Abstract
In order to develop a test battery based on a variety of neurological systems in fish, three sensory systems (vision, olfaction, and lateral line) as well as nerve transmission (acetylcholine esterase) were analyzed in zebrafish (Danio rerio) embryos with respect to their suitability as a model for the screening of neurotoxic trace substances in aquatic ecosystems. As a selection of known or putative neurotoxic compounds, amidotrizoic acid, caffeine, cypermethrin, dichlorvos, 2,4-dinitrotoluene, 2,4-dichlorophenol, 4-nonylphenol, perfluorooctanoic acid, and perfluorooctane sulfonic acid were tested in the fish embryo test (OECD test guideline 236) to determine EC10 values, which were then used as maximum test concentration in subsequent neurotoxicity tests. Whereas inhibition of acetylcholinesterase was investigated biochemically both in vivo and in vitro (ex vivo), the sensory organs were studied in vivo by means of fluorescence microscopy and histopathology in 72- or 96-h-old zebrafish embryos, which are not regarded as protected developmental stages in Europe and thus — at least de jure — represent alternative test methods. Various steps of optimization allowed this neurotoxicity battery to identify neurotoxic potentials for five out of the nine compounds: Cypermethrin and dichlorvos could be shown to specifically modulate acetylcholinesterase activity; dichlorvos, 2,4-dichlorophenol, 4-nonylphenol, and perfluorooctane sulfonic acid led to a degeneration of neuromasts, whereas both vision and olfaction proved quite resistant to concentrations ≤ EC10 of all of the model neurotoxicants tested. Comparison of neurotoxic effects on acetylcholinesterase activity following in vivo and in vitro (ex vivo) exposure to cypermethrin provided hints to a specific enzyme-modulating activity of pyrethroid compounds. Enhancement of the neuromast assay by applying a simultaneous double-staining procedure and implementing a 4-scale scoring system (Stengel et al. 2017) led to reduced variability of results and better statistical resolution and allowed to differentiate location-dependent effects in single neuromasts. Since acetylcholinesterase inhibition and neuromast degeneration can be analyzed in 72- and 96-h-old zebrafish embryos exposed to neurotoxicants according to the standard protocol of the fish embryo toxicity test (OECD TG 236), the fish embryo toxicity test can be enhanced to serve as a sensitive neurotoxicity screening test in non-protected stages of vertebrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water plays a central role in human life, and the provision of high-quality drinking water has become a major challenge in human societies (Stengel et al. 2017). Given the multitude of anthropogenic compounds found in surface and groundwaters, anthropogenic impact may be a major risk for water quality (Pal et al. 2014; Postigo and Barceló 2015). An increasing number of anthropogenic pollutants associated with neurotoxic effects have been identified in water bodies including groundwater (Fox et al. 2012; Grandjean and Landrigan 2006), and there is increasing evidence that chronic exposure to such neurotoxicants may cause neurodevelopmental disorders such as autism, attention-deficit hyperactivity disorder, dyslexia, and others (Fox et al. 2012; Grandjean and Landrigan 2014).
Since so far only a limited number of priority pollutants are being considered by the Water Framework Directive (EU 2008), various research programs have been initiated to develop acute and mechanism-specific bioassays to identify the putative damage potential within a hazard-based risk assessment. Within the scope of RiSKWa, a joint research program by the German Federal Ministry of Education and Research (Huckele and Track 2013), the major task of the “Tox-Box” project was to enhance the existing health-related indicator value concept (HRIV; German: GOW-Konzept – Gesundheitlicher Orientierungswert) through development and prioritization of additional endpoint-related testing strategies for genotoxicity, neurotoxicity, germ cell damage, and endocrine effects (Grummt et al. 2013).
Among the endpoints covered within Tox-Box, neurotoxicity is, by far, the least developed, and the present study was designed to develop a battery of assays for the identification of neurotoxicity in aquatic vertebrates. Over the last few years, with respect to techniques to reveal neurotoxicity, major emphasis has been given to behavioral assays (Chen et al. 2011; de Esch et al. 2012; Klüver et al. 2015; Noyes et al. 2015; Selderslaghs et al. 2013; Tierney 2011), and zebrafish has become a favorite alternative to mammalian models (Cowden et al. 2012; Dishaw et al. 2014; Irons et al. 2010; Jarema et al. 2015; Padilla et al. 2011; Sipes et al. 2011; Tanguay and Reimers 2008; Tilton et al. 2006; Truong et al. 2011, 2014; Zellner et al. 2011). Since, however, all of these approaches are quite time-consuming and require sophisticated equipment, an attempt was made within Tox-Box to develop simple methodologies suited for rapid screening purposes. For this end, together with the classical biochemical acetylcholinesterase assay (Kais et al. 2015), major sensory organs — namely eyes (retina), nose (olfactory epithelium), and the lateral line (neuromasts) — were investigated in zebrafish (Danio rerio) embryos as a major model for aquatic toxicity assessment. As test substances, a set of known or suspected model neurotoxicants (amidotrizoic acid, caffeine, cypermethrin, dichlorvos, 2,4-dinitrotoluene, 2,4-dichlorphenol, 4-nonylphenol, perfluorooctanoic acid, and perfluorooctane sulfonic acid) was selected.
Acetylcholinesterase inhibition as a classical mode of neurotoxicity
Inhibition of the enzyme acetylcholinesterase (EC 3.1.1.7) is by far the best-documented mode of action related to certain neurotoxic compounds such as organophosphate- and carbamate-type pesticides (Kais et al. 2015). Competitive binding to acetylcholinesterase results in an accumulation of acetylcholine within the synaptic cleft and, thus, to an overstimulation of signal transduction across cholinergic synapses (Russom et al. 2014), and acetylcholinesterase inhibition has been developed as a well-known biomarker of neurotoxicity (for a recent review, see Kais et al. 2015). However, chemicals other than carbamates and organophosphates have also been documented to alter acetylcholinesterase activities, e.g., polyaromatic hydrocarbons (PAHs; Kang and Fang 1997) or dioxins (Fan et al. 2013), and acetylcholinesterase enzymatic activity may also be altered following degradation of cholinergic neurons by, e.g., bisphenol A (Miyagawa et al. 2007). In zebrafish, the determination of acetylcholinesterase activity (Ellman et al. 1961) has been adapted and successively optimized for adult and embryonic zebrafish by Guilhermino et al. (1996), Küster (2005), as well as Küster and Altenburger (2006, 2007). For the sake of animal welfare, the number of individual zebrafish embryos could recently be reduced significantly (Kais et al. 2015; Perez et al. 2013).
Vision: chemical modification of the retina
For most fish species, vision plays a central role for orientation, schooling, capturing of prey and avoidance of predators, as well as for reproduction. The general architecture of the retina into defined layers is common to all vertebrates and covers three nuclear and two plexiform layers (Schmitt and Dowling 1994). In the development of zebrafish embryos, retinal layers develop from 50 hours post-fertilization (hpf; Schmitt and Dowling 1994), and all cell types are functional from 72 hpf. Sensory motor response could be documented from 68 to 79 hpf, and tracking eye movements was recorded from 73 to 80 hpf (Easter and Nicola 1996). Since the correct arrangement of cell types is vital for functionality, chemical-induced abnormalities in retinal architecture may severely corrupt the performance of exposed embryos (Lee et al. 2012; Mishra and Devi 2014; Wang et al. 2012) and can be tracked by means of histopathology (Huang et al. 2014; Kim et al. 2013; Mecklenburg and Schraermeyer 2007; Mela et al. 2012; Triebskorn et al. 1994; Wang et al. 2012). Since the basic structure of the vertebrate retina can easily be documented by conventional histological staining techniques, the present study used routine hematoxylin-eosin staining to detect the effects of neurotoxic substances.
Olfaction
A functional olfactory system most likely plays a crucial role in the life of fish (Hara 1975, 1994; Spehr et al. 2006). Since, among vertebrates, most fish species have superior olfactory capacities, chemical-induced corruption of olfaction may be assumed to have a profound impact on orientation and migration, predator-prey relationships and social behavior (e.g., via alarm pheromones), feeding, mating, and other population-relevant processes. This seems even more important, since, in contrast to the cells of the retina, receptor cells of the olfactory epithelium are directly exposed to potential toxicants in the surrounding medium. As a consequence, studies into chemical-related changes of the olfactory system have a long tradition in ecotoxicology. In their review on environmentally relevant laboratory studies, Tierney et al. (2010) listed multiple methods and thus documented the fundamental usability of the olfactory system in ecotoxicology.
With the advent of the first olfaction-dependent behaviors, functionally mature olfactory neurons may be assumed to be present in zebrafish from 96 hpf (Lindsay and Vogt 2004). As the main odorant-receiving units in the olfactory epithelium, the olfactory receptor neurons (ORNs) can be divided into ciliary (cORNs) and microvillus olfactory receptor neurons (mORNs) as well as crypt cells (Byrd and Brunjes 1995; Doving and Lastein 2009). These receptor neurons can be differentiated by either expressing the G protein subunit Gαolf/s (cORC) or the subunits Gα0 and Gαq/11 (mORC and crypt cells; Hansen and Zielinski 2005). Since in the embryonic olfactory epithelia Gαolf/s seemed most promising for the detection of intact ciliary olfactory receptor neurons, the present study used fluorescence-labeled antibodies against Gαolf/s for the visualization of adverse effects of chemicals on the olfactory epithelium.
The neuromast assay: alterations of the lateral line as a tool to screen for ototoxicity
The lateral line system in aquatic organisms is a sensory system related to the inner ear that consists of interconnected neuromasts, which detect changes of mechanical pressure in the surrounding medium (Stengel et al. 2017). Morphologically, it is divided into the anterior lateral line system (ALL) located along the head and the posterior lateral line system (PLL) spanning the trunk (Bleckmann and Zelick 2009; Ghysen and Dambly-Chaudiere 2004; Metcalfe et al. 1985; Raible and Kruse 2000; Stengel et al. 2017). Given that the receptor cells are homologous to neurons in the inner ear of humans (Fritzsch et al. 2007; Hayashi et al. 2015; Ton and Parng 2005) and are located close to the epidermal surface of the fish, the lateral line system is of particular interest for the determination of neurotoxic effects in vivo (Froehlicher et al. 2009a). As a consequence, it has not only been used in neurotoxicology (Linney et al. 2004), but also in ecotoxicology (for review, see Stengel et al. 2017). Although the homology of hair cells in the lateral line and in the human inner ear makes the former a potential model for human neurotoxicology (Fritzsch et al. 2007), data on the neurotoxicity of specific substances are rare, and there are only a limited number of ecotoxicological studies using the lateral line for the monitoring of neurotoxic compounds in aquatic ecosystems (Coffin et al. 2009, 2010; Froehlicher et al. 2009b; Harris et al. 2003; Linbo et al. 2006, 2009). Only recently, however, the method has been further optimized by implementing a simplified double-staining procedure and a 4-scale scoring system (Stengel et al. 2017).
Material and methods
Chemicals and toxicants
All toxicants were purchased from Sigma-Aldrich (Deisenhofen, Germany) at the highest purity available, unless stated otherwise. As model neurotoxicants, amidotrizoic acid, caffeine, copper sulfate (Merck, Darmstadt), cypermethrin, 3,4-dichloroaniline, dichlorvos, 2,4-dinitrotoluene, neomycin, 4-nonylphenol, paraoxon-methyl, perfluorooctanoic acid, and perfluorooctane sulfonic acid were used. For test concentrations in the fish embryo tests and neurotoxicity assays, see Table 1. All toxicant stock solutions were prepared in artificial water according to OECD TG 236 (OECD 2013), unless stated otherwise. Cypermethrin was dissolved in dimethyl sulfoxide (DMSO) and diluted to final concentrations of 0.1% DMSO in each treatment.
The primary antibody against rabbit Gαolf was purchased from Santa Cruz Biotechnology (Heidelberg, Germany; order number: sc-383; polyclonal Gαs/olf heterotrimeric IgG (C-18) from rabbit; 200 μg/ml). The fluorescence-labeled secondary antibody against rabbit IgG was purchased from Thermo Fisher-Invitrogen (Carlsbad, CA, USA; order number: A10042; Alexa Fluor 568 donkey anti-rabbit IgG dk α rb A568; 2 mg/ml). Primary and secondary antibodies were used at dilutions of 1:200 and 1:1000 in phosphate-buffered saline (PBS), respectively.
As fluorescent stains in the neuromast assay, 2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide (DASPEI) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma Aldrich: The 0.05% DASPEI stock solution in 5% DMSO was diluted with artificial water to a final strength of 0.005% DASPEI (50 μg/ml) in 0.5% DMSO. The 1-mg/ml DAPI stock solution was prepared in artificial water and diluted to a working strength of 1 ng/ml.
Fish
All adult zebrafish used for breeding were wild-type descendants of the “West Aquarium” strain obtained from the Aquatic Ecology and Toxicology breeding facilities at the University of Heidelberg (licensed under no. 35-9185.64/BH). Details of zebrafish maintenance, egg production, and embryo rearing have repeatedly been described in detail (Kimmel et al. 1995, 1988; Nagel 2002; Spence et al. 2006; Wixon 2000) and have been updated for the purpose of the zebrafish embryo toxicity test by Lammer et al. (2009).
Zebrafish embryo toxicity test (FET)
In order to avoid indirect effects by systemic acute toxicity potentially mimicking or concealing neurotoxic effects, the highest test concentrations in the neurotoxicity tests were set at the EC10 values as determined in the fish embryo test according to OECD TG 236 (OECD 2013) for any observable effect in zebrafish embryos. In brief, embryos were exposed at latest from 1 hpf in glass vessels, which had been preincubated (saturated) for at least 24 h, to a series of dilutions of the respective toxicants (Table 1). After control of the fertilization success, embryos were transferred to 24-well plates (TPP Renner, Dannstadt, Germany), which had been preincubated with 2 ml of the test solutions per well for 24 h prior to the test initiation, and kept in an incubator at 26.0 ± 1.0 °C under a 10:14-h dark:light regime. In order to prevent evaporation or cross-contamination between wells, the plates were sealed with self-adhesive foil (Nunc, Wiesbaden, Germany). Embryo tests were classified as valid, if the mortality in the negative control was ≤ 10%, and the positive control (3,4-dichloroaniline) showed mortalities between 20 and 80% (Lammer et al. 2009; OECD 2013). All fish embryo tests were run in at least three independent replicates.
Zebrafish embryos were exposed to the test substances until 96 hpf in a semistatic fashion, i.e., the medium was changed every 24 h subsequent to the daily inspection of the embryos. Every 24 h, the embryos were controlled for lethal effects according to OECD TG 236 (coagulation, nonformation of somites, non-detachment of the tail, and lack of heartbeat) as well as for sublethal changes using the endpoints specified by Bachmann (2002), Schulte and Nagel (1994) as well as Nagel (2002). Both lethal and sublethal effects were used for the determination of EC values. For subsequent neurotoxicity tests, EC10 concentrations of all toxicants as revealed by the FETs were used (Table 1).
In vivo acetylcholinesterase inhibition assay
In the acetylcholinesterase inhibition tests, five embryos per concentration were exposed to the model neurotoxicants for 96 h and processed as whole-body homogenates according to Kais et al. (2015). In brief, 96-h-old embryos were rinsed 3 × 10 min in artificial water, anesthetized by rapid cooling on ice, blotted dry on filter paper, and euthanized by shock-freezing in liquid nitrogen for subsequent storage at −20 °C. For homogenization, five embryos per concentration were thawed on ice, and 400 μl ice-cold 0.1 M phosphate buffer in 1% Triton X-100 was added prior to homogenization with a TissueLyzer II® (Quiagen, Hilden, Germany) at 30 Hz for 1 min. Homogenates were centrifuged at 10,000g and 4 °C for 15 min, and supernatants were used for enzyme kinetics and protein determination in 96-well plates using a GENios® microtiter plate reader (Tecan, Crailsheim, Germany) as described by Küster (2005) and Küster and Altenburger (2006)). Each experiment was run in triplicate; all enzyme measurements were conducted in 8 reads per concentration per replicate. Enzyme activities were calculated as changes in optic density ΔOD/min/mg protein contents according to Ellman et al. (1961)). Paraoxon-methyl served as a positive control at a concentration of 2 mg/L (Kais et al. 2015).
In vitro (ex vivo) acetylcholinesterase inhibition assay
For measurement of acetylcholinesterase activity after in vitro exposure, five untreated control embryos were pooled and homogenized as described above (Kais et al. 2015). After transfer of 50 μl homogenate to 96-well microtiter plates, each well was incubated for 15 min at room temperature with 50 μl of the model neurotoxicants dichlorvos or cypermethrin at concentrations of 0.75, 1.5, and 3 mg/L or 1, 5, 10, and 20 ng/L (in phosphate buffer), respectively. Determination of enzyme activities and protein contents followed the protocol described above.
Retina toxicity
Experiments into retina toxicity were carried out with artificial water as a negative control. After 96 h exposure to model neurotoxicants, embryos were rinsed with artificial water for 3 × 10 min prior to transfer of 20 embryos per concentration in an Eppendorf tube. Embryos were immobilized by rapid cooling (Wilson et al. 2009) and fixed in Davidson’s fixative (Fournie et al. 2000; OECD 2010) for at least 24 h at 4 °C. After triplicate rinsing in PBS, groups of 20 embryos were embedded in 1% agarose in multimolds according to Sabaliauskas et al. (2006). After solidification, agarose blocks were transferred to histocassettes (Leica Microsystems, Wetzlar, Germany) and stored overnight in 70% ethanol. Following dehydration in a graded series of ethanol, agarose blocks were infiltrated with paraffin using a 48-h program on a Leica TP 1020 tissue processor (Leica Microsystems, Wetzlar, Germany) and embedded in Histoplast® with a Leica EG 1140 H paraffin-dispensing module. After solidification, paraffin blocks were stored at room temperature. Paraffin sections were cut at 4 μm on a Reichert-Jung (Nussloch, Germany) HN 40 microtome, mounted on glass slides, and stained with hematoxylin and eosin (Romeis 2010).
Olfactory toxicity
Experiments into retina toxicity were carried with artificial water as a negative control, and 1.6 mg/L copper sulfate served as a positive control. After 96 h exposure to model neurotoxicants, embryos were rinsed in artificial water for 3 × 10 min. For preparation of whole mounts, embryos were immobilized via rapid cooling according to Wilson et al. (2009) and fixed in 4% paraformaldehyde in PBS for 4 h at 4 °C. After rinsing for 3 × 10 min in PBS supplemented with 0.1% Triton X-100 (PBST), embryos were permeabilized in ice-cold acetone for 7 min at −20 °C.
After rehydration with distilled water for 5 min and rinsing for 3 × 10 min with PBST, embryos were incubated for 4 h in blocking solution (2% bovine serum albumin (BSA) in PBST) at room temperature prior to overnight incubation with the primary antibody (anti-GαSolf; dilution 1:200) in blocking solution at 4 °C. On the following day, embryos were rinsed in PBST for 5 × 10 min and incubated in the dark overnight with the secondary antibody (dk α rb A568; dilution 1:1000) in PBS at 4 °C. All subsequent steps were carried out in the dark to prevent photobleaching of fluorescence-labeled secondary antibodies. Following another 3 × 10 min rinse with PBST, embryos were incubated with 1 ng/L DAPI in PBS for 30 min at room temperature. For microscopy, embryos were rinsed for 3 × 10 min in PBS and whole-mounted in 0.7% low-melting Seakem Gold agarose (Biozym, Oldendorf, Germany) on glass bottom culture dishes (MatTek, Ashland, USA).
Alternatively, immobilized and paraformaldehyde-fixed embryos were rinsed for 3 × 10 min in PBS and gradually incubated in a 10% sucrose solution in PBS for 3 h at 20 °C and in a 30% sucrose solution overnight at 4 °C to prevent formation of ice crystals in the following freezing procedure. Embryos were transferred into molding cups and embedded with tissue freezing medium (Leica, Nussloch, Germany). After positioning for coronal sectioning using forceps, embryos were frozen at −20 °C in a Leica cryostat CM 3050S and stored in a freezer at −20 °C. Coronal cryosections of 25 μm thickness were made using the Leica cryostat CM 3050S, collected on gelatinized microscope slides, and stored in a freezer at −20 °C.
For staining, cryosections were marked with Super PAP Pen (Daido Sangyo, Osaka, Japan) as a liquid barrier and dried at room temperature in a moist chamber for 10 min. Sections were postfixed with 4% paraformaldehyde for 5 min and rinsed for 3 × 10 min in PBS. Sections were blocked with 2% BSA in PBST for 1 h at 20 °C and incubated for 2 h with the primary antibodies as described above. After rinsing for 3 × 10 min in PBS, slides were incubated overnight at 4 °C in the dark with the secondary antibodies as described above. After a 3 × 10-min rinse in PBS, slides were incubated with 70 μl of 1 μg/ml DAPI per section for 1 min under light exclusion. After rinsing for 3 × 5 min in PBS, slides were coverslipped in GelMount Aqueous Mounting Medium and stored at 4 °C in a light-proof box until microscopy.
Neuromast assay
Neuromast assays were performed according to Stengel et al. 2017: Four embryos per concentration were exposed to the model neurotoxicants for 96 h in three replicates with artificial water as a negative control and 1.6 mg/L copper sulfate as a positive control. Embryos were stained with 0.005% DASPEI in 0.5% DMSO and 1 ng/ml DAPI for 30 min each. Nine specific neuromasts per embryo and four embryos per replicate were stained and scored in a weighted 3-step and 4-step scoring approach. Each neuromast was scored from grade 0 (unchanged control: all hair cells well-stained) over grade 1 (minor damage: reduced mitochondrial staining intensity and/or less hair cells) and grade 2 (strong damage: mitochondria only faintly stained and/or strongly reduced number of hair cells) to grade 3 (very strong damage: mitochondria and hair cells barely detectable) in the 4-step approach or according to Harris et al. (2003) for the 3-step scoring approach. For data presentation, the overall score of an embryo was determined by forming the average of the scores for single neuromasts of the respective embryo. For each replicate, the average score for all 4 embryos per replicate was computed, and for each treatment the average score of the 3 replicates was calculated. For differential sensitivity of the neuromasts of the anterior and the posterior lateral line systems, the neuromasts of either system were calculated separately (detailed results presented in Stengel et al. 2017).
Microscopic analysis
Slides for olfactory toxicity (immunohistochemistry) were analyzed with a Nikon ECLIPSE 90i microscope (Nikon, Düsseldorf, Germany). Micrographs were taken with a Nikon DS-Ri1 camera for frozen and paraffin sections and a Nikon C1 camera for confocal laser scanning microscopy of whole mounts and neuromast scoring (Stengel et al. 2017). Alexa Fluor 568 was excited at 561 nm, and emission was recorded at 605 nm. DAPI was excited at 405 nm, and emission was recorded at 500 nm. A water immersion objective with a ×60 magnification was used for imaging of whole mounts and neuromasts with scan speed set to 1/16, whereas for frozen and paraffin sections, a plan oil emersion objective at ×60 magnification was used.
Quantification of results and statistical analyses
With respect to quantification, effects are usually given in percent of either positive or negative control activity. Statistical analysis for embryo toxicity was accomplished with ToxRat® 2.10.03 (ToxRat Solutions, Alsdorf, Germany). For neurotoxicity assays, statistical analyses were carried out with Sigma Plot 12.3 (Systat-Jandel, Erkrath, Germany) using one-way ANOVA in combination with Dunn’s test by comparing replicates for significant differences.
Results
Fish embryo toxicity tests
Prior to the neurotoxicity assays, zebrafish embryo toxicity tests were conducted according to OECD TG 236 (OECD 2013) to screen for ranges of acute toxicity of the model neurotoxicants and to determine EC10 values (Table 2). EC10 values were later used as the maximum test concentrations to exclude unspecific secondary effects on neuronal systems.
Amidotrizoic acid and cypermethrin failed to induce any lethal or sublethal effects within solubility limits; thus, LC and EC values could not be derived. As a consequence, the solubility limit of amidotrizoic acid (8.9 mg/L) and an assumed effect level for cypermethrin of 20 ng/L were used as maximum test concentrations in subsequent neurotoxicity tests. Since 2,4-dinitrotoluene only produced sublethal effects, only EC values, but no LC values could be determined. For all other substances, EC10 values could be directly determined as maximum test concentrations for neurotoxicity testing.
Acetylcholinesterase inhibition
Embryos were exposed to the EC10 concentrations (Table 2) of the nine selected model compounds, as determined in range-finding FETs. DMSO (0.1%; solvent control for cypermethrin) did not show any influence on acetylcholinesterase activity (Fig. 1). Likewise, except for cypermethrin and dichlorvos, none of the test compounds showed significant effects. Acetylcholinesterase activities in embryos exposed to dichlorvos ranged between 45% of the activity in the negative control and 73% of the inhibitory effects of the positive control paraoxon-methyl. Unexpectedly, acetylcholinesterase activities in embryos exposed to cypermethrin showed a slight, but statistically significant increase to 107% of the negative control (Fig. 1).
Specific acetylcholinesterase activities in pools of five 4-day-old zebrafish (Danio rerio) embryos following in vivo exposure to DMSO, caffeine, cypermethrin, dichlorvos, 2,4-dinitrotoluene, 2,4-dichlorophenol, amidotrizoic acid, perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and 4-nonylphenol for 96 h. Inhibition is expressed as percent of the negative control (100%; open column). The positive control (2 mg/L paraoxon-methyl) is given as the black column. N = 3. Statistical difference from negative controls: *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA, Dunn’s test)
The potential existence of systemic regulatory effects leading to different amounts of enzyme available was excluded by adding an in vitro (ex vivo) exposure scenario for cypermethrin and dichlorvos (Fig. 2). Embryos were exposed to concentrations similar to those used for in vivo exposure (cypermethrin — 1, 5, 10, and 20 ng/L; dichlorvos — 0.75, 1.5, and 3 mg/L); 2 mg/L paraoxon-methyl served as a positive control. Embryos exposed to cypermethrin did not show any effect on acetylcholinesterase after in vitro exposure. In contrast, in vitro exposure to dichlorvos resulted in even more pronounced acetylcholinesterase inhibition than in vivo exposure (Fig. 2).
Specific acetylcholinesterase activities in pools of five 4-day-old zebrafish (Danio rerio) embryos following in vitro (ex vivo) exposure to cypermethrin (left) and dichlorvos (right). Inhibition is expressed as percent of the negative control (100%; open columns). Positive control: 2 mg/L paraoxon-methyl (black columns). N = 3. Statistical difference from negative controls: ***p < 0.001 (one-way ANOVA, Dunn’s test)
Neurotoxicant-induced changes in the structure of the retina
Both hematoxylin/eosin (Fig. 3) and combined paraformaldehyde/DAPI fluorescence staining (Fig. 4) of zebrafish embryo eyes allowed a well-defined visualization of retinal cell layers. However, none of the selected model neurotoxicants induced any abnormalities in the retinal structure after 96 h exposure to EC10 concentrations (Figs. 3 and 5).
Hematoxylin/eosin-stained 5-μm sections of paraffin-embedded eyes of zebrafish (Danio rerio) embryos after 96 h exposure to c 2,4-dichlorophenol, d amidotrizoic acid, e 4-nonylphenol, and f perfluorooctanoic acid. a, b Negative controls, with b enlargement of white frame in a. Note that small cracks especially within the inner nuclear layer should be regarded as artifacts of sectioning and not be interpreted as adverse effects of neurotoxicants. Abbreviations: RCG — receptor cell layer, ONL — outer nuclear layer, OPL — outer plexiform layer, INL — inner nuclear layer, IPL — inner plexiform layer, GCL — ganglion cell layer. Bar = 20 μm
Fluorescence imaging of the eye of a 96-h control zebrafish (Danio rerio) embryo with paraformaldehyde autofluorescence (green, a) and DAPI-staining for nuclei (blue, b) as well as merged (c). d Higher magnification of white frame in c. Arrows in a indicate areas with high paraformaldehyde autofluorescence (1: INL, 2: ONL, 3: RCL). d Erratic structural irregularities are indicated by dots in the inner plexiform layer and asterisk/hash for the inner nuclear layer. Abbreviations: RCG — receptor cell layer, ONL — outer nuclear layer, OPL — outer plexiform layer, INL — inner nuclear layer, IPL — inner plexiform layer, GCL — ganglion cell layer. All bars: 20 µm
Fluorescence imaging of the eyes of 96-h zebrafish (Danio rerio) embryos stained by paraformaldehyde autofluorescence (green) and DAPI nuclei (nuclei: blue): negative control, amidotrizoic acid, dichlorvos, 2,4-dinitrotoluene, and perfluorooctanoic acid at EC10, EC5, and EC2.5 concentrations (as derived from the 96-h fish embryo test results). At exposure concentrations ≤ EC10 values, the selected model neurotoxicants fail to induce significant changes in the architecture of the retina
Neurotoxicant-induced modifications of the olfactory epithelium
If compared to standard cryotechniques, structural preservation of cilia in the whole-mount approach was far better (details not shown). Whole-mount staining of embryonic zebrafish with dk α rb A568 detecting anti-GαSolf revealed a strong fluorescence signal in the cilia of olfactory receptor neurons; whereas the nuclei of the olfactory epithelium itself could well be visualized, DAPI failed to stain the underlying tissues (Fig. 6), most likely due to restricted permeability of the olfactory epithelium for the dye.
Fluorescence staining of the olfactory epithelium of 96-h-old zebrafish (Danio rerio) embryos with dk α rb A568 detecting GαSolf (red) and DAPI (blue) as a counterstaining. a Olfactory epithelium of a 96-h-old zebrafish embryo prepared in cryotechnique. b Olfactory epithelium of zebrafish embryo prepared in whole-mount. The tissue presentation in the whole-mount presentation is clearly superior to that after cryopreparation. White arrow indicates location of cilia. Bars = 25 μm
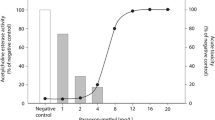
In a full dose-response relationship experiment with copper sulfate up to 40 mg/L, a clear-cut concentration-dependent destruction of receptor cilia could be documented, however, only at concentrations above the EC10 concentration in the FET (Fig. 7), indicating that adverse effects on the olfactory epithelium occur, however only at elevated concentrations. Likewise, none of the nine selected neurotoxicants produced any changes in the ciliary architecture of the embryonic olfactory epithelium at concentrations ≤ EC10 (Fig. 8). Only the positive controls copper sulfate and zinc sulfate led to a decrease in the number of cilia or to structural changes in the olfactory epithelium.
Olfactory epithelium of 96-h-old zebrafish (Danio rerio) embryos exposed to different concentrations of the positive control copper sulfate for 30 min, stained for cilia (red) with dk α rb A568 detecting GαSolf and counterstained with DAPI (nuclear layer: blue). With increasing concentrations of copper sulfate, an increasing amount of cilia undergoes destruction. a Negative control. b 2.5 mg/L. c 5 mg/L. d 10 mg/L. e 20 mg/L. f 40 mg/L CuSO4. Bars = 50 μM
Fluorescence staining of the olfactory epithelium of 96-h-old zebrafish (Danio rerio) embryos with dk α rb A568 detecting GαSolf (red) and DAPI (blue) as a counterstaining after exposure to selected neurotoxicants. a Negative control. b 39.9 mg/L CuSO4 (positive control): complete destruction of cilia (#). c 15 g/L ZnSO4 (positive control): partial destruction of cilia. d 3.9 mg/L 2,4-dinitrotoluene (EC10). e 1.6 mg/L 2,4-dichlorophenol (EC10); 2.8 mg/L dichlorvos (EC10). *: intact cilia of olfactory receptor neurons. Bar = 50 μm
Alterations in the lateral line (neuromast assay)
As documented in detail by Stengel et al. (2017), combined DASPEI/DAPI staining leads to a well-defined fluorescence signal from both nuclei and mitochondria in neuromasts of both the anterior and the posterior lateral line (Fig. 9). The combined DAPI/DASPEI staining strongly improved the identification of neuromasts, if compared to single DASPEI staining, thus leading to a better detection of effects especially at higher test concentrations. For substances such as the positive control, copper sulfate, overall fluorescence intensity of the neuromasts gradually decreases with increasing concentrations (Fig. 9; for details, see Stengel et al. 2017).
Live-imaging of neuromasts in the head region of a 96-h-old zebrafish (Danio rerio) embryo: 30 min double-staining with 0.005% DASPEI (2-[p-(dimethylamino) styryl]ethyl-pyridiniumiodite (pink) as a marker of mitochondria-rich cells) in 0.5% DMSO and 0.1% DAPI (4′,6-diamidine-2-phenylindol; blue: nuclei) in artificial water mounted in 1% low-melting agarose supplemented with 0.016% MS222 for anesthesia. Among potential positive controls (copper sulfate, neomycin, and paraoxon-methyl), 96 h exposure to copper sulfate (lower series of micrographs) leads to the most prominent concentration-dependent decline in stainable neuromasts (with permission from Braunbeck et al. 2015)
As an enhancement of the 3-grade scoring scheme by Harris et al. (2003), quantification of neuromast fluorescence intensity by a 4-grade system allowed a significant reduction of variability of scoring results for, e.g., neomycin (Fig. 10). Thus, in contrast to the 3-grade scoring system, which failed to show statistical significance of effects by up to 36.8 mg/L neomycin, the 4-grade scoring system resulted in a statistically significant reduction of neuromast integrity from 9.2 mg/L neomycin (Fig. 10).
Left: Comparison of results obtained from 3-step and 4-step scoring for neuromast modification after exposure of zebrafish (Danio rerio) embryos to 2.3 – 36.8 mg/L neomycin for 96 h; n = 3. Statistical difference from negative controls: *p < 0.05 (one-way ANOVA, Dunn’s test). Right: Scoring visualization of neuromast damage in chosen neuromasts (from left to right): 0 — unchanged control: all hair cells well stained; 1 — minor damage: reduced mitochondrial staining intensity and/or less hair cells; 2 — strong damage: mitochondria only faintly stained and/or strongly reduced number of hair cells; 3 — very strong damage: mitochondria and hair cells barely detectable. Data for fluorescence intensity are given as the average of the individual fluorescence scores of 12 embryos per replicate
Among the selected model neurotoxicants, dichlorvos, 2.4-dinitrotoluene, 4-nonylphenol, and perfluorooctane sulfonic acid could be documented to produce statistically significant adverse effects in the hair cells of the lateral line organ. Effects were shown to depend on the substance, exposure time, and neuromast localization (Stengel et al. 2017).
Discussion
Except for acetylcholinesterase inhibition (for review of the recent literature, see Kais et al. (2015) and a certain trend to visualize the consequence of neurotoxicity via behavioral studies (Beauvais et al. 2001; Chen et al. 2011; Eddins et al. 2010; Kienle et al. 2009; Klüver et al. 2015; Kokel et al. 2010; Rihel and Schier 2012; Selderslaghs et al. 2009, 2012, 2013; Topping et al. 2007), neurotoxicity testing in fish has not yet reached standardization. Nevertheless, neurotoxicity has already been implemented in national regulations for the protection of, e.g., drinking water resources (Dieter 2003; UBA 2003). Namely, the German health-related indicator value (HRIV) concept (Grummt et al. 2013) lists neurotoxicity as the second endpoint to be addressed (Fig. 11): In case there are data to indicate that a substance is neurotoxic, a maximum concentration (HRIV3) of 0.3 μg/L may not be exceeded. If data on neurotoxicity of a given substance are lacking, neurotoxicity is assumed following a worst-case scenario and the same HRIV3 (0.3 μg/L) applies. If groundwater used for drinking water exceeds 0.3 μg/L for a proven or suspected neurotoxicant, measures have to be taken to reduce the concentration to < 0.03 μg/L; otherwise, the water may no longer be used for drinking water purposes.
Summary of the German health-related indicator value (HRIV) concept (adapted from Grummt et al. (2013) based on UBA (2003). HRIV values depend on the availability of toxicological data sets. Since HRIV1 was the initial value for substances with no toxicological data available, and since HRIV2 was defined after HRIV1, the numbering does not appear logical. In case a substance has been proven to be neurotoxic, a maximum concentration (HRIV) of 0.3 μg/L may not be exceeded. If data on neurotoxicity of a given substance are not available, neurotoxicity is assumed following a worst-case scenario, and the same HRIV3 (0.3 μg/L) applies
As a consequence, there is considerable regulatory pressure on the development and standardization of methodologies to measure neurotoxicity in aquatic ecosystems, and several research projects have been imitated to provide techniques to reveal, among other parameters, neurotoxicity (Grummt et al. 2013; Huckele and Track 2013). The purpose of the present communication is to introduce a tool box to measure neurotoxicity in fish; zebrafish embryos were picked as experimental models due to the high level of standardization of the fish embryo test (OECD 2013) and since, up to an age of 120 h post-fertilization, zebrafish embryos are not regarded as protected according to current EU animal welfare regulations (EU 2010; Strähle et al. 2011).
The fish embryo toxicity test (OECD 2013) has repeatedly been reviewed (Belanger et al. 2013; Braunbeck et al. 2005, 2015; Embry et al. 2010; Halder et al. 2010; Lammer et al. 2009; Nagel 2002), and its potential to incorporate endpoints beyond such indicating acute toxicity has repeatedly been highlighted (Braunbeck et al. 2015; Di Paolo et al. 2015; Driessen et al. 2013; Kanungo et al. 2014; Nishimura et al. 2015; Sipes et al. 2011; Weigt et al. 2011). In the present study, all experimental procedures were carried out on the basis of OECD test guideline 236 (OECD 2013), and the dose-response relationships for acute toxicity were used to derive EC10 values, which were then used as the highest test concentration in subsequent assays for neurotoxicity. As already emphasized by Kais et al. (2015), Stengel et al. (2017) as well as Weigt et al. (2012), specific toxicity can only reliably be monitored, if systemic effects in consequence of acute toxicity are strictly avoided, i.e., if tests are conducted at concentrations well below acute toxicity.
Acetylcholinesterase inhibition is a classical marker of neurotoxicity by organophosphorus- and carbamate-type pesticides as well some PAHs (Chen et al. 2014; Kang and Fang 1997; Mwila et al. 2013; Russom et al. 2014; Tilton et al. 2011; Wogram et al. 2001; Yen et al. 2011) and has already been standardized for the zebrafish embryo (Kais et al. 2015; Küster 2005; Küster and Altenburger 2006; Perez et al. 2013). Results of the present study further confirm the selectivity of the acetylcholinesterase assay, since only the synthetic organophosphate pesticide dichlorvos inhibited acetylcholinesterase activities (Table 3; Assis et al. 2007, 2012; Silva et al. 2013). Likewise, although its primary targets are voltage-gated sodium channels (Casida et al. 1983), pyrethroid pesticides such as cypermethrin have repeatedly been shown to modulate acetylcholinesterase activities (Arora et al. 2017; Bonansea et al. 2016; Das and Mukherjee 2003; Narra 2016; Reddy et al. 1991; Vani et al. 2012), however, usually in an inhibitory fashion. In contrast, in the zebrafish embryo, cypermethrin caused a small, yet significant increase of acetylcholinesterase activity, which corroborates conflicting observations by other authors (Assis et al. 2012; Gioda et al. 2013; Samanta et al. 2014). Pyrethroids may also bind to the acetylcholine receptor (Soderlund et al. 2002), which, however, can at least be excluded as a mode of action in the ex vivo (in vitro) acetylcholinesterase assay used.
Likewise, alteration of neuromasts by neurotoxicants as the functional units of the lateral line in fish has repeatedly been documented in zebrafish (Buck et al. 2012; Coffin et al. 2010; Froehlicher et al. 2009a; Harris et al. 2003; Johnson et al. 2007; Owens et al. 2008; Ton and Parng 2005) and has recently been further optimized by Stengel et al. (2017): The combination of DASPEI and DAPI staining resulted in a longer persistence of fluorescence activity during degenerative processes such as apoptosis or necrosis and thus significantly reduced variability of observations between replicates. Enhancement from a 3-step (Harris et al. 2003) to a 4-step scoring scheme further reduced variability and not only allows a better differentiation of effects but also increases the statistical resolution of the assay. Finally, consideration of neuromasts located in both the anterior and the posterior lateral line systems clearly improves the coverage of effects by neurotoxicants.
In contrast to the acetylcholinesterase and neuromast assays, both vision and olfaction proved quite resistant to the action of neurotoxicants at levels up to EC10 concentrations (Table 3). Morphological alterations in the architecture of the fish retina have only very rarely been documented: Chandrasekar et al. (2011) revealed increased rates of apoptosis in the retina of 33-h-old zebrafish embryos associated with strong malformations of the notochord for a concentration of 440 μg/L nonylphenol, which, however, exceeds the EC10 of nonylphenol of ~ 140 μg/L (Table 2) by a factor of more than 3 and can, thus, be questioned as a specifically neurotoxic effect. For concentrations between 0.5 and 1.8 μg/L chlorpyrifos, an organophosphorus pesticide with a similar mode of action as dichlorvos, Mishra and Devi (2014) observed an induction of vacuolization in the tectum opticum of the snakehead fish (Channa punctatus). Although tested at similar concentrations, effects by dichlorvos in the retina of zebrafish are apparently less sensitive than those observed in the tectum opticum.
The specificity of the rabbit GαSolf antibodies to detect embryonic olfactory epithelia in fish has repeatedly been documented (Braubach et al. 2012; Ferrando et al. 2011, 2014; Hansen et al. 2003). For copper sulfate, effects on ciliary olfactory neurons from 2.5 mg/L corroborate observations by Bettini et al. (2006), who illustrated effects by copper sulfate from 15 μg/L. More general effects by copper sulfate on the structure and functionality of the olfactory system have been shown by Baldwin et al. (2003, 2011), Sandahl et al. (2006), Moran et al. (1992), Julliard et al. (1996), as well as Hansen and Zeiske (1998), however, with variable effect thresholds. For the other model neurotoxicants 2,4-dinitrolouene, dichlorvos, and 2,4-dichorophenol, no effect on the olfactory system could be shown.
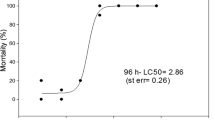
With respect to the lower detection limits of responsive endpoints at the molecular, morphological, and apical levels, it appears of interest to note that EC10 values for acetylcholinesterase inhibition (0.18 mg/L), neuromast degeneration (0.75 mg/L), and the fish embryo test (2.86 mg/L) consistently increased with the level of biological organization (Fig. 12 for dichlorvos) lending further support to the concept of correlations between endpoint sensitivity and both the specificity of effects and the hierarchy of biological organization (Braunbeck 1992, 1998).
Comparison of lower detection limits of effects by the model neurotoxicant dichlorvos: IC10 for acetylcholinesterase inhibition (red): 0.18 mg/L; EC10 values in the neuromast assay (green): 0.75 mg/L; and EC10 value in the standard fish embryo test (OECD TG 236; blue): 2.86 mg/L). The higher the level of biological organization, the less sensitive the tests tends to be for specifically acting (i.e. neurotoxic) compounds
Conclusions
Whereas both the visionary and the olfactory system proved quite resistant, the acetylcholinesterase and the neuromast assays proved highly sensitive for effects by neurotoxicants and should, therefore, be incorporated into a test battery for neurotoxic action in fish. Acetylcholinesterase inhibition/stimulation and neuromast degeneration address different modes of action, which can easily be combined with observations already routinely recorded in the standardized protocol for the fish embryo test (OECD test guideline no. 236; OECD 2013) such as movement (somite formation) and heartbeat to form a comprehensive battery of endpoints addressing the effects of potentially neurotoxic compounds. Since acetylcholinesterase inhibition and neuromast degeneration in both the anterior and the posterior lateral line systems can easily be analyzed in 72- or 96-h-old zebrafish embryos exposed to neurotoxicants according to the standard protocol of the fish embryo toxicity test (OECD TG 236), the fish embryo toxicity test can be enhanced to serve as a sensitive neurotoxicity screening test. Care should be taken, however, to not categorize observations made at concentrations higher than EC10 values as specific neurotoxic effects. In any case, the test battery for neurotoxicity should be validated by additional experiments with neurotoxicants with other modes of action.
References
Arora S, Balotra S, Pandey G, Kumar A (2017) Binary combinations of organophosphorus and synthetic pyrethroids are more potent acetylcholinesterase inhibitors than organophosphorus and carbamate mixtures: an in vitro assessment. Toxicol Lett 268:8–16
Assis CR, Amaral IP, Castro PF, Carvalho LB, Bezerra RS (2007) Effect of dichlorvos on the acetylcholinesterase from tambaqui (Colossoma macropomum) brain. Environ Toxicol Chem 26:1451–1453
Assis CR, Linhares AG, Oliveira VM, Franca RC, Carvalho EV, Bezerra RS, de Carvalho LB (2012) Comparative effect of pesticides on brain acetylcholinesterase in tropical fish. Sci Total Environ 441:141–150
Bachmann J 2002 Entwicklung und Erprobung eines Teratogenitäts-Screening Testes mit Embryonen des Zebrabärblings Danio rerio, Dresden Techn. Univ. http://nbn-resolving.de/urn:nbn:de:swb:14-1034346530531-90116, 214 pp
Baldwin DH, Sandahl JF, Labenia JS, Scholz NL (2003) Sublethal effects of copper on coho salmon: impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environ Toxicol Chem 22:2266–2274
Baldwin DH, Tatara CP, Scholz NL (2011) Copper-induced olfactory toxicity in salmon and steelhead: extrapolation across species and rearing environments. Aquat Toxicol 101:295–297
Beauvais SL, Jones SB, Parris JT, Brewer SK, Little EE (2001) Cholinergic and behavioral neurotoxicity of carbaryl and cadmium to larval rainbow trout (Oncorhynchus mykiss). Ecotoxicol Environ Saf 49:84–90
Belanger SE, Rawlings JM, Carr G (2013) Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environ Toxicol Chem 32:1768–1783
Bettini S, Ciani F, Franceschini V (2006) Cell proliferation and growth-associated protein 43 expression in the olfactory epithelium in Poecilia reticulata after copper solution exposure. Eur J Histochem 50:141–146
Bleckmann H, Zelick R (2009) Lateral line system of fish. Integr Zool 4:13–25
Bonansea RI, Wunderlin DA, Amé MV (2016) Behavioral swimming effects and acetylcholinesterase activity changes in Jenynsia multidentata exposed to chlorpyrifos and cypermethrin individually and in mixtures. Ecotoxicol Environ Saf 129:311–319
Braubach OR, Fine A, Croll RP (2012) Distribution and functional organization of glomeruli in the olfactory bulbs of zebrafish (Danio rerio). J Comp Neurol 520:2317–2339
Braunbeck T (1992) Umweltcytologie – zelluläre Reaktionen zum Nachweis einer Schadstoffbelastung von Organismen. Ruperto Carola 85:61–85
Braunbeck T (1998) Cytological alterations in fish hepatocytes—in vivo and in vitro biomarkers of environmental contamination. In: Braunbeck T, Hinton DE, Streit B (eds) Fish ecotoxicology. Experientia, Suppl. Ser. Birkhäuser, Basel, pp 61–140
Braunbeck T, Lammer E, Leist E, Rudolf M (2005) Towards an alternative for the acute fish LC50 test in chemical assessment: the zebrafish (Danio rerio) embryo toxicity test—an update. ALTEX 22:87–102
Braunbeck T, Kais B, Lammer E, Otte J, Schneider K, Stengel D, Strecker R (2015) The fish embryo test (FET): origin, applications, and future. Environ Sci Pollut Res 22:16247–16261
Buck LM, Winter MJ, Redfern WS, Whitfield TT (2012) Ototoxin-induced cellular damage in neuromasts disrupts lateral line function in larval zebrafish. Hear Res 284:67–81
Byrd CA, Brunjes PC (1995) Organization of the olfactory system in the adult zebrafish: histological, immunohistochemical, and quantitative analysis. J Comp Neurol 358:247–259
Casida JE, Gammon DW, Glickman AH, Lawrence LJ (1983) Mechanisms of selective action of pyrethroid insecticides. Ann Rev Pharmacol Toxicol 23:413–438
Chandrasekar G, Arner A, Kitambi SS, Dahlman-Wright K, Lendahl MA (2011) Developmental toxicity of the environmental pollutant 4-nonylphenol in zebrafish. Neurotoxicol Teratol 33:752–764
Chen J, Huang C, Zheng L, Simonich M, Bai C, Tanguay R, Dong Q (2011) Trimethyltin chloride (TMT) neurobehavioral toxicity in embryonic zebrafish. Neurotoxicol Teratol 33:721–726
Chen C, Wang Y, Zhao X, Wang Q, Qian Y (2014) The combined toxicity assessment of carp (Cyprinus carpio) acetylcholinesterase activity by binary mixtures of chlorpyrifos and four other insecticides. Ecotoxicology 23:221–228
Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW (2009) Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res 253:42–51
Coffin AB, Ou H, Owens KN, Santos F, Simon JA, Rubel EW, Raible DW (2010) Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish 7:3–11
Cowden J, Padnos B, Hunter D, Mac Phail R, Jensen K, Padilla S (2012) Developmental exposure to valproate and ethanol alters locomotor activity and retino-tectal projection area in zebrafish embryos. Reprod Toxicol 33:165–173
Das BK, Mukherjee SC (2003) Toxicity of cypermethrin in Labeo rohita fingerlings: biochemical, enzymatic and haematological consequences. Comp Biochem Physiol 134C:109–121
de Esch C, Slieker R, Wolterbeek APM, Woutersen RA, de Groot D (2012) Zebrafish as potential model for developmental neurotoxicity testing: a mini review. Neurotox Teratol 34:545–553
Di Paolo C, Seiler TB, Keiter S, Hu M, Muz M, Brack W, Hollert H (2015) The value of zebrafish as an integrative model in effect-directed analysis—a review. Environ Sci Eur 27:8. https://doi.org/10.1186/s12302-015-0040-y
Dieter HH (2003) Kommentar zur Bewertung der Anwesenheit nicht oder nur teilbewertbarer Stoffe im Trinkwasser aus gesundheitlicher Sicht. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 46:245–248
Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM (2014) Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio). Toxicol Sci 142:445–454
Doving KB, Lastein S (2009) The alarm reaction in fishes—odorants, modulations of responses, neural pathways. Ann N Y Acad Sci 1170:413–423
Driessen M, Kienhuis AS, Pennings JLA, Pronk TE, van de Brandhof E-J, Roodbergen M, Spaink HP, van de Water B, van der Ven LTM (2013) Exploring the zebrafish embryo as an alternative model for the evaluation of liver toxicity by histopathology and expression profiling. Arch Toxicol 87:807–823
Easter JSS, Nicola GN (1996) The development of vision in the zebrafish (Danio rerio). Dev Biol 180:646–663
Eddins D, Cerutti D, Williams P, Linney E, Levin ED (2010) Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotox Teratol 32:99–108
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Embry MR, Belanger SE, Braunbeck T, Galay-Burgos M, Halder M, Hinton DE, Léonard MA, Lillicrap A, Norberg-King T, Whale G (2010) The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquat Toxicol 97:79–87
EU (2008) Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the council. Off J EU L 348/84:14. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32008L0105
EU (2010) Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Lex 276:33–79
Fan Y, Ding S, Ye X, Manyande A, He D, Zhao N, Yang H, Jin X, Liu J, Tian C, Xu S, Ying C (2013) Does preconception paternal exposure to a physiologically relevant level of bisphenol A alter spatial memory in an adult rat? Hormon Behav 64:598–604
Ferrando S, Gallus L, Gambardella C, Amaroli A, Vallarino M, Tagliafierro G (2011) Immunolocalization of G protein alpha subunits in the olfactory system of Polypterus senegalus (Cladistia, Actinopterygii). Neurosci Lett 499:127–131
Ferrando S, Gallus L, Gambardella C, Marchesotti E, Ravera S, Franceschini V, Masini MA (2014) Effects of urea on the molecules involved in the olfactory signal transduction: a preliminary study on Danio rerio. Fish Physiol Biochem 40:1793–1800
Fournie JW, Krol RM, Hawkins WE (2000) Fixation of fish tissues. In: Ostrander GK (ed) The laboratory fish. Academic, San Diego, pp 569–578
Fox DA, Grandjean P, de Groot D, Paule MG (2012) Developmental origins of adult diseases and neurotoxicity: epidemiological and experimental studies. Neurotoxicology 33:810–816
Fritzsch B, Beisel KW, Pauley S, Soukup G (2007) Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol 51:663–678
Froehlicher M, Liedtke A, Groh K, Neuhauss SC, Segner H, Eggen RI (2009a) Estrogen receptor subtype beta2 is involved in neuromast development in zebrafish (Danio rerio) larvae. Dev Biol 330:32–43
Froehlicher M, Liedtke A, Groha KJ, Neuhauss SCF, Segner H, Eggen RIL (2009b) Zebrafish (Danio rerio) neuromast: promising biological endpoint linking developmental and toxicological studies. Aquat Toxicol 95:307–319
Ghysen A, Dambly-Chaudiere C (2004) Development of the zebrafish lateral line. Curr Opin Neurobiol 14:67–73
Gioda CR, Loro VL, Pretto A, Salbego J, Dressler V, Flores EM (2013) Sublethal zinc and copper exposure affect acetylcholinesterase activity and accumulation in different tissues of Leporinus obtusidens. Bull Environ Contam Toxicol 90:12–16
Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals. Lancet 368:2167–2178
Grandjean P, Landrigan PJ (2014) Neurobehavioural effects of developmental toxicity. Lancet Neurol 13:330–338
Grummt T, Kuckelkorn J, Bahlmann A, Baumstark-Khan C, Brack W, Braunbeck T, Feles S, Gartiser S, Glatt H, Heinze R, Hellweg C, Hollert H, Junek R, Knauer M, Kneib-Kissinger B, Kramer M, Krauss M, Kuster E, Maletz S, Meinl W, Noman A, Prantl E-M, Rabbow E, Redelstein R, Rettberg P, Schadenboeck W, Schmidt C, Schulze T, Seiler TB, Spitta L, Stengel D, Waldmann P, Eckhardt A (2013) Tox-Box: securing drops of life—an enhanced health-related approach for risk assessment of drinking water in Germany. Environ Sci Eur 25:27 http://www.enveurope.com/content/25/1/27
Guilhermino L, Celeste Lopes M, Carvalho AP, Soares AM (1996) Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere 32:727–738
Halder M, Léonard M, Iguchi T, Oris JT, Ryder K, Belanger SE, Braunbeck T, Embry MR, Whale G, Norberg-King T, Lillicrap A (2010) Regulatory aspects on the use of fish embryos in environmental toxicology. Integr Environ Assess Manag 6:484–491
Hansen A, Zeiske E (1998) The peripheral olfactory organ of the zebrafish, Danio rerio: an ultrastructural study. Chem Senses 23:39–48
Hansen A, Zielinski BS (2005) Diversity in the olfactory epithelium of bony fishes: development, lamellar arrangement, sensory neuron cell types and transduction components. J Neurocytol 34:183–208
Hansen A, Rolen SH, Anderson K, Morita Y, Caprio J, Finger TE (2003) Correlation between olfactory receptor cell type and function in the channel catfish. J Neurosci 23:9328–9339
Hara TJ (1975) Olfaction in fish. Prog Neurobiol 5:271–335
Hara TJ (1994) The diversity of chemical stimulation in fish olfaction and gustation. Fish Biol Fish 4:1–35
Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW (2003) Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol 4:219–234
Hayashi L, Sheth M, Young A, Kruger M, Wayman GA, Coffin AB (2015) The effect of the aquatic contaminants bisphenol-A and PCB-95 on the zebrafish lateral line. Neurotoxicology 46:125–136
Huang L, Zuo Z, Zhang Y, Wu M, Lin JJ, Wang C (2014) Use of toxicogenomics to predict the potential toxic effect of benzo(a)pyrene on zebrafish embryos: ocular developmental toxicity. Chemosphere 108:55–61
Huckele S, Track T (2013) Risk management of emerging compounds and pathogens in the water cycle (RiSKWa). Environ Sci Eur 25:1 http://www.enveurope.com/content/25/1/1
Irons TD, MacPhail RC, Hunter DL, Padilla S (2010) Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol Teratol 32:84–90
Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S (2015) Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol 52:194–209
Johnson AC, Carew E, Sloman KA (2007) The effects of copper on the morphological and functional development of zebrafish embryos. Aquat Toxicol 84:431–438
Julliard AK, Saucier D, Astic L (1996) Time-course of apoptosis in the olfactory epithelium of rainbow trout exposed to a low copper level. Tissue Cell 28:367–377
Kais B, Stengel D, Batel A, Braunbeck T (2015) Acetylcholinesterase in zebrafish embryos as a tool to identify neurotoxic effects in sediments. Environ Sci Pollut Res 22:16329–16339
Kang JJ, Fang HW (1997) Polycyclic aromatic hydrocarbons inhibit the activity of acetylcholinesterase purified from electric eel. Biochem Biophys Res Comm 238:367–369
Kanungo J, Cuevas E, Ali SF, Paule MG (2014) Zebrafish model in drug safety assessment. Curr Pharm Des 20:5416–5429
Kienle C, Köhler H-R, Gerhardt A (2009) Behavioural and developmental toxicity of chlorpyrifos and nickel chloride to zebrafish (Danio rerio) embryos and larvae. Ecotox Environ Saf 72:1740–1747
Kim KT, Zaikova T, Hutchison JE, Tanguay RL (2013) Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci 133:275–288
Kimmel CB, Sepich DS, Trevarrow B (1988) Development of segmentation in zebrafish. Development 104(Suppl):197–207
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310
Klüver N, König M, Ortmann J, Massei R, Paschke A, Kuehne R, Scholz S (2015) The fish embryo toxicity test (FET)—identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ Sci Technol 49:7002–7011
Kokel D, Bryan J, Laggner C, White R, Cheung CYJ, Mateus R, Healey D, Kim S, Werdich lAA, Haggarty SJ, MacRae CA, Shoichet B, Peterson RT (2010) Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol 6:231–237
Küster E (2005) Cholin- and carboxylesterase activities in developing zebrafish embryos (Danio rerio) and their potential use for insecticide hazard assessment. Aquat Toxicol 75:78–95
Küster E, Altenburger R (2006) Comparison of cholin- and carboxylesterase enzyme inhibition and visible effects in the zebra fish embryo bioassay under short-term paraoxon-methyl. Biomarkers 111:341–343
Küster E, Altenburger R (2007) Suborganismic and organismic effects of aldicarb and its metabolite aldicarb-sulfoxide to the zebrafish embryo (Danio rerio). Chemosphere 68:751–760
Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T (2009) Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol 149C:196–209
Lee W, Kang CW, CK S, Okubo K, Nagahama Y (2012) Screening estrogenic activity of environmental contaminants and water samples using a transgenic medaka embryo bioassay. Chemosphere 88:945–952
Linbo TL, Stehr CM, Incardona JP, Scholz NL (2006) Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ Toxicol Chem 25:597–603
Linbo TL, Baldwin DH, McIntyre JK, Scholz NL (2009) Effects of water hardness, alkalinity, and dissolved organic carbon on the toxicity of copper to the lateral line of developing fish. Environ Toxicol Chem 28:1455–1461
Lindsay SM, Vogt RG (2004) Behavioral responses of newly hatched zebrafish (Danio rerio) to amino acid chemostimulants. Chem Senses 29:93–100
Linney E, Dobbs-McAuliffe B, Sajadi H, Malek RL (2004) Microarray gene expression profiling during the segmentation phase of zebrafish development. Comp Biochem Physiol 138(C):351–362
Mecklenburg L, Schraermeyer U (2007) An overview on the toxic morphological changes in the retinal pigment epithelium after systemic compound administration. Toxicol Pathol 35:252–267
Mela M, Grotzner SR, Legeay A, Mesmer-Dudons N, Massabuau JC, Ventura DF, de Oliveira Ribeiro CA (2012) Morphological evidence of neurotoxicity in retina after methylmercury exposure. Neurotoxicology 33:407–415
Metcalfe WK, Kimmel CB, Schabtach E (1985) Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol 233:377–389
Mishra A, Devi Y (2014) Histopathological alterations in the brain (optic tectum) of the fresh water teleost Channa punctatus in response to acute and subchronic exposure to the pesticide chlorpyrifos. Acta Histochem 116:176–181
Miyagawa K, Narita M, Narita M, Akama H, Suzuki T (2007) Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci Lett 418:236–241
Moran DT, Rowley JC 3rd, Aiken GR, Jafek BW (1992) Ultrastructural neurobiology of the olfactory mucosa of the brown trout, Salmo trutta. Microsc Res Tech 23:28–48
Mwila K, Burton MH, Van Dyk JS, Pletschke BI (2013) The effect of mixtures of organophosphate and carbamate pesticides on acetylcholinesterase and application of chemometrics to identify pesticides in mixtures. Environ Monitor Assess 185:2315–2327
Nagel R (2002) DarT: the embryo test with the Zebrafish Danio rerio—a general model in ecotoxicology and toxicology. ALTEX 19(Suppl 1):38–48
Narra MR (2016) Single and cartel effect of pesticides on biochemical and haematological status of Clarias batrachus: a long-term monitoring. Chemosphere 144:966–974
Nishimura Y, Murakami S, Ashikawa Y, Sasagawa S, Umemoto N, Shimada Y, Tanaka T (2015) Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit Anom (Kyoto) 55:1–16
Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL (2015) Advanced morphological – behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol Sci 145:177–195
OECD (2010) Guidance document on the diagnosis of endocrine-related histopathology in fish gonads. OECD Series on Testing and Assessment No. 123. Organization for Economic Cooperation and Development, Paris
OECD (2013) OECD guidelines for the testing of chemicals. Section 2: effects on biotic systems test no. 236: fish embryo acute toxicity (FET) test. Organization for Economic Cooperation and Development, Paris
Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW (2008) Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet 4:e1000020
Padilla S, Hunter DL, Padnos B, Frady S, MacPhail RC (2011) Assessing locomotor activity in larval zebrafish: influence of extrinsic and intrinsic variables. Neurotox Teratol 33:624–630
Pal A, He Y, Jekel M, Reinhard M, Gin KY (2014) Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ Int 71:46–62
Perez J, Domingues I, Monteiro M, Soares AM, Loureiro S (2013) Synergistic effects caused by atrazine and terbuthylazine on chlorpyrifos toxicity to early-life stages of the zebrafish Danio rerio. Environ Sci Pollut Res Int 20:4671–4680
Postigo C, Barceló D (2015) Synthetic organic compounds and their transformation products in groundwater: occurrence, fate and mitigation. Sci Total Environ 503-504:32–47
Raible DW, Kruse GJ (2000) Organization of the lateral line system in embryonic zebrafish. J Comp Neurol 421:189–198
Reddy AT, Ayyanna K, Yellamma K (1991) Sensitivity of brain cholinesterase to cypermethrin toxicity in freshwater teleost Tilapia mossambica. Biochem Int 23:959–962
Rihel J, Schier AF (2012) Behavioral screening for neuroactive drugs in zebrafish. Dev Neurobiol 72:373–385
Romeis (2010) Romeis – Mikroskopische Technik. Springer Spektrum Publ, Heidelberg 603 pp
Russom CL, LaLone CA, Villeneuve DL, Ankley GT (2014) Development of an adverse outcome pathway for acetylcholinesterase inhibition leading to acute mortality. Environ Toxicol Chem 33:2157–2169
Sabaliauskas NA, Foutz CA, Mest JR, Budgeon LR, Sidor AT, Gershenson JA, Joshi SB, Cheng KC (2006) High-throughput zebrafish histology. Methods 39:246–254
Samanta P, Pal S, Mukherjee AK, Ghosh AR (2014) Biochemical effects of glyphosate based herbicide, Excel Mera 71, on enzyme activities of acetylcholinesterase (AChE), lipid peroxidation (LPO), catalase (CAT), glutathione-S-transferase (GST) and protein content on teleostean fishes. Ecotoxicol Environ Saf 107:120–125
Sandahl JF, Miyasaka G, Koide N, Ueda H (2006) Olfactory inhibition and recovery in chum salmon (Oncorhynchus keta) following copper exposure. Can J Fish Aquat Sci 63:1840–1847
Schmitt EA, Dowling JE (1994) Early eye morphogenesis in the zebrafish, Brachydanio rerio. J Comp Neurol 344:532–542
Schulte C, Nagel R (1994) Testing acute toxicity in the embryo of zebrafish, Brachydanio rerio, as an alternative to the acute fish test: preliminary results. ATLA 22:12–19
Selderslaghs IWT, Van Rompay AR, De Coen W, Witters HE (2009) Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod Toxicol 28:308–332
Selderslaghs IWT, Blusb R, Witters HE (2012) Feasibility study of the zebrafish assay as an alternative method to screen for developmental toxicity and embryotoxicity using a training set of 27 compounds. Reprod Toxicol 33:142–154
Selderslaghs I, Hooyberghs J, Blust R, Witters HE (2013) Assessment of the developmental neurotoxicity of compounds by measuring locomotor activity in zebrafish embryos and larvae. Neurotoxicol Teratol 37:44–56
Silva KC, Assis CR, Oliveira VM, Carvalho LB Jr, Bezerra RS (2013) Kinetic and physicochemical properties of brain acetylcholinesterase from the peacock bass (Cichla ocellaris) and in vitro effect of pesticides and metal ions. Aquat Toxicol 126:191–197
Sipes NS, Padilla S, Knudsen TB (2011) Zebrafish as an integrative model for twenty-first century toxicity testing. Birth Defects Rev 93C:256–267
Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML (2002) Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171:3–59
Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F (2006) Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci 26:1961–1970
Spence R, Jordan WC, Smith C (2006) Genetic analysis of male reproductive success in relation to density in the zebrafish, Danio rerio. Front Zool 3:5. https://doi.org/10.1186/1742-9994-3-5
Stengel D, Zindler F, Braunbeck T (2017) An optimized method to assess ototoxic effects in the lateral line of zebrafish (Danio rerio) embryos. Comp Biochem Physiol 193C:18–29
Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T (2011) Zebrafish embryos as an alternative to animal experiments. A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 33:245–153
Tanguay RL, Reimers MJ (2008) Analysis of ethanol developmental toxicity in zebrafish. Methods Mol Biol 447:63–74
Tierney KB (2011) Behavioural assessments of neurotoxic effects and neurodegeneration in zebrafish. Biochim Biophys Acta 1812:381–389
Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, Kennedy CJ (2010) Olfactory toxicity in fishes. Aquat Toxicol 96:2–26
Tilton F, La Du JK, Vue M, Alzarban N, Tanguay RL (2006) Dithiocarbamates have a common toxic effect on zebrafish body axis formation. Toxicol Appl Pharmacol 216:55–68
Tilton FA, Bammler TK, Gallagher EP (2011) Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately or as mixtures. Comp Biochem Physiol 153C:9–16
Ton C, Parng C (2005) The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res 208:79–88
Topping DC, Bernard LG, JL O’D, English JC (2007) Hydroquinone: acute and subchronic toxicity studies with emphasis on neurobehavioral and nephrotoxic effects. Food Chem Toxicol 45:70–78
Triebskorn R, Köhler H-R, Körtje K-H, Negele R-D, Rahmann H, Braunbeck T (1994) Evaluation of bis(tri-n-butyltin)oxide (TBTO) neurotoxicity in rainbow trout (Oncorhynchus mykiss). II. Ultrastructural diagnosis and tin localization by energy filtering transmission electron microscopy (EFTEM). Aquat Toxicol 30:199–213
Truong L, Harper SL, Tanguay RL (2011) Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol 691:271–279
Truong L, Mandrell D, Mandrell R, Simonich M, Tanguay RL (2014) A rapid throughput approach identifies cognitive deficits in adult zebrafish from developmental exposure to polybrominated flame retardants. Neurotoxicology 43:134–142
UBA (2003) Bewertung der Anwesenheit teil- oder nicht bewertbarer Stoffe im Trinkwasser aus gesundheitlicher Sicht: Empfehlung des Umweltbundesamtes nach Anhörung der Trinkwasserkommission beim Umweltbundesamt. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 46:249–251
Vani T, Saharan N, Roy S, Ranjan R, Pal A, Siddaiah G, Kumar R (2012) Alteration in haematological and biochemical parameters of Catla catla exposed to sub-lethal concentration of cypermethrin. Fish Physiol Biochem 38:1577–1584
Wang YP, Hong Q, Qin DN, Kou CZ, Zhang CM, Guo M, Guo XR, Chi X, Tong ML (2012) Effects of embryonic exposure to polychlorinated biphenyls on zebrafish (Danio rerio) retinal development. J Appl Toxicol 32:186–193
Weigt S, Huebler N, Strecker R, Braunbeck T, Broschard TH (2011) Zebrafish (Danio rerio) embryos as a model for testing proteratogens. Toxicology 281:25–36
Weigt S, Huebler N, Strecker R, Braunbeck T, Broschard TH (2012) Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reprod Toxicol 33:133–141
Wilson JM, Bunte RM, Carty AJ (2009) Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio). J Am Ass Laborat Anim Sci 48:785–789
Wixon J (2000) Featured organism: Danio rerio, the zebrafish. Yeast 17:225–231
Wogram J, Sturm A, Segner H, Liess M (2001) Effects of parathion on acetylcholinesterase, butyrylcholinesterase and carboxylesterase in three-spined stickleback (Gasterosteus aculeatus) following short-term exposure. Environ Toxicol Chem 20:1528–1531
Yen J, Donerly S, Levin ED, Linney EA (2011) Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotox Teratol 33:735–741
Zellner D, Padnos B, Hunter DL, MAcPhail RC, Padilla S (2011) Rearing conditions differentially affect the locomotor behavior of larval zebrafish, but not their response to valproate-induced developmental neurotoxicity. Neurotox Teratol 33:674–679
Funding
The authors gratefully acknowledge the financial support of the project Tox-Box (“A holistic approach towards a harmonized testing strategy for exposure- and hazard-based risk management of anthropogenic trace substances in drinking water to secure long-term drinking water supplies”) by the German Federal Ministry of Education and Research (BMBF) under grant no. 02WRS1282G within the RiSKWa funding scheme (“Risk management of emerging compounds and pathogens in the water cycle”), which is a constitutive part of the BMBF action plan NaWaM (“Sustainable water management”) and integrated into the BMBF framework program FONA (“Research for sustainable development”). The excellent collaboration within the Tox-Box project, especially with the team led by Dr. Tamara Grummt at the Federal Environment Agency at Bad Elster (Germany), is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Stengel, D., Wahby, S. & Braunbeck, T. In search of a comprehensible set of endpoints for the routine monitoring of neurotoxicity in vertebrates: sensory perception and nerve transmission in zebrafish (Danio rerio) embryos. Environ Sci Pollut Res 25, 4066–4084 (2018). https://doi.org/10.1007/s11356-017-0399-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0399-y