Abstract
Although metallic mineral resources are most important in the economy of Mongolia, mining activities with improper management may result in the pollution of stream waters, posing a threat to aquatic ecosystems and humans. In this study, aiming to evaluate potential impacts of metallic mining activities on the quality of a transboundary river (Selenge) in central northern Mongolia, we performed hydrochemical investigations of rivers (Tuul, Khangal, Orkhon, Haraa, and Selenge). Hydrochemical analysis of river waters indicates that, while major dissolved ions originate from natural weathering (especially, dissolution of carbonate minerals) within watersheds, they are also influenced by mining activities. The water quality problem arising from very high turbidity is one of the major environmental concerns and is caused by suspended particles (mainly, sediment and soil particles) from diverse erosion processes, including erosion of river banks along the meandering river system, erosion of soils owing to overgrazing by livestock, and erosion by human activities, such as mining and agriculture. In particular, after passing through the Zaamar gold mining area, due to the disturbance of sediments and soils by placer gold mining, the Tuul River water becomes very turbid (up to 742 Nephelometric Turbidity Unit (NTU)). The Zaamar area is also the contamination source of the Tuul and Orkhon rivers by Al, Fe, and Mn, especially during the mining season. The hydrochemistry of the Khangal River is influenced by heavy metal (especially, Mn, Al, Cd, and As)-loaded mine drainage that originates from a huge tailing dam of the Erdenet porphyry Cu-Mo mine, as evidenced by δ34S values of dissolved sulfate (0.2 to 3.8 ‰). These two contaminated rivers (Tuul and Khangal) merge into the Orkhon River that flows to the Selenge River near the boundary between Mongolia and Russia and then eventually flows into Lake Baikal. Because water quality problems due to mining can be critical, mining activities in central northern Mongolia should be carefully managed to minimize the transboundary movement of aquatic contaminants (in particular, turbidity, dissolved organic carbon, Fe and Al) from mining activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the centuries, rivers in the world have been extensively used by mankind and, therefore, very few of them have preserved their natural condition (Wetzel 2001). The quantity and quality of river water are intimately linked to land use in catchment; agricultural activities, industrialization, and urbanization have intensively degraded river water. In particular, poor land-use practices in catchment owing to the population increase and economic growth are rapidly declining the availability of usable fresh water in terms of both quality and quantity of water (Calder 1992; Luis et al. 2011).
Especially in Mongolia, a country of desert and steppes with the total population of about 2.7 million, the overall dry climatic condition with low rainfall (mostly <200 mm/year) causes the vital water shortage problem; most nationwide water resources are only found in the land of about 30 % of the country’s territory (JAICA 2006; Davaa and Odgarav 2012). The drinking water supply in Mongolia depends on groundwater (about 80 %) and surface water. Moreover, under the influence of economic stimulation, surface water pollution increases progressively (JAICA 2006). While mining and livestock industry are most important for Mongolian economy, these industries, together with wastewater effluents, are the main sources of water pollution (JAICA 2003; Kelderman and Batima 2006). Mongolia is among the top 10 richest countries in mineral resources in the world, with an estimated 1 to 3 trillion US dollars of copper, gold, coal, oil, and other resources (IMF 2015). Among these resources, gold mining amounts to about 70 % of total foreign currency.
Considerable water quality issues in relation to mining activities have recently been raised in Mongolia (JAICA 2003; Choi et al. 2004; Stubblefield et al. 2005; KEI 2006, 2008; Kelderman and Batima 2006; World Bank 2006; Hofmann et al. 2010; Thorslund et al. 2012, 2016; Battogtokh et al. 2014; Batbayar et al. 2015; Chalov et al. 2015; Nadmitov et al. 2015; Pfeiffer et al. 2015; Shinkareva et al. 2015). These issues include the following: (1) turbidity of surface water, resulting from the dredging of riverside alluvial sediments in search for placer gold; (2) water pollution due to the inflow of mine drainage with heavy metals from sulfide-bearing tailings in the areas of base metals and gold mining; and (3) changes of hydrogeological characteristics and water quality by dewatering. Most mining operations in Mongolia are the surface mining from placers, open-casts, and open pits, which requires the excavation and/or washing of metal-bearing alluvial or other geologic strata. Therefore, surface mining results in a significant landscape disturbance (Tarras-Wahlberg 2002; Stubblefield et al. 2005; Thorslund et al. 2012; Chalov 2014; Chalov et al. 2015). Placer gold mining by mining companies and illegal miners called “Ninja” causes severe disturbances of landscape and aquatic environments (Choi et al. 2004; KEI 2006). In addition, open-pit mining of copper-molybdenum ores from porphyry-type Cu-Mo deposits results in environmental problems arising from tailing storage, dust, and water contamination (Battogtokh et al. 2014; Ziadat et al. 2015). However, a better understanding of the mining-related water quality problems in Mongolia, based on a regional hydrochemical and isotopic survey, is still needed.
The main aims of this study are (1) to investigate the status of water quality and chemistry of rivers (i.e., Orkhon, Haraa, Khangal, Tuul, and Selenge) in the Baikal sub-watershed (or Selenge Basin) of central northern Mongolia and (2) to assess the influences of mining activities and watershed geology on surface water quality in the region. For this purpose, seeking to examine the effects of mining activities on surface water chemistry in central northern Mongolia on the basis of systematic surface water sampling, we performed a 3-year (2004–2006) Korea-Mongolia Joint Project (KEI 2006). The data and interpretation of the results of the present study will be used as hydrochemical baseline that defines the present-day background as the starting point for a reasonable water quality evaluation and monitoring in future. This will be a crucial step in sustainable watershed management in central northern Mongolia.
Study area
The study area in the Selenge Basin of central northern Mongolia covers a wide pasture land (steppe) between the latitudes of 47° 20′–50° 40′ N and the longitudes of 103° 50′–108° 50′ E and includes three provinces (Tov, Bulgan, and Selenge) among 28 administrative provinces (“Aimag”) of Mongolia. There are several important cities in the area, including Ulaanbaatar (the capital), Darkhan (the second largest city), Erdenet (the third largest city), and Bulgan (Fig. 1a). The study area belongs to the typical semi-arid continental climate zone, characterized by highly variable temperatures, low range of air humidity, non-uniform distribution of precipitation within a year, and cold and long-lasting winters and warm summers. The annual fluctuation range of air temperatures is up to 74 °C (−39.6 °C to +34.5 °C) and the monthly average surface temperatures are +21 °C in July, 0 °C in October, and −24 °C in January (KEI 2006). The average annual precipitation ranges from about 230–290 mm/year in the Tov and Selenge provinces to about 340 mm/year in the Bulgan province. Over 80–90 % of the annual precipitation occurs during the growing season between May and September, typically in the form of thunderstorms, while the rainfall in the other months (dry and cold season) is generally below 50 mm (Semenova and Myagmarjav 1977; Davaa and Odgarav 2012). According to Li et al. (2007), the estimated cumulative evapotranspiration in a steppe under grazing in central Mongolia is about 66 % of the precipitation. Total annual precipitation in 2005 was 137 mm at Zaamar, 193 mm at Ulaanbaatar, and 270 mm at Erdenet and Darkhan. In the study area, northerly to northwesterly winds are dominant, with the annual mean wind speed of 2.8 m/s. Annual mean air humidity is 62 %, with the ranges from 70 to 74 % in December and January to 48 % in April. It starts snowing in September. Snow covers the land until melting in middle May (Choi et al. 2004).
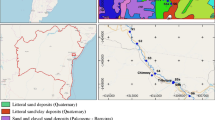
a Locality map showing water sampling sites in central northern Mongolia. Crosses (N = 29): October 2005; circles (N = 27): June 2006. b Distribution of metallic ore mines in central northern Mongolia (modified after www.miram.gov.mn)
Relatively large-sized rivers, such as Tuul, Orkhon, Haraa, and Selenge, and their tributaries flow through the study area (Fig. 1a). The Tuul River drains from the south-western slope of the Khentei Mountain located northeast of Ulaanbaatar in the Tov province, flows south-westward, and meanders near Ulaanbaatar. The total length of the Tuul River is about 900 km with the catchment area of 49,800 km2 (Davaa and Odgarav 2012). The Tuul River flows through the Ulaanbaatar city and then the Zaamar gold mining district (a largest gold mining town in Mongolia) to finally adjoin the Orkhon River. Hence, the Tuul River is the most polluted river in Mongolia. The Orkhon River is one of the main tributaries of the Selenge River which forms a major watershed of Lake Baikal, Russia. The Selenge river basin contributes approximately 50 % of the total lake inflow to Lake Baikal (Falkner et al. 1997). Granina et al. (1998) also reported that at least 75 % of the input of terrigenous materials to Lake Baikal originates from the Selenge river basin.
The Tuul River is moderate sized in Mongolia, with flow rates of 20–100 m3/s (Davaa and Odgarav 2012). Although the Tuul River sub-basin occupies about 3.2 % of the country’s territory, it is most important, as over a half of the national population lives in the basin. The Zaamar gold mining district is located along the banks of the Tuul River and its tributaries. The Khangal River drains from the Erdenet city that is well known for a huge porphyry Cu-Mo mine and flows south-eastward down to the confluence with Orkhon River (Fig. 1a).
The geology of central northern Mongolia consists of metamorphic, igneous, and sedimentary complexes of Early Archean to Late Cenozoic ages. Several plutonic and volcanic activities occurred until recent age (Takahashi 2004). Major parts of the watersheds of the Tuul and Orkhon rivers are mostly composed of sedimentary rocks and igneous rocks, respectively (Tomurtogoo et al. 1998). Geology of the Zaamar mining district in the Tuul River sub-basin is composed of Cambrian-Ordovician to Carboniferous sedimentary rocks, while the Erdenet porphyry Cu-Mo mine area in the Orkhon river sub-basin is occupied by Mesozoic intrusive rocks. Figure 1b shows the distribution of various types of mineral deposits in the study area (www.miram.gov.mn). Gold deposits of both hydrothermal quartz-calcite veins and placers occur in the Zaamar area, and porphyry Cu-Mo deposits occur in Erdenet. Other types of mineral deposits, including Fe-Cu skarns and hydrothermal W-Sn veins, also occur in the study area, but those are minor in economic importance.
The Zaamar gold mining district is situated on a grassy pasture land located about 240 km west of Ulaanbaatar (Fig. 1b) and has been the largest and most economically important area in Mongolia since the 1970s. In the Zaamar district, over 30 mining companies and numerous illegal miners called Ninja are exploiting gold from placers and/or hydrothermal veins of an approximately 20-km-long belt along the banks of Tuul River and its small tributaries. The river channel sediments vary from 15 to 30 m in total thickness and are composed of alluvial to colluvial rubble-clay deposits (1 to 10 m thick) overlying 5- to 20-m-thick, yellow to red-colored Quaternary gravels interbedded with sand, loam, and clay. Gold occurs as coarse- to medium-grained particles in sand and gravel (Choi et al. 2004; Karpoff and Roscoe 2005).
The Erdenet porphyry Cu-Mo deposit is one of the largest mines in Mongolia and has been operated since 1978 by a Mongol-Russian joint company. The deposit is located 365 km northeast of Ulaanbaatar and 165 km southwest of the Trans-Mongolian railroad junction at Darkhan. The Erdenet mine annually produces approximately 20 million metric tons of Cu ore, from which approximately 354,000 metric tons of copper concentrate and 3500 tons of molybdenum concentrate are produced annually (World Bank 2006). The copper concentrate from Erdenet contains 27–35 % of copper with trace amounts of selenium (50–60 g/t), silver (50–70 g/t), tellurium (8–9 g/t), and gold (0.3–0.5 g/t). The molybdenum concentrate typically contains 47–54 % of molybdenum with trace amounts of rhenium (450 g/t), selenium (90 g/t), and tellurium (15 g/t). The main stockwork ore zone of the Erdenet deposit has the surface area of about 2 km2 (Gerel and Munkhtsengel 2005).
Sampling and analytical procedure
Two sampling campaigns for the present study were performed in October 7 to 10, 2005 (corresponding to the start of cold dry season), and June 10 to 15, 2006 (corresponding to the early rainy season) (Davaa and Odgarav 2012). Twenty-nine and 27 representative water samples were collected during the first and second sampling campaigns, respectively. Before the first sampling, there was no rainfall at least for 20 days. Then, mining activities were stopped due to cold temperatures. On the other hand, there was a rainfall event during the second sampling.
The samples collected in October 2005 (the first sampling campaign) include surface waters of the Tuul River (N = 9), Orkhon River (N = 7), Khangal River (N = 9), Haraa River (N = 2), and a tributary of the Orkhon River (sample no. 25) (Fig. 1a). Groundwater used for domestic drinking in the Erdenet city was also collected (sample no. ERD). The samples of June 2006 (second sampling campaign) include the surface waters of the Tuul River (N = 9), Orkhon River (N = 7), Khangal River (N = 6), Haraa River (N = 2), Selenge River (N = 2), and a tributary of the Orkhon River (sample no. 25). Samples from some localities (seven in the Tuul River, six in the Orkhon River, six in the Khangal River, two in the Haraa River, and one of a tributary of the Orkhon River) were collected repeatedly to examine the temporal variation of water chemistry. All of the sampling and analyzing procedures undertaken in the present study followed the standard method (APHA et al. 1995).
At the time of sampling, unstable parameters such as pH, redox potential (Eh), temperature, electrical conductivity (EC), dissolved oxygen (DO), and turbidity were measured in situ by portable meters. EC (μS/cm) and water temperature (°C) were measured by a portable multi-parameter meter (Model Orion 130A). The collected samples were filtered by a 0.45-μm cellulose membrane filter to remove most of the suspended particles such as sediments and particulate organic matters. Water samples for the analyses of ions and dissolved organic carbon (DOC) were collected in 70-ml polyethylene bottles pre-washed with deionized water. The samples for cation analysis were further acidified to pH <2 by adding a few drops of ultrapure nitric acid. Before analysis, all collected samples were stored at 4 °C.
In the laboratory, the concentrations of major dissolved constituents, such as Na, K, Ca, Mg, Si, Cl, NO3, SO4, and F, were analyzed by the combination of ICP-AES (Perkin Elmer OPTIMA 3000 XL) and IC (Dionex 120) at the Center for Mineral Resources Research (CMR) of Korea University. The analysis of minor and trace elements, such as Fe, Li, Al, Cr, Mn, Cu, Zn, As, Se, Sr, Cd, Ba, Pb, and Hg, was also performed using ICP-MS (Perkin Elmer ELAN 3000). Careful quality controls were undertaken in order to obtain reliable datasets with a charge balance error below 5 %. The detection limits (μg/l) of the analysis were 2 for Al and Fe; 0.8 for Cu, Ba, Pb, and Hg; 1 for Zn, Mn, and Sr; 0.6 for Se, Cr, and As; 0.5 for Cd; 0.9 for Li; 0.2 for Ca and Na; 0.3 for K and Si; and 0.1 for Mg. Duplicate, blanks, and standards were used to obtain accurate and precise analytical data. The percentage relative standard deviation (RSD) values of duplicate or triplicate sample analyses were below 5 %. The concentration of DOC was analyzed with the total organic carbon (TOC) analyzer.
Some river water samples (n = 7) collected in June 2006 from the Tuul and Khangal rivers were analyzed for nitrogen isotope compositions of nitrate to evaluate the origin. In addition, a total of 15 river water samples collected in June 2006 were prepared for the sulfur isotope analysis of dissolved sulfate. The isotopic analysis was conducted in the Isotope Science Laboratory of University of Calgary using the elemental analyzer continuous flow isotope ratio mass spectrometry. Stable isotope data of samples are reported in the δ notation (‰) with respect to air N2 (AIR) and the Canyon Diablo Troilite (V-CDT).
Results and discussion
Basic statistics of the physicochemical data of collected water samples from investigated rivers in the study area are summarized in Tables 1 and 2 (see also the Supplementary Information (SI) for further details regarding analytical results).
pH, dissolved organic carbon, and turbidity
In general, the chemistry of surface water depends on several factors, including (1) the relative contribution of surface runoff and groundwater (i.e., base flow); (2) the mixing of water of different quality from tributaries; (3) biogeochemical reactions within the river system; and (4) inputs of anthropogenic contaminants (Berner and Berner 1987; Meybeck et al. 1989). The pH values of river waters in the study area are slightly alkaline (7.2–9.0, average 8.1; Table 1). In semi-arid climate zones, such as the one of the study area, surface water may exhibit relatively high pH values, since streams are much fed by groundwater base flow for much of the year (Meybeck et al. 1989; Pietroń et al. 2015). A moderately high pH of surface water in the study area can be explained by several processes that include the following (Appelo and Postma 1996): (1) dissolution of carbonate minerals in rocks and sediments/soils of watersheds and (2) algal activities (especially, photosynthetic assimilation). Bicarbonate is predominant (average 2.5 mmol/l) among the dissolved anions in the collected river waters and is much higher than dissolved silica (average 0.1 mmol/l). This suggests that weathering of carbonate minerals, rather than weathering of silicate minerals, as proposed by Kelderman and Batima (2006), is likely to be the major control of river water chemistry overall in the study area. The pH of river waters in the study area also tends to slightly increase in June (Fig. 2a), possibly due to the contribution of groundwater (i.e., base flow with higher pH) to the stream flow until the start of the rainy period (Pietroń et al. 2015). However, algal blooming was not considerable enough to potentially increase the pH during the two sampling campaigns.
The concentrations of DOC vary with the time and locality (rivers) of sampling. Overall, the relationship between pH and DOC is not distinct, but rather shows a tendency of DOC to increase in June with warm temperatures (Fig. 2b). This may indicate that, while the decay of organic matter can be enhanced within streams to form organic acids under higher temperatures, it is not sufficient to markedly decrease the pH of river water. In October 2005, the DOC values were generally below 3 mg/l, except for Khangal river waters (2.9 to 11.3 mg/l). In June 2006 with active placer mining, the DOC values in the Tuul River characteristically increased up to 4.7–13.4 mg/l (Table 1). Such substantial increase of DOC in the Khangal and Tuul rivers is certainly due to a larger influx of sewage effluents from heavily populated mining towns.
Turbidity of surface water bodies is caused by suspended and colloidal matter, such as clay, silt, and finely divided organic and inorganic matter, as well as plankton and other microscopic organisms (APHA et al. 1995). In particular, suspended sediment loads are largely mobilized during the high flow periods in small and intermittent streams and may constitute an important water quality problem in relation to algal primary productivity and gill clogging of aquatic organisms (Kirk 1994; Vondracek et al. 2003). Sediments also facilitate the transport of aquatic pollutants, including heavy metals (Hatje et al. 2001; Dalai and Ishiga 2013; Pietroń et al. 2015; Thorslund et al. 2016) and organic compounds, such as polychlorinated biphenyls (PCBs), dioxins, and pesticides (Walters et al. 1989; Götz et al. 1994; Borah et al. 2003). Turbidity of river waters in the study area was generally very high and variable, ranging from 2.1 to 742 NTU (Table 1). The high turbidity in the study area is induced by the influx of soil/sediment particles into rivers that emerges due to the erosion of river banks preferentially in meandering streams, the overgrazing by animals, and human activities such as mining, cultivation, and land exploitation (Hartwig et al. 2012; Pietroń et al. 2015; Priess et al. 2015) (see Fig. S1 in SI).
In particular, very high turbidity values (>500 NTU) were observed especially in the Tuul River near the Zaamar gold mining area during June 2006 when placer mining was active with the increased surface water availability due to proceeding rainfalls. The effect of turbidity problems predominantly arising from the Zaamar area was recognized even at the downstream site of the Orkhon River (sample site no. 31; 104 NTU). By contrast, turbidity of the Tuul River significantly decreased (<60 NTU) during October 2005 when the mining was being closed due to cold weather (<10 °C). Considering that the Zaamar mining area is situated in a semi-arid region with an average precipitation of only 260 mm/year, mining operations heavily rely on river water. To separate gold grains from alluvial sediments near the river, both the mechanical separation with a bucket-line dredge and the washing with pressurized water spraying are performed. During the dredging and washing with water, clay- and silt-sized particles of alluvial sediments are suspended to produce a very high turbidity of brownish-colored water. These turbid waters are retained in settling and filtration dams and are reused for mining. Due to the negative impacts on drinking water quality, the direct release of turbid waters generated from mining is not prohibited; thus, the turbidity of water is periodically monitored at several localities above and below the Zaamar mining district. Even so, high turbidity of the Tuul River is frequently observed during summer months with active mining; the turbidity problem becomes less severe during the winter season with the closure of mining (Chalov et al. 2015; Pietroń et al. 2015). Chalov et al. (2015) also pointed out that the turbidity of river waters of the Selenge river basin is largely arising from suspended sediments from mining activities, since the contents of particulate organic matter in the rivers are relatively low (1 to 16 %). In addition, the riparian zone along the bank of the Tuul River is also destroyed by placer gold mining, which may affect the aquatic and terrestrial wildlife and may lead to flooding during the rainy season.
General hydrochemistry
The concentrations of total dissolved solids (TDS) in river waters range widely from 46 to 1446 mg/l and show no distinct change with the sampling period (Table 1). In the Tuul River, low TDS values (46 to 133 mg/l) were typically observed at upstream sites (sample nos. 29, 30, 34, 35) near Ulaanbaatar, while the TDS values substantially increased to 149–196 mg/l at downstream sites (sample nos. 3, 5, 7, 10-1, 12) near the Zaamar mining area. The Khangal river waters were very high in TDS (666–1446 mg/l), exceeding the EPA drinking water standard (500 mg/l). This trend is certainly due to the effect of mine drainage and partly sewage. At the sample point no. 20 of the Orkhon River after the confluence with the Tuul River (see Fig. 1a for locality), the increase of the TDS values up to 280 and 296 mg/l is certainly due to the influence of the TDS-rich Khangal River.
On the Piper diagram (Piper 1944), chemical compositions of the collected water samples are graphically shown (Fig. 3). The cation composition is dominated by Ca and does not significantly vary as a function of sampling time or locality (rivers), whereas the anion composition is overall dominated by bicarbonate. The anion composition is clearly different for the sulfate-rich Khangal and, locally, Tuul river waters, whereas the sulfate levels for the other river waters (mostly <25 mg/l) are similar to the worldwide average (11.2 mg/l; Berner and Berner 1987). The Khangal river waters mostly exceed the EPA drinking water standard of sulfate (250 mg/l) (see Table S1 in SI). These observations at Khangal reflect the effect of different land uses, especially of mining activity which accompanies the inflow of both mine drainage and sewage from populated mining areas. For the Khangal River, anion composition tends to gradually change from the sulfate dominance at upstream sites to the bicarbonate-sulfate dominance toward the downstream sites near the river’s confluence with the Orkhon River. Such spatial pattern of the sulfate-rich Khangal river water is certainly due to the oxidation of sulfides in huge mine tailings of the Erdenet Cu-Mo mine. Surface waters from downstream sites of the Tuul River near the Zaamar mining area are also dominated by bicarbonate among anions; however, the proportions of nitrate, chloride, and sulfate are clearly higher than those for other rivers, such as Orkhon, Selenge, and Haraa, especially during June 2006 (Table S1 in SI). These findings suggest a larger inflow of anthropogenic contaminants (esp., sulfate and chloride) into the Tuul River, especially near the Zaamar mining area.
Piper plots (Piper 1944) of the chemical composition of surface water samples collected in a October 2005 and b June 2006
Spatio-temporal changes of hydrochemical data
The temporal hydrochemical change of river waters was examined by comparing the data between October 2005 and June 2006 (see Fig. S2 in SI). In the samples of June 2006, anthropogenic constituents, such as chloride, nitrate, and sulfate, tended to increase. Together with the higher turbidity and TDS, these observations indicate the larger impacts of anthropogenic contaminants on river water during June, due to a larger inflow of anthropogenic effluents (especially, sewage) and mine drainage. During summer months, temperatures and rainfall amount are high. Therefore, human activities, such as mining and cultivation, become more active during summer, resulting in a more pronounced deterioration of river water quality in the study area.
The spatial variation of water chemistry was also examined according to the investigated sites (rivers) (Fig. 4). Among the studied rivers, Khangal river waters are most saline (i.e., highest TDS) with highest concentrations of all major dissolved ions; in particular, the concentrations of sulfate are very high (up to 770 mg/l). Surface waters of the Tuul River are most turbid and are also enriched in nitrate, chloride, and sulfate (Fig. 4). As described above, the highly saline nature of the Khangal river water stems from the influences from mine drainage and partly sewage from the Erdenet Cu-Mo mine area. The Tuul River drains mainly through the semi-arid climate zone with little vegetation and rainfall and through the Zaamar gold mining area with its dense population. Therefore, higher extents of sediment/soil loss are expected to result in a high turbidity in the Tuul river watershed, due to placer gold mining (Pietroń et al. 2015). In addition, sewage effluents cause high concentrations of nitrate and chloride. On top of that, lower degrees of chemical weathering resulting from the interplay of the characteristic watershed geology (i.e., predominance of sedimentary rocks with lower degrees of chemical weathering) and little vegetation are likely to result in the lowest concentrations of bicarbonate (<96 mg/l) in the Tuul river water (Fig. 4).
Nitrate-N and sulfate-S isotopes
To further examine the origin of sulfate and nitrate in river waters, δ15N values of nitrate and δ34S values of sulfate were determined for selected samples (Table 3). Except for the Khangal River, the overall nitrate levels in rivers of northern Mongolia are relatively low (mostly <5 mg/l; Table 1). The δ15N values of nitrate in water samples (N = 7) from the Tuul and Khangal rivers range from 4.8 to 10.8 ‰. Except for sample no. 1 (δ15Nnitrate = 4.8 ‰), all other samples have the nitrogen isotopic values above 8.7 ‰. This indicates that nitrate largely originates from sewage and manure in the populated areas, such as Zaamar and Erdenet (Kendall 1998). In particular, higher levels of nitrate (up to 26.7 mg/l) in the whole range of the Khangal River undoubtedly originate from sewage and manure from the Erdenet mine area (see Fig. S3 in SI).
The δ34S values of sulfate for 15 river water samples range from 0.2 to 6.9 ‰ (Table 3). The range excludes the origin of sulfate from evaporate in rocks, while potential sources are sulfate from atmospheric deposition, soil, and oxidation of reduced sulfur minerals, such as pyrite (Krouse and Mayer 2000; Fitzhugh et al. 2001). It is noteworthy that sulfate from the Khangal river waters is clearly lower (0.2 to 3.8 ‰, mostly 0.2 to 1.7 ‰) than the corresponding values from other rivers (Table 3). The values for the Khangal river waters cohere well with the reported δ34S values of ores from the Erdenet Cu-Mo mine (−3.3 to 2.7 ‰, average 0.4 ‰; Tugarinov et al. 1974). Therefore, it is obvious that the sulfate-rich nature of the Khangal river waters stems from oxidation of pyrite in porphyry Cu-Mo ores. The additional determination of oxygen isotope composition of sulfate will be needed for a better interpretation of sulfate origin in central northern Mongolian rivers.
Water-rock interaction
Among the investigated rivers, the water chemistry of the Khangal River is particularly significantly controlled by the inflow of mine drainage, which possibly enhances water-rock interaction to result in the enrichments of various solutes, such as Na, K, Ca, Mg, SO4, HCO3, and dissolved silica (Table 1). To evaluate the characteristics of water-rock interaction controlling river water chemistry in central northern Mongolia, we examined the ionic compositions used for source rock deduction (Fig. 5).
The plots on the Na versus Cl diagram (Fig. 5a) mostly fall near the 1:1 line, except for the Khangal river waters with their higher Cl concentrations. However, a careful examination shows that sodium concentrations are slightly above the 1:1 line. This suggests the presence of another source of Na (possibly, plagioclase weathering) in addition to NaCl in sewage. As discussed above, the excessive Na in the Khangal river waters is likely to be due to the enhanced dissolution of plagioclase by mine drainage (Chae et al. 2004).
Except for the Khangal river waters, plots falling near or along the 1:1 line on the [Ca + Mg] versus [HCO3 + CO3] diagram (Fig. 5b) indicate that dissolution of carbonate minerals is an important source of alkali earths and alkalinity in water. The excessive concentrations of Ca + Mg, as compared to the equivalent amounts of SO4 (Fig. 5c), indicate that sulfate minerals cannot be a potential source of sulfate. Considering pyrite dissolution as suggested by sulfur isotope data, remarkably high concentrations of Ca + Mg in the Khangal river waters are the result of a substantial dissolution of carbonate minerals.
In other to further investigate the subsequent occurrence of pyrite and plagioclase weathering, the plots on the [Na − Cl] versus [HCO3 + CO3 + SO4] − [Ca + Mg] diagram were examined (Fig. 5d). The concentrations of “excess” Na were calculated by subtracting the equivalent Cl concentrations; the values are remarkably high for the Khangal river waters, likely indicating the enhanced weathering of plagioclase due to pyrite oxidation. Since Ca and Mg can be assumed to be originated from dissolution of carbonates such as calcite and dolomite, the values of the y-axis in Fig. 5d may account for the concentrations of major anions that were originated solely from plagioclase weathering. As expected, most water samples from the Khangal River plot along and near the 1:1 line. Therefore, it is likely that excess Na in the Khangal river waters is mainly originated from plagioclase weathering that buffers sulfuric acid from oxidation of pyrite in mining areas. In summary, dissolution of carbonate minerals together with pyrite and plagioclase acts to primarily regulate the natural chemistry of the rivers in northern Mongolia (Kelderman and Batima 2006). Such enhanced dissolution of silicate and carbonate minerals is likely the major sources of trace harmful elements in river water.
Contamination by trace elements
The Erdenet Cu-Mo ores contain primary ore minerals, such as pyrite (FeS2), chalcopyrite (CuFeS2), molybdenite (MoS2), tennantite (Cu 12 As 4 S 13), galena (PbS), and sphalerite (ZnS) with supergene ore minerals, including bornite (Cu 5 FeS4), chalcocite (Cu2S), covellite (CuS), and elemental copper (Munkhtsengel et al. 2006). Primary vein-type ores in the Zaamar gold mining area also contain ore minerals, including pyrite, sphalerite, and galena. Therefore, in surface waters influenced by mine drainage, oxidation dissolution of ore minerals in mine wastes and/or ores may release hazardous trace elements, such as Cu, Pb, Zn, As, and Fe, which are important micronutrients and/or toxic elements (if present in high concentrations) in aquatic systems.
The concentration ranges of some trace elements in the river waters of the study area are 5.3–997 μg/l for Fe, 5.4–728 μg/l for Al, 1.2–570 μg/l for Mn, 0.5–252 μg/l for Cu, 1.5–24 μg/l for Zn, 0.2–14 μg/l for As, and <0.1–6.2 μg/l for Cd (Table 2). The concentrations of Pb and Hg are very low: <1.2 μg/l for Pb and “not detected” (< 0.1 μg/l) for Hg. Compared to the WHO drinking water quality guidelines, the observed levels of the examined trace elements are generally low. However, the concentrations of dissolved Al, Mn, and Fe exceed the standards in a few samples: in particular, Al (>50–200 μg/l) in the Tuul river waters and several Orkhon, Khangal, and Selenge river water samples; Mn (>50 μg/l) in many Khangal and Tuul river water samples; and Fe (>300 μg/l) in several Tuul river water samples (Table S1 in SI). The high levels of Al, Fe, and Mn in the Tuul river water samples were typically observed in June 2006, which was certainly due to the dissolution of sediment particles (i.e., silicates) supplied by placer mining into the river. In June 2006, a significant Al contamination from the Zaamar gold mining area was observed even after the confluence with the Orkhon River (sample nos. 21 and 22; see Fig. 1a for localities). Similarly, enrichments of Mn exceeding the drinking water standard are distinct in the upstream parts of the Khangal River in both sampling periods, but are diminished in the downstream parts. This clearly indicates the influence of mine drainage from the Erdenet Cu-Mo mine.
High levels of heavy metals in exceedance of the WHO drinking water quality guidelines are observed in several Khangal river water samples (Table 2): Cd up to 6.2 μg/l (>3 μg/l), As up to 14.1 μg/l (>10 μg/l), and Mn up to 570.2 μg/l (>400 μg/l). High levels of Cu (up to 252 μg/l), Zn (up to 23.9 μg/l), and Se (up to 4.9 μg/l) are also observed in the Khangal river water samples. By contrast, enrichments of heavy metals are not distinct in other studied rivers.
However, the levels of Cu, Zn, and As tend to markedly increase in the Tuul river water samples during June 2006 (Table S1 in SI). Nadmitov et al. (2015) studied the concentrations of trace metals (Mn, Fe, Ni, Cr, Cu, Pb, Cd, As, and Zn) in surface water samples collected in 2007–2009 from the Selenge river basin; the reported levels of investigated metals are generally similar to our data. The authors suggested that possible harmful effects on aquatic ecosystems by Zn, Cu, Fe, and As should be carefully monitored in the Selenge river basin. Thorslund et al. (2016) studied the speciation and transport of metals (Al, As, Cd, Cr, Cu, Fe, Mn, Mo, Pb, V, and Zn) in surface water samples collected in 2012–2013 from the Tuul River; the authors found that (1) the total (unfiltered) concentrations of several metals such as Al, Fe, Mn, As, Cd, Cr, Cu, and Pb remarkably increase across the Zaamar gold mining area; (2) the differences between the particulate and dissolved concentrations are greatest for Al, Fe, and Mn; and (3) among toxic metals, arsenic shows the total concentrations exceeding the WHO standard (10 μg/l). Pfeiffer et al. (2015) also reported local enrichments of arsenic (up to 2820 μg/l) in river waters of northern Mongolia, which are arising from mining activities.
The calculations of Pearson correlation coefficients show the significant correlations between turbidity and immobile elements such as Al and Fe (Table 4). This result indicates that dissolved Al and Fe in river waters are largely originated from turbidity materials (i.e., suspended and/or colloidal particles) that are supplied by erosional processes preferentially during the season of high flow and active mining activity.
It is also noticeable that, except for a few elements, such as Al, Mn, Fe, Cd, and As, the observed levels of most trace elements in this study are not particularly high. This is possibly due to the sorption of trace elements onto suspended particles, such as metal oxides, clay minerals, and organic matter (Battogtokh et al. 2014; Thorslund et al. 2016). The study on trace element concentrations of bottom sediments from the Khangal River (Baljinnyam et al. 2014) showed significant enrichments of Fe (up to 27,100 mg/kg), Mn (up to 900 mg/kg), Cu (up 1630 mg/kg), Zn (up to 52.8 mg/kg), As (up to 19.1 mg/kg), and Se (up to 1.2 mg/kg). Thus, a further study of the partitioning of trace metals between river waters and sediments is needed.
Transboundary movements of aquatic contaminants: potential impacts on Lake Baikal
A careful monitoring on the spatio-temporal variation of aquatic substances, such as dissolved ions and suspended particles in the Orkhon River, is essential to evaluate the transboundary movement of aquatic contaminants from Mongolia to Lake Baikal. Figure 6 depicts the spatial change of some physicochemical parameters of river water as a function of the upstream distance from the confluence (sample site no. 32; see Fig. 1a for locality) between the Orkhon and Selenge rivers. Locations of the confluence between the studied rivers and the Orkhon River are also shown. The turbidity and DOC of the Orkhon River rapidly increase after the mixing with the Tuul River, and the increased levels are maintained or further increase down to the confluence with the Selenge River (Fig. 6a). As discussed above, this observation is the result of the influx of suspended particles and untreated sewage, especially from the Zaamar gold mining area. The remarkable increase of trace elements is not generally observed. However, substantial increases of Fe and Al are remarkable (Fig. 6b). Therefore, the results of this study suggest that the input of suspended particles, sewage water, Fe, and Al, originating especially from mining areas, should be carefully monitored for managing the transboundary movement of contaminants finally to Lake Baikal.
Conclusion
The major results and implications of this study are summarized as follows:
-
1.
The hydrochemistry of surface waters in the study area is characterized by Ca-HCO3 to Ca-HCO3-SO4 types with slightly alkaline pH (7.2 to 9.1) and variable TDS values (46–1446 mg/l). The natural (pristine) water chemistry is observed at upstream sites of the Tuul River and is characterized by low TDS (<133 mg/l). The natural water chemistry is mainly controlled by the dissolution of carbonate minerals in rocks and can be effectively used as a geochemical baseline for managing surface water quality in the study area.
-
2.
Anthropogenic contamination of river water in the study area is largely the result of mining and is discernible by the increases of turbidity, DOC, TDS, some anions (SO4, NO3, Cl), and some trace elements (such as Al, Fe, Mn, Cd, As, Cu, Zn, and Se).
-
3.
The very high turbidity (up to 742 NTU) is the most serious water quality problem, due to the influx of sediment and soil particles, especially resulting from mining and land devastation. The turbidity problem is typically observed in the Tuul River near the Zaamar placer gold mining area. The increase of DOC is arising from sewage effluents from the populated mining areas (Zaamar and Erdenet). The TDS is locally exceeding the drinking water standard (500 mg/l), especially in Khangal River under the influence from saline mine drainage that originates from a huge tailing dam with mine wastes from the Erdenet porphyry Cu-Mo mine.
-
4.
The Khangal river waters and, locally, Tuul river waters are highly enriched in sulfate, locally exceeding the drinking water standard (250 mg/l). The sulfate-rich nature results from oxidation of pyrite in wastes and ores, as indicated by the sulfur isotope values of dissolved sulfate (0.2 to 3.8 ‰) that cohere well with the reported δ34S values of ore sulfur. The enrichments of nitrate and chloride are also typical of anthropogenic contamination, especially by the influx of sewage effluents from mining areas.
-
5.
The substantial increase of turbidity, TDS, chloride, nitrate, sulfate, and some trace elements is typically observed in the samples from June 2006 with active mining, as well as in the samples collected in the Tuul and Khangal rivers. High levels of trace elements (especially, Al, Mn, and Fe) are typically observed at the downstream sites of the Tuul River near the Zaamar mining area, occasionally exceeding drinking water standards. This is likely due to the dissolution of sediment particles supplied by placer mining. The Khangal river water is also very high in Mn, and some heavy metals, such as Cd (up to 6.2 μg/l) and As (up to 14.1 μg/l), are observed. The enrichments of Cu, Zn, and Se are also found in the Khangal river waters. However, except for Al, Mn, Fe, Cd, and As, the observed levels of most trace elements are not significantly higher in the studied rivers and tend to be restricted in each water course. This trend is likely to be mainly due to the sorption of metals onto particulate matters before mixing with the Orkhon River.
-
6.
Furthermore, several contaminants, such as turbidity, DOC, Fe, and Al, influence the river water quality down to the confluence between the Orkhon and Selenge rivers, potentially affecting the water quality of Lake Baikal. These transboundary contaminants originate from suspended particles and sewage effluents from the Zaamar gold mining area. Therefore, careful and continuous monitoring of the water quality, especially in the Tuul River, is crucial for managing the transboundary movement of aquatic contaminants. The hydrochemical data of this study will be effectively used as baselines to interpret water quality monitoring data.
References
APHA, AWWA, WEF (1995) Standard methods for the examination of water and wastewater, 19th edn. APHA Publications, Maryland
Appelo CAJ, Postma D (1996) Geochemistry, groundwater and pollution, 3rd edn. Balkema, Rotterdam/Brookfield
Baljinnyam N, Frontasyeva MV, Aleksiayenak YV (2014) INAA for determination of trace elements in bottom sediments of the Selenga river basin in Mongolia. Phys Particles and Nuclei Lett 11:199–208. doi:10.1134/S1547477114020149
Batbayar B, Karthe D, Pfeiffer M, von Tűmpling W, Kappas M (2015) Influence of urban settlement and mining activities on surface water quality in northern Mongolia, Water and environment in the Selenga-Baikal basin. International Research Cooperation for an Ecoregion of Global Relevance. ISSN 1614-4716
Battogtokh B, Lee JM, Woo N (2014) Contamination of water and soil by the Erdenet copper-molybdenum mine in Mongolia. Environ Earth Sci 71:3363–3374
Berner EK, Berner RA (1987) The global water cycle, geochemistry and environment. Prentice-Hall, Upper Saddle River 397 pp
Borah DK, Bera M, Shaw S (2003) Water, sediment, nutrient, and pesticide measurements in an agricultural watershed in Illinois during storm events. Trans ASAE 46:657–674
Calder IR (1992) Hydrologic effects of land-use change. In: Maidment DR (ed) Handbook of hydrology. McGraw-Hill, USA
Chae GT, Yun ST, Kim KH, Lee PK, Choi BY (2004) Atmospheric versus lithogenic contribution to the composition of first- and second-order stream waters in Seoul and its vicinity. Environ Int 30:73–85
Chalov SR (2014) Effects of placer mining on suspended sediment budget: case study of north of Russia’s Kamchatka Peninsula. Hydrol Sci J 59:1–14
Chalov SR, Jarsjo J, Kasimov NS, Romanchenko AO, Pietroń J, Thorslund J, Promakhova EV (2015) Spatio-temporal variation of sediment transport in the Selenga River Basin, Mongolia and Russia. Environ Earth Sci 73:663–680
Choi J, Badarch M, Lee J, Lee YJ, Badarch EO (2004) Project report on joint pilot studies between Korea and Mongolia on assessment of environmental management system in gold mining industry of Mongolia (I). Korea Environment Institute, Seoul 107 pp
Dalai B, Ishiga H (2013) Geochemical evaluation of present-day Tuul River sediments, Ulaanbaatar basin, Mongolia. Environ Monit Assess 185:2869–2881
Davaa G, Odgarav J (2012) Tuul River. In: Chikamori H, Liu H, Daniell T (eds), Catalogue of rivers for Southeast Asia and the Pacific, Vol. VI UNESCO-IHP Regional Steering Southeast Asia and the Pacific Committee for Southeast Asia and the Pacific
Falkner KK, Church M, Measures CI, LeBaron G, Thouron D, Jeandel C, Stordal MC, Grill GA, Mortlock R, Froelich P, Chan LH (1997) Minor and trace element chemistry of lake Baikal, its tributaries, and surrounding hot springs. Limnol Oceanogr 42:329–345
Fitzhugh RD, Furman T, Korsak AK (2001) Sources of stream sulphate in head-water catchments in Otter Creek Wilderness, West Virginia, USA. Hydrol Process 15:541–556
Gerel O, Munkhtsengel B (2005) Erdenetiin Ovoo porphyry copper-molybdenum deposit in northern Mongolia. In: Porter TM (ed) Super porphyry copper and gold deposits: a global perspective, vol 2. PGC Publishing, Adelaide, pp. 525–554
Götz R, Enge P, Friesel K, Roch K, Kjeller LO, Kulp SE, Rappe C (1994) Sampling and analysis of waters and suspended particulate matter of the River Elbe for polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs). Chemosphere 28:63–74
Granina LZ, Callender E, Grachev AM, Grachev MA (1998) Input of particulate elements with riverine waters into Lake Baikal and their role in chemical balance (Ti, Cr, Sr, Cu, Zn, Pb, Br). Doklaly RAN 362:391–395 (in Russian)
Hartwig M, Theuring P, Rode M, Borchardt D (2012) Suspended sediments in the Kharaa River catchment (Mongolia) and its impact on hyporheic zone functions. Environ Earth Sci 65:1535–1546
Hatje V, Rae K, Birch GF (2001) Trace metal and total suspended solids concentration I freshwater: the importance of small-scale temporal variability. J Environ Monit 3:251–256
Hofmann J, Venohr M, Behrendt H, Opitz D (2010) Integrated water resources management in central Asia: nutrient and heavy metal emissions and their relevance for the Kharaa River Basin, Mongolia. Water Sci Technol 62:353–363
IMF (International Monetary Fund) (2015) IMF country report no. 15/109 (2015 article IV consultation staff report, press release, and statement by the executive director for Mongolia). IMF, Washington DC
JAICA (Japan International Cooperation Agency) (2003) Project report “action research on mercury pollution in Boroo area, Mongolia”. JAICA, Japan 37 pp
JAICA (Japan International Cooperation Agency) (2006) Project report “the river basin management model project for the conservation of wetland and ecosystem and its sustainable use in Mongolia, pre-evaluation survey report”. JAICA, Japan 21 pp
Karpoff BS, Roscoe WE (2005) Report on placer gold properties in the Tuul Valley, Zaamar Goldfield, Mongolia. Roscoe Postle Associates Inc, Canada 67 pp
KEI (Korea Environment Institute) (2006) Project report on “joint research between Korea and Mongolia on water quality and contamination of transboundary watershed in northern Mongolia”. KEI, Seoul 61 pp
KEI (Korea Environment Institute) (2008) Project report on “integrated water management model on the Selenge River basin status survey and investigation (phase I)”. KEI, Seoul 419 pp
Kelderman P, Batima P (2006) Water quality assessment of rivers in Mongolia. Water Sci Technol 53:111–119
Kendall C (1998) Tracing nitrogen sources and cycling in catchments. In: Kendall C, McDonnell JJ (eds) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp. 521–576
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems, 2nd edn. Cambridge University Press, NY
Krouse HR, Mayer B (2000) Sulphur and oxygen isotopes in sulphate. In: Cook PG, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer Academic, Dordrecht, pp. 195–231
Li SG, Asanuma J, Kotani A, Davaa G, Oyunbaatar D (2007) Evapotranspiration from a Mongolian steppe under grazing and its environmental constraints. J Hydrol 333:133–143
Luis AT, Teixeira P, Almeida SFP, Matos JX, Silva EF (2011) Environmental impact of mining activities in the Lousal area (Portugal): chemical and diatom characterization of metal contaminated stream sediments and surface water of Corona stream. Sci Tot Environ 409:4312–4325
Meybeck M, Chapman D, Helmer R (1989) Global freshwater quality: a first assessment. Blackwell, Oxford 307 pp
Munkhtsengel B, Ohara M, Gerel O, Dandar S, Tsuchiya N (2006) Preliminary study of formation mechanism of the Erdenetiin Ovoo porphyry copper-molybdenum deposit and environmental effects of Erdenet mine, northern Mongolia. Proceedings of CP833, Water Dynamics: 3rd International Workshop on Water Dynamics, pp 204–207
Nadmitov B, Hong S, Kang SI, Chu JM, Gomboev B, Janchivdorj L, Lee CH, Khim JS (2015) Large-scale monitoring and assessment of metal contamination in surface water of the Selenga River Basin (2007–2009). Environ Sci Pollut Res 22:2856–2867
Pfeiffer M, Batbayar G, Hofmann J, Siegfried K, Karthe D, Hahn-Tomer S (2015) Investigating arsenic (As) occurrence and sources in ground, surface, waste and drinking water in northern Mongolia. Environ Earth Sci 73:649–662
Pietroń J, Jarsjo J, Romanchenko AO, Chalov SR (2015) Model analyses of the contribution of in-channel processes to sediment concentration hysteresis loops. J Hydrol 527:576–589
Piper AM (1944) A graphical procedure in the geochemical interpretation of water-analyses. Am Geophys Union Trans 25:914–923
Priess JA, Schweitzer C, Batkhishig O, Koschitzki T, Wurbs D (2015) Impacts of agricultural land-use dynamics on erosion risks and options for land and water management in Northern Mongolia. Environ Earth Sci 73:697–708
Semenova BA, Myagmarjav B (1977) Hydrological regime of the Selenge river basin, Leningrad
Shinkareva GL, Kasimov NS, Lychagin MY (2015) Heavy metal fluxes in the rivers of the Selenga basin, water and environment in the Selenga-Baikal basin. International Research Cooperation for an Ecoregion of Global Relevance. ISSN 1614-4716
Stubblefield A, Chandra S, Eagan S, Tuvshinjargal D, Davaadorzh G, Gilroy D, Sampson J, Thorne J, Allen B, Hogan Z (2005) Impacts of gold mining and land use alterations on the water quality of central Mongolian rivers. Integr Environ Assess Manag 1:365–373
Takahashi Y (2004) Introduction to geology of Mongolia based upon GIS. Geological Society of Japan (GSJ) Open File, no. 413
Tarras-Wahlberg NH (2002) Environmental management of small-scale and artisanal mining: the Portovelo-Zruma gold mining area, southern Ecuador. J Environ Manag 65:165–179
Thorslund J, Jarsjo J, Chalov SR, Belozerova EV (2012) Assessment of the gold mining impact on riverine heavy metal transport in a sparsely monitored region: the upper Lake Baikal Basin case. J Environ Monit 14:2780–2792
Thorslund J, Jarsjő J, Wällstedt T, Mőrth CM, Lychagin MY, Chalov SR (2016) Speciation and hydrological transport of metals in non-acidic river systems of the Lake Baikal basin: field data and model predictions. Reg Environ Change (in press). doi:10.1007/s10113-016-0982-7
Tomurtogoo O, Byamba J, Badarch G, Minjin C, Orolmaa D, Khosbayar P, Chuluun D (1998) Geologic map of Mongolia, scale 1:1, 000, 000 and summary. Mineral Resources Authority of Mongolia, Ulaanbaatar
Tugarinov AI, Voinkov DM, Grinenko LN, Pavlenko AS (1974) Isotopic composition and sources of molybdenum-copper mineralization of Mongolia. Geochemistry 2:173–178 (in Russian)
USAWorld Bank (2006) The Mongolia: a review of environmental and social impacts in the mining sector. http://www.worldbank.org/eapenvironment
Vondracek B, Zimmerman JKH, Westra JV (2003) Setting an effective TMDL: sediment loading and effects of suspended sediment on fish. J Am Water Resour Assoc 39:1005–1015
Walters RW, Ostazeski SA, Guiseppi-Elie A (1989) Sorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin from water by surface soils. Environ Sci Technol 23:480–484
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press, Cambridge
Ziadat AH, Jiries A, Berdanier B, Batarseh M (2015) Bio-monitoring of heavy metals in the vicinity of copper mining site at Erdenet, Mongolia. J Appl Sci 15(11):1297–1304
Acknowledgments
This work was initially supported by Korea Environment Institute (KEI) as the Korea-Mongolia collaboration project “Joint Research Between Korea and Mongolia on Water Quality and Contamination of Transboundary Watershed in Northern Mongolia.” Mr. Ochirsukh Ayur (Mongolian Nature and Environment Consortium) helped us in field surveys. The preparation of this manuscript was supported by KEITI through the Korea CO2 Storage Environmental Management (K-COSEM) Research Center.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Stuart Simpson
Electronic supplementary material
ESM 1
(DOC 1079 kb)
Rights and permissions
About this article
Cite this article
Batsaikhan, B., Kwon, JS., Kim, KH. et al. Hydrochemical evaluation of the influences of mining activities on river water chemistry in central northern Mongolia. Environ Sci Pollut Res 24, 2019–2034 (2017). https://doi.org/10.1007/s11356-016-7895-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7895-3