Abstract
The accumulation of hydrophobic compounds by phytoplankton plays a crucial role in the biogeochemical cycle of persistent organic pollutants (POPs) in aquatic environments. We studied the accumulation of polycyclic aromatic hydrocarbons (PAHs) in the freshwater diatom Synedra acus subsp. radians during its cultivation with crude oil hydrocarbons, using epifluorescent and laser confocal microscopy as well as gas chromatography–mass spectrometry (GC/MS) analysis. Our results revealed that in the presence of crude oil or an extract of a crude oil/n-hexane solution (light oil), S. acus subsp. radians accumulated PAHs in its lipid bodies. During cultivation in the presence of a crude oil/n-hexane solution, the cells selectively accumulated C12–C18 alkanes, with a preference for C15 and C16 homologues. The length of n-alkane hydrocarbon chains accumulated in cells was similar to the acyl chains of fatty acids of the diatom. We therefore suggest that the insertion of n-alkanes into the membrane lipid bilayer promotes the transmembrane transport of PAH in diatoms. Our results confirm the hypothesis that diatoms play a role in the elimination of hydrophobic hydrocarbons from aquatic systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplanktons play a key role in the accumulation of hydrocarbons, including polycyclic aromatic hydrocarbons (PAHs), entering aquatic ecosystems (Lei et al. 2007; Hong et al. 2008; Nizzetto et al. 2012). By accumulation and transformation of hydrophobic hydrocarbons, phytoplankton participates in the transfer of these substances to higher trophic levels and the involvement into the carbon cycle, which may have harmful effects on the higher consumers in the food web (Kingston 2002; Soto et al. 2014) and generally impact ecosystem functioning.
To determine the pathway of hydrocarbons in the aquatic system, an evaluation of the bioconcentration factors (BCFs) for these compounds is crucial. The bioconcentration level depends on the physical and chemical properties of hydrophobic compounds (Swackhamer and Skoglund 1993; Gerofke et al. 2005; Ko et al. 2012), cell surface hydrophobicity (Katagi 2010), and the qualitative and quantitative lipid content of the cells (Berglund et al. 2001).
Mechanisms of hydrocarbon accumulation and biodegradation were mainly studied using prokaryotic organisms (Watkinson and Morgan 1990) with biosurfactant activity (Beal and Betts 2000; Bouchez-Naïtali and Vandecasteele 2008). Based on kinetic analyses, active and passive transmembrane [C14]phenanthrene transport has been found in the bacterium Anthrobacter sp. (Kallimanis et al. 2007). A temperature-independent benzo(a)pyrene transport has been shown in the filamentous fungus Fusarium solari (Verdin et al. 2005). Epifluorescence microscopic studies have revealed passive diffusion of benzo(а)pyrene into the lipid bodies of F. solari cells. The authors proposed that the mechanism of passive benzo(а)pyrene uptake can be explained by the properties of destabilization of the lipid mosaic by lipophilic molecules. Uptake and accumulation of phenanthrene in lipid vesicles using two-photon excitation microscopy (TPEM) were demonstrated in the filamentous fungus Pythium ultimum (Furuno et al. 2012). The location and the aggregation of benzo(a)pyrene in the lipid bodies of Chlorella sp. were characterized with fluorescence confocal microscopy and fluorescence lifetime imaging using the phasor approach (Subashchandrabose et al. 2014).
To the best of our knowledge, there are no studies examining the role of lipid bodies in diatoms during PAH accumulation. However, in freshwater ecosystems used as drinking water sources, this aspect is of vital importance. For example, in Lake Baikal, Siberia, the world’s largest freshwater reservoir, diatoms contribute significantly to lake primary production (Popovskaya 2000). Through accumulation of organic hydrophobic compounds along with bacteria capable of degrading hydrocarbons, diatoms take part in the bioremediation of the surface water of the lake (Pavlova, et al., 2008; Gorshkov et al. 2010) and transport this compounds upwards in the food chain of Baikal (Kucklick et al. 1996), including sites of natural oil seeps (Khlystov et al. 2007; Kontorovich et al. 2007).
Freshwater diatom Synedra acus subsp. radians (Kütz.) Skabitschevsky is one of the dominant species in the phytoplankton of Lake Baikal (Popovskaya et al. 2016) and can serve as a good model organism for ecological and ecotoxicological studies. The main aim of this work was to investigate the accumulation and storage of petroleum hydrocarbons by this freshwater diatom. Our specific objectives were to (1) determine the concentrations crude oil and light oil causing inhibitory effects on diatom growth, (2) quantify the kinetics of PAH accumulation in the diatom during cultivation with light oil, and (3) evaluate the importance of n-alkanes for PAH accumulation in the diatom’s lipid bodies. We address these objectives through detailed chemical analyses of PAHs and n-alkanes using gas chromatography–mass spectrometry (GC/MS) and a combination of epifluorescent and scanning confocal microscopy to characterize PAH accumulation in intracellular lipid bodies.
Materials and methods
Preparation of crude oil and light oil solutions
Crude oil was supplied by the Angarsk refinery (deposits of western Siberia) and contained 15.3 % of n-alkanes and 1.2 % of PAHs. To remove bacterial contaminants, the crude oil sample was filtered through a polycarbonate filter with a pore diameter of 0.2 μm (Millipore, USA). Light oil was obtained by mixing crude oil with n-hexane (1:4 m/v) using sonication at 40 kHz for 5 min (Ferroplast Medical ultrasonic bath, Russia), followed by separation of the n-hexane supernatant from the lighter hydrocarbon fraction by centrifugation at 1000g for 3 min. Quantitative analysis of the residual showed that the mass of the heavy asphaltene fraction was gravimetrically quantified after solvent evaporation to constant weight (Capelli et al. 2001) which did not exceed 6 %.
Experimental growth conditions for the diatom S. acus subsp. radians
S. acus subsp. radians was isolated from a natural population sampled in Listvenichniy bay, Lake Baikal, in 2010, during the season of its dominance in the phytoplankton. An axenic culture of S. acus subsp. radians (Shishlyannikov et al. 2011) was grown in 1-l Erlenmeyer flasks with 250 ml of sterile DM medium (Beakes et al. 1988) of the following composition (concentration given in mg l−1: Ca(NO3)2 · 4H2O, 20; KH2PO4, 12.4; MgSO4 · 7H2O, 25; NaHCO3, 16; Na2EDTA, 2.25; H3BO3, 2.48; MnCl2 · 4H2O, 1.39; (NH4)6Mo7O24 · 4H2O, 1.0; Na2SiO3 · 9 H2O 11.4; cyanocobalamin, 0.04; thiamine hydrochloride, 0.04; biotin, 0.04). Crude oil or light oil at concentrations of 10, 50, 100, and 250 mg l−1 of the medium was added to the axenic culture during the exponential growth phase (6000–7000 cells ml−1). The diatom was grown for 13 days at 6 °C and a 14:10-h light/dark cycle. Samples were shaken occasionally. Each experiment was performed in triplicate.
For determination of the maximum cell division rates in the presence crude oil and light oil, subsamples for cell counting (1 ml) were taken at approximately the same time every day. The abundance of diatoms (cells ml−1) was estimated by enumerating cells on a glass slide with an epifluorescence microscope Axiovert 200 (Carl Zeiss, Germany). Maximum cell division rates (μ) were calculated using the following formula:
where N t2 and N t1 are cell numbers in 1 ml at times t 2 and t 1 (Thomas and Dodson 1974). We used the average value of three successive cell counts in the calculations.
Determination of PAHs and n-alkanes
We quantified the concentrations of PAHs and n-alkanes in the cells and supernatant separately. Before the chemical analysis, 50 μl of the surrogate internal standards (naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, pyrelene-d12, 5 ng μl−1 each, in acetonitrile) and squalane (130 ng μl−1 dissolved in n-hexane) were added to the fractions containing cells or supernatant. Using ultra-sonic treatment, PAHs and n-alkanes were extracted from the culture medium with n-hexane (20 ml, twice) and from wet cell biomass (weight sample ~25 mg) with n-hexane/acetone mixture (1:1 v/v, 10 ml, twice). Obtained extracts were dried under anhydrous K2SO4, centrifuged as described above, and aliquots of the part of extract (0.5 ml) were taken for determination of naphthalene’s. Then, the residual extracts were concentrated to 1–2 ml with a rotor evaporator at 40 °С (IKA, Germany). The final extracts were reduced to a volume of about 0.5 ml under argon stream and analyzed using a gas chromatograph (Agilent GC system 6890) equipped with a HT-8 capillary column (30 m length, 0.25 mm internal diameter, and 0.25 μm film thickness, SGE, Austin, TX, USA) with an autosampler, a split/splitless injector, and coupled with a mass selective detector (Agilent MSD 5973 Network) with an electron impact mode at 70 eV. Helium was used as carrier gas at a constant flow of 1.0 ml min−1 with injector, quadrupole detector, and ion source temperatures of 290, 150, and 230 °C, respectively. The extracts (2 μl) were injected in splitless mode into the column. The oven temperature was programmed to increase from 80 to 310 °C at a rate of 7 °C min−1 and kept constant for 3 min. Peaks of PAHs and n-alkanes were detected in the selected ion monitoring mode. Selected recording ions were as follows: m/z values of 128, 136, 142, 152, 154, 164, 166, 178, 188, 202, 228, 240, 252, 264, 276, and 278 (for PAHs), and 57, 71, and 183 (for n-alkanes). PAH and n-alkane peaks were identified by their relative retention times using the external standard (Polynuclear Aromatic Hydrocarbons Mix, Supelco, USA), chromatograms of light oil, and mass-spectrums n-alkanes.
The quantification of PAHs was based on the internal standard method using deuterated surrogate internal standards. The calibration was done for each PAH by internal calibration of certified mixture PAHs. Five concentrations of calibration standard in the range expected in sample were analyzed to obtain a linear curve fit with R 2 value of 0.997. Surrogate standard was used to monitor losses during extraction. Consistent recovery of PAHs (≥90 %) was observed during analysis. The reliability of the calibration was checked by injecting known standard and solvent blanks into the column. Values below the mean plus three standard deviations of the blank values were considered below limit of quantification (LOQ). Instrument limit of detection (ILOQ) was calculated as three times the chromatogram baseline noise level and for PAHs is 10–20 ng g−1 of wet weight (ww).
Determination of PAH bioconcentration factor
PAH bioconcentration factors were calculated according to the following formula (Sijm et al. 1995):
where С cells is the PAH concentration in diatom cells after centrifugation (mg kg−1 of wet weight) and С water is the PAH concentration in the supernatant of the culture medium (mg kg−1).
Epifluorescence microscopy for detection of PAHs in lipid bodies
The intracellular uptake of PAHs in lipid bodies of diatom was monitored by epifluorescent microscopy during cultivation of diatom in the presence of petroleum hydrocarbons (Verdin et al. 2005). Ten microliters of Nile red dye (Sigma, USA, 2 mg ml−1 acetone solution) was added to 1 ml of the cell culture at different growth phases (Shishlyannikov et al. 2014), incubated for 5 min in the dark, and analyzed using an epifluorescence microscope Axiovert 200 (Carl Zeiss, Germany) equipped with a mercury lamp Osram HBO 50 W/AC, Penguin 600CL digital camera (Pixera Corp., USA) and the software “AxioSet” (Carl Zeiss, Germany). Fluorescence analysis was carried out at the excitement wavelengths of 365 nm for PAHs and 450–490 nm for Nile red and at the emission wavelengths of 420 and 515 nm, respectively.
Laser confocal microscopy for localization of PAHs in lipid bodies
The intracellular localization of PAH in lipid bodies was determined using laser confocal microscopy (Furuno et al. 2012).
S. acus subsp. radians cells were isolated from the culture medium by centrifugation at 1000g for 10 min and washed twice with 400 μl of sterile DM medium. The cells were then fixed with 2 % paraformaldehyde solution (Sigma, USA) for 15 min. Subsequently, diatom cells were placed into ProLong® Gold antifade reagent (Life Technologies, USA) and observed with a laser confocal microscope LSM 710 (Carl Zeiss, Germany) equipped with a Plan-Apochromat 63×/1.40 Oil DIC M27 objective lens (Carl Zeiss, Germany).
The fluorescence excitation and emission wavelengths for lipid bodies were 405 and 420–550 nm; those for chloroplast fluorescence were 561 and 650–723 nm, respectively. Microscopic images were processed using the ZEN 2010 program (Carl Zeiss, Germany).
Statistical analysis
The experimental values for maximum cell division rates of S. acus subsp. radians to crude oil and light oil hydrocarbons and data for PAH accumulation by S. acus subsp. radians cultivated in the presence of light oil were calculated as average \( \overline{x} \) from two replicates with confidence interval Δx. The 95 % confidence interval values Δx for\( \overline{x} \) were derived from Student’s t distribution with symmetric probability distribution using the formula (Smagunova and Karpukova 2008):
where S is the standard deviation (SD) of the concentration values, average values \( \overline{x} \), α = 1 − P = 0.05 (P is the level of confidence of 95 %), n is the number of measurements, f = n − 1 = 2 is the degree of freedom to calculate the S values, and t (α, f) is the t table value.
Results and discussion
Toxic effect of crude oil on S. acus subsp. radians growth
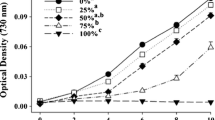
The toxic effect of crude oil hydrocarbons on the growth of S. acus subsp. radians was studied using crude oil (Fig. 1) and an extract of a crude oil/n-hexane solution (light oil) without fractions of high molecular asphaltene hydrocarbons (Fig. 1).
We observed no growth inhibition of S. acus subsp. radians at the crude oil concentration of 10 mg l−1. However, cell growth rates decreased by 1.5 times when the concentration was at 50 mg l−1. A further increase in the concentration resulted in cell death (Fig. 1). The results obtained for the light oil inhibition were similar to the crude oil inhibition of diatom cell growth.
The relative standard deviation (RSD) values describing the precision of the estimates of maximum division rates in the presence of crude oil were 8 % (for 0–50 mg l−1 concentration) and 31 % (for 100–250 mg l−1 concentration). A significant precision decline of the results obtained in conditions of relatively high hydrocarbon concentrations (100–250 mg l−1) in the medium was noted. The RSD values for light oil concentrations were estimated as 13 % (for 0–100 mg l−1 concentration) and 22 % (for 250 mg l−1 concentration).
The toxic effect of crude oil and light oil on the growth of freshwater and marine microalgae depends on the microalgae species, the crude oil type, the concentrations of hydrocarbons in the aqueous phase, and the time and temperature of exposure (Corner 1978, Ozhan et al. 2014). Among the hydrocarbons in crude oil, PAHs are the most toxic ones for different representatives of planktonic and benthic algae (Djomo et al. 2004; Gamila and Ibrahim 2004; Wang et al. 2008; Ozhan and Bargu 2014; Croxton et al. 2015). Previous studies have revealed contradicting effects on the influence of crude oil hydrocarbons on different species of microalgae. In particular, hydrocarbons in the aqueous phase at a concentration of 1 mg l−1 inhibited the growth of the arctic diatom Melosira moniliformis and stimulated the growth of Ditylum brightwellii (Mironov and Lanskaja 1967). However, the rate of cell division in D. brightwellii declined at a crude oil concentration of 10 mg l−1. In addition, recent study has shown that the two diatom species, D. brightwellii and Chaetoceros socialis, demonstrate higher tolerance to crude oil than the three dinoflagellates species, Pyrocystis lunula, Scrippsiella trochoidea, and Heterocapsa triquetra , and the larger species of phytoplankton were more tolerant to crude oil than the smaller ones (Ozhan et al. 2014). Our results show that inhibition of S. acus subsp. radians takes place at a crude oil hydrocarbon concentration between 10.0 and 50.0 mg l−1, which corresponds to a concentration toxic for the most known microalgae (Anderson et al. 1974; Kusk 1981; Fabregas et al. 1984; Tukaj 1987) and cyanobacteria (Obaidy et al. 2014).
Localization and identification of PAHs in lipid bodies
Blue fluorescence of 1–2-μm-sized oval formations was observed by epifluorescence microscopy in the diatom cytoplasm after day 5 of growth (exponential phase) in the presence of 10 mg l−1 of crude oil or light oil (PAH concentration in the medium was 120 μg l−1). These formations were located in the center and in the periphery of the cells, reaching a size of 4–5 μm on day 13 (stationary growth phase) (Fig. 2a). Control experiments with diatoms cultivated without crude oil hydrocarbons resulted in no fluorescence (Fig. 2b).
PAH accumulation in lipid bodies of S. acus subsp. radians after 13 days of cultivation. a, b, d Epifluorescence microscopy. c Laser confocal microscopy (orthogonal slice view). a In the presence of crude oil (PAH concentration 120 μg l−1). b Without crude oil (control). c In the presence of light oil (PAH concentration 120 μg l−1). d Lipid bodies stained with Nile red (after cultivation of diatom in the presence of light oil). White arrows show lipid bodies. Chloroplast—red, PAHs—blue, stained lipid bodies—yellow. Scale bar—5 μm
To confirm the intracellular localization of PAHs, we used laser confocal microscopy (Furuno et al. 2012). Under these conditions, the image (Fig. 2c) clearly demonstrated the spatial location of blue-colored fluorescent vesicles within limits of cell (between two chloroplasts within cell limits).
The nature of the cellular compartment involved in PAH storage investigated using Nile red dye is specific to neutral lipids (Greenspan et al. 1985). We used this dye to identify the lipid nature of orange-red formations found in cells growing in the presence of light oil (Fig. 2d). Nile red has also been used for the detection of benzo(a)pyrene accumulation in lipid bodies of Chlorella sp. (Subashchandrabose et al. 2014).
PAHs are the main components of crude oil and are capable to fluoresce (Konstantinova-Schlesinger 1961). PAHs are excited generally in the 200–400 nm range and are also strongly fluoresce. Multicomponent mixtures of PAHs have spectral overlap excitation and emission wavelengths (Patra 2003). The data obtained by laser confocal microscopy (Fig. 3) shows that the fluorescence of lipid bodies in S. acus subsp. radians ranged from 450 to 550 nm, which is a characteristic for PAH emission (Warner et al. 1977). This observation leads to the conclusion that diatoms accumulated PAHs in intracellular lipid bodies.
Laser confocal microscopy of S. acus subsp. radians after 5 days in the presence of light oil (10 mg l−1). a Fluorescence spectrum of lipid bodies (green line) and chloroplast (red line). b 2D optical section; green arrow shows a lipid body. The scanning emission spectrum zones are shown with marks (green cross—lipid body, white cross—chloroplast)
It may be assumed that PAHs also accumulated in the chloroplast because the membranes of chloroplasts have the highest contents of lipids. Nevertheless, we did not identify the emission spectrum of PAH in diatom chloroplasts. This was probably caused by the wide spectrum of autofluorescent substances found in chloroplasts, and thus, it is difficult to distinguish PAHs’ fluorescence from additional molecules.
Assessment of bioconcentration factors for PAHs in S. acus subsp. radians
Crude oil usage for the estimation of bioconcentration factors (BCF ww) can lead to an incorrect interpretation of results due to the presence of an insoluble asphaltene fraction in the water-organic phase of the culture medium, which is the concurrent adsorption site for hydrocarbons dissolved in the medium. Therefore, we used the light oil in the experiments of BCF ww determination for PAHs in S. acus subsp. radians. We identified 17 PAHs in light oil using GC/MS techniques. Twelve PAHs are listed in Table 1; the remaining five compounds were present only in minimal quantities (below 1.5 %).
In rapidly growing diatoms, PAH concentrations in cells are reduced by the accumulation of new biomass, a phenomenon known as growth dilution (Swackhamer and Skoglund 1993), so BCF ww values for PAHs are best determined in conditions of slow growth. Therefore, analysis of PAH concentration factor was performed at pre-stationary growth phase (0.09 cells day−1) and in the asphaltene-free light oil treatment.
Total PAH concentration in the diatom biomass cultivated in medium containing 10 mg l−1 of light oil was 45 μg g−1 wet weight (ww) after the first incubation day at the pre-stationary growth phase and did not change until day 5 (Table 1). We used these data for an assessment of factors influencing water-diatom partitioning.
The partitioning coefficient becomes invariant (reaches a steady state) theoretically at the point at which the fugacity ratio equals 1 (Mackay 1982). If bioconcentration of hydrophobic compounds in microalgae primarily concerns partitioning into the lipid fraction (Swackhamer and Skoglund 1993), it can be assumed to be proportional to K ow (octanol-water partition coefficient):
where α is dependent on the lipid content and lipid class and therefore depends on different species and their physiological states.
BCF PAH values were calculated from diatom and aqueous phase measurements (Fig. 4). Plots for logarithmic dependencies of BCF PAH on K ow (for BCF PAH < 5) had maxima at logK ow = 4.5 to 5.0. BCF PAH values for benzo(a)anthracene (11) and chrysene (12) (logK ow 5.6 and 5.7, respectively) were not taken into consideration due to lower solubility of these compounds in water and their high molecular weights which likely prevent their transport through the diatom plasmalemma (Dimitrov et al. 2002). Figure 4b shows that α values BCF PAH < 5 are about 1.3 times higher after day 5 compared to day 1 when they were cultivated in the presence of light oil (0.75 and 0.58, respectively). This finding can be explained by an increase of the lipid impact on the accumulation of PAHs by the diatom during cultivation. The lipid content of S. acus subsp. radians is composed of fatty acids in the lipid membrane bilayer and intracellular lipid bodies (Shishlyannikov et al. 2014). PAH accumulation in lipid bodies observed by cytochemical methods (Fig. 2c) supports this hypothesis. The importance of the lipid content in bioaccumulation of hydrophobic compounds by aquatic organisms has been ascribed recently (Katagi 2010; Croxton et al. 2015). According to the previously published data by Croxton et al. (2015), increasing the lipid mean value in diatom for all РАН-exposed treatments was significantly higher than control treatments. It was suggested that “more energy to maintain cell growth or for diluting the PAH compound within a larger cellular lipid pool” (Croxton et al. 2015). In the present study, we did not observe similar results. This is probably related to the differences of the concentrations of individual PAHs both in culture mediums and diatom’s species. In addition, we used light oil, which included both PAHs and n-alkanes, whereas Croxton et al. (2015) used the individual PAHs.
LogBCF ww versus logK ow for S. acus subsp. radians cultivated in the presence of light oil (10 mg l−1). a After 1 day. b After 5 days. The logBCF ww = f(logK ow) plots were calculated for PAHs with logK ow <5. The plots were made and the correlation coefficients R were calculated using all points with the exception of 11 and 12
n-alkane accumulation by S. acus subsp. radians
The composition of n-alkanes found in S. acus subsp. radians cells after cultivation in the presence of light oil was significantly different compared to the composition of n-alkanes in the culture medium (Fig. 5a). Several n-alkanes with a hydrocarbon chain length from С12 to С18 were accumulated, with a preference for n-alkanes with a chain length of С15 and С16 (Fig. 5b). Patterns of n-alkane or PAHs in the control groups were not detected. It should be noted that n-alkanes that accumulated in the diatom cells have a chain length similar to that of acyl chains of fatty acids in the lipid component of S. acus subsp. radians (Shishlyannikov et al. 2014). The same selectivity in n-alkane accumulation with a preference for chain lengths of С13 to С16 was observed when the seawater diatom Cyclotella cryptica was exposed to crude oil (Karydis 1980).
Sikkema et al. (1995) showed that the accumulation of lipophilic substances and their interaction with the phospholipid layer of the cytoplasmic membrane caused its modification and changed cytoplasmic membrane functioning. Since hydrocarbons are non-polar compounds, they can be incorporated into the membrane and enter the cell by passive diffusion. PAH and n-alkane insertion into the membrane bilayer was demonstrated using artificial vesicles where n-alkanes penetrated into the vesicles by intercalating with chains of fatty acids of similar lengths (Hunt and Tipping 1978; Castelli et al. 2002). Studies on gene expression and transcriptomics responses of the diatom Thalassiosira pseudonana exposed to PAHs have shown that PAHs inhibited the formation of new silica valves to decrease diatom growth rates (Bopp and Lettieri 2007, Carvalho et al. 2011). According to these works, PAHs impair the lipid metabolism and silica shell formation. The growth inhibition and the subsequent death of S. acus subsp. radians at a PAH concentration higher than 600 μg l−1 (at concentration of light oil in medium—50 mg l−1) also can be explained by an increasing negative effect of PAH accumulation in lipid bodies on diatom metabolism and morphogenesis of new cell valves. We propose that n-alkanes with a chain length similar to acyl chains of fatty acids in lipid bodies of diatoms inserted into the plasmalemma bilayer contribute to the passive hydrocarbon diffusion into the cell, including PAHs which are accumulated in the lipid bodies, leading to an inhibition of the intracellular metabolism of the diatom.
Conclusion
In this work, we demonstrate that intracellular accumulation of PAHs into lipid bodies of S. acus subsp. radians takes places during cell growth in the presence of crude oil hydrocarbons. In the process of intracellular PAH accumulation, diatom cells selectively accumulate n-alkanes with a chain length from С12 to С18 from the culture medium, with a preference for the chain lengths С15 and С16. The hydrocarbon chain length of accumulated n-alkanes is similar to that of the acyl chains of fatty acids in the lipid content of diatom. We therefore suggest that n-alkanes contribute to PAH transmembrane transport and that PAH transport from the aquatic environment into the cell and accumulation in lipid bodies is a universal process. Our study shows that diatoms can participate in PAH bioaccumulation with the subsequent removal of these substances from the surface layer water by precipitation of the cells or incorporation in the trophic web in crude oil-polluted sites.
References
Anderson JW, Neff JM, Cox BA, Tatem HE, Hightower GM (1974) Characteristics of dispersions and water-soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish. Mar Biol 27:75–88
Beakes G, Canter HM, Jaworski GHM (1988) Zoospore ultrastructure of Zygorhizidium affluens Canter and Z. planktonicum Canter, two chytrids parasitizing the diatom Asterionella formosa Hassall. Can J Bot 66:1054–1067
Beal R, Betts WB (2000) Role of rhamnolipid biosurfactants in the uptake and mineralization of hexadecane in Pseudomonas aeruginosa. J Appl Microbiol 89:158–168
Berglund O, Larsson P, Ewald G, Okla L (2001) The effect of lake trophy on lipid content and PCB concentrations in planktonic food webs. Ecology 82:1078–1088
Bopp SK, Lettieri T (2007) Gene regulation in the marine diatom Thalassiosira pseudonana upon exposure to polycyclic aromatic hydrocarbons (PAHs). Gene 396:293–302
Bouchez-Naïtali M, Vandecasteele JP (2008) Biosurfactants, an help in the biodegradation of hexadecane? The case of Rhodococcus and Pseudomonas strains. World J Microbiol Biotechnol 24:1901–1907
Capelli SM, Busalmen JP, De Sanchez SR (2001) Hydrocarbon bioremediation of a mineral-base contaminated waste from crude oil extraction by indigenous bacteria. Int Biodeterior Biodegrad 47:233–238
Carvalho RN, Bopp SK, Lettieri T (2011) Transcriptomics responses in marine diatom Thalassiosira pseudonana exposed to the polycyclic aromatic hydrocarbon benzo [a] pyrene. PLoS One 6:e26985
Castelli F, Librando V, Sarpietro MG (2002) Calorimetric approach of the interaction and absorption of polycyclic aromatic hydrocarbons with model membranes. Environ Sci Technol 36:2717–2723
Corner EDS (1978) Pollution studies with marine plankton. Part I. Petroleum hydrocarbons and related compounds. Adv Mar Biol 15:289–380
Croxton AN, Wikfors GH, Schulterbrandt-Gragg RD (2015) The use of flow cytometric applications to measure the effects of PAHs on growth, membrane integrity, and relative lipid content of the benthic diatom, Nitzschia brevirostris. Mar Pollut Bull 91:160–165
Dimitrov SD, Dimitorova NC, Walker JD, Veith GD, Mekenyan OG (2002) Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size. Pure Appl Chem 74:1823–1830
Djomo JE, Dauta A, Ferrier V, Narbonne JF, Monkiedje A, Njine T, Garrigues P (2004) Toxic effects of some major polyaromatic hydrocarbons found in crude oil and aquatic sediments on Scenedesmus subspicatus. Water Res 38:1817–1821
Fabregas J, Herrero C, Veiga M (1984) Effect of oil and dispersant on growth and chlorophyll a content of the marine microalga Tetraselmis suecica. Appl Environ Microb 47:445–447
Furuno S, Foss S, Wild E, Jones KC, Semple KT, Harms H, Wick LY (2012) Mycelia promote active transport and spatial dispersion of polycyclic aromatic hydrocarbons. Environ Sci Technol 46:5463–5470
Gamila HA, Ibrahim MBM (2004) Algal bioassay for evaluating the role of algae in bioremediation of crude oil: I-isolated strains. Bull Environ Contam Toxicol 73:883–889
Gerofke A, Kömp P, McLachlan MS (2005) Bioconcentration of persistent organic pollutants in four species of marine phytoplankton. Environ Toxicol Chem 24:2908–2917
Gorshkov A, Marinayte I, Zemskaya T, Khodzher T (2010) Modern level of petroleum products in water of Lake Baikal and its tributaries. Chem Sustain Dev 18:711–718 in Russian
Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100:965–973
Hong YW, Yuan DX, Lin QM, Yang TL (2008) Accumulation and biodegradation of phenanthrene and fluoranthene by the algae enriched from a mangrove aquatic ecosystem. Mar Pollut Bull 56:1400–1405
Hunt GR, Tipping LR (1978) A 1H NMR study of the effects of metal ions, cholesterol and n-alkanes on phase transitions in the inner and outer monolayers of phospholipid vesicular membranes. BBA-Biomembranes 507:242–261
Kallimanis A, Frillingos S, Drainas C, Koukkou AL (2007) Taxonomic identification, phenanthrene uptake activity, and membrane lipid alterations of the PAH degrading Arthrobacter sp. strain Sphe3. Appl Microbiol Biot 76:709–717
Karydis M (1980) Uptake of hydrocarbons by the marine diatom Cyclotella cryptica. Microb Ecol 5:287–293
Katagi T (2010) Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. In: Whitacre DM (ed) Reviews of environmental toxicology. Springer, New York, pp. 1–132
Khlystov OM, Gorshkov AG, Egorov AV, Zemskaya TI, Granin NG, Kalmychkov GV, Vorob’eva SS, Pavlova ON, Yakup MA, Makarov MM, Moskvin VI, Grachev MA (2007) Oil in the lake of world heritage. Dokl Earth Sci 415:682–685
Kingston PF (2002) Long-term environmental impact of oil spills. Spill Sci Technol B 7:53–61
Ko FC, Baker JE, Tew KS (2012) Kinetics of polychlorinated biphenyl partitioning to marine Chrysophyte Isochrysis galbana. Sci Total Environ 416:410–417
Konstantinova-Schlesinger MA (1961) Luminescent analysis. Physmatgiz, Moscow in Russian
Kontorovich AE, Kashirtsev VA, Moskvin VI, Burshtein LM, Zemskaya TI, Kostyreva EA, Kalmychkov GV, Khlystov OM (2007) Petroleum potential of Baikal deposits. Russ Geol Geophys 48:1046–1053
Kucklick JR, Harvey HR, Baker JE, Ostrom PH, Ostrom NE (1996) Organochlorine dynamics in the pelagic food web of Lake Baikal. Environ Toxicol Chem 15:1388–1400
Kusk KO (1981) Effects of hydrocarbons on respiration, photosynthesis and growth of the diatom Phaeodactylum tricornutum. Bot Mar 24:413–418
Lei AP, ZL H, Wong YS, Tam NFY (2007) Removal of fluoranthene and pyrene by different microalgal species. Bioresour Technol 98:273–280
Mackay D (1982) Correlation of bioconcentration factors. Environ Sci Technol 16:274–278
Mironov OG, Lanskaja LA (1967) The influence of oil on the development of marine phytoplankton. In: Vodyanitskiy, V.A. (ed.), Journal of Biooceanography. Moscow, pp 161–164 (in Russian)
Nizzetto L, Gioia R, Li J, Borga K, Pomati F, Bettinetti R, Dachs J, Jones KC (2012) Biological pump control of the fate and distribution of hydrophobic organic pollutants in water and plankton. Environ Sci Technol 46:3204–3211
Obaidy A, Hameed A, Jawad M, Lami M (2014) The toxic effects of crude oil in some freshwater cyanobacteria. J Environ Protect 5:359–367
Ozhan K, Bargu S (2014) Can crude oil toxicity on phytoplankton be predicted based on toxicity data on benzo (a) pyrene and naphthalene? Bull Environ Contam Toxicol 92:225–230
Ozhan K, Miles SM, Gao H, Bargu S (2014) Relative Phytoplankton growth responses to physically and chemically dispersed South Louisiana sweet crude oil. Environ Monit Assess 186:3941–3956
Patra D (2003) Applications and new developments in fluorescence spectroscopic techniques for the analysis of polycyclic aromatic hydrocarbons. Appl Spectrosc Rev 38:155–185
Pavlova ON, Zemskaya TI, Gorshkov AG, Parfenova VV, Suslova MY, Khlystov OM (2008) Study on the Lake Baikal microbial community in the areas of the natural oil seeps. Appl Biochem Micro+ 44:287–291
Popovskaya GI (2000) Ecological monitoring of phytoplankton in Lake Baikal. Aquat Ecosyst Health Manag 3:215–225
Popovskaya GI, Genkal SI, Likhoshway YV (2016) Diatoms of the plankton of Lake Baikal: atlas and key, 2nd edn. Nauka, Novosibirsk
Shishlyannikov SM, Zakharova YR, Volokitina NA, Mikhailov IS, Petrova DP, Likhoshway YV (2011) A procedure for establishing an axenic culture of the diatom Synedra acus subsp. radians (Kütz.) Skabibitsch. from Lake Baikal. Limnol Oceanogr-Meth 9:478–484
Shishlyannikov SM, Klimenkov IV, Bedoshvili YD, Mikhailov IS, Gorshkov AG (2014) Effect of mixotrophic growth on the ultrastructure and fatty acid composition of the diatom Synedra acus from Lake Baikal. J Biol Res-Thessalon 21:15
Sijm DT, Middelkoop J, Vrisekoop K (1995) Algal density dependent bioconcentration factors of hydrophobic chemicals. Chemosphere 31:4001–4012
Sikkema J, De Bont JA, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Smagunova AN, Karpukova OM (2008) Methods of mathematical statistic in analytical chemistry. Irkutsk State University, Irkutsk in Russian
Soto LA, Botello AV, Licea-Durán S, Lizárraga-Partida ML, Yáñez-Arancibia A (2014) The environmental legacy of the Ixtoc-I oil spill in Campeche Sound, southwestern Gulf of Mexico. Front Mar Sci 1:57
Subashchandrabose SR, Krishnan K, Gratton E, Megharaj M, Naidu R (2014) Potential of fluorescence imaging techniques to monitor mutagenic PAH uptake by microalga. Environ Sci Technol 48:9152–9160
Swackhamer DL, Skoglund RS (1993) Bioaccumulation of PCBs by algae: kinetics versus equilibrium. Environ Toxicol Chem 12:831–838
Thomas WH, Dodson AN (1974) Effect of interactions between temperature and nitrate supply on the cell-division rates of two marine phytoflagellates. Mar Biol 24:213–217
Tukaj Z (1987) The effects of crude and fuel oils on the growth, chlorophyll ‘a’content and dry matter production of a green alga Scenedesmus quadricauda (Turp.) Bréb. Environ Pollut 47:9–24
Verdin A, Lounes-Hadj-Sahraoui A, Newsam R, Robinson G, Durand R (2005) Polycyclic aromatic hydrocarbons storage by Fusarium solani in intracellular lipid vesicles. Environ Pollut 133:283–291
Wang L, Zheng B, Meng W (2008) Photo-induced toxicity of four polycyclic aromatic hydrocarbons, singly and in combination, to the marine diatom Phaeodactylum tricornutum. Ecotoxicol Еnviron Saf 71:465–472
Warner IM, Christian GD, Davidson ER, Callis JB (1977) Analysis of multicomponent fluorescence data. Anal Chem 49:564–573
Watkinson RJ, Morgan P (1990) Physiology of aliphatic hydrocarbon degrading microorganisms. Biodegradation 1:79–92
Acknowledgments
We thank Yu. Zakharova and N. Volokitina (Limnological Institute SB RAS) for their kind gift of the diatom culture and Prof. E. V. Likhoshway (Limnological Institute SB RAS) for her assistance in the preparation of this manuscript. This research was funded by the Federal Agency for Scientific Organizations (FASO). We would like to thank Dr. Teofil Nakov (University of Arkansas) and anonymous reviewers for their constructive comments. We also thank the United Instrumental Center (Irkutsk Scientific Center) and the Center of Ultramicroanalysis (Limnological Institute, Irkutsk) for providing the equipment. The experiments on S. acus subsp. radians growth in the presence of petroleum hydrocarbons as well as microscopic analyses were carried out within the Project VI.50.1.3. Qualitative and quantitative evaluations of hydrocarbons in samples were fulfilled within the Project VIII.76.1.5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Shishlyannikov, S.M., Nikonova, A.A., Klimenkov, I.V. et al. Accumulation of petroleum hydrocarbons in intracellular lipid bodies of the freshwater diatom Synedra acus subsp. radians . Environ Sci Pollut Res 24, 275–283 (2017). https://doi.org/10.1007/s11356-016-7782-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7782-y