Abstract
In the present study, metal-facilitated free radical generation in particulate matter (PM) and its association with deoxyribonucleic acid (DNA) damage were studied. The examined data showed that the concentration of fine PM in Pune exhibited seasonal variations. Inductively coupled plasma-atomic emission spectrometry (ICP-AES) was used to examine the metal composition, which showed the presence of metals such as Cu, Zn, Mn, Fe, Co, Cr, Pb, Cd, and Ni. Fe metal was present in the highest concentrations in both the seasons, followed by Zn. The scanning electron microscopy-energy-dispersive spectrometer (SEM-EDS) results also demonstrated that the fine PM particles deposited in summer samples were less than those of winter samples, suggesting that the PM load in winter was higher as compared to that in summer. Elemental mapping of these particles substantiates deposition of metals as Fe, Zn, etc. on particles. The electron paramagnetic species (EPR) technique was utilized for free radical detection, and plasmid DNA assay was utilized to study the genotoxicity of ambient fine PM. Obtained g values show the presence of radicals in PM samples of Pune. PM contains the C-centered radical with a vicinal oxygen atom (g = 2.003). In addition to this, the g value for Fe was also observed. Therefore, we intend that the radicals related with fine PM comprise metal-mediated radicals and produce DNA damage. The plasmid DNA assay results indicated that the TM50 values (toxic mass of PM causing 50 % of plasmid DNA damage) of PM exhibited seasonal variations with higher TM50 values for summer and lower TM50 values during winter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Particulate matter (PM) is one of the precarious air pollutants, which consists of a mixture of solid and liquid particles suspended in the air, varying in size, shape, surface area, morphology, solubility, origin, and chemical, physical, and biological characteristics (Mouli et al. 2003; Satsangi and Yadav 2014; Pipal et al. 2014). Epidemiological studies have shown the relationship between PM exposure and increased mortality and morbidity due to cardiovascular and respiratory diseases (Fierro et al. 1996, Abbey et al. 1995, Pope et al. 1995; Ghauri et al. 2012; Pope et al. 2002; Nawrot et al. 2007; Gotschi et al. 2008; Hsieh et al. 2011; Hung et al. 2012). Researchers have agreed that for a health-related aerosol study, probably fine (PM2.5) particles would be the more appropriate size than coarse mode particles (PM10) (Kupelwieser et al. 2010). Fine PM is considered to be more hazardous than larger-size PM due to its longer dwelling time in the atmosphere, and also, it can easily be deposited in the lungs in the course of respiration consequentially leading to many diseases. These PM2.5 particles generally are of alveolar size range, thus able to penetrate into the deep area of the lungs, i.e., the alveolar region, leading to several well-documented health effects including cardiovascular diseases. Moreover, more toxic substances are absorbed by fine particles as compared to coarse particles (Ormstad 2010; Peters et al. 2001; Makkonen et al. 2010). Once deposited in the respiratory system, the action of PM depends on their chemical composition and toxicity.
According to some researchers, more than one constituent of PM may be responsible for its toxicity (Donaldson et al. 1998; Dreher 2000). These toxic substances include transition metal content, organic content, particle size and surface, free radicals, presence of endotoxins, or other biologicals (Dreher 2000; Donaldson et al. 1998; Monn and Becker 1999; Monn et al. 2002; Molinelli et al. 2006; Niu et al. 2010; Massey et al. 2013). According to the WHO nationwide analysis report in 2002, the main cause of death was chronic respiratory diseases (WHO 2002). Maharashtra State Health Resource Centre also published a report in March 2010 to examine the peak ten death causes in Maharashtra in which they found that chronic obstructive pulmonary disease (COPD) was the highest cause of death (Health Status Maharashtra 2010). Smoking is the major reason for COPD, but one of the reasons for COPD is also the fine particulate matter emitted by combustion sources and vehicular emissions. A significant association between outdoor air pollution and COPD admissions has been observed by several studies in the USA, in Europe, and worldwide (Ko and Hui 2012).
Pune is one of the most rapidly growing metropolitan cities of the country in Maharashtra, India, due to the establishments of various small- and large-scale industries like glasswork, electronic items, steel, rubber, agricultural products, etc. The industrial area covers nearly 10,000 industries that include TATA, Bajaj, Thermax, L&T, etc. (Sonaje et al. 2013). In addition to the faster rate of industrialization, it is emerging as an IT park city because it provides a source of employment and education resulting in the immigration of people from other states to it (Gidde and Sonawane 2012). Thus, Pune now has developed into a big city with more than three million inhabitants. The rapid rise in urbanization and population has tremendously increased the vehicular count by 54 % in the city from 16,22,675 (2008) (Gidde and Sonawane 2012) to 29,83,749 (2015) (Times City, 9 Dec 2015). Due to these facts, the air quality of Pune is getting worse especially in terms of particulate matter.
Although there are several studies on the particulate pollution of Pune, the toxicological studies from this city are almost lacking, clearly pointing towards the existence of a research gap from this city. Keeping this in view, the present study was framed with the aim to assess the genotoxicity of fine PM-bound metals in Pune City by characterizing their concentrations and quantifying the free radical generation and plasmid DNA damage. The study provides significant information on the baseline data and the importance of transition metals in the generation of free radicals from ambient PM and their potential health risks in the Pune urban area.
Material and methods
Sampling and analysis
Pune (18° 32′ N, 73° 51′ E) is located on the western margin of the Deccan plateau, 560 m (1840 ft) above sea level. It is situated on the leeward side of the Sahyadri mountain range, which forms a barrier between the Arabian Sea (Satsangi and Yadav 2014). The city is surrounded by hills and mountains. Just outside the city, Sinhagad fort is located at an altitude of 1300 m. Pune has a tropical wet and dry climate with average temperatures ranging between 20 and 33 °C (Satsangi and Yadav 2014). In summer, the temperature range was from 28.3 to 39.5 °C and in winter from 10.5 to 23.0 °C. Figure 1 shows the map of the sampling site. A detailed site description has been given elsewhere (Satsangi and Yadav 2014).
Sampling of fine PM was carried out at the terrace of the Department of Chemistry, Savitribai Phule Pune University (SPPU), Pune. A major traffic junction (i.e., university circle) and a railway line are nearly 1 and 3 km from the sampling site, respectively. The principal sources of contamination are traffic, biomass burning, and construction activities in and around the vicinity of the sampling site (Yadav and Satsangi 2013). The sampling was carried out at a height of ~12 m from the ground level, from December 2012 to May 2013. PM samples were collected for a period of 24 h on pre-conditioned (48 h at 25 °C and constant relative humidity around 55 %) and pre-weighed polytetrafluoroethylene (PTFE) filters (Whatman, 47 mm diameter, 2 μm pore size) using a Mini Vol - TAS air sampler (Airmetrics Co. Inc., 5 L min−1 flow rate, sample volume of 7.2 m3) provided with PM2.5 cutoff inlets. A total of 49 samples were collected in winter and 53 samples in the summer season (Yadav et al. 2015). After particle collection, the filters were preserved in the refrigerator at 4 °C till the analysis. In the present study, the filter was cut into two equal halves; one half of the filter was used for trace metal analysis and the other half for plasmid DNA assay, electron paramagnetic species (EPR) study, and scanning electron microscopy (SEM) analysis.
Gravimetric analysis
PM masses were calculated gravimetrically by subtracting the initial mass of the blank filter from the final mass of the sampled filter. The filters were weighed repeatedly (Shimadzu AUX 220; sensitivity ±1 μg) till three reproducible values were obtained (Yadav et al. 2015). The filters were equilibrated in silica gel desiccators at 25 °C temperature at a relative humidity of 35–45 % for 24 h before weighing to eradicate the effect of humidity and also to gain accurate particulate matter measurements. The filters were handled with tweezers coated with Teflon tape to reduce the risk of contamination (Yadav et al. 2015).
Trace metal concentration analysis
In this study, half of the filter samples were extracted using Milli-Q water at room temperature. The filters were sonicated for 1 h with 15 mL of Milli-Q water (pH ~5.8). This extract was then acidified using ~10 % nitric acid to prevent the loss of metals and stored in clean polypropylene bottles to determine water-soluble metal concentration. In recent years, water is chosen as the simplest and most universal extraction solvent for evaluating the water-extractable fraction of total metals in PM (Heal et al. 2005; Shi et al. 2003; Yadav and Satsangi 2013). An inductively coupled plasma-atomic emission spectrometer (ICP-AES) (ARCOS, Spectro, Germany) was used for analysis of nine metals (Cu, Zn, Mn, Fe, Co, Cr, Pb, Cd, Ni) within a month of extraction.
SEM analysis
Scanning electron microscopy (JEOL JSM-6330F, JEOL Ltd., Akishima, Tokyo, Japan) was used for morphological characterization of fine particles. The samples were cut in sizes of 1 cm2 from the PM collected sample filter. To make the samples electrically conductive, a thin film of platinum (Pt) was deposited on the surface of the samples (Satsangi and Yadav 2014). These samples were then placed and fixed on electron microprobe stubs. A computer-controlled field emission SEM equipped with an energy-dispersive spectrometer (EDS) was used for analyses of PM samples. In the present investigation, the SEM was used in its most common mode, i.e., emissive mode. Instrumental parameters were as detector, Si (Li); accuracy, 10 %; limit of detection, 500,000; and energy, 20 kV (accelerating voltages, 0.5–30 kV) (Satsangi and Yadav 2014). Particle morphology and particle count were assessed by a quantitative image analyzer (IA) using ImageJ software (Satsangi and Yadav 2014).
EPR analysis
Electron paramagnetic resonance (EPR) was utilized to evaluate the free radicals generated by PM. The filters containing the PM samples were cut into strips, rolled, and inserted in such a way that the PM collected side was the outer part into quartz EPR tubes. The EPR analysis of the same samples was done in triplicate for better detection of g values. A g value is a dimensionless quantity that describes the magnetic moment and gyromagnetic ratio of a particle or nucleus. The EPR spectra were recorded at low temperature using a JES-FA200 ESR spectrometer with the following instrumental conditions: microwave frequency, 9.3 GHz; X-band, 100 kHz; modulation amplitude, 2 G; attenuation, 7 dB; scan range, 400 G; receiver gain, 2500; time constant, 1 s; and scan time, 2 min. The g values were assessed using standard 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Roy et al. 2015).
DNA toxicity analysis
This assay examines the influence of extracts of fine PM to bring in strand breaks of phage DNA (Dellinger et al. 2001). Particles were separated from the filter substrate by immersing half of the filter in 500 μL phosphate-buffered saline (PBS) in microcentrifuge tubes. The sample was shaken evenly for 6 h and then vortexed for 30 min to discharge the PM into the solution from the filter. This was considered as the stock PM solution. This stock solution was further diluted with PBS to the required concentration. This concentration could be between 50 and 400 μg mL−1 depending on the original mass concentration of the PM (Shi et al. 2004; Xiao et al. 2013). Another two blank Eppendorf (EP) microcentrifuge tubes were taken and filled with the negative (unexposed blank filter extracted in PBS) and positive (0.04 % H2O2) as controls, respectively. To all the experiment solutions as well as control solutions, 1 μL (200 ng mL−1) of phiX174 supercoiled phage DNA (Bangalore Genie) was added. The microcentrifuge tubes were then incubated at room temperature by gently vibrating for 1 h onto a vortex (Vortex Genie2). After this, 3 μL of loading dye (bromophenol blue) was added to the above solution and homogenized. Then this solution was loaded in the gel wells and was run on 0.6 % agarose gel in 1 % tris-borate-EDTA (TBE) buffer for 6 h at 35 V. The gel was stained with ethidium bromide. Finally, the gels were visualized and photographed by computerized Geldoc (EC3 Chemi HR 410 Imagining System). The degradation and strand breaks of DNA were determined by the conversion of supercoiled øX174 DNA to the linear forms as previously reported by others (Kumagai et al. 1996, Senlin et al. 2008, Xiao et al. 2013). A quantitative procedure was set up to measure the relative proportion of the DNA damaged in each lane (in terms of a percentage of the total DNA in each lane). Visionworks life science software was used for calculating the percent of DNA in each lane. The PM-induced DNA damage rate was calculated by subtracting the damage caused by the negative control. A regression equation was used to calculate the PM mass causing 50 % damage (i.e., TM50 toxic mass causing 50 % damage) to supercoiled DNA. The experiment was performed in triplicate to reduce the uncertainty on the PM dose.
Quality control parameters
A blank test for background contamination was performed by using unexposed filter papers, which were handled with field samples. The blank filters were stored and taken to the field to replicate the same atmosphere as the actual field samples used in the measurements. The blank filters were taken thrice during the season (Yadav and Satsangi 2013). After particle collection, the filters were well preserved in the refrigerator at 4 °C till the analysis. In this study, the background contamination was used to correct measurements. Field blank values were subtracted from the metal concentration to determine background contamination. Field blank values were very low, usually below or near the method detection limits. The recovery efficiencies were also calculated by spiking 10 % of the samples with a known amount of metal. The minimum detection limit and recovery test results are presented in Table 1 for all the metals. The recovery efficiency range was 92.3–108.2 %. Analysis for the same standard solution was repeated ten times for the reproducibility test, which verified the constancy of instruments.
For DNA assay, several batches of experiment were performed with different concentrations of samples collected throughout the study along with the negative (DNA + PBS) and positive (DNA + PBS + H2O2) controls. The experiment was performed in triplicate to reduce the uncertainty on the PM dose.
Results and discussion
Mass concentration of particulate matter
Mass concentrations of the exposed PM sample were gravimetrically determined during the period of Dec 2012 to May 2013 in the Department of Chemistry, Savitribai Phule Pune University (SPPU). The daily mass concentrations of particulate matter at this site varied from 59.8 ± 27.2 to 139.6 ± 38.6 μg m−3. As depicted in Fig. 2, the concentrations of PM in the winter season (average = 111.1 ± 32.2 μg m−3) (Dec–Feb) were about 1.4 times higher than the concentrations in summer months (average = 77.15 ± 24.03 μg m−3) (March–May). The seasonal and monthly variabilities of the PM concentration were influenced by both the local anthropogenic activities and meteorological factors. The PM concentration may go up to numerous times higher than the normal level under poor meteorological conditions, i.e., inversion conditions (Yadav et al. 2015; Bathmanabhan and Madanayak 2010; Elminir 2005). The higher mass concentration was observed in the winter season as compared to the summer season which can be explained on the basis of physical parameters like temperature (temp.), relative humidity (RH), and other meteorological variations (i.e., wind speed and direction) (Table 2). The wind speed and temperature were found to be low in the winter and gradually increased during summer. In winter, the average relative humidity was also comparatively high (67.36 %), contributing to higher collection of fine PM. Moreover, due to a lower mixing height, the dispersion of fine and submicron particulates is restricted and results in trapping of the particles nearer to the ground level (Salve et al. 2006; Yadav and Satsangi 2013) which leads to an elevated concentration of fine PM in the winter season. In addition, a high concentration of PM during winter can be due to biomass burning (excess of solid waste viz. waste paper, fallen leaves, and garden cuttings are burned by the side of roads) and increased heating activities in the proximity of the site (Yadav et al. 2015) The National Air Quality Standards (NAAQS) for ambient fine PM (PM2.5) in India are set at 60 μg m−3. The average concentration of the present study (94.1 ± 31.2 μg m−3) was found to be 1.5 times higher than the NAAQS. The PM concentrations are much higher (3.7 times) than WHO standards (25 μg m−3).
Fine PM mass concentrations in the present study were compared with earlier reported mass concentrations in India with similar locations (i.e., urban) (Table 3). The PM mass concentrations reported by Pipal et al. (2014) at Agra and Deshmukh et al. (2013) at Raipur were higher as compared to those of the present study, whereas the PM2.5 mass concentration reported by Srimuruganandam and Nagendra (2011) in Chennai were less than those of the present study.
Trace metal concentrations and their source apportionment
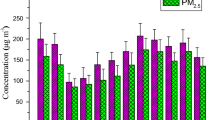
A total of nine elements (Cu, Zn, Mn, Fe, Co, Cr, Pb, Cd, Ni) in the fine PM were analyzed by ICP-AES. Figure 3 shows the concentrations of metals distributed in PM during winter (Dec to Feb) and summer months (March–May). The concentration of trace metals in the winter season ranked in the following order: Fe > Zn > Cr > Cu > Pb > Mn > Cd > Co > Ni while in summer as Fe > Zn > Pb > Cr > Cu > Mn > Co > Ni > Cd. Fe was the most abundantly present metal in both the seasons with concentrations of 2.41 and 1.54 μg m−3 in winter and summer, respectively. Principal component analysis was applied on the dataset of metals in PM for the source identification. Table 4 represents three rotated factor loadings explaining more than 82.4 % of total variance for PM. Factor 1 corresponds to 34.1 % loading with the components of Cu, Co, and Cr which can be accredited to road traffic emission. Cu is associated with the wearing of brakes and an automobile’s oil pump or corrosion of metal parts coming in contact with oil. Cr is an important constituent of many metal alloys. It is also contained in cement and originating from construction activities (Chen et al. 2011), which were carried out in the vicinity of the sampling sites as new buildings are under construction in the university. The second factor 27.5 % shows high loading for Zn, Fe, Ni, and Cd which can be accredited to road dust. Its large milieu is soil and some other sources from vehicles such as tier stride, drum brake graze, etc. (Yadav and Satsangi 2013). Galvanized materials, wear of tires, and the usage of zinc compounds in rubber production could be probable sources of Zn. Fe is mostly emitted from soil (Yadav and Satsangi 2013). Ni is used as a fuel oil marker. Cd is linked with diesel engines and the taxing of brakes. The third factor 20.8 % shows high loading of Pb, Fe, and Zn which may be associated with resuspended road dust and fossil fuel combustion from vehicles. From the year 2000, Pb has been phased out of gasoline in India, but still, it perseveres in road dust from previous vehicular exhaust release due to its extensive dwelling time in the environment (Yadav and Satsangi 2013; Kulshrestha et al. 2009).
PM induced oxidative stress and DNA damage
To determine the PM-induced oxidative stress on DNA, phage phiX174 was selected and analyzed by plasmid assay. Percent DNA damage and its TM50 (by linear regression) values were calculated for each plot. The term TM50 can be defined as particle mass causing 50 % damage to DNA. A lower TM50 value expresses a higher oxidative capacity or genotoxicity due to PM (Table 5 ). Several batches of experiment were performed with different concentrations (50 to 400 μg mL−1) of samples collected throughout the study along with the negative (DNA + unexposed filter in PBS) and positive (DNA + PBS + H2O2) controls. The representative images of the DNA assay are shown in Fig. 4a, b which depicts the in vitro effects of PM extracts on phage phiX174 supercoiled DNA. Lane 1 in Fig. 4a, b shows the experiment with untreated DNA + PBS, lane 2 shows the positive control (i.e., with 0.04 % H2O2 which shows the entire DNA damage), lane 3 shows extracts from an unexposed filter incubated with DNA (negative control), and lanes 4 to 6 depict DNA treated with different concentrations (100, 200, 300 μg mL−1) of extracts of winter and summer fine PM.
In general, overall DNA damage for winter PM particles was between 40 and 90 % whereas for summer it was between 20 and 70 %. For comparison purposes, such PM extracts from both winter and summer seasons were selected and diluted to nearly same concentrations to detect the toxic content in PM samples. As depicted in Fig. 4a, b, at 300 μg mL−1 concentration, the DNA damage was found to be 90 and 68 % by PM samples collected in winter and summer seasons, respectively. In winter, 78 % of DNA damage was observed at 200 μg mL−1 PM concentration while summer samples exhibited 1.8 times (i.e., 45 %) lower damage than winter samples. At 100 μg mL−1 of PM concentration, DNA damage was found to be 42 and 25 % for winter and summer, respectively. Meager DNA damage was observed even at 400 μg mL−1 for some summer samples (data not shown). All these data showed that at the same dosage of particles, the overall oxidative ability of the winter PM samples was higher than that of the summer samples. One of the possible reasons for this damage can be corroborated with the presence of a high concentration of metal content in the PM. The total concentration of metals was found to be 3.9 and 2.7 μg m−3 in winter and summer seasons, respectively. SEM-EDS analysis of several samples was done and representative graphs are shown in Fig. 5. The SEM-EDS results also demonstrated that the number and portions of fine PM particles in the summer samples (Fig. 5a–c) were less than those of the winter samples (Fig. 5d–f) suggesting that the PM load in winter was higher as compared to that of summer. The SEM-EDS mapping technique also confirmed the presence of different metals such as Fe, Zn, etc., in PM. As depicted in Fig. 5, masses of aggregated particles were observed which contained minerals with various shapes such as spherical, cuboids, soot aggregates, and fly ashes as main components in PM which can easily enter the respiratory tract. Elemental mapping of these particles substantiates deposition of metals as Fe, Zn, and Ti on the particles (Fig. 6). Metals such as Fe, Cu, Cr, and Ni can act as pro-oxidants and can produce Fenton-type reaction products along with the oxidative stress in biological systems, thus leading to oxygen-promoted DNA damage (Shi et al. 2003; Donaldson et al. 2005; Bräuner et al. 2007) leading to heritable mutations (Risom et al. 2005; Peters et al. 2006). These mutations may result from the chemical modification of histones (or binding proteins that back the structure of supercoiled DNA) which opens the coiled DNA to linear and allows its alteration (Donaldson and Ston 2003). The pyrimidine and purine base modifications are the major steps towards DNA damage by radicals, which leads to breaking of the DNA strand, cross-linkage of DNA-protein, and its basic site (Ji et al. 2005; Schyman et al. 2008). In addition to soluble metals, PAH or quinones also play an imperative role in oxidative damage (Li et al. 2008). Radical generation may be facilitated by metals in PM. As the present study is focused on metal concentrations and their genotoxicity, EPR was employed to assess which metal plays a vital role in the generation of free radicals. All EPR analyses were done at low temperature (77 K). Several samples were analyzed by EPR and showed similar peak patterns and g values, so representative graphs are shown in Fig. 7a–d. Figure 7a shows EPR spectra of field blank filters with no EPR signals (Roy et al. 2015). Figure 7b–d shows the EPR spectra of atmospheric fine PM along with their g values as 1.99 (i.e., ~2.0), 2.024, 2.0031, 2.0032, and 2.0036. EPR g values may change from one sample to another; this change depends on the number of paramagnetic species interactions present in that particular sample. The intensity of the signal is proportional to the amount of radicals released. A g value corresponding to 2.0031 (Fig. 7b) and 2.0032 (Fig. 7c) in fine fraction suggests the presence of free radicals in it. In the present study, a C-centered radical with a vicinal oxygen atom (g = 2.0031, 2.0036) was observed. It has been reported that C-centered radicals have a g value approximately near that of a free electron whereas C-centered radicals with a neighboring oxygen atom have slightly high g factors in the range of 2.003–2.004 (Tian et al. 2009; Dellinger et al. 2007). In general, carbon-centered free radicals are reactive when are in the company of living cells and are thus important for a biological system. Carbon-centered radicals are found to cause DNA alkylation, thus leading to DNA damage (Augusto 1993). In addition to this, a g value of 1.9 (~2.00) shows the presence of Fe (Fig. 7c). This suggests that in current study metals, particularly, Fe plays a vital role in the oxidant-generating activity. Available Fe can generate hydroxyl radicals via the Fenton reaction. The Fe concentration (2.41 μg m−3 in winter and 1.54 μg m−3 in summer) in Pune fine PM samples was higher than that of other metals which suggests that Fe may be responsible for DNA damage through radical generation. Many studies also reported that Fe is frequently related to oxidative damage (Valavanidis et al. 2000; Han et al. 2001; Di Pietro et al. 2009; Charrier and Anastasio 2011), and soluble Fe influences the oxidative capacity by the Fenton reaction (Shi et al. 2003; Risom et al. 2005; Nawrot et al. 2009). Numerous other “Fenton active” transition metals like Cr, Cu, etc. which typically arise in ambient PM are also known to induce OH· formation (Donaldson and Ston 2003; Shi et al. 1999). As the EPR study did not show any g value related to these metals, it has been ruled out from the discussion.
Moreover, plasmid assays of PM samples in the present study illustrate that TM50 (i.e., toxic mass causing 50 % DNA damage) of the majority of the winter samples are lower than those of the summer samples. Table 5 depicts the mean TM50 values of particulate matter in winter and summer seasons. It is inferred from the table that TM50 for the winter season: 132.5 ± 47.6 μg mL−1 (Dec 2013), 117.7 ± 28.2 μg mL−1 (Jan 2014), and 112.5 ± 9.2 μg mL−1 (Feb 2014) are lower than the TM50 values observed in the summer season: 171.3 ± 44.1 μg mL−1 (March 2014), 184.2 ± 53.7 μg mL−1 (April), and 230 ± 29.4 μg mL−1 (May). In general, low TM50 values show the high bioreactivity of PM (Shi et al. 2003; Xiao et al. 2013). Thus, it can be inferred that the bioreactivity of the winter PM samples is in general higher than that of the summer samples of Pune. These data further support the air quality of Pune was gravely polluted in winter (as shown in Fig. 2). When compared with other studies, we found that our study is in accordance with Shi et al. (2003) and Xiao et al. (2013) who also reported high oxidative damage and toxicities by winter samples. TM50 values were correlated with concentrations of total metals analyzed, and significant negative correlation was observed (Spearman correlation coefficient of −0.860) which suggests that metals in PM are responsible for DNA damage to a great extent. Further, the TM50 was also correlated with the individual metals in fine PM to assess the contribution of particular metals in DNA damage (Table 6). Strong negative correlation was observed between TM50 and Fe which further indicates that Fe is responsible for the plasmid DNA damage. Beside Fe, Zn is another metal which showed statistical negative correlation (TM50: Zn = −0.79). Zn has been considered as a bioactive element (Adamson et al. 2000; Richards et al. 1989) which can affect enzyme formation in an organism (Senlin et al. 2006). In the present study, Cu, Cr, Mn, and other such metal ions behave analogous in oxidant engendering activity but they are present in low concentration. Thus, the data shown by ICP-AES, EPR, SEM, and plasmid DNA assay together suggest that Pune PM shows significant genotoxicity.
The present study has some limitations. PM sources contribute to the total PM exposure as other pollutants viz. PAH, black carbon/soot particles, and mixture effects have not been assessed. Thus, further work on the effects of pollutant mixtures and secondary aerosols should allow better understanding of the pollution components and sources responsible for the genotoxic effects of air pollution exposure with more data points. Overall, this particular study is expected to present an important database for genotoxicity of PM which would be essential for the policy makers and environmentalists for proper planning.
Conclusion
The average daily mass concentrations of fine (PM2.5) particulate matter in the present study varied from 59.8 ± 27.2 to 139.6 ± 38.6 μg m−3. The concentrations of fine PM in the winter season (Dec–Feb) were about 1.4 times higher than the concentrations in the summer months (Mar–May). The lowest physical concentration was observed in the month of April (59.8 μg m−3). High temperature and wind speed might be the cause of the lower levels of fine PM at these particular months. During the winter season, average relative humidity was also comparatively high (67 %), contributing to higher collection of fine PM. Beside the meteorological parameters, the high concentration of PM during winter can be due to biomass burning (excess of solid waste together with mostly garden cuttings, fallen leaves, and paper are burned by the side of roads) and increased heating activities. The ICP-AES analysis indicated that, in the Pune PM samples, the Fe concentration was much higher than other metals in both winter (2.41 μg m−3) and summer (1.54 μg m−3) seasons. Strong negative correlation was observed between TM50 with Fe and Zn. Thus, this suggests that mainly Fe may be responsible for DNA damage through radical generation including other metals such as Zn and Cu. Various sources which have been found responsible for a higher mass and metal concentration were vehicular pollution and combustion of fossil fuel, resuspension of road dust, biomass burning, soil-emitted particles, etc. EPR analysis shows the g value for free radicals and Fe in PM samples of Pune. The g values for a C-centered radical with a vicinal oxygen atom were also observed, which are reactive when they are in the company of DNA and cause DNA alkylation, thus leading to DNA damage. This suggests that in the current study, metals play a vital role in oxidant-generating activity. Therefore, we conclude that the radicals linked with fine PM comprise metal-mediated radicals and produce DNA damage.
References
Abbey DE, Lebowitz MD, Mills PK (1995) Long-term ambient concentrations of particulates and oxidants and development of chronic disease in a cohort of nonsmoking California residents. Inhal Toxicol 7:19–34
Adamson IYR, Prieditis H, Hedgecock C, Vincent R (2000) Zinc is the toxic factor in the lung response to an atmospheric particulate sample. Toxicol Appl Pharmacol 166:111–119
Augusto O (1993) Alkylation and cleavage of DNA by carbon-centered radical metabolites. Free Radic Biol Med 15(3):329–336
Bathmanabhan S, Madanayak SNS (2010) Analysis and interpretation of particulate matter—PM10, PM2.5 and PM1 emissions from the heterogeneous traffic near an urban roadway. Atmos Pollut Res 1:184–194
Brauner EV, Forchhammer L, Moller P, Simonsen J, Glasius M, Wahlin P, Raaschou-Nielsen O, Loft S (2007) Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environ Health Perspect 115(8):1177–1182
Charrier JG, Anastasio C (2011) Impacts of antioxidants on hydroxyl radical production from individual and mixed transition metals in a surrogate lung fluid. Atmos Environ 45:7555–7562
Chen D, Pan X, Xiao P, Farwell MA, Zhang B (2011) Evaluation and identification of reliable reference genes for pharmacogenomics, toxicogenomics, and small RNA expression analysis. J Cell Physiol 226:2469–2477
Dellinger B, Lomnicki S, Khachatryan L, Maskos Z, Hall RW, Adounkpe J, McFerrin C, Truong H (2007) Formation and stabilization of persistent free radicals. Proc Combust Inst 31(1):521–528
Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA (2001) Role of free radicals in the toxicity of airborne fine particulate matter. Chemical Res in Toxicol 14(10):1371–1377
Deshmukh DK, Deb MK, Mkoma SL (2013) Size distribution and seasonal variation of size-segregated particulate matter in the ambient air of Raipur city, India. Air Qual Atmos Health 6:25–276
Di Pietro A, Visalli G, Munaò F, Baluce B, La Maestra S, Primerano P, Corigliano F, De Flora S (2009) Oxidative damage in human epithelial alveolar cells exposed in vitro to oil fly ash transition metals. Int J Hyg Environ Health 212:196–208
Donaldson K, Ston V (2003) Current hypotheses on mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita 39(3):405–410
Donaldson K, Li XY, MacNee W (1998) Ultrafine (nanometre) particle mediated lung injury. J Aerosol Sci 29(5–6):553–560
Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N (2005) Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol 2:10
Dreher K (2000) Particulate matter-physicochemistry and toxicology: in search of causality—a critical perspective. Inhal Toxicol 2(3):45–57
Elminir HK (2005) Dependence of urban air pollutants on meteorology. Sci Total Environ 350(1–3):225–237
Fierro F, Kosalkov K, Gutierrez S, Martın JF (1996) Autonomously replicating plasmids carrying the AMA1 region in Penicillium chrysogenum. Curr Genet 29:482–489
Ghauri B, Mansha M, Khalil C (2012) Characterization of cytotoxicity of airborne particulates from urban areas of Lahore. J Environ Sci 24(11):2028–2034
Gidde MR, Sonawane PP (2012) Assessment of traffic related air pollution and ambient air quality of metropolitan cities (case study of Pune City). IOSR J Engineering (IOSRJEN) 29(6):1382–1390
Gotschi T, Heinrich J, Sunyer J, Kunzli N (2008) Long-term effects of ambient air pollution on lung function. A Review Epidemiology 19:690–701
Han JY, Takeshita K, Utsumi H (2001) Noninvasive detection of hydroxyl radical generation in lung by diesel exhaust particles. Free Radic Biol Med 30:516–525
Heal MR, Hibbs LR, Agius RM, Beverland LJ (2005) Total and water soluble trace metal content of urban background PM10, PM2.5 and black smoke in Edinburgh, UK. Atmos Environ 39:1417–1430
Health Status Maharashtra (2010) Maharashtra state health resource centre, national rural health mission (2005–2012). https://www.nrhm.maharashtra.gov.in/Health_Status_2010.pdf
Hsieh LT, Wu EMY, Wang LC, Chien GPC, Yeh YF (2011) Reduction of toxic pollutants emitted from heavy-duty diesel vehicles by deploying diesel particulate filters. Aerosol Air Qual Res 11:709–715
Hung LJ, Tsai SS, Chen PS, Yang YH, Liou SH, Wu TN, Yang CY (2012) Traffic air pollution and risk of death from breast cancer in Taiwan fine particulate matter (PM2.5) as a proxy marker. Aerosol Air Qual Res 12:275–282
Ji YJ, Xia YY, Zhao MW, Huang BD, Li F (2005) Theoretical study of the % OH reaction with cytosine. J Mol Struct THEOCHEM 723:123–129
Ko FW, Hui DS (2012) Air pollution and chronic obstructive pulmonary disease. Respirology 17:395–401
Kulshrestha A, Satsangi PG, Masih J, Taneja A (2009) Metal concentration of PM2.5 and PM10 particles and seasonal variations in urban and rural environment of Agra, India. Sci Total Environ 407:6196–6204
Kumagai Y, Kato JI, Hoshino K, Akasaka T, Sato K, Ikeda H (1996) Quinolone-resistant mutants of Escherichia coli DNA topo-isomerase IV par C gene. Antimicrob Agents Chemother 40:710–714
Kupelwieser NS, Horvath H, Szymanski WW (2010) Urban aerosol studies of PM1 size fraction with reference to ambient conditions and visibility. Aerosol and Air Quality Res 10:425–432
Li N, Xia T, Nel AE (2008) The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med 44:1689–1699
Maharashtra State Health Resource centre, National Rural Health Mission (2005–2012) Health Status Maharashtra:2010
Makkonen U, Hellen H, Anttila P, Ferm M (2010) Size distribution and chemical composition of airborne particles in south-eastern Finland during different seasons and wildfire episodes in 2006. Sci of the Total Environ 408:644–651
Massey DD, Kulshrestha A, Taneja A (2013) Particulate matter concentrations and their related metal toxicity in rural residential environment of semi-arid region of India. Atmos Environ 67:278–286
Molinelli AR, Santacana GE, Madden MC, Jimenez BD (2006) Toxicity and metal content of organic solvent extracts from airborne particulate matter in Puerto Rico. Environ Res 102:314–325
Monn C, Becker S (1999) Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10–2.5) in outdoor and indoor air. Toxicol Appl Pharmacol 155:245–252
Monn C, Fendt R, Koller T (2002) Ambient PM (10) extracts inhibit phagocytosis of defined inert model particles by alveolar macrophages. Inhal Toxicol 14(4):369–385
Mouli PC, Mohan SV, Reddy SJ (2003) A study on major inorganic ion composition of atmospheric aerosols at Tirupati. Journal of Hazard Materials 96:217–228
Nawrot TS, Kuenzli N, Sunyer J, Shi T, Moreno T, Viana M, Heinrich J, Forsberg B, Kelly F, Sughis M, Nemery B, Borm P (2009) Oxidative properties of ambient PM2.5 and elemental composition: heterogeneous associations in 19 European cities. Atmos Environ 43:4595–4602
Nawrot TS, Torfs R, Fierens F, De HS, Hoet PH, Van KG, De BG, Nemery B (2007) Stronger associations between daily mortality and fine particulate air pollution in summer than in winter: evidence from a heavily polluted region in Western Europe. J Epidemiol Community Health 61:146–149
Niu JJ, Rasmussen PE, Wheeler A, Williams R, Chenier M (2010) Evaluation of airborne particulate matter and metals data in personal, indoor and outdoor environments using ED-XRF and ICP-MS and CO-located duplicate samples. Atmos Environ 44:235–245
Ormstad H (2010) Suspended particulate matter in indoor air: adjuvants and allergen carriers. Toxicology 152:53–68
Peters A, Dockery DW, Muller JE, Mittleman MA (2001) Increased particulate air pollution and the triggering of myocardial infraction. Circulation 103:804–807
Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, Reed W, Rothen-Rutishauer B, Schurch S, Schultz H (2006) Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol 3:13
Pipal AS, Jan R, Satsangi PG, Tiwari S, Taneja A (2014) Study of surface morphology, elemental composition and origin of atmospheric aerosols (PM 2.5 and PM10) over Agra, India. Aerosol and Air Quality Res 14:1685–1700
Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287(9):1132–1141
Pope CA, Thun M, Namboodiri M, Dockery D, Evans J, Speizer F, Heath C (1995) Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. American J Respir Critical Care Med 151:669–674
Richards R, Atkins L, Marrs TC (1989) The biochemical and pathological changes produced by the intra-tracheal instillation of certain components of zinc hexachloroethane smoke. Toxicology Letter 54:79–88
Risom L, Moller P, Loft S (2005) Oxidative stress- induced DNA damage by particulate air pollution. Mutat Res 592:119–137
Roy R, Jan R, Yadav S, Vasave MH, Satsangi PG (2015) Study of metals in radical-mediated toxicity of particulate matter in indoor environments of Pune. India Air Qual Atmos Health. doi:10.1007/s11869-015-0376-x
Salve PR, Maurya A, Wale SR (2006) Distribution of trace metal in atmosphere particulate matter in rural environment. Indian J Environ Protect 26(B):673–667
Satsangi PG, Yadav S (2014) Characterization of PM2.5 by X-ray diffraction and scanning electron microscopy-energy dispersive spectrometer: its relation with different pollution sources. Int J Environ Sci Technol 11:217–232
Schyman, P., Eriksson, L.A., Zhang, R., Laaksonen, A., 2008. Hydroxyl radical–thymine adduct induced DNA damages. Chemical Physics Letters. 458, 186–189.
Senlin L, Longyi S, Minghong W, Jones TP, Merolla L, Richard RJ (2006) Correlation between plasmid DNA damage induced by PM10 and trace metals in inhalable particulate matters in Beijing air. Sci in China Series D: Earth Sci 49(12):1323–1331
Senlin L, Zhenkun Y, Xiaohui C, Minghong W, Guoying S, Jiamo F, Paul D (2008) The relationship between physicochemical characterization and the potential toxicity of fine particulates (PM2.5) in Shanghai atmosphere. Atmos Environ 42:7205–7214
Shi JP, Harrisonn RM, Brear F (1999) Particle size distribution from a modern heavy duty diesel engine. The Sci Total Environ 235:305–317
Shi T, Schins RPF, Knaapen AM, Kuhlbush T, Pitz M, Heinrich J, Brom PJA (2003) Hydroxy radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J Environ Monit 5:550–556
Shi Z, Shao L, Jones TP, Whittaker AG, Richards RJ, Zhang P (2004) Oxidative stress on plasmid DNA induced by inhalable particles in the urban atmosphere. Chin Sci Bull 49(7):692–697
Sonaje NP, Mane SJ, Kote AS (2013) Modelling of respirable suspended particulate matter concentration using artificial neural networks in an urban area. Eng Sci Technol 3(4):694
Srimuruganandam SM, Nagendra S (2011) Characteristics of particulate matter and heterogeneous traffic in the urban area of India. Atmos Environ 45:3091–3102
Tian L, Koshland CP, Yano J, Yachandra VK, Yu ITS, Lee SC, Lucas D (2009) Carbon-centered free radicals in particulate matter emissions from wood and coal combustion. Energy Fuel 23:2523–2526
Valavanidis A, Salika A, Theodoropoulou A (2000) Generation of hydroxyl radicals by urban suspended particulate air matter: the role of iron ions. Atmos Environ 34:2379–2386
WHO report (2002) Reducing risk promoting healthy life, World Health Organization.
Xiao Z, Shao L, Zhang N, Wang J, Wang J (2013) Heavy metal compositions and bioreactivity of airborne PM10 in a valley-shaped city in northwestern China. Aerosol and Air Qual Res 13:1116–1125
Yadav S, Satsangi PG (2013) Characterization of particulate matter and its related metal toxicity in an urban location in South West India. Environ Monit and Assess 185:7365–7379
Yadav S, Praveen OD, Satsangi PG (2015) The effect of climate and meteorological changes on particulate matter in Pune, India. Environmental Monitoring and Assessment 187:402. doi:10.1007/s10661-015-4634-z
Acknowledgments
The authors wish to thank the Department of Science and Technology (DST No. SR/FTP/ES-91/2009) and UGC (F. No. 41-324/2012 (SR)), New Delhi, for financial assistance. The authors also express their gratitude to the Head, Department of Chemistry, Savitribai Phule Pune University, for her encouragement. IIT, SAIF—Mumbai is also acknowledged for analyzing the samples on ICP-AES and EPR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Research highlights
•Among the metals detected in fine PM, Fe concentration was found to be highest.
•EPR showed the presence of free radicals, Fe, carbon-centered radicals, etc.
•DNA damage corroborated the high concentration of metal content and free radicals in the PM.
•TM50 value of winter samples are lower than the summer samples.
•Oxidative ability of the winter PM samples was higher than that of the summer samples
Rights and permissions
About this article
Cite this article
Yadav, S., Jan, R., Roy, R. et al. Role of metals in free radical generation and genotoxicity induced by airborne particulate matter (PM2.5) from Pune (India). Environ Sci Pollut Res 23, 23854–23866 (2016). https://doi.org/10.1007/s11356-016-7494-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7494-3