant of 81.92 ms, and conversion time of 41 ms. The peak intensities of DMPO-SO4 and DMPO-OH were used to determine the concentrations of SO4 −•and HO• by measuring the height of the main low-field peak (in arbitrary units determined by instrument parameters) corrected for background noise.
Chemical analysis
TCA and TCP were quantified by using liquid-liquid extraction coupled with GC-MS (QP2010, Shimadzu, Japan). After filtering through glass fiber, each 5 mL of the sample was put into a 10-mL extraction bottle. Then, 1 g of sodium chloride, 1 mL of n-hexane, and an internal standard (2-isobutyl-3-methoxypyrazine) were added to the sample. The extraction process was performed on a rotating oscillator for 10 min. After 10-min stewing, 0.5 mL of n-hexane at the top of the solution was taken for GC-MS analysis. The details of GC and MS operational conditions are available in previous publications (Zhang et al. 2016).
All the experiments were conducted in duplicate and the data were averaged. The general experimental errors were all within 10% and an error analysis example was shown in Table S1 (Supplementary Information). For most figures, the error bars are too short to be seen, so they are all omitted in the graphs.
Results and discussion
Degradation of TCA by persulfate activated with Fe2+
Influences of Fe2+ and PS initial concentrations
The kinetics of TCA evaporation and degradation by Fe2+ and PS only in buffered solution (pH = 7) are shown in Fig. S1 (Supplementary Information). TCA evaporation in the experiments could be neglected because only 3.9% loss of TCA was detected. Very low degradation was observed in the Fe2+/TCA system, while only 12% of TCA was removed in the PS/TCA system. Although PS has strong oxidizing power (E 0 = 2.01 V), it cannot remove TCA efficiently.
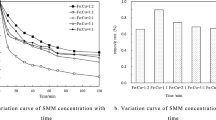
The influence of the initial Fe2+ concentration on TCA degradation is shown in Fig. 1(a). For all PS/Fe2+ molar ratios, TCA degradation appeared to occur instantaneously in the first 10 min and became gentle in the next 50 min. In the case of a PS/Fe2+ molar ratio of 1:0.5, TCA degradation (at 1-h reaction time, same as below) was 56.2%. The TCA removal efficiency enhanced constantly with the increase of Fe2+ content from a Fe2+/PS molar ratio of 0.5:1 to 2:1. TCA degradation was almost 87.5% at an Fe2+/PS molar ratio of 2:1. However, further increase of Fe2+ content to an Fe2+/PS molar ratio of 4:1 resulted in no improvement of TCA degradation, but it slightly dropped (4.4%) compared to the best one. Theoretically, the increase of Fe2+ concentration should produce more S2O8 2− and therefore increase TCA destruction. However, if the Fe2+ concentration is too high, the produced SO4 −• may react with excess Fe2+ as shown in Eq. (2) or even other chemicals (Peyton 1993) rather than TCA. A similar phenomenon has been reported by Liang et al. (2004a); i.e., in the case of TCE oxidation by Fe2+-activated PS, further increases in Fe2+ concentration resulted in decreased oxidation rate. In consideration of saving Fe2+ as well as acquiring good removal efficiency, the Fe2+/PS molar ratio of 1.5:1 was chosen for the rest of the experiments in this work.
Fig. 1(b) shows the influence of PS dosage on TCA degradation. Increases in PS concentration (from 0.12 to 0.48 mM) at one level of PS/Fe2+ molar ratio (i.e., 1:1.5) resulted in increases in TCA degradation. TCA degradation at 0.12 and 0.48 mM PS initial concentration was 80 and 93.7%, respectively. Obviously, TCA oxidation is directly related to SO4 −•, which originates the activation of PS. Therefore, the SO4 −• concentration increases with the increase in PS concentration at a fixed PS/Fe2+ molar ratio. However, too much PS in the reaction system may cause quenching of free radicals (see Eqs. (8) and (9)), which inhibits the removal of organics (Olmez-Hanci et al. 2013).
It should be noted that, in the case of different PS concentrations, TCA degradation by Fe2+-activated PS during the first 10 min was a pseudo-first-order reaction, as shown in Fig. S2 (a) and Eq. (10):
where [TCA] is the TCA concentration, t is the reaction time, and k TCA is a pseudo-first-order rate constant. It was interesting to find out that k TCA exhibits a linear trend as a function of PS dosage as shown in Fig. S2(b) and Table S1. The same linear relationship between reaction rate constant and PS dosage was also reported by the study of Gao et al. (2015), where carbamazepine was degraded by UV/PS.
Influence of pH and free radical identification
The pH is an important factor affecting the degradation of contaminants in PS activation processes (Liang et al. 2007) as is the distribution of free radicals. To investigate the influence of pH on TCA degradation, batch experiments were conducted at pH 3, 7, and 9 with buffer solution (10 mM of phosphate). As shown in Fig. 2(a), 63.5 and 80% of TCA was degraded within 60 min by Fe2+/PS at pH 3 and 7, respectively, while it decreased to only 5.1% at pH 10. These results indicated that the degradation of TCA by Fe2+/PS was effective at both acidic and neutral pH rather than alkaline pH.
To investigate the role of free radicals in the reactions with various pH values, the free radicals generated in the Fe2+/PS system at 1-min reaction time were identified by EPR using DMPO as the spin-trapping agent. As shown in Fig. 2(b), DMPO-SO4 (six lines, 1:1:1:1:1:1, pink circle) and DMPO-OH (four lines, 1:2:2:1, green square) signals can be attributed to the formation of SO4 −• and, respectively, as reported in another study (Fang et al. 2015). The hyperfine splitting constants were as follows: a H = a N = 14.9 G for DMPO-OH; a N = 13.2 G, a H = 9.5 G, 1.47 G, and 0.75 G for DMPO-SO4. It should be noted that there were also other signals (three lines, 1;1;1, purple triangle) in the spectra. Hypothetically, these might be signals from phosphate radical spin adducts (DMPO-H2PO4, DMPO-HPO4, DMPO-PO4), as phosphate was used as the pH-adjusting agent in the experiments. However, the oxidation abilities of these phosphate radicals were much lower than SO4 −• and •OH (Buxton et al. 1988; Neta et al. 1988), so their contribution to TCA oxidation was neglected in this study.
SO4 −• and •OH have comparable reaction activities with TCA (k SO4-•, TCA = (3.72 ± 0.1) × 109 M−1 s−1; k HO•, TCA = 5.1 × 109 M−1 s−1 (Buxton et al. 1988; Neta et al. 1988), so it is important to verify their contributions in different pH values. According to the peak intensities of DMPO-SO4 and DMPO-OH, it can be demonstrated that the concentrations of SO4 −• and •OH decreased and increased, respectively, when pH increased. This can be explained by Eq. (11):
where, OH− promotes the formation of •OH. This phenomenon was consistent with Liang and Su’s study (Liang and Su 2009) that in PS system, SO4 −• is the predominant radical at acidic pH, while HO• is the predominant radical at more basic pH levels (e.g., pH > 9). In our study, the poor TCA degradation at pH 10 (Fig. 2(a)) could be explained by the precipitation of iron at high pH value. The precipitation of Fe2+ at pH 10 resulted in less free Fe2+-activating PS to generate enough SO4 −•. Although the concentration of •OH was higher, the total radical amounts still could not compete with those at pH 3 and 7 and perform comparable oxidation ability. At lower pH values, however, SO4 −• is the main radical instead of •OH and plays an important role in oxidation (Liang and Su 2009; Wu et al. 2015). According to the peak intensity of DMPO-SO4, the SO4 −• concentration was much larger at pH 3 than at pH 7, which can explain the better TCA degradation at pH 3 than at pH 7 within the first 2 min. However, as the reaction proceeded, the degradation rate at pH 3 decreased faster than at pH 7, and the final degradation at pH 3 was exceeded by that at pH 7 (63.5 < 80%). This can be explained by radical quenching happening when too many SO4 −• are produced in the acidic system (see Eq. (8)).
Influence of dosing method of Fe2+
To ascertain the influence of the Fe2+-dosing method, an experiment was conducted where Fe2+ was added sequentially in small increments (0.12 mM) to the reaction system at 0, 10, and 30 min, as opposed to all at once. As shown in Fig. 3(a), after three successive additions of Fe2+, the overall TCA removal was 90.5%, which was better than 80% in the experiment where all the Fe2+ was added at once. Furthermore, when comparing the TCA degradation of the two experiments within 10 min (see Fig. 3(b)), the addition of 0.12 mM of Fe2+ at initial time resulted in even better removal efficiency than the addition of 0.36 mM of Fe2+ at once (56.2 > 46.7%). The results showed that gradually providing the available Fe2+ in low concentrations slowly generates SO4 −•, which can attack TCA preferentially instead of Fe2+ (see Eq. (2)). This phenomenon is similar to the observations by Pignatello and Baehr (1994) who noted in Fenton’s reaction that gradual addition of Fe2+, in dilute form, could minimize the oxidation of Fe2+ by •OH. It appears that slow and steady production of free radicals is most desirable and that Fe2+ availability plays an important role in controlling the ferrous ion-activated PS reaction.
Influence of Fe2+-dosing method on TCA degradation. Conditions: a batch addition, [PS]0 = 0.24 mM, [TCA]0 = 0.47 μM, [Fe2+]0 = 0.36 mM, [TCA]0 = 0.47 μM, temperature: 20 °C; b gradual addition: [PS]0 = 0.24 mM, [TCA]0 = 0.47 μM, temperature: 20 °C. 0.12 mM of Fe2+ was added to the solution at each addition time (0, 10, and 30 min)
Influence of chelating agents
The previous section showed that excess Fe2+ could quench radicals and inhibit the oxidation reaction. Therefore, the strategy of preventing contact between Fe2+ and SO4 −• can effectively promote the removal efficiency of TCA. One of the methods of inhibiting the SO4 −• scavenging by excess Fe2+ is to add organic chelating agents to the Fe2+/PS system (Liang et al. 2004b). In this study, three different chelating agents (CA, EDTA, and OA) were introduced into the Fe2+/PS system to investigate their possible impact on TCA degradation. As shown in Fig. 4(a)–(c), different chelating agents had different impacts on TCA oxidation.
In CA experiments, an obvious increase of degradation rate was observed during the first 10-min reaction (see Fig. 4(a)), which demonstrated that the generation of Fe2+ (CA) complex successfully retarded the SO4 −• scavenging by Fe2+ as well as the possible precipitation of Fe2+, which resulted in more SO4 −• generation and its subsequent reaction with TCA. After 10 min, however, the reaction with CA became instantaneously stalled, and finally, the TCA degradation was 61.4, 50, and 57.6% at CA/Fe2+ molar ratio of 0.5:1, 1:1, and 1.5:1, respectively, lower than that without CA (80%). It can be explained by the competition for \( {\mathrm{SO}}_4^{\bullet \hbox{-} } \) between CA and TCA, and the same observation between CA and trichloroethylene has been reported in another study (Liang et al. 2004b).
In the case of EDTA (see Fig. 4(b)), a test with an EDTA/Fe2+ molar ratio of 0.5:1 achieved better TCA degradation (87.5%) than that without EDTA (80%). This better performance of EDTA than CA with the same chelate/Fe2+ molar ratio might be due to the higher chelating ability of EDTA with iron than CA (Xu et al. 2011). The high stability of Fe2+ (EDTA) resulted in steady generation and less scavenging of SO4 −•. Nevertheless, the increase of EDTA/Fe2+ molar ratio (over 0.5:1) inhibited the TCA degradation. The increase of EDTA content might result in over chelating of Fe2+, which inhibited the PS activation by Fe2+. Moreover, excess EDTA can also chelate with Fe3+, which may cut the Fe3+-Fe2+ cycle as mentioned above.
As for OA (see Fig. 4(c)), no increase but an obvious reduction of TCA removal occurred compared to the experiment with no OA. This phenomenon demonstrated that the addition of OA would inhibit the degradation of TCA, and this inhibition increased as the molar ratio of CA/Fe2+ increased. This might be due to two reasons: (i) the stability of Fe2+ (OA) complex is not high enough to prevent the reaction with Fe2+ and SO4 −•; (ii) OA may react with SO4 −• preferentially instead of TCA (Huie and Clifton 1996), resulting in the decrease of TCA degradation.
In conclusion, the influence of chelating agents on TCA degradation depends on their type and content.
Influence of HA
Some of the matrix species (e.g., HA) that are commonly present in water can react with radical species, such as SO4 −•, in competition with the target pollutants (Criquet and Leitner 2009), which impacts the treatment efficiency. Thus, to simulate the real water matrix, HA was introduced to the Fe2+/PS system. Surprisingly, the TCA degradation increased from 80 to 85.4% as the HA increased from 0 to 1 mg/L, but it gradually dropped to 80.9 and 75.8% when HA increased to 3 and 5 mg/L, respectively (see Fig. 5). Actually, as a natural organic matter, HA contains various organic groups like hydroxyl and carboxyl, which can chelate with Fe2+ (Theis and Singer 1974). At low levels of HA, Fe2+ was steadily released by HA-Fe2+ complexity and less Fe2+ was scavenged by SO4 −•. Therefore, for experiment with 1 mg/L of HA dosage, TCA degradation was slightly promoted compared to that with no HA addition. However, excessive HA might compete for SO4 −• with TCA so that the TCA degradation slowed down. Fang et al. (2017) reported a similar finding in the double roles of HA during oxidation of PCB28 with V(V)/PS treatment, where the rate constant (k obs) increased from 0.0228 to 0.0263 h−1 as HA increased from 0 to 1 mg/L, but it decreased to 0.0153 h−1 when HA increased to 10 mg/L.
Product analysis and proposed pathway
To identify the oxidation products of TCA degradation by Fe2+/PS, a batch experiment was conducted amplifying the concentrations of TCA, PS, and Fe2+ for ten times ([TCA]0 = 9.4 μM, [PS]0 = 2.4 mM, [Fe2+]total = 3.6 mM). Concerning the increasing Fe2+ content in the solution, Fe2+ was (0.72 mM at a time) added to the solution at five reaction times (0, 10, 20, 30, 40, and 50 min) to avoid unnecessary quenching of SO4 −• with Fe2+. As shown in Fig. 6, TCA (retention time, 15.955 min) and trichlorophenol (TCP) (retention time, 16.510 min) were found in samples at reaction times of 5, 30, and 60 min. The results showed that TCA was rapidly degraded within 30 min and transformed to TCP. Luo et al. (2016) found the products TCP and 2,6-dichloro-1,4-benzoquinone in TCA degradation by UV/PS treatment. Unfortunately, none of the other products were detected in our study.
A possible mechanism for TCA degradation based on SO4 −• was proposed by Luo et al. (2016) (see Fig. S3). The initial step dominating TCA oxidation is the abstraction of an electron from the aromatic ring resulting in the formation of a cation substrate intermediate •TCA (Liu et al. 2016). Then, an ipso-hydroxylation pathway was followed; •TCA reacts with H2O to form a cation substrate intermediate hydroxyl-cyclohexadienyl (HCHD) radical, and this radical then reacts with O2 to produce organic peroxy radical, which subsequently eliminates methanol, O2, and •OH to produce the primary product TCP (Liu et al. 2016).
Degradation of TCA by persulfate activated with Fe0
It is believed that Fe0 can gently release Fe2+ via various types of reactions (i.e., Eq. (3)–(6)), which can prevent excess Fe2+ reacting with SO4 −•. Therefore, Fe0 was also chosen as an activator of PS to treat TCA in this study.
Influence of S2O8 2− initial concentration and Fe0 dosage
To investigate the proper oxidant dosages in the reaction, batch experiments were conducted with different Fe0 and PS concentrations as shown in Fig. 7(a). The results showed that the TCA degradation was not desirable (less than 25%) until the PS concentration and iron dosage increased greatly compared to the control experiment ([Fe2+]0 = 0.36 mM, [PS]0 = 0.24 mM, TCA degradation = 80%). The TCA degradation was 84.3% when the [PS]0 = 0.48 mM and the Fe0 dosage was 2.4 mM. In this case, the [PS]0 and the iron dosage were 2 and 6.67 times compared to those of the control test. This means more PS and iron were needed to achieve similar TCA degradation when Fe2+ was replaced with Fe0. In general, the interaction of Fe0 with oxidants is a heterogeneous reaction (Liang and Guo 2010), and only the surface of iron particles could be oxidized to Fe2+. Therefore, the available iron was limited in the PS-activated experiments. In conclusion, more PS and far more Fe0 (10~44 μm in particle size) were needed to acquire favorable TCA removal efficiency compared to the Fe2+/PS system. In other research, an Fe0/PS system could acquire better oxidation efficiency compared to Fe2+/PS with the same iron dosage at low pH values (Oh et al. 2010; Oh et al. 2009). However, in this experiment, the reaction pH was 7, which might result in a lower reaction rate of TCA with Fe0/PS. The effect of pH on TCA oxidation by Fe0/PS was investigated next.
Influence of S2O8 2− concentration and Fe0 dosage (a), pH (b), temperature (c), and chelating agents (d) on TCA degradation by Fe0/PS. Conditions: a pH = 7, [TCA]0 = 0.47 μM, temperature: 25 °C; b [TCA]0 = 0.47 μM, [PS]0 = 0.48 mM, [Fe0]0 = 2.4 mM, temperature: 25 °C; c pH = 7, [TCA]0 = 0.47 μM, [PS]0 = 0.48 mM, [Fe0]0 = 2.4 mM; d pH = 7, [TCA]0 = 0.47 μM, [PS]0 = 0.48 mM, [Fe0]0 = 2.4 mM, [chelating agent]control test = 0 mM, [CA]0 = [OA]0 = [EDTA]0 = 0.96 mM, temperature: 25 °C
Influence of pH
To elucidate the effect of pH on TCA degradation by the Fe0/PS system, experiments were conducted in phosphate-buffered solutions with different pH values, and the data are presented in Fig. 7(b). It can be seen that TCA degradation was 96.6, 84.3, and 5.7% for pH 2.5, 7, and 11.6, respectively. In acidic conditions, TCA was more easily degraded by the Fe0/PS system, which can be explained by more Fe2+ releasing from Fe0 according to Eq. (5). More Fe2+ in low pH conditions could activate PS to produce more SO4 −•. Because the Fe2+ concentration was not high enough to scavenge SO4 −•, the inhibition of TCA degradation did not happen at low pH values compared with the Fe2+/PS system as discussed in section 3.1.2. At pH 7 and 11.6, the corrosion of Fe0 would slow down and less Fe2+ was released, which inhibited the oxidation of TCA. A similar observation was made by Le et al. (2011), who obtained 99% removal efficiency of anthraquinone dye Reactive Blue 19 by Fe0/PS at pH 3 within 30 min, but only 60 and 32% at pH 7 and 10, respectively.
Influence of temperature
The temperature of the reaction may have an impact on its rate or extent. Given that TCA is a volatile organic compound, temperatures of 10, 15, and 25 °C were selected to investigate the influence of temperature on the reaction. As shown in Fig. 7(c), in the first 10 min, TCA degradation rates slightly increased as temperature changed from 10 to 25 °C and the final TCA degradation was 81.7, 83.4, and 84.1% at 10, 15, and 25 °C, respectively. The results demonstrated that in this temperature range, temperature could promote TCA degradation slightly but not significantly. The degradation rate constant (k TCA) in the first 10 min increased when the temperature changed from 10 to 25 °C. In view of faster rate constants at higher temperatures, the relationship between temperature and k TCA is further correlated via the Arrhenius equation to estimate the activation energy (E a, kj mol−1) of TCA degradation using the following equation (Eq. (12)):
where A denotes the pre-exponential factor (min−1); R represents the universal gas constant (8.3145 J mol−1 K−1); and T is the solution temperature in Kelvin (K). According to Eq. (12), a plot of 1/T versus ln k TCA is shown in Fig. S4, and the data points are fitted by a linear regression with R 2 = 0.954. This indicates that the reaction kinetics of TCA degradation by Fe0/PS in the first 10 min can be appropriately associated with temperature via the Arrhenius equation. The slope of the fitting line is then adopted to estimate the activation energy as 11.82 kJ mol−1. However, the temperature did not promote TCA degradation further after 10 min. Therefore, temperature may not be an important factor in real applications.
Influence of chelating agents
To explore the effect of chelating agents on the reaction, CA, OA, and EDTA were added to the solution with a fixed chelating agent/Fe0 molar ratio of 2:5. It can be seen from Fig. 7(d) that the final TCA degradation was 90.5, 48.0, and 28.0% for CA, OA, and EDTA, respectively, compared to the control test (81.5%). For all the chelating agents, their functions in the Fe0/PS system can be concluded in two ways: (i) chelating with Fe2+, which inhibits the scavenging of free radicals by Fe2+; (ii) competing with TCA for free radicals. They have totally different impacts on TCA degradation. In these experiments, CA was likely to act in the first way while OA and EDTA preferred to act in the latter one. Therefore, it can be concluded that with a CA/Fe0 molar ratio of 2:5, CA promoted the oxidation of TCA by Fe0/PS, but OA and EDTA retarded the reaction.
Degradation of TCA by persulfate activated with Fe3+
To investigate the degradation of TCA under an Fe3+/PS system, an experiment was conducted with a PS/Fe3+ molar ratio of 1:2. As shown in Fig. 8, 67% TCA was degraded within 60 min by the Fe3+/PS system. This removal rate was 20% lower than that under Fe2+/PS with the same Fe/PS molar ratio. The degradation of organic pollutants by Fe3+/PS or chelated Fe3+/PS has been investigated by several researchers (Liang and Su 2009; Rodriguez et al. 2014). Liang and Su 2009 investigated the oxidation of TCE by an EDTA/Fe(III)/PS system where EDTA played an important role in the reduction of Fe3+ to Fe2+, which served as an activator of PS. Rodriguez et al. (2014) found that oxidation intermediates of Orange G could reduce Fe3+ to Fe2+ at low pH. In our experiment, however, no chelating agents were added to the reaction and Fe3+ was difficult to be reduced by TCA due to the poor oxidation capability of Fe3+, so the activation of PS by Fe3+ should be illustrated in another way. Wu et al. (2017) proposed that S2O8 2− could reduce Fe3+ to Fe2+, as shown in Eq. (13), which might provide a good explanation for how Fe3+ initiated the activation of PS in our experiment.
After Fe3+ was reduced to Fe2+, Fe2+ would continue to activate PS to generate SO4 −•. In conclusion, Fe3+ was capable of activating PS to generate SO4 −• via a two-step reaction (Eqs. (1) and (13)), which further proved the existence of an Fe2+-Fe3+ circle in iron-activated PS systems.
Conclusions
In this study, the application of iron (Fe2+, Fe0, and Fe3+) was successfully used to activate PS and degrade TCA in solution under various conditions. An increase of Fe2+ (or Fe0) and PS dosage promoted the degradation of TCA, but excess Fe2+ inhibited the reaction instead. Fe2+/PS exhibited the greatest removal efficiency at pH 7, while Fe0/PS had the best performance at pH 2.5. The EPR results proved that SO4 −• was easy to generate at low pH values and was the main free radical responsible for TCA oxidation. TCA degradation was improved by gradual addition of Fe2+ and adding chelating agents (i.e., CA and EDTA) and humic acid at proper dosages. However, the addition of OA and too much CA, EDTA, or humic acid would decrease the removal rate of TCA. Additionally, temperature influenced the reaction insignificantly. The only oxidation product of TCA detected in this study was TCP, which might be oxidized to other products afterwards. Finally, Fe3+ was proved to be an efficient activator for PS, which confirmed the existence of an Fe2+-Fe3+ redox circle in iron-activated PS systems.
References
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Berlin AA (1986) Kinetics of radical-chain decomposition of persulfate in aqueous-solutions of organic-compounds. Kinet Catal 27:34–39
Bruce D, Westerhoff P, Brawley-Chesworth A (2002) Removal of 2-methylisoborneol and geosmin in surface water treatment plants in Arizona. J Water Supply Res T 51:183–197
Bruchet A, Laine JM (2005) Efficiency of membrane processes for taste and odor removal. Water Sci Technol 51:257–265
Bu L, Zhou S, Shi Z, Deng L, Gao N (2017) Removal of 2-MIB and geosmin by electrogenerated persulfate: performance, mechanism and pathways. Chemosphere 168:1309–1316
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (.OH/.O−) in aqueous solution. J Phys Chem Ref Data 17:513–886
Chestnutt TE Jr, Bach MT, Mazyck DW (2007) Improvement of thermal reactivation of activated carbon for the removal of 2-methylisoborneol. Water Res 41:79–86
Criquet J, Leitner NKV (2009) Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis. Chemosphere 77:194–200
Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49:5645–5653
Fang G, Wu W, Deng Y, Zhou D (2017) Homogenous activation of persulfate by different species of vanadium ions for PCBs degradation. Chem Eng J 323:84–95
Gao Y, Gao N, Deng Y, Yin D, Zhang Y (2015) Degradation of florfenicol in water by UV/Na2S2O8 process. Environ Sci Pollut R 22:8693–8701
Guan Y, Ma J, Li X, Fang J, Chen L (2011) Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/Peroxymonosulfate system. Environ Sci Technol 45:9308–9314
Huie RE, Clifton CL (1996) Kinetics of the reaction of the sulfate radical with the oxalate anion. Int J Chem Kinet 28:195–199
Johnson RL, Tratnyek PG, Johnson RO (2008) Persulfate persistence under thermal activation conditions. Environ Sci Technol 42:9350–9356
Kolthoff IM, Medalia AI, Raaen HP (1951) The reaction between ferrous iron and peroxides .4. Reaction with potassium persulfate. J Am Chem Soc 73:1733–1739
Lalezary S, Pirbazari M, Mcguire MJ (1986) Oxidation of 5 earthy musty taste and odor compounds. J Am Water Works Ass 78:62–69
Le C, Wu J, Li P, Wang X, Zhu N, Wu P, Yang B (2011) Decolorization of anthraquinone dye Reactive Blue 19 by the combination of persulfate and zero-valent iron. Water Sci Technol 64:754–759
Liang C, Guo Y (2010) Mass transfer and chemical oxidation of naphthalene particles with zerovalent iron activated persulfate. Environ Sci Technol 44:8203–8208
Liang C, Su H (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562
Liang C, Bruell CJ, Marley MC, Sperry KL (2004a) Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 55:1213–1223
Liang C, Bruell CJ, Marley MC, Sperry KL (2004b) Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion. Chemosphere 55:1225–1233
Liang C, Wang Z, Bruell CJ (2007) Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 66:106–113
Lin Y, Liang C, Chen J (2011) Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chem Aust 82:1168–1172
Liu H, Bruton TA, Li W, Van Buren J, Prasse C, Doyle FM, Sedlak DL (2016) Oxidation of benzene by persulfate in the presence of Fe(III)- and Mn(IV)-containing oxides: stoichiometric efficiency and transformation products. Environ Sci Technol 50:890–898
Luo C, Jiang J, Ma J, Pang S, Liu Y, Song Y, Guan C, Li J, Jin Y, Wu D (2016) Oxidation of the odorous compound 2,4,6-trichloroanisole by UV activated persulfate: kinetics, products, and pathways. Water Res 96:12–21
Maillet L, Lenes D, Benanou D, Le Cloirec P, Correc O (2009) The impact of private networks on off-flavour episodes in tap water. J Water Supply Res T 58:571–579
Marquez-Sillero I, Aguilera-Herrador E, Cardenas S, Valcarcel M (2011) Determination of 2,4,6-tricholoroanisole in water and wine samples by ionic liquid-based single-drop microextraction and ion mobility spectrometry. Anal Chim Acta 702:199–204
Matzek LW, Carter KE (2016) Activated persulfate for organic chemical degradation: a review. Chemosphere 151:178–188
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous-solution. J Phys Chem Ref Data 17:1027–1284
Oh S, Kim H, Park J, Park H, Yoon C (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater 168:346–351
Oh S, Kang S, Chiu PC (2010) Degradation of 2,4-dinitrotoluene by persulfate activated with zero-valent iron. Sci Total Environ 408:3464–3468
Olmez-Hanci T, Arslan-Alaton I, Genc B (2013) Bisphenol a treatment by the hot persulfate process: oxidation products and acute toxicity. J Hazard Mater 263:283–290
Park N, Lee Y, Lee S, Cho J (2007) Removal of taste and odor model compound (2,4,6-trichloroanisole) by tight ultrafiltration membranes. Desalination 212:28–36
Peyton GR (1993) The free-radical chemistry of persulfate-based total organic-carbon analyzers. Mar Chem 41:91–103
Pignatello JJ, Baehr K (1994) Ferric complexes as catalysts for Fenton degradation of 2,4-D and metolachlor in soil. J Environ Qual 23:365–370
Prat C, Besalu E, Baneras L, Antico E (2011) Multivariate analysis of volatile compounds detected by headspace solid-phase microextraction/gas chromatography: a tool for sensory classification of cork stoppers. Food Chem 126:1978–1984
Qi F, Xu B, Chen Z, Ma J (2009a) Catalytic ozonation for degradation of 2,4,6-Trichloroanisole in drinking water in the presence of gamma-AlOOH. Water Environ Res 81:592–597
Qi F, Xu B, Chen Z, Ma J, Sun D, Zhang L (2009b) Influence of aluminum oxides surface properties on catalyzed ozonation of 2,4,6-trichloroanisole. Sep Purif Technol 66:405–410
Qi F, Xu B, Chen Z, Ma J, Sun D, Zhang L, Wu F (2009c) Ozonation catalyzed by the raw bauxite for the degradation of 2,4,6-trichloroanisole in drinking water. J Hazard Mater 168:246–252
Rodriguez S, Vasquez L, Costa D, Romero A, Santos A (2014) Oxidation of Orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI). Chemosphere 101:86–92
Song W, O’Shea KE (2007) Ultrasonically induced degradation of 2-methylisoborneol and geosmin. Water Res 41:2672–2678
Tan C, Gao N, Chu W, Li C, Templeton MR (2012) Degradation of diuron by persulfate activated with ferrous ion. Sep Purif Technol 95:44–48
Theis TL, Singer PC (1974) Complexation of iron(II) by organic-matter and its effect on iron(II) oxygenation. Environ Sci Technol 8:569–573
Urase T, Sasaki Y (2013) Occurrence of earthy and musty odor compounds (geosmin, 2-methylisoborneol and 2,4,6-trichloroanisole) in biologically treated wastewater. Water Sci Technol 68:1969–1975
Vlachos P, Stathatos E, Lyberatos G, Lianos P (2008) Gas-phase photocatalytic degradation of 2,4,6-trichloroanisole in the presence of a nanocrystalline Titania film. Applications to the treatment of cork stoppers. Catal Commun 9:1987–1990
Wang Q, You W, Li X, Yang Y, Liu L (2014) Seasonal changes in the invertebrate community of granular activated carbon filters and control technologies. Water Res 51:216–227
Wu Y, Bianco A, Brigante M, Dong W, de Sainte-Claire P, Hanna K, Mailhot G (2015) Sulfate radical Photogeneration using Fe-EDDS: influence of critical parameters and naturally occurring scavengers. Environ Sci Technol 49:14343–14349
Wu Y, Prulho R, Brigante M, Dong W, Hanna K, Mailhot G (2017) Activation of persulfate by Fe(III) species: implications for 4-tert-butylphenol degradation. J Hazard Mater 322:380–386
Xie P, Ma J, Liu W, Zou J, Yue S, Li X, Wiesner MR, Fang J (2015) Removal of 2-MIB and geosmin using UV/persulfate: contributions of hydroxyl and sulfate radicals. Water Res 69:223–233
Xu J, Xin L, Huang T, Chang K (2011) Enhanced bioremediation of oil contaminated soil by graded modified Fenton oxidation. J Environ Sci 23:1873–1879
Zhang K, Zhou X, Zhang T, Mao M, Li L, Liao W (2016) Kinetics and mechanisms of formation of earthy and musty odor compounds: chloroanisoles during: water chlorination. Chemosphere 163:366–372
Funding
This project was supported by the National Natural Science Foundation of China (No. 51778561, 51408518), the Natural Science Foundation of Zhejiang Province (No. LY17E080014), Xiamen Urban Water Environmental Eco-Planning and Remediation Engineering Research Center (No. XMERC-201701), and the Fundamental Research Funds for the Central Universities (No. 2016FZA4017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Santiago V. Luis
Electronic supplementary material
ESM 1
(DOCX 101 kb)
Rights and permissions
About this article
Cite this article
Zhang, K., Zhou, X., Zhang, T. et al. Degradation of the earthy and musty odorant 2,4,6-tricholoroanisole by persulfate activated with iron of different valences. Environ Sci Pollut Res 25, 3435–3445 (2018). https://doi.org/10.1007/s11356-017-0452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0452-x