Abstract
We evaluated the acute lethal and sublethal effects of technical-grade glyphosate (GLY) and the GLY-based commercial formulation Roundup ULTRA MAX® (RU) on two Gosner stages (Gss) 25 and 36 of the South-American Creole frog, Leptodactylus latrans. Bioassays were performed following standardized methods within a wide range of concentrations (0.0007–9.62 mg of acid equivalents per liter—a.e./L—of RU and 3–300 mg/L of GLY). The endpoints evaluated were mortality, swimming activity, growth, development, and the presence of morphologic abnormalities, especially in the mouthparts. No lethal effects were observed on larvae exposed to GLY during either Gs-25 or Gs-36. The concentrations inducing 50 % lethality in RU-exposed larvae at different exposure times and Gss ranged from 3.26 to 9.61 mg a.e./L. Swimming activity was affected by only RU. Effects on growth and development and the induction of morphologic abnormalities—like oral abnormalities and edema—were observed after exposure to either GLY or RU. Gs-25 was the most sensitive stage to both forms of the herbicide. The commercial formulation was much more toxic than the active ingredient on all the endpoints assessed. Effects on growth, development, and the induction of morphologic abnormalities observed in the range of environmental concentrations reported for agroecosystems of Argentina constitute an alert to the potential detrimental effects of the herbicide that could be affecting the fitness and survival of anurans in agroecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of their high sensitivity to the type of toxicants used in agriculture, amphibians are good indicators of the potential impact of pesticides in agroecosystems (Berrill et al. 1993; Sparling and Fellers 2009; Egea-Serrano et al. 2012), while, as a result of their life cycle, they can be considered representative organisms of both aquatic and terrestrial environments (Stebbins and Cohen 1997; Young et al. 2004; Sparling et al. 2010). Leptodactylus latrans (Anura: Leptodactylidae), commonly known in Argentina as rana criolla (Creole frog), is a widespread species in South America (Heyer et al. 2010) and as such is traditionally used for culinary purposes in Argentina. The species has been categorized as “Not Threatened” according to Vaira et al. (2012) and as “Least Concern” according to the International Union for Conservation of Nature ( 2016). The frog’s breeding season coincides with the beginning of the spring rains, while breeding takes place in both temporary and permanent ponds, in which 5000–35,000 eggs are released within floating foam nests. This species exercises parental care of the eggs and larvae, where the latter are gregarious and nektonic (Cei 1980; Natale 2006).
Glyphosate (N-[phosphonomethyl]glycine; GLY) is a postemergence herbicide widely employed to control weeds in crops primarily of soybean but also of corn, wheat, and sunflower, among others. GLY has been considered one of the most widely used herbicides worldwide in the last decades (Duke and Powles 2008). After being applied, the compounds can either first reach the soil and then be transported to other areas or else adsorbed directly into the soil to be degraded there by microorganisms. This latter possibility leads to a rather low mobility of both GLY and its main metabolite, the aminomethylphosphonic acid (AMPA; Giesy et al. 2000; Borggaard and Gimsing 2008; Al-Rajab and Hakami 2014). Alternatively, the herbicide can be transported to deeper soil layers via macropore flow or to surface water through runoff (Borggaard and Gimsing 2008; Coupe et al. 2012).
In recent years, the use of GLY in Argentina has increased owing to the extension in the cultivated area of genetically modified soybean, engineered to be GLY-resistant (Domingo Yagüez et al. 2011; López et al. 2012). In addition, Argentina is the third largest producer of soybeans in the world, after the USA and Brazil (Benbrook 2016), and according to the Cámara de Sanidad Agropecuaria y Fertilizantes, Buenos Aires (CASAFE 2012), the GLY-based herbicides are those most commonly used in the country. Previous reports for Argentina had registered concentration levels of GLY at sites near agricultural areas ranging from 0.035 to 5.0 mg/kg for soils (Aparicio et al. 2013), 0.0005 to 0.700 mg/L for surface waters, and 0.006 to 5.0 mg/kg for sediments (Peruzzo et al. 2008; Aparicio et al. 2013; Primost 2013; Lupi et al. 2015; Ronco et al. 2016). In contrast, the concentrations of AMPA ranged from 0.30 to 38.9 mg/kg for soils, 0.0002 to 0.002 mg/L for surface waters, and 0.002 to 7.29 mg/kg for sediments (Aparicio et al. 2013; Primost 2013; Lupi et al. 2015; Ronco et al. 2016).
The lethal effects of several GLY formulations and their corresponding active ingredients have been studied for different species of anuran larvae. The concentrations producing 50 % lethality (LC50 values) range from approximately 1 to 20 mg of the acid equivalent per liter (a.e./L) for commercial formulations (Mann and Bidwell 1999; Howe et al. 2004; Govindarajulu 2008; Bernal et al. 2009; Relyea and Jones 2009; Fuentes et al. 2011; Moore et al. 2012; Güngördü 2013; Yadav et al. 2013; Annett et al. 2014). Since technical-grade GLY has not been shown to have lethal effects on amphibian larvae, several authors have ascribed this difference in toxicity to the surfactants present in the formulations (Giesy et al. 2000; Edginton et al. 2004; Howe et al. 2004; Puglis and Boone 2011; Moore et al. 2012). In addition, the sublethal effects of GLY commercial formulations and the active ingredient have been studied in anuran larvae. For example, effects on growth, swimming activity, behavior, and cardiac function along with the formation of cranial and oral abnormalities, DNA damage, and alterations in enzyme activities have been reported for commercial formulations of GLY within the range of 1 to 27 mg a.e./L after acute exposure (Clements et al. 1997; Lajmanovich et al. 2003; Lajmanovich et al. 2011, 2013; Edginton et al. 2004; Costa et al. 2008). In addition, effects on growth, development, tadpole morphology, and the levels of messenger RNAs (mRNAs) encoded by genes involved in metamorphosis and development have been reported for commercial formulations of GLY within the range of 0.2 to 3 mg a.e./L after chronic exposure (Howe et al. 2004; Relyea 2004, 2012; Lanctôt et al. 2013, 2014). Although fewer reports are available containing data for technical-grade GLY, the chronic effects that have been documented are somewhat similar—i.e., growth, alteration of the sex ratio, and suppression of levels of mRNAs encoded by genes involved in metamorphosis and development (Lanctôt et al. 2014).
The monitoring of sublethal effects—such as those on behavior, growth, development, and morphology, among others—in anuran larvae is of relevance to an evaluation of the fitness of the adult amphibians (Berven and Gill 1983; Smith 1987). Deleterious effects on swimming performance have been demonstrated to be detrimental to foraging and the ability of the tadpoles to actively avoid predators (Semlitsch 1993; Stauffer and Semlitsch 1993; Horat and Semlitsch 1994; Jung and Jagoe 1995; Semlitsch et al. 1995; Rist et al. 1997; Broomhall and Shine 2003; Denoël et al. 2013). That environmental conditions can impair the growth and development of anuran larvae, resulting in higher rates of development during a shorter time and in smaller body sizes at metamorphosis, is certainly well known. These changes affect anuran survival by increasing the age at which sexual maturity is reached, with a decrease in the size of individuals at the time of their first reproduction and a consequent decline in fecundity (Wilbur and Collins 1973; Smith-Gill and Berven 1979; Morey and Reznick 2000; Denver and Crespi 2006). Morphologic abnormalities, accordingly, have been shown to occur in the amphibian populations of agroecosystems (Cooke 1981; Peltzer et al. 2011; Agostini et al. 2013). These anatomical changes can impair swimming (e.g., axial abnormalities and edema) and in many instances decrease the chances of normal foraging and other behavior (e.g., oral deformities; Rowe et al. 1996; Rowe et al. 2001; Venesky et al. 2010a, 2010b; Venesky et al. 2013; Tolledo et al. 2014; Babini et al. 2015).
The effects of GLY on native Argentine species have been reported only for two anuran larvae: Scinax nasicus (Hylidae: Lajmanovich et al. 2003) and Rhinella arenarum (Bufonidae: Lajmanovich et al. 2011, 2013; Junges et al. 2013). Despite the previously mentioned wide distribution and particular characteristics of L. latrans, the effects of different pesticides and other contaminants on the tadpoles of this species have been poorly studied (Araújo et al. 2014a, 2014b; Lajmanovich et al. 2015). Within this context, the aim of the present study was to assess the lethal and sublethal effects of the GLY-based herbicide Roundup ULTRA MAX® (RU) and technical-grade GLY on the Gosner stages (Gss) 25 and 36 (Gosner 1960) of L. latrans larvae through the use of acute-toxicity bioassays (96 h of exposure). The sublethal effects assessed were on swimming ability, growth, and development and on the occurrence of morphologic deformations, with particular emphasis on oral-disc abnormalities.
Materials and methods
Chemicals
Test solutions were prepared with the GLY-based formulation Roundup ULTRA MAX® (Monsanto Argentina S.A.I.C., Maipú, Buenos Aires, Argentina), containing 74.7 % of the monoammonium salt of N-(phosphonomethyl)glycine (GLY acid equivalent to 67.9 % [w/w]) and inert adjuvants quantum satis, and technical-grade GLY acid of 95.1 % purity (Gleba, La Plata, Buenos Aires, Argentina). Dilutions were made from a 740-mg a.e./L stock solution for RU and a 1500-mg/L stock solution for GLY with filtered and dechlorinated tap water (pH 7.7; hardness 180–250 mg CaCO3/L). The stock solution of GLY acid was adjusted to neutral pH with 0.1 N NaOH. Samples of test solutions were taken at low, intermediate, or high concentrations, according to the experimental design, immediately after preparation (0 h) and after 24 h of exposure to confirm the concentrations. The GLY concentrations in test solutions were determined by liquid-chromatography–mass-spectrometry (LC-MS; Agilent 1100 system, Agilent Technologies Inc., Miami, FL, USA) following derivatization with fluorenylmethyloxycarbonyl chloride according to the standardized method described by Meyer et al. (2009). The solvents used in chromatographic analysis were high-performance liquid chromatography quality grade, while the salts were analytical grade (J.T. Baker-Mallinckrodt Baker Inc., USA). Nanopure water was obtained in the laboratory by means of a Sartorius arium water purification system (Sartorius AG, Göttingen, The Netherlands). The standard of GLY (99 %) was acquired from Sigma-Aldrich (St. Louis, MO, USA).
Test species
Small portions (about 10 %) of five recently laid (8–10 h), foam nests of L. latrans were collected from temporary ponds from two fairly well-preserved areas; one located in Berisso (34° 55.723′ S, 57° 43.131′ W), in an uninhabited coastal sector of the Río de la Plata estuary, and a second located in a rural area of the El Pescado-Stream floodplain, La Plata (35° 1.262′ S, 57° 51.423′ W), Buenos Aires province, Argentina (Demetrio 2012). Once in the laboratory, the organisms were maintained in tanks with 500 L of dechlorinated tap water (as detailed above) with continuous aeration at 25 ± 1 °C and a 16:8 light/dark cycle. The larvae were fed ad libitum with blended lettuce until the individuals reached the stage needed according to our experimental design. The tadpoles were maintained under laboratory conditions according to the Guide for Care and Use of Laboratory Animals (National Research Council 2011).
Experimental design: toxicity bioassays
The bioassays of toxicity were performed on tadpoles at two developmental stages: Gs-25 (±0) and 36 (±2) following standardized methods proposed by the US Environmental Protection Agency ( 1975) and the American Society for Testing and Materials ( 2007) with minor modifications by Natale et al. (2006).
Toxicity bioassays with Gosner-stage 25
The bioassays were carried out in glass chambers with five individuals and 500 mL of the corresponding test solution under semistatic conditions (with medium replacement every 24 h) at four replicates per concentration. According to our previous experiments, we decided to feed the organisms in certain bioassays since a 72-h starvation could become a significant condition in the survival of young tadpoles. Hence, two types of toxicity bioassays were performed—involving feeding and nonfeeding—to compare the effects under both experimental conditions. Tadpoles were fed with 1 mL of blended lettuce every 24 h, 1 h before medium replacement, in the feeding bioassay and were not fed throughout the experiment in the nonfeeding bioassay. Preliminary tests were performed in order to arrive at a wide GLY-concentration range for assessing lethal and sublethal effects. Definitive bioassays were conducted with 23 concentrations, ranging from 0.0007 to 9.62 mg a.e./L of RU (encompassing both sublethal and lethal concentrations), 7 concentrations at between 3 and 300 mg/L of GLY, and a control group with merely dechlorinated tap water.
Toxicity bioassays with Gosner-stage-36 tadpoles
Bioassays were performed based on the results of Gs-25 tests, with seven concentrations between 0.37 and 9.62 mg a.e./L of RU (involving both lethal and sublethal concentrations) and seven concentrations between 3 and 300 mg/L of GLY (those being only sublethal concentrations). Once 50 % of the tadpoles reached Gs-36, the individuals were placed in test chambers according to the experimental procedure cited above. Testing conditions were the same as those explained for bioassays with the Gs-25 larvae. The tadpoles were not fed throughout the bioassays with the Gs-36 larvae because those individuals did not manifest indications of starvation during the later stages.
Endpoints measured
Lethal endpoints
Mortality was evaluated every 24 h and determined by the absence of movement after gently prodding the tadpoles with a polypropylene rod as well as by the change in their color and overall appearance. Dead individuals were removed and fixed in 10 % (v/v) aqueous formaldehyde.
Sublethal endpoints
Swimming activity was registered every 24 h by gently swirling the water three times with a polypropylene rod and observing for 1 min the swimming of each individual. The effects on swimming were classified according to the descriptions made by Brunelli et al. (2009) with minor modifications (Ruiz de Arcaute et al. 2012) and involving three categories: regular swimming, irregular swimming (erratic swimming, body twisting, and convulsions), and immobility (complete stillness for the whole observation period, but with slight movement observed after gently prodding with the propylene rod).
At the end of the experiments, all the tadpoles still alive were anesthetized in benzocaine solution (250 mg/L) according to recommendations of the European Commission (Close et al. 1996), then fixed in Bouin’s solution, and finally preserved in 70 % (v/v) aqueous ethanol for subsequent evaluation of growth, development, and the occurrence of morphologic deformities. Growth was determined by measuring the body length—i.e., snout-vent length (SVL)—according to Mc Diarmid and Altig (2000) with a digital caliper of 0.01 mm. Development and morphologic characteristics were observed under a Nikon SMZ745T binocular microscope (Nikon Instruments, Inc., Melville, NY, USA) equipped with a 519CU 5.0 ROM-CMOS camera (Micrometrics®, Unitron, Commack, NY, USA), the stages of development identified according to Gosner (1960), and the abnormalities classified as proposed by Cooke (1981), Bantle et al. (1996), Peltzer et al. (2013), Altig (2007), and Tolledo et al. (2014) with minor modifications. A total of 14 types of deformities were considered. Axial abnormalities were classified as lateral flexure of the tail (tail flexure <60°), severe lateral flexure of the tail (tail flexure ≥60°), dorsal flexure of the tail (the tail is bent in the vertical plane to the dorsal region of the body), ventral flexure of the tail (ibidem to the ventral region), wavy tail (the tail is more than once curved in the horizontal plane), and coiled tail (tail curls on itself by forming a spiral). The presence of edema was classified as abdominal edema (appearing in the intestinal region of the body), thoracic edema (appearing in the cardiac region of the body), and facial edema (appearing in the head, particularly in the facial region). Gut abnormalities were recorded as an abnormal coiling of intestine. Finally, oral abnormalities were defined as a loss (i.e., the absence) of the upper and/or lower jaw sheath, depigmentation of the upper and/or lower jaw sheath (jaw sheaths grayish), loss (i.e., the absence) of anterior and/or posterior tooth rows, and tooth-ridge abnormalities (anterior and/or posterior tooth ridge having abnormal gaps, or being absent altogether).
Statistical analysis
A regression analysis was performed between nominal and measured concentrations of GLY in the water, and the regression coefficient (b) was accordingly used to correct the concentrations presented in this study. The measured concentrations at the initial time (0 h) and after 24 h were compared by a paired Student t test according to Zar (2010). Swimming activity data were recorded in the three categories defined above as regular swimming, irregular swimming, and immobility. Each category was measured and recorded as a binary response (i.e., present or absent). Mortality and swimming activity data were analyzed by the Probit method (Finney 1971) through the use of the Probit Analysis Program, version 1.5 (USEPA 1999) in order to estimate LC50 and the concentrations producing 50 % effect (EC50), respectively. Concentration–response (C–R) curves at different times (24, 48, 72, 96 h) were estimated along with their 95 % confidence limits. Regression (a and b) and correlation (r) coefficients were calculated for each C–R curve and comparisons between different regression lines made according to Zar (2010). In addition, the swimming activity, growth, and abnormality data were analyzed by a one-way ANOVA with the Dunnett post hoc test (Zar 2010) to estimate the concentrations for no observed effect (NOEC) and the lowest observed effect (LOEC). The ANOVA assumptions were corroborated by Barlett’s test for homogeneity of variances and the Shapiro-Wilk test for normality. Effects on development compared to control group were evaluated by means of the Kruskal-Wallis test along with the Dunn post hoc test (Zar 2010). The incipient lethal concentration was estimated (according to LC50 values) as the point at which the curve began to run parallel to the x-axis according to Newman (2015). Comparisons between the LC50 values for the conditions of feeding and nonfeeding were made with a paired Student t test (Zar 2010) and between the lethal C–R curves for the Gs-25 and Gs-36 at each exposure time by simple linear regression comparison according to Zar (2010), while comparisons of the sublethal data at 96 h between GLY and RU bioassays or between the Gs-25 and Gs-36 were made with a paired Student t test according to Zar (2010).
Results and discussion
Chemical analysis
The GLY concentrations throughout this entire report are given after corrections were made on the basis of the measurements on the test solutions of RU and GLY. Moreover, the concentrations measured at the initial time (0 h) and after 24 h were not significantly different (p = 0.507), thus indicating that the GLY concentrations remained constant throughout the bioassays.
Lethal effects
Table 1 summarizes the lethal effects of RU on Gss 25 and 36 of L. latrans tadpoles. Because of the high mortality at 96 h in the nonfed group, the results demonstrate that L. latrans requires feeding during the early stages of development, though such feeding is not necessary if tests are performed with more advanced developmental stages. In this regard, that previous reports evaluating the effects of contaminants on Gs-25 L. latrans larvae were performed during 12-h (Araujo et al. Araújo et al. 2014a, Araújo et al. 2014b) and 48-h exposures (Lajmanovich et al. 2015) is indeed notable. In contrast, the paired t test of the LC50 values between the fed and nonfed groups after 24, 48, and 72 h of exposure indicated no significant differences (t = 2.767; df = 2; p = 0.109), thus suggesting that food supply does not influence the lethal effects of GLY under conditions of acute exposure. Since certain species of anuran larvae used for toxicity testing cannot reach 96 h of exposure under the conditions required in the standard protocols (in nonfeeding tests) because of starvation (Mann and Bidwell 1999), the present results could be relevant when testing toxicants on species in captivity with similarly stringent feeding requirements.
Previous studies evaluating the lethal effects of technical-grade GLY on anuran larvae demonstrated that the compound is not lethal at high concentrations (Bidwell and Gorrie 1995; Mann and Bidwell 1999; Howe et al. 2004; Moore et al. 2012). Our results support the absence of toxicity by GLY at up to 300 mg/L for both the Gs-25 and Gs-36 larvae, since we observed no lethal effects upon treatments with GLY (Table 2).
The formulation RU exhibited toxic effects with LC50 values at different exposure times and Gosner stages that ranged from 3.26 to 9.61 mg a.e./L. These results (cf. Table 1 and Table 2) are consistent with those obtained from the literature for other anuran species, ranging from 0.8 to 20.3 mg a.e./L with commercial GLY-containing formulations (Lajmanovich et al. 2003; Edginton et al. 2004; Howe et al. 2004; Bernal et al. 2009; Relyea and Jones 2009; Fuentes et al. 2011; Güngördü 2013; Yadav et al. 2013).
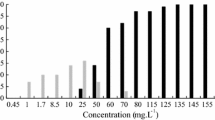
Lajmanovich et al. (2011) reported that four GLY formulations—Roundup ULTRA MAX®, Infosato®, Glifoglex®, and C-KYuyos®—achieve the LC50 stabilization at 24 h of exposure in R. arenarum tadpoles. In the present work, a multiple comparison of the various C–R curves for Gs-25 larvae exposed to RU for different lengths of time indicated significant differences (F = 30.90; df = 4; p < 0.0001) except between 72 and 96 h (Tukey’s post hoc test p > 0.05). The data suggest that commercial formulations of GLY exhibit a lethal effect within the first days of exposure and reach the incipient value after 72 h (Fig. 1). Comparisons among C–R curves between Gs-25 and Gs-36 tadpoles exposed to RU revealed significant differences at 72 (t = 10.571; df = 1; p < 0.001) and 96 h of exposure (t = 10.296; df = 1; p < 0.001), indicating that Gs-25 was more sensitive to toxicity by RU than Gs-36 (Table 1).
Sublethal effects
Tables 2 and 3 summarize the results of bioassays on the effects of both GLY and RU on the swimming activity, growth, and development of Gs-25 and Gs-36 tadpoles of L. latrans and on the induction of morphologic deformities in those stages. Swimming activity was affected by exposure to only RU, while effects on growth, development, and abnormalities were observed for both GLY and RU. A paired t test between LOEC values for sublethal effects of both GLY and RU bioassays showed significant differences (t = 4.427; df = 2; p = 0.047), indicating that RU was definitively more toxic than GLY. In addition, a paired t test between sublethal NOEC values for tadpoles of those same stages showed significant differences (t = 5.529; df = 2; p = 0.015), indicating Gs-25 tadpoles to be more sensitive than those of Gs-36 in all the endpoints tested. Because of the low number of survivors, and the failure of larvae to tolerate 96 h of exposure in the nonfed-group bioassays, sublethal effects were examined only for bioassays of the fed group.
Effects on swimming activity
The effects of GLY on the swimming activity of anuran larvae have been poorly studied and mostly associated with the pesticide formulations (Wojtaszek et al. 2004; Wood and Welch 2015). To the best of our knowledge, no previous reports evaluating the effects of technical-grade GLY on the swimming activity of anuran larvae exist. In our study, no effects on the swimming activity were observed in either Gs-25 or Gs-36 tadpoles exposed to GLY (Table 2).
By contrast, the effects of commercial formulations have been evaluated in tadpoles of certain anuran species. For example, Wood and Welch (2015) reported that the commercial formulation of GLY Roundup Weed & Grass Killer Super Concentrate® did not affect the behavior (activity) of Anaxyrus terrestris larvae, whereas Wojtaszek et al. (2004) observed a paralysis or inability to move away during the first 24 h of exposure to the GLY formulation Vision® in Gs-25 tadpoles of Lithobates pipiens and Lithobates clamitans. In the present study, a one-way ANOVA performed on the swimming activity data indicated that irregular swimming was observed in Gs-25 larvae after 24 h of exposure (F = 12.360; df = 5; p < 0.0001) with a LOEC value of 5.92 mg a.e./L (the Dunnet post hoc test; p = 0.003), although no significant effects were observed upon exposure of Gs-25 tadpoles subsequent to that time (F = 1.498; df = 4; p = 0.282). In addition, irregular swimming was also noted in Gs-36 larvae exposed to RU with a 96-h EC50 of 7.99 mg a.e./L (7.63–31.07). Swimming activity data on the Gs-36 tadpoles revealed significant differences with respect to irregular swimming (F = 10.710; df = 6; p = 0.0002), with a LOEC value of 5.18 mg a.e./L (the Dunnet post hoc test; p = 0.0003).
In summary, our results have demonstrated that tadpoles of different Gosner stages were affected in their ability to swim. This finding can have particular relevance in the field, since swimming has been demonstrated to be related to foraging so that an impairment in swimming may result in a decrease in feeding and as a consequence in nutrition, development, and growth (Semlitsch 1993; Horat and Semlitsch 1994; Semlitsh et al. Semlitsch et al. 1995; Rist et al. 1997; Broomhall and Shine 2003; Denöel et al. Denoël et al. 2013), which undernourishment and stunted growth would have an impact on fitness in adulthood (Berven and Gill 1983; Smith 1987). In addition, a reduction in swimming activity may also lead to an inability to escape from predators, thus resulting in a risk of nonsurvival for the larvae (Jung Jung and Jagoe 1995, Feder 1983; Stauffer and Semlitsch 1993; Broomhall and Shine 2003).
Effects on growth and development
The effects of GLY on growth of anuran larvae have been poorly studied. Howe et al. (2004) reported that GLY does not affect the growth of Gs-25 anuran larvae, while Lanctôt et al. (2014) reported that the compound causes growth inhibition in tadpoles exposed at Gs-25 upon reaching Gs-36–Gs-38. In this study, a one-way ANOVA demonstrated that larvae at Gs-25 exhibit a significant increment in growth after exposure to GLY (F = 3.050; df = 7; p = 0.005) with a LOEC value of 15 mg/L (the Dunnet post hoc test; p = 0.012). In contrast, no significant effect (F = 0.678, df = 7; p = 0.690) on growth was observed for Gs-36 tadpoles when exposed to GLY (Tables 2 and 3).
In comparison, a test of the effect of exposure to RU on the growth of Gs-25 larvae by a one-way ANOVA indicated a significant difference (F = 3.422; df = 14; p < 0.0001) with a LOEC value of 0.37 mg a.e./L (the Dunnet post hoc test; p = 0.003; Tables 2 and 3), but the same one-way ANOVA performed on the growth data for the Gs-36 larvae showed no significant effects by RU (F = 2.081; df = 7; p = 0.053; cf. Tables 2 and 3). These results are in agreement with previous reports that revealed a significant increment in the growth of anuran larvae when exposed to GLY formulations (i.e., for the SVL: Wojtaszek et al. 2004; mass: Jones et al. 2011; Gahl et al. 2011; for both the SVL and the mass: Navarro-Martín et al. 2014). Nevertheless, other studies indicated that GLY formulations had no significant effects on anuran-larval growth (i.e., for the SVL: Edginton et al. 2004; Edge et al. 2012; for the mass: Smith 2001; Williams and Semlitsch 2010), whereas still other studies reported a growth inhibition upon exposure of anuran larvae to several GLY formulations (i.e., for the SVL: Howe et al. 2004; for the mass: Relyea 2004, 2012; Cauble and Wagner 2005; Jones et al. 2010; for the SVL and mass: Lanctôt et al. 2014). Clearly, no consensus whatsoever can be found among the existing data on the effects of GLY formulations on growth of anuran larvae. The discrepancies among the above GLY effects could result from differences in the biology of different species, or even differences among GLY formulations.
Previous studies evaluating the effects of GLY on the development of anuran larvae have shown that the isopropylamine salt of the compound does not induce significant effects on development (i.e., with respect to the time to reach metamorphosis: Howe et al. 2004; Lanctôt et al. 2014). Our results on the development of Gs-36 individuals showed no significant effects (the Kruskal-Wallis test; H = 2.251; df = 7; p = 0.945) after exposure to GLY, though differences were observed in Gs-25 larvae exposed to the compound (the Kruskal-Wallis test; H = 19.38; df = 7; p = 0.007), resulting in a significant increase in growth over that of the control group, with a LOEC value of 15 mg/L (Dunn’s post hoc test; p = 0.016; Table 3).
Several authors have already evaluated the effects of GLY commercial formulations on the development of anuran larvae. The most frequently observed effect was a reduction in the rate of metamorphosis (late completion of metamorphosis: Howe et al. 2004; Williams and Semlitsch 2010; Gahl et al. 2011; Navarro-Martín et al. 2014), but even the absence of an effect on metamorphosis has been reported (Smith 2001; Lanctôt et al. 2014; Wood and Welch 2015). In addition, Cauble and Wagner (2005) observed that tadpoles exposed to commercial formulations of GLY developed faster than controls. In the present study, although Gs-36 larvae exposed to RU evidenced no significant differences (Kruskal-Wallis’s test H = 3.657; df = 6; p = 0.723) in development, Gs-25 tadpoles similarly exposed manifested a significant increment in the rate of metamorphosis (Kruskal-Wallis test; H = 64.28; df = 14; p < 0.0001) with a LOEC value of 0.0007 mg a.e./L (Dunn’s post hoc test; p = 0.014; Table 3).
As stated above, growth and development are directly related. Larval growth is characterized by three main features: an initial period of nearly exponential growth, a deceleration, and finally a loss of body size at the climax of metamorphosis (Wilbur and Collins 1973). Development consists of both growth and differentiation, and this relationship varies with environmental conditions (Wilbur and Collins 1973). That, as an adaptive response to stressful conditions, larvae may metamorphose earlier at a smaller body size has, for example, been well documented (Wilbur and Collins 1973; Smith-Gill and Berven 1979; Morey and Reznick 2000; Denver and Crespi 2006). Moreover, a minimum, or threshold, body size must be achieved by a larva to undergo metamorphosis, whereas upon attaining a maximum body size, all larvae will necessarily metamorphose (Wilbur and Collins 1973, Collins Collins and Lewis 1979, Morey and Reznick 2000). In these experiments, we observed that exposure to either GLY or RU at early developmental stages induced an impairment of growth and further development. In this regard, our results could be indicating that GLY can be considered a stress-producing agent for tadpoles by causing an accelerated development that reaches metamorphosis at a precocious age. Within this context, the absence of an effect on Gs-36 individuals as opposed to the developmental acceleration of Gs-25 larvae can be explained by the minimum body size required to achieve metamorphosis.
Morphologic abnormalities
The proportion of abnormalities observed in the control group in all the bioassays was below 10 %, except for the loss of tooth rows in Gs-36 larvae observed in 20 % of the controls. Previous studies evaluating gonadal abnormalities and tail damage in anuran larvae exposed to GLY had indicated that the compound did not induce deformities (Howe et al. 2004; Lanctôt et al. 2014), whereas another report (Paganelli et al. 2010) had observed that embryos exposed to GLY presented craniofacial and ocular abnormalities. Our results indicated that GLY induced a loss of upper- and/or lower-jaw sheaths in tadpoles of Gs-25 (F = 3.801; df = 7; p = 0.006) and Gs-36 (F = 8.062; df = 7; p = 0.0003), with the same LOEC value of 30 mg/L being detected for both Gosner stages (the Dunnet post hoc test; p = 0.007 for Gs-25 and p = 0.045 for Gs-36; Tables 2 and 3; Fig. 2a, b). Moreover, a one-way ANOVA with Gs-36 tadpoles revealed significant differences in the incidence of edema (F = 3.917; df = 7; p = 0.011), from 300 mg/L of GLY (the Dunnet post hoc test; p = 0.006; Tables 2 and 3; Fig. 2g, h).
Morphologic abnormalities induced by GLY and RU on Gs-25 and Gs-36 larvae of L. latrans. a The oral disc of Gs-25 control larvae. b The oral disc of a GLY-exposed Gs-25 larva showing a loss of the lower-jaw sheath. c, e The oral discs of Gs-36 control larvae. d The oral disc of an RU-exposed Gs-36 larva illustrating tooth-ridge abnormalities. f The oral disc of an RU-exposed Gs-36 larva exemplifying the loss of tooth rows. g The body of a Gs-25 control larva. h The body of an RU-exposed Gs-25 larva with pronounced edema. The black arrows mark abnormalities. Scale bars, 1 mm
The occurrence of morphologic alterations upon exposing organisms to commercial formulations of GLY has been reported previously. Among those citations, we can mention oral, craniofacial, and ocular abnormalities, flexiured tails, branchial-cartilage reduction (i.e., larvae: Lajmanovich et al. 2003; embryos: Edginton et al. 2004; Paganelli et al. 2010), intestinal abnormalities (i.e., embryos: Edginton et al. 2004; larvae: Lenkowski et al. 2010), tail damage, and the presence of intersex gonads (Howe et al. 2004). Notwithstanding, other studies have not detected the presence of gonadal or morphologic alterations (i.e., in larvae: Edginton et al. 2004; Lanctôt et al. 2014; Navarro-Martín et al. 2014). A one-way ANOVA revealed that the presence of edema in Gs-25 tadpoles exposed to RU exhibited significant differences in occurrence from control values (F = 7.957; df = 14; p < 0.0001), at a LOEC value of 2.96 mg a.e./L (the Dunnet post hoc test p < 0.0001; Tables 2 and 3; Fig. 2g, h). In addition, Gs-36 data demonstrated significant differences in the incidence of tooth-ridge abnormalities (F = 9.12; df = 6; p = 0.0004; Tables 2 and 3; Fig. 2c, d) and the loss of tooth rows (F = 3.965; df = 5; p = 0.023; Tables 2 and 3; Fig. 2e, f) from control values, with LOEC values of 2.22 mg a.e./L for both determinations (the Dunnet post hoc test; p = 0.013 and p = 0.023, respectively).
The presence of edema results in a shifting of the center of gravity and a twisting of the body’s axis that can be also become manifest as an abnormality in swimming (Uthpala et al. 2010). This pathology could lead to a difficulty in escaping from predators or an impossibility in foraging, thus affecting the survival of tadpoles in such agroecosystems.
The oral disc of tadpoles in all but three families is composed of keratinized mouthparts (the jaw sheaths and tooth rows) that are surrounded by soft mouthparts (the labia and papillae). Leptodactylus latrans has the most common configuration of labial tooth rows corresponding to the 2/3 formula (Cei 1980; Mc Diarmig and Altig Mc Diarmid and Altig 2000; Altig 2007b). During feeding, the tooth rows function in anchoring the mouth to the substrate and the jaw sheaths in rasping surfaces to generate a particulate suspension of food (Wassersug and Yamashita 2001; Venesky et al. 2010a; Venesky et al. 2013). Therefore, the presence of oral anomalies (particularly in the jaw sheaths, tooth ridges, and tooth rows) must necessarily affect the capacity for feeding in tadpoles so as to limit the type and quantity of food that can be consumed. Those individuals will accordingly be expending more energy resources in the mere acquisition of food, thus affecting their growth and development. Such morphologic alterations in the tadpoles have well established impacts on the fitness of the adult frogs that ultimately affect their survival (Rowe et al. 1996; Rowe et al. 2001; Venesky et al. 2010a, 2010b; Venesky et al. 2013; Tolledo et al. 2014; Babini et al. Babini et al. 2015).
Conclusions
Experimental use of the species L. latrans enabled the detection of adverse effects induced by exposure to a formulation of GLY and to the active form of the herbicide. Lethal and sublethal effects were reproducible under standardized laboratory conditions, pointing to the use of this species as a surrogate anuran in toxicity testing for ecotoxicological surveys in regions where the frog inhabits—especially in those agroecosystems where L. latrans develops and reproduces that are being continuously impacted by pesticide use in extensive agriculture. In addition—and with respect to the methodology of testing—the present study shows for the first time that food supply to early developmental stages of L. latrans does not influence the lethal effects of GLY under conditions of acute exposure.
The GLY-based formulation Roundup ULTRA MAX® induced acute lethal and sublethal effects on L. latrans tadpoles. According to the categories established by the US Environmental Protection Agency, the Roundup ULTRA MAX® formulation can be classified as a slightly toxic (class III) agent, while GLY can be classified as a practically nontoxic (class IV) compound.
Technical-grade GLY produced sublethal effects on growth and development and induced morphologic deformities, such as oral abnormalities and edema. We wish to emphasize that, to the best of our knowledge, this report is the first one demonstrating oral abnormalities in anuran larvae exposed to technical-grade GLY. Moreover, the commercial formulation induced sublethal effects on all the experimental endpoints evaluated (i.e, swimming activity, growth, development, and morphologic abnormalities). Although growth and development proved to be the most sensitive of those endpoints, all of them would affect the fitness and survival of frogs in agroecosystems since the presence of, for example, edema can lead to difficulties in swimming. In addition, the occurrence of oral abnormalities and alterations in swimming activity must necessarily decrease feeding with deleterious consequences on growth and development.
The more sensitive developmental stage was Gs-25. The difference in sensitivity among Gosner stages observed here is in agreement with the findings from several studies reporting that Gs-25 tadpoles are the most sensitive (Mann and Bidwell 1999; Edginton et al. 2004; Smith 2001; Howe et al. 2004; Brodeur et al. 2009). This difference can be attributed to (1) the presence of protective membranes in embryos (Berril et al. 1993; Berrill et al. 1998), (2) the lack of target organs in embryos and very young larvae (Edginton et al. 2004), and (3) the size of the developing individual, which characteristic appears to be a parameter mitigating toxicity in advanced developmental stages (Mann and Bidwell 1999). Nevertheless, other studies have shown that the relationship between development and sensitivity did not follow a clear pattern (Berrill et al. 1998; Howe et al. 1998; Natale et al. 2000). This lack of consensus may be related to differences in the experimental protocols or the particular anuran species involved.
Both forms of the herbicide induce similar sublethal effects, though at different concentrations, with RU being five orders of magnitude more toxic than GLY. This difference suggests the conclusion that adjuvants in the formulation may favor bioavailability of the active ingredient or else also consist of additives that contribute to toxicity, as some authors have suggested (Giesy et al. 2000; Edginton et al. 2004; Howe et al. 2004; Puglis and Boone 2011; Moore et al. 2012).
On the basis of the environmental concentrations of GLY reported for Argentina and upon consideration of the worst-case scenario that has been detected of 0.7 mg a.e./L—a level at which sublethal effects on growth and development and the induction of morphologic abnormalities were observed in the present work—adverse impacts on the fitness of anuran larvae inhabiting agroecosystems of that region would necessarily be expected, as would such sequelae elsewhere in accordance with the levels of that toxic compound present.
References
Agostini MG, Kacoliris F, Demetrio P, Natale GS, Bonetto C, Ronco AE (2013) Abnormalities in amphibian populations inhabiting agroecosystems in northeastern Buenos Aires Province, Argentina. Dis Aquat Org 104:163–171
Al-Rajab AJ, Hakami OM (2014) Behavior of the non-selective herbicide glyphosate in agricultural soil. Am J Environ Sci 10:94–101
Altig R (2007) Comments on the descriptions and evaluations of tadpole mouthpart anomalies. Herpetol Conserv Biol 2:1–4
Annett R, Habibi HR, Hontela A (2014) Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J Appl Toxicol 34:458–479
Aparicio VC, De Gerónimo E, Marino D, Primost J, Carriquiriborde P, Costa JL (2013) Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 93:1866–1873
Araújo CVM, Shinn C, Moreira-Santos M, Lopes IG, Espíndola EL, Ribeiro R (2014a) “Copper-driven avoidance and mortality in temperate and tropical tadpoles. Aquat Toxicol 146:70–75
Araújo CVM, Shinn C, Vasconcelos AM, Ribeiro R, Espíndola ELG (2014b) “Preference and avoidance responses by tadpoles: the fungicide pyrimethanil as a habitat disturber. Ecotoxicology 23:851–860
ASTM (2007) ASTM E729-96(2007), Standard guide for conducting acute toxicity tests on test materials with fishes, macroinvertebrates and amphibians. ASTM International, Pennsylvania, USA
Babini MS, Bionda Cde L, Salas NE, Martino AL (2015) Health status of tadpoles and metamorphs of Rhinella arenarum (Anura, Bufonidae) that inhabit agroecosystems and its implications for land use. Ecotoxicol Environ Saf 118:118–125
Bantle, J. A., Dumont, J. N., Finch, R. A., Linder, G., and Fort, D. J. (1996). Atlas of abnormalities. A guide for the performance of FETAX. Second ed. Oklahoma State University. Oklahoma, USA.
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environmental Science Europe 28:3–18
Bernal MH, Solomon KR, Carrasquilla G (2009) Toxicity of formulated glyphosate (glyphos) and cosmo-flux to larval Colombian frogs 1. Laboratory acute toxicity. J Toxic Environ Health A 72:961–965
Berril M, Bertram S, Wilson A, Louis S, Brigham D, Stromberg C (1993) Lethal and sublethal impacts of pyrethroid insecticides on amphibian embryos and tadpoles. Environ Toxicol Chem 12:525–539
Berrill M, Coulson D, McGillivray D, Pauli B (1998) Toxicity of endosulfan to aquatic stages of anuran amphibians. Environ Toxicol Chem 17:1738–1744
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Am Zool 23:85–97
Bidwell, J. R., and Gorrie, J. R. (1995). “Acute toxicity of a pesticide to selected frog species.” Final Report. Department of Environmental Protection. Perth, Western Australia.
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456
Brodeur JC, Svartz G, Perez-Coll CS, Marino DJG, Herkovits J (2009) Comparative susceptibility to atrazine of three developmental stages of Rhinella arenarum and influence on metamorphosis: non-monotonous acceleration of the time to climax and delayed tail resorption. Aquat Toxicol 91:161–170
Broomhall S, Shine R (2003) Effects of the insecticide endosulfan and presence of congeneric tadpoles on Australian treefrog (Litoria freycineti) tadpoles. Arch Environ Contam Toxicol 45:221–226
Brunelli E, Bernabò I, Berg C, Lundstedt-Enkel K, Bonacci A, Tripepi S (2009) Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles. Aquat Toxicol 91:135–142
CASAFE. (2012). Argentine Plant Protection Products Market. 2012. Chamber of Agricultural Health and Fertilizers. Buenos Aires, Argentina. (in Spanish)
Cauble K, Wagner RS (2005) Sublethal effects of the herbicide glyphosate on amphibian metamorphosis and development. Bull Environ Contam Toxicol 75:429–435
Cei, J. M. (1980). Amphibians of Argentina. Monograph. University of Florence. Firenze, Italy. (in Spanish).
Clements C, Ralph S, Petras M (1997) Genotoxicity of select herbicides in Rana catesbeiana tadpoles using the alkaline single-cell gel DNA electrophoresis (comet) assay. Environ Mol Mutagen 29:277–288
Close B, Banister K, Baumans V, Bernoth E, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H (1996) Recommendations for euthanasia of experimental animals: part 1. Lab Anim 30:293–316
Collins JP, Lewis MA (1979) Overwintering tadpoles and breeding season variation in the Rana pipiens complex in Arizona. Southwest Nat 24:371–373
Cooke AS (1981) Tadpoles as indicators of harmful levels of pollution in the field. Environmental Pollution Series A 25:123–133
Costa MJ, Monteiro DA, Oliveira-Neto AL, Rantin FT, Kalinin AL (2008) Oxidative stress biomarkers and heart function in bullfrog tadpoles exposed to Roundup Original®. Ecotoxicology 17:153–163
Coupe RH, Kalkhoff SJ, Capel PD, Gregoire C (2012) Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag Sci 68:16–30
Demetrio, P. M. (2012). “Study of biological effects of pesticides used in soybean RR and evaluation of adverse impacts on agro-ecosystems of the Pampas.” PhD Dissertation. National University of La Plata. (in Spanish).
Denoël M, Libon S, Kestemont P, Brasseur C, Focant JF, De Pauw E (2013) Effects of a sublethal pesticide exposure on locomotor behavior: a video-tracking analysis in larval amphibians. Chemosphere 90:945–951
Denver RJ, Crespi EJ (2006) Stress hormones and human developmental plasticity. NeoReviews 7:183–188
Domingo Yagüez, J., Ferreyra, A., Langhi, R., Pausich, G., Pezzola, A., and Coma, C. (2011). Soy Campaign 2010–2011. Buenos Aires, Argentina: National Institute of Agricultural Technology, Ministry of Agriculture, Livestock and Fisheries. (in Spanish).
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64:319–325
Edge CB, Thompson DG, Hao C, Houlahan JE (2012) A silviculture application of the glyphosate-based herbicide Vision MAX to wetlands has limited direct effects on amphibian larvae. Environ Toxicol Chem 31:2375–2383
Edginton AN, Sheridan PM, Stephenson GR, Thompson DG, Boermans HJ (2004) Comparative effects of pH and Vision® herbicide on two life stages of four anuran amphibian species. Environ Toxicol Chem 23:815–822
Egea-Serrano A, Relyea RA, Tejedo M, Torralva M (2012) Understanding of the impact of chemicals on amphibians: a meta-analytic review. Ecology and Evolution 2:1382–1397
Feder ME (1983) The relation of air breathing and locomotion to predation on tadpoles, Rana berlandieri, by turtles. Physiol Zool 56:522–531
Finney DJ (1971) Probit Analysis, 3rd edn. Cambridge University Press, Cambridge, England
Fuentes L, Moore LJ, Rodgers JH, Bowerman WW, Yarrow GK, Chao WY (2011) Comparative toxicity of two glyphosate formulations (original formulation of Roundup® and Roundup WeatherMAX®) to six North American larval anurans. Environ Toxicol Chem 30:2756–2761
Gahl MK, Pauli BD, Houlahan JE (2011) Effects of chytrid fungus and a glyphosate-based herbicide on survival and growth of wood frogs (Lithobates sylvaticus. Ecol Appl 21:2521–2529
Giesy, J. P., Dobson, S., and Solomon, K. R. (2000). “Ecotoxicological risk assessment for Roundup® herbicide.” In Reviews of environmental contamination and toxicology, ed. G. W. Ware. Springer, New York USA. 35–120.
Gosner K (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Govindarajulu, P. P. (2008). Literature review of impacts of glyphosate herbicide on amphibians: what risks can the silvicultural use of this herbicide pose for amphibians in B.C.? Wildlife Report No. R-28. B.C. Ministry of Environment. Victoria, BC.
Güngördü A (2013) Comparative toxicity of methidathion and glyphosate on early life stages of three amphibian species: Pelophylax ridibundus, Pseudepidalea viridis, and Xenopus laevis. Aquat Toxicol:140–141
Heyer, R., Langone, J., La Marca, E., Azevedo-Ramos, C., di Tada, I., Baldo, D., Lavilla, E., Scott, N., Aquino, L., and Hardy, J. (2010). Leptodactylus latrans. In The IUCN Red List of Threatened Species. The IUCN Red List of Threatened Species. Version 2015-4. www.iucnredlist.org. Accessed on 16 April 2016
Horat P, Semlitsch RD (1994) Effects of predation risk and hunger on the behavior of two species of tadpoles. Behav Ecol Sociobiol 34:393–401
Howe CM, Berrill M, Pauli BD, Helbing CC, Werry K, Veldhoen N (2004) Toxicity of glyphosate-based pesticides to four North American frog species. Environ Toxicol Chem 23:1928–1938
Howe G, Gillis R, Mowbray RC (1998) Effect of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ Toxicol Chem 17:519–525
IUCN (2016). The IUCN Red List of Threatened Species. Version 2016–1. www.iucnredlist.org. Accessed on 7 July 2016.
Jones DK, Hammond JI, Relyea RA (2010) Roundup® and amphibians: the importance of concentration, application time, and stratification. Environ Toxicol Chem 29:2016–2025
Jones DK, Hammond JI, Relyea RA (2011) Competitive stress can make the herbicide Roundup® more deadly to larval amphibians. Environ Toxicol Chem 30:446–454
Jung RE, Jagoe CH (1995) Effects of low pH and aluminum on body size, swimming performance, and susceptibility to predation of green tree frog (Hyla cinerea) tadpoles. Can J Zool 73:2171–2183
Junges CM, Vidal EE, Attademo AM, Mariani ML, Cardell L, Negro AC, Cassano A, Peltzer PM, Lajmanovich RC, Zalazar CS (2013) Effectiveness evaluation of glyphosate oxidation employing the H2O2/UVC process: toxicity assays with Vibrio fischeri and Rhinella arenarum tadpoles. Journal of Environmental Science and Health, Part B 48:163–170
Lajmanovich RC, Attademo AM, Peltzer PM, Junges CM, Cabagna MC (2011) Toxicity of four herbicide formulations with glyphosate on Rhinella arenarum (Anura: Bufonidae) tadpoles: B-esterases and glutathione S-transferase inhibitors. Arch Environ Contam Toxicol 60:681–689
Lajmanovich RC, Junges CM, Attademo AM, Peltzer PM, Cabagna-Zenklusen MC, Basso A (2013) Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water Air Soil Pollut 224:1–13
Lajmanovich RC, Junges CM, Cabagna-Zenklusen MC, Attademo AM, Peltzer PM, Maglianese M, Marquez VE, Beccaria AJ (2015) Toxicity of Bacillus thuringiensis var. israelensis in aqueous suspension on the South American common frog Leptodactylus latrans (Anura: Leptodactylidae) tadpoles. Environ Res 136:205–212
Lajmanovich RC, Sandoval MT, Peltzer PM (2003) Induction of mortality and malformation in Scinax nasicus tadpoles exposed to glyphosate formulations. Bull Environ Contam Toxicol 70:0612–0618
Lanctôt C, Navarro-Martín L, Robertson C, Park B, Jackman P, Pauli BD, Trudeau VL (2014) Effects of glyphosate-based herbicides on survival, development, growth and sex ratios of wood frog (Lithobates sylvaticus) tadpoles. II: agriculturally relevant exposures to Roundup WeatherMax® and Vision® under laboratory conditions. Aquat Toxicol 154:291–303
Lanctôt C, Robertson C, Navarro-Martín L, Edge C, Melvin SD, Houlahan J, Trudeau VL (2013) Effects of the glyphosate-based herbicide Roundup WeatherMax® on metamorphosis of wood frogs (Lithobates sylvaticus) in natural wetlands. Aquat Toxicol:140–141
Lenkowski JR, Sanchez-Bravo G, McLaughlin KA (2010) Low concentrations of atrazine, glyphosate, 2,4-dichlorophenoxyacetic acid, and triadimefon exposures have diverse effects on Xenopus laevis organ morphogenesis. J Environ Sci 22:1305–1308
López S, Aiassa D, Benítez-Leite S, Lajmanovich R, Mañas F, Poletta G, Sánchez N, Simoniello MF, Carrasco AE (2012) Pesticides used in south American GMO-based agriculture: a review of their effects on humans and animal models. In: Advances in molecular toxicology, ed. J. C. Fishbein and J. M. Heilman. Elsevier, Amsterdam The Netherlands, pp. 41–75
Lupi L, Miglioranza KSB, Aparicio VC, Marino D, Bedmar F, Wunderlin DA (2015) Occurrence of glyphosate and AMPA in an agricultural watershed from the southeastern region of Argentina. Sci Total Environ 536:687–694
Mann RM, Bidwell JR (1999) The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch Environ Contam Toxicol 36:193–199
Mc Diarmid RW, Altig R (2000) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, USA
Meyer, M. T., Loftin, K. A., Lee, E. A., Hinshaw, G. H., Dietze, J. E., and Scribner, E. A. (2009). Determination of glyphosate, its degradation product aminomethylphosphonic acid, and glufosinate, In Water by isotope dilution and online solid-phase extraction and liquid chromatography/tandem mass spectrometry: U.S. Geological Survey Techniques and Methods. Techniques and Methods 5–A10. U.S. Geological Survey. Virginia, USA.
Moore LJ, Fuentes L, Rodgers JH Jr, Bowerman WW, Yarrow GK, Chao WY, Bridges WC Jr (2012) Relative toxicity of the components of the original formulation of Roundup® to five North American anurans. Ecotoxicol Environ Saf 78:128–133
Morey S, Reznick D (2000) A comparative analysis of plasticity in larval development in three species of spadefoot toads. Ecology 81:1736–1749
Natale, G. S. (2006). “Ecotoxicological analysis of a community of anurans in the Pampa region: effect of Cr (VI) on embryos and larvae of different species of a taxo-community.” PhD Dissertation, National University of La Plata. (in Spanish).
Natale GS, Ammassari LL, Basso NG, Ronco AE (2006) Acute and chronic effects of Cr (VI) on Hypsiboas pulchellus embryos and tadpoles. Dis Aquat Org 72:261–267
Natale GS, Basso NE, Ronco AE (2000) Effect of Cr(VI) on early life stages of three species of Hylid frogs (Amphibia, Anura) from South America. Environ Toxicol 15:509–512
National Research Council of the National Academies (2011) Guide for care and use of laboratory animals, 8th edn. The National Academies Press, Washington, DC, USA
Navarro-Martín L, Lanctôt C, Jackman P, Park BJ, Doe K, Pauli BD, Trudeau VL (2014) Effects of glyphosate-based herbicides on survival, development, growth and sex ratios of wood frogs (Lithobates sylvaticus) tadpoles. I: chronic laboratory exposures to VisionMax®. Aquat Toxicol 154:278–290
Newman MC (2015) Fundamentals of ecotoxicology, The science of pollution. 4th Edition. CRC Press. Taylor & Francis group, Florida USA
Paganelli A, Gnazzo V, Acosta H, López SL, Carrasco AE (2010) Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. Chem Res Toxicol 23:1586–1595
Peltzer PM, Lajmanovich RC, Sanchez LC, Attademo AM, Junges CM, Bionda CL, Martino A, Basso A (2011) Morphological abnormalities in amphibian populations. Herpetol Conserv Biol 6:432–442
Peltzer PM, Lajmanovich RC, Attademo AM, Junges CM, Cabagna-Zenklusen MC, Repetti MR, Sigrist ME, Beldoménico H (2013) Effect of exposure to contaminated treefrog (Trachycephalus typhonius) tadpoles. Ecotoxicol Environ Saf 98:G142–G151
Peruzzo PJ, Porta AA, Ronco AE (2008) Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ Pollut 156:61–66
Primost, J. (2013). “Study of environmental levels of glyphosate and AMPA in a model of intensive agriculture area around Urdinarrain, Entre Rios”. Graduate Dissertation, National University of La Plata. (in Spanish)
Puglis HJ, Boone MD (2011) Effects of technical-grade active ingredient vs. commercial formulation of seven pesticides in the presence or absence of UV radiation on survival of green frog tadpoles. Arch Environ Contam Toxicol 60:145–155
Relyea RA (2004) Growth and survival of five amphibian species exposed to combinations of pesticides. Environ Toxicol Chem 23:1737–1742
Relyea RA (2012) New effects of Roundup on amphibians: predators reduce herbicide mortality; herbicides induce antipredator morphology. Ecol Appl 22:634–647
Relyea RA, Jones DK (2009) The toxicity of Roundup Original Max® to 13 species of larval amphibians. Environ Toxicol Chem 28:2004–2008
Rist L, Semlitsch RD, Hotz H, Reyer H-U (1997) Feeding behaviour, food consumption, and growth efficiency of hemiclonal and parental tadpoles of the Rana esculenta complex. Funct Ecol 11:735–742
Ronco AE, Marino DJG, Abelando M, Almada P, Apartin CD (2016) Water quality of the main tributaries of the Paraná Basin: glyphosate and AMPA in surface water and bottom sediments. Environ Monit Assess 188:1–13
Rowe CL, Hopkins WA, Congdon JD (2001) Integrating individual-based indices of contaminant effects. How multiple sublethal effects may ultimately reduce amphibian recruitment from a contaminated breeding site.” The. Scientific World 1:703–712
Rowe CL, Kinney OM, Fiori AP, Congdon JD (1996) Oral deformities in tadpoles (Rana catesbeiana) associated with coal ash deposition: effects on grazing ability and growth. Freshw Biol 36:723–730
Ruiz de Arcaute C, Salgado Costa C, Demetrio PM, Natale GS, Ronco AE (2012) Influence of existing site contamination on sensitivity of Rhinella fernandezae (Anura, Bufonidae) tadpoles to Lorsban® 48E formulation of chlorpyrifos. Ecotoxicology 21(8):2338–2348
Semlitsch RD (1993) Adaptive genetic variation in growth and development of tadpoles of the hybridogenetic Rana esculenta complex. Evolution 47:1805–1818
Semlitsch RD, Foglia M, Mueller A, Steiner I, Fioramonti E, Fent K (1995) Short_term exposure to triphenyltin affects the swimming and feeding behavior of tadpoles. Environ Toxicol Chem 14:1419–1423
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68:344–350
Smith GR (2001) Effects of acute exposure to a commercial formulation of glyphosate on the tadpoles of two species of anurans. Bull Environ Contam Toxicol 67:483–488
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–585
Sparling DW, Fellers GM (2009) Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environ Toxicol Chem 28:1696–1703
Sparling DW, Linder G, Bishop CA, Krest S (2010) Ecotoxicology of amphibians and reptiles, Second edn. Society of Environmental Toxicology and Chemistry Press, Florida USA
Stauffer HP, Semlitsch RD (1993) Effects of visual, chemical and tactile cues of fish on the behavioural responses of tadpoles. Anim Behav 46:355–364
Stebbins RC, Cohen NW (1997) A natural history of amphibians. Princeton University Press, Princeton, New Jersey
Tolledo J, Silva ET, Nunes-de-Almeida CHL, Toledo LF (2014) Anomalous tadpoles in a Brazilian oceanic archipelago: implications of oral anomalies on foraging behaviour, food intake and metamorphosis. Herpetol J 24:237–243
U.S. EPA, (1975). Methods for acute toxicity tests with fish, macroinvertebrates, and amphibians. USEPA 660/3–75-009. Ecological Research series, U.S. Environmental Protection Agency. Washington DC, USA, pp 62.
U.S. E.P.A. (1999). EPA Probit Analysis Program used for calculating LC/EC values. Version 1.5. Ecological Monitoring Research Division, Environmental Monitoring Systems Laboratory, U.S. Environmental Protection Agency. OHIO, USA.
Uthpala AJ, Rupika SR, Ayanthi NN, Priyani HA (2010) Toxicity of agrochemicals to common hourglass tree frog (Polypedates cruciger) in acute and chronic exposure. IJAB Online Issues 12:641–648
Vaira M, Akmentins M, Attademo M, Baldo D, Barrasso DA, Barrionuevo S, Basso N, Blotto B, Cairo S, Cajade R, Céspedez J, Corbalán V, Chilote P, Duré M, Falcione C, Ferraro D, Gutierrez R, Ingaramo Md, Junges C, Lajmanovich R, Lescano JN, Marangoni F, Martinazzo L, Marti R, Moreno L, Natale GS, Perez Iglesias JM, Peltzer P, Quiroga L, Rosset S, Sanabria E, Sanchez L, Schaefer E, Úbeda C, Zaracho V (2012) Categorización del estado de conservación de los anfibios de la República Argentina. Cuadernos de herpetología 26:131–159 in Spanish
Venesky MD, Rossa-Feres DC, Nomura F, Vasconcellos de Andrade G, Leite PT, de Sousa V. TT, Anderson CV, Wassersug RJ (2013) Comparative feeding kinematics of tropical hylid tadpoles. J Exp Biol 216:1928–1937
Venesky MD, Wassersug RJ, Parris MJ (2010a) . “How does a change in labial tooth row number affect feeding kinematics and foraging performance of a Ranid tadpole (Lithobates sphenocephalus)? Biol Bull 218:160–168
Venesky MD, Wassersug RJ, Parris MJ (2010b) “The impact of variation in labial tooth number on the feeding kinematics of tadpoles of southern leopard frog (Lithobates sphenocephalus. Copeia 3:481–486
Williams BK, Semlitsch RD (2010) Larval responses of three Midwestern anurans to chronic, low-dose exposures of four herbicides. Arch Environ Contam Toxicol 58:819–827
Wassersug RJ, Yamashita M (2001) Plasticity and constraints on feeding kinematics in anuran larvae. Comparative Biochemistry and Physiology Part A 131:183–195
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314
Wojtaszek BF, Staznik B, Chartrand DT, Stephenson GR, Thompson DG (2004) Effects of Vision® herbicide on mortality, avoidance response, and growth of amphibian larvae in two forest wetlands. Environ Toxicol Chem 23:832–842
Wood L, Welch AM (2015) Assessment of interactive effects of elevated salinity and three pesticides on life history and behavior of southern toad (Anaxyrus terrestris) tadpoles. Environ Toxicol Chem 34:667–676
Yadav SS, Giri S, Singha U, Boro F, Giri A (2013) Toxic and genotoxic effects of Roundup on tadpoles of the Indian skittering frog (Euflictis cyanophlyctis) in the presence and absence of predator stress. Aquat Toxicol:132–133
Young BE, Stuart SN, Chanson JS, Cox NA, Boucher TM (2004) Disappearing jewels: the status of new world amphibian. Nature Serve, Virgina, USA
Zar JH (2010) Biostatistical analysis. Prentice Hall, New Jersey, US
Acknowledgments
We would like to thank Dr. Damian Marino for chemical analyses. The study was supported by Agencia Nacional de Promoción Científica y Tecnológica under Grants PICT-2010-0891 and PICT-2014-0919. Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The tadpoles were maintained under laboratory conditions according to the Guide for Care and Use of Laboratory Animals (National Research Council 2011).
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bach, N.C., Natale, G.S., Somoza, G.M. et al. Effect on the growth and development and induction of abnormalities by a glyphosate commercial formulation and its active ingredient during two developmental stages of the South-American Creole frog, Leptodactylus latrans . Environ Sci Pollut Res 23, 23959–23971 (2016). https://doi.org/10.1007/s11356-016-7631-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7631-z