Abstract
Assessment of levels of polybrominated diphenyl ethers (PBDEs) from the sediment of Asunle stream, an adjourning stream of the Obafemi Awolowo University dumpsite, has been carried out. Sediment samples were collected from the stream at six locations for a period of 8 months, composed of 4 months each of wet (May–Aug) and dry (Nov–Feb) seasons. Soxhlet extraction was employed for the isolation of all the target compounds from the sediment samples. Extracts were further subjected to multi-layer column chromatography employing different forms of silica gel. The prepared samples were analyzed using GC-MS. The overall mean concentrations of the total PBDEs ranged from 1.80 to 9.46 ng/g. The results showed that the concentrations of the PBDEs were slightly higher during the wet season than those during the dry season. In all the studied locations, BDE28, BDE47, BDE99, BDE100, BDE153, and BDE154 were detected in all the sediment samples at concentrations that ranged from 0.73 to 10.43 ng/g. Results of this study indicated that BDE153 was the major pollutant of the Asunle stream sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Technological advancements during the last century have led to the use of synthetic carbon-based polymers for everyday household and office items such as furniture, fabrics, automotive parts, housings for electronic equipment and surface coatings for other materials (de Wit 2002; Guerra et al. 2011). The high tendency of these materials to act as a source of fuel is an indication that their very presence could portend danger, particularly in instances where a high risk of ignition is associated with the item’s use. Fire outbreak is a major cause of damage to both private and public properties. In most cases, fire outbreak could involve loss of lives and public expenses. For instance, in the USA in 2007, over 1.5 million fires were reported, which resulted in 17,675 injuries, 3430 deaths and direct losses of over $14 billion (Karter 2008). Similarly, in 2013, 1.24 million fires were reported, which resulted in 15, 925 injuries, 3240 deaths and direct loss of $11.5 billion (Karter 2014). In Lagos State of Nigeria, 2342, 1774 and 1499 fire cases were reported in 2012, 2013, and 2014, respectively, and direct property losses worth N54bn during the 3 years were reported (Vanguard, January 20, 2015). Also, 262 people were reportedly lost in 368 fire incidents in 2011, while 185 lives were lost in 470 fire incidents in 2012 (Premium Times 2013). In Nigeria, about N50bn were lost annually due to fire disasters (The Punch Newspaper 2013).
Flame retardants (FRs) play an important role in safeguarding life and property. Today, in order to prevent fire outbreak, FRs are incorporated into combustible materials such as plastics, wood, textiles, electronic products and paper materials. Combustion involves four stages: preheating, decomposition and evolution of volatiles, ignition and propagation (Troitzch 1990). Prevention of any of these four stages should lead to the suppression of fire (Zhang et al. 2016). Halogens are very effective in trapping free radicals produced during the combustion process (Guerra et al. 2011). Approximately 25 % of all FRs contain bromine as the active ingredient, which is very effective in trapping free radicals, hence removing the capability of the flame to propagate. Organobromine compounds have become more popular due to their stability, higher trapping efficiency, and lower decomposing temperature (Guerra et al. 2011. More than 80 different aliphatic, cyclo-aliphatic, aromatic, and polymeric compounds are used as brominated flame retardants (BFRs). BFRs, such as polybrominated biphenyls (PBBs), polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD), and tetrabromobisphenol A (TBBPA), have been used in different consumer products in large quantities, and consequently, they have been detected in environmental matrices (Guerra et al. 2011). Of all the four common BFRs, PBDEs are the most commonly reported. Plastics in electrical and electronic equipment and related wastes are considered to contain the largest share of PBDEs followed by polyurethane foams found in car, furniture, mattress or baby products (Stockholm Convention 2011).
PBDEs are used as additives in combustible materials; they are capable of being released into the environment, thereby settling in dust, water, sediment and biota (de Wit 2002). PBDEs are toxic and persistent environmental xenobiotics that may undergo long range transport and are capable of bioaccumulating and biomagnifying along the food chain (Kuriyama et al. 2005; Moon et al. 2007). These properties and their diffusion patterns in air have triggered concern about the possible general environmental effects of this group of substances. In Africa, several studies on PBDEs have been carried out and have confirmed the presence of PBDEs at significant levels in environmental matrices such as the eggs of African ibis Polder et al. (2008), crocodile eggs (Bouwman et al. 2014), leachate (Odusanya et al. 2009; Daso et al. 2013a), human serum (Linderholm et al. 2010), cow milk (Asante et al. 2010), human milk (Darnerud et al. 2010; Hassine et al. 2012), sludge (Daso et al. 2011b), bivalves (Bodin et al. 2011), fish (Wepener et al. 2012), dust (Kefeni and Okonkwo 2012; 2014), altitudinal soil (Parolini et al. 2013), water (Daso et al. 2013b), air and precipitation (Arinaitwe et al. 2014). Olukunle et al. 2012b and Daso et al. 2013c carried out the evaluation of various extraction methods for the analysis of PBDEs and their application to aqueous environmental samples. The presence of PBDEs in river sediments has been reported by many researchers (Bodin et al. 2011; Daso et al. 2011a; Olukunle et al. 2012a; La Guardia et al. 2013; Adewuyi and Adeleye 2013),
Research concerning the presence of PBDEs as new, emerging, and ubiquitous contaminants released into the Nigerian environment from the breakdown of combustible materials is still in its infancy, and information on production and usage of PBDEs in Africa is scarce generally. The data on cathode ray tubes (CRT) in the inventory of electrical and electronic equipment and related wastes in Nigeria is reported as 791,325 t, out of which, 23,7000 t was plastics with 216, 000 t obtained from TV casings and 21,000 t from computer CRT casings (Ogunbiyi et al. 2012). From these figures, average concentrations of c-Octa-BDE, deca-BDE, and TBBPA were estimated by Sindiku et al. (2014) to be 594, 1880, and 1450 t, respectively. Sindiku et al. (2014) reported that average PBDE levels of c-Octa- and deca-BDE in Nigerian stockpiled CRT casings were 1.1 % for television and 0.13 % for computer, and these values were above the hazardous substance limit. There is virtually no environmental data regarding PBDE levels in stream water and sediment with the exception of the study conducted by Adeleye and Adewuyi (2013) on sediment of Lagos Lagoon. This study was therefore aimed at determining the occurrence and distribution of some PBDEs in the sediments of Asunle stream of the Obafemi Awolowo University, Ile-Ife, Nigeria.
Materials and methods
Study area
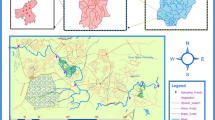
Obafemi Awolowo University dumpsite has been in operation since the inception of the university in 1971. The dumpsite serves as the final destination where all refuse collected within the university community are dumped and subjected to open-air incineration. Among the wastes commonly dumped are medical wastes, domestic and household wastes, television and computer monitors, X-ray cathode lamps, florescent tubes, discarded refrigerators, metallic materials from engineering and mechanic workshops, waste papers, cosmetic products, expired drugs, and chemicals. The university dumpsite lacks appropriate solid waste treatment facility, which leads to dumping and subsequent open-air burning of hazardous wastes, thereby causing environmental pollution. The destruction of these wastes via incineration is capable of adding potentially toxic metals and persistent organic pollutants to the surrounding and the adjoining stream. This can happen through leaching, as residues of incinerated waste materials are washed down into the adjoining stream, thereby adversely impacting the aquatic environment. Very close to the dumpsite is Asunle stream, a perennial stream whose source is located about 0.25 km uphill from the Obafemi Awolowo University Ile-Ife refuse dumpsite (Fig. 1). The stream serves as the water source for several farmlands of the study area and runs a stretch of more than 10 km, cutting across human communities such as Abagbooro, Agbogbo and Amuta (Ogunfowokan et al. 2013). Commonly planted crops on the farmlands around the stream include cash crops such as cocoa, cola nut, palm trees, etc. and food crops such as cassava, maize, yam, cocoyam, plantain, banana, pineapple, oranges, pepper, and various types of vegetables. This makes human activities around the stream vigorous. The rural dwellers living along this stream rely on the water from the stream for household purposes, irrigation and agricultural applications, palm oil processing, and mixing and dilution of pesticides used for spraying cocoa and other crops. These factors were considered in deciding to monitor the level and distribution of PBDEs in the sediments of Asunle stream.
Sample collection, preparation and extraction
Samples were collected on a monthly basis over 8 months spanning November, 2012, through February, 2013, representing the dry season, and May through August, 2013, representing the wet season. Sediment samples were collected at five locations along the course of the stream. The bottom sediment samples were collected in aluminium foil previously cleaned with pure acetone and wrapped in a black cellophane nylon. The samples were air dried to constant weight in an aerated cupboard to prevent cross-contamination. The dried samples were ground and sieved with 500-μm stainless steel sieve. The sieved samples were stored in air-tight cellophane bags kept in clean 250-mL-capacity amber-coloured bottles and preserved in the refrigerator at 4 °C until further analysis was required.
Approximately 10 g of the prepared soil sample was weighed and transferred into a pre-extracted cellulose thimble for Soxhlet extraction. The soil sample was spiked with 100 μL each of 100 ng/mL of two surrogate standards, pentachloronitrobenzene (PCNB) and BDE77, to monitor the analytical recovery of the target analyte. The sample was then extracted with 150 mL of n-hexane/dichloromethane (2:1 v/v) for 3 h. Prior to extraction, about 2 g of copper powder was added to each sample to remove traces of elemental sulphur that could possibly interfere with the analyte’s determination.
Sample cleanup
Before extract cleanup, sample extracts were concentrated to approximately 1 ml using a rotary evaporator. Purification of extract in this study was done using a modified multilayer silica gel column technique of Yun et al. (2008) and Kupper et al. (2008). The concentrated extracts were then cleaned on a preconditioned multi-layer silica gel column using n-hexane as the eluting solvent. The glass column was packed from the bottom with 1 g activated silica gel, 4 g of basic silica gel (30 % NaOH, w/w), 1 g of activated silica gel, 8 g of acidic silica gel (44 % H2SO4 w/w), 2 g activated silica gel, and 4 g of anhydrous sodium sulphate. The packed column was preconditioned with 10 mL of doubly distilled n-hexane to remove trapped air and background contaminants within the column. The n-hexane layer over the uppermost layer in the column was maintained at 2 mm to prevent further infiltration of air into the column.
The concentrated extract was then quantitatively transferred into the column and eluted with 100 mL of n-hexane. The eluate was concentrated to approximately 1 mL using a rotary evaporator. This was followed by addition of 1 mL of isooctane serving as keeper before final concentration to a suitable volume in an amber sample vial under a gentle stream of nitrogen. The prepared samples were kept in the refrigerator prior to final instrumental analysis.
Instrumental analysis
Analysis of target compounds was performed on a Trace 1300 Series GC coupled to a TSQ8000 Mass Spectrometer equipped with a TriPlus RSH Auto Sampler available at Stellenbosch University, South Africa. The chromatographic separation of analyte was performed using a ZB 274305 semi-volatile column (30-m length, 0.25-mm id, 0.25-mm film thickness). Helium gas was employed as a carrier gas with a flow rate of 1.0 mL/min using a constant flow mode. High-purity nitrogen gas was used as make-up gas with a flow rate of 20 mL/min. The injector and detector temperatures were set at 280 and 320 °C, respectively. The oven temperature was programmed as follows: 150 °C held for 2 min, ramped at 8 °C/min to 320 °C and held for 2 min. One microlitre each of the mixed standard solutions containing the target compounds and extracts of the experimental samples was injected using a splitless injection mode.
Quantification was based on examination of the peaks of target compounds using external calibration curve technique. The calibration plots were composed of six levels of 5, 10, 20, 50, 75, and 100 ng/mL for all the target compounds. The linearity (r 2) of the calibration plots for each target compound was greater than 0.9914. Identification of analyte was done by comparing the retention times with those of reference standards.
The quality assurance and quality control (QA/QC) samples included the use of amber-coloured bottles for sampling storage, regular injection of solvent blank and standard solutions of the target compounds. Matrix spiked experiments were also performed. The results of the recoveries of the target compounds ranged from 92 % (BDE100) to 105 % (BDE28), while recovery of surrogate standards ranged from 72 % (BDE77) to 87 % (PCNB). A calibration check of 5 ng/mL standard was run after every five sample runs to ensure that less than 20 % variation was found from initial calibration standards.
TOC determination
The total organic carbon (TOC) content of sediment samples was determined by weight loss on ignition method described by Dean (1974). Prior to the gravimetric determination, the sediment samples were dried to constant weight at 105 °C to ensure total removal of moisture. In each case, about 5 g of sediment samples was weighed into crucible and ignited in a Vescar muffle furnace model ECF 3 at 440 °C for 4 h. The setup was allowed to cool and later transferred into a dessiccator, followed by weighing of the ignited sediment samples. The final weight of the ignited sediment was then determined to estimate the total organic content of each sample.
Chemicals used, their sources and purification
The chemicals and reagent used included dicholoromethane, n-hexane, isooctane, acetone (Merck, South Africa); silica gel 60–200 mm, anhydrous sodium sulphate, and copper powder (Sigma-Aldrich, South Africa); PBDEs and PCNB standards (Cambridge Isotope Laboratories, Andover, MA, USA, via Industrial Analytical (Pty), Midrand, Gauteng, South Africa); helium; and nitrogen (99.99 %) were supplied by Afrox (Pty) Ltd., Cape Town, South Africa.
All the organic solvents were triply distilled to obtain pure solvent that precluded all trace organic contaminants. Other materials such as glass wool, anhydrous sodium sulphate, silica gel, and copper powder were all heated in a muffle furnace model ECF 3 at 450 °C for 4 h prior use.
Statistical analysis
All data were managed with Microsoft Excel software (2007 version). The various data obtained were analyzed using descriptive statistics and one-way analysis of variance along with the Duncan multiple range test to determine significant difference between means. The two-tailed Pearson correlation coefficient was used to determine the strength of association between PBDE congeners. Principal component analysis was used to predict and identify possible sources of these contaminants. Statistical analysis of the data was performed using the Statistical Package for the Social Science (SPSS) software 15.0 for Windows Evaluation version.
Results and discussion
Monthly levels of PBDEs in bed sediment
The result obtained from the analysis of the bed sediment for the selected PBDE congeners, as presented in Table 1, indicated that all the congeners investigated in this study were present in the sediments. Polybrominated diphenyl ethers (PBDEs), like most persistent organic pollutants, bind to suspended materials in the aqueous phase and settle along with the suspended materials into the bed sediments, thus leading to accumulation of these contaminants in the bed sediments. Organic matter in the bed sediments thus acts as a repository for persistent organic contaminants.
The overall mean concentrations of the total PBDEs ranged from 1.80 to 9.46 ng/g. The highest concentration was recorded in August. This period was characterized by heavy rainfall. Thus, high precipitation during the rainy season was probably responsible for increased levels of these contaminants during this period as a result of their mobilization from dumpsite soil into the stream through surface runoff. With respect to the variation of these contaminants at different sampling periods, only BDE47 showed statistically different (p < 0.05) values from the others with analysis of variance (ANOVA). The overall mean concentrations of the total Ʃ6PBDEs throughout the period of study did not show any significant difference. Stream hydrodynamics such as flow rate and the degree of turbulence might have affected the settling rates and deposition of suspended matter in the river (Forstner 2004).
Levels of PBDEs in bed sediment of Asunle stream at various locations
The distributions of PBDE congeners at various locations in the bed sediments were investigated and are presented in Table 2. Total PBDEs ranged from 0.73 to 10.43 ng/g. The lowest mean value was recorded at the upstream (control) site (L0), while location 5 had the highest recorded mean concentrations. Location 1 was the site closest to the dumpsite where open incineration takes place from time to time. The concentration of PBDEs showed an increasing trend with the distance from L0 to L5. It appeared that the PBDE congeners were retained more by river sediments as the distance increased from the dumpsite. This could be as a result of larger volume of water with corresponding slower transportation rate of the river body, which resulted in deposition of larger PBDE-bearing bottom sediments over time. Dumping of e-wastes and their open-air combustion is likely responsible for the addition of PBDE contamination to the soil and river (Zhang et al. 2016). Leung et al. (2006) and Leung et al. (2007) affirmed that PBDEs are present in soil and river sediments as a result of insufficient destruction resulting from dumping, open burning, and acid leaching. In some instances, atmospheric deposition could become an important route for PBDE transportation to the sediments (Hale et al. 2003; Song et al. 2006). The presence and levels of PBDEs recorded upstream, about 0.25 km away from the dumpsite, could be explained in keeping with such previous findings, while prevailing wind and stormy weather during the rainy season could enhance the transport and re-distribution of PBDEs to other regions far away from the point of generation. This observation was in agreement with Zhao et al. (2009) who found that PBDEs from e-waste recycling area diffused into ambient regions and resulted in a halo pattern of PBDE contamination of at least 74 km in radius. The elevated mean concentration value obtained at location 5 could be attributed to the pronounced sedimentation that occurred at this site since these contaminants could bind to humic substances in the sediment.
To further investigate the behaviour of the target compounds in the stream sediments investigated, ANOVA was conducted. Results indicated that BDEs 47 and 154 did not exhibit significant difference (p < 0.05) in all the locations unlike other PBDE congeners in the bed sediments although the flow rate as well as the pattern of stream flow may affect the settling of suspended matter and sediment deposition in the river bed.
Total organic carbon (TOC) is a measure of the organic carbon in the sediment sample. Since particles rich in organic carbon have the greatest potential to bind PBDEs and since PBDEs preferentially bind to particulates in water (Environment Canada 2009), measuring the TOC concentration in the sediment samples was used to establish whether there was any correlation between the measured PBDE and TOC. The TOC measured in this study ranged from 0.87 ± 0.28 % at L1 to 1.80 ± 1.67 % at L5. The TOC showed an increasing trend with the distance from L0 to L5.
Seasonal variations of PBDE in bed sediment of Asunle stream
Extensive monitoring of contaminants in different environmental matrices for a period of 2 years would be most appropriate to predict seasonal variability of the target compounds (Chapman 1996). However, assessment of the total PBDEs in different seasons could also be used to predict seasonal characteristics of most contaminants in a given environmental matrix. Seasonal variations of PBDE congeners in sediment samples collected from Asunle stream are presented in Fig. 2. The results show that the concentrations of these contaminants were slightly higher in the sediment during the wet season. However, BDE153 demonstrated an exceptionally higher concentration in the wet season. During the wet season, the leaching of these contaminants into the aquatic system could have been a contributive factor to their high levels as detected and recorded in the sediments during the wet season. The lower values of these contaminants during the dry season might be due to decreased rate of precipitation and consequently lower amount of the contaminants transported via erosion during this period.
Principal component analysis of PBDEs in bed sediment of Asunle stream
To predict and identify the possible sources of the PBDE contaminants, the results obtained were subjected to principal component analysis to establish whether the contaminants were actually arising from the same source or not. The principal component analysis of PBDEs in the bed sediment of Asunle stream is presented in Fig. 3. The analysis revealed that there were varied sources of these contaminants. The hexa-BDE technical formulation did not cluster with the other congeners. The total variance for these two components came up to 57.87 %. The first component accounted for 36.89 % of the recorded variance. Congeners BDE99 and BDE28 had high positive loadings of 0.845 and 0.742, respectively. The second component accounted for 20.98 % of the recorded variance, in which case, BDE47 had high positive loadings of 0.786.
In addition, looking at the association pattern of the various congeners, one could suggest that various materials that contain different proportion of PBDEs may be the attributive factor that made different congeners behave differently given that they came from the same source-dump. Studies have shown that the higher the vapour pressure and the lower the octanol-air partition coefficient, the more likely the BDE congeners will volatilize (USEPA 2010). Consequently, lower brominated congener BDEs have the greatest tendency to volatilize from PBDE-containing consumer products. Coated plastics used in electrical products and other electronic wastes, polymer products such as polyurethane foam in car/transport accessories, furniture, construction, mattress and baby care products could also be sources from where PBDEs are derived (Ogungbuyi et al. 2012; Stockholm Convention 2011). Generally, the main sources of PBDEs were probably from leachates emanating from the PBDE-containing consumer products and other domestic wastes from the dumpsite that found their way into the stream.
Correlation analysis of PBDEs in bed sediment of Asunle stream
Correlation analysis of PBDE congeners in the bed sediment of Asunle stream is presented in Table 3. The results showed that there were positive correlations among the lower congeners in particular. BDE28 was significantly positively correlated with BDE47 and BDE99 at p < 0.01 and with BDE153 at p < 0.05. BDE47 correlated positively with BDE99 at p < 0.05, while BDE99 correlated positively with BDEs 100 and 153 at p < 0.01. Those PBDEs with positive correlation values are probably traceable to the same source, such as BFR-containing products in the dumpsite.
The Pearson correlation carried out to establish possible relationship between target PBDE congeners and total organic carbon (TOC) in the bottom sediment of Asunle stream showed that BDE28, BDE47, BDE99, and BDE100 congeners were positively correlated with TOC, but the correlation was weak. BDE153 and BDE154 were negatively correlated with TOC, thus suggesting that the presence of organic matter might not influence their levels in the stream. However, none of the congeners had a significant correlation at either 0.01 or 0.05 levels (two tailed) with the TOC.
Comparison of PBDE levels in sediment of the study area with levels in other countries
Polybrominated diphenyl ether (PBDE) concentrations in river sediments have been measured and reported in several regions of the world. A comparison of the results obtained in this study with similar studies conducted around the world is shown in Table 4. The levels of the target compounds found in the bed sediment of Asunle stream were lower than those reported elsewhere. This may be attributed to the more pronounced industrial activities in the catchments of waste water treatment plant and high population density of the areas where the previous studies were conducted. For instance, PBDE demand figures for 2001 suggested that North America accounted for 95 % of the global penta-BDE consumption (Law et al. 2006). The two rivers of the Pearl River delta, the Zhujiang and Dongjiang, flow through the world’s most densely urbanized region of about 120 million people and a major electronic manufacturing centre. Durban Bay and most of the rivers studied in South Africa were associated with residential and industrialized areas, while Lagos Lagoon is highly polluted as a result of population density with a high percentage (over 75 %) of Nigeria’s industry located in Lagos (Friends of the Environment 2006; La Guardia et al. 2013). However, it is important to note that accurate and direct comparisons of PBDE concentration levels are impossible as a result of difference in test parameters and congeners from one study to another.
Findings in the present study suggested that as local demands of polymer products and electronics are increasing within the university community, there is the tendency for used polymer and electrical and electronic wastes at the dumpsite to accumulate, and hence, levels of BFR concentrations may continue to increase. This will inadvertently lead to increased contamination of Asunle water body with BFR. Hence, the rural dwellers using the water and the aquatic habitat within the stream vicinity might be negatively impacted. This underlines the need to further investigate the environmental burdens on plants and animals, as well as risks associated with BFR exposure among the rural dwellers and farmers depending on this water body for farm produce processing and drinking purposes. Although the current levels of PBDEs detected in the sediments of Asunle stream were below the levels detected in sediments of rivers located within highly urbanized regions, it is pertinent to put in place measures that could prevent heightened presence of PBDEs in the aquatic ecosystem of the study area in the future. This will go a long way to protecting those who rely on water from Asunle stream for various day-to-day uses. Trash should be properly disposed, and conventional ways of getting rid of waste should be employed. Proper leachate management is an important factor for any dumpsite; hence, a typical landfill/dumpsite should be constructed to contain leachate collection and treatment, either internally or externally.
Conclusion
The inappropriate disposal and open incineration of solid waste and e-waste have become important sources of environmental contamination and pollution by a number of toxic chemicals including PBDEs. This study is one of the few studies in Nigeria conducted on the contamination of PBDEs in stream sediment adjoining the Obafemi Awolowo University dumpsite. The results suggest that past uncontrolled waste disposal most likely resulted in the occurrence and migration of PBDEs into the surrounding environment. The leaching of PBDEs along with high levels of suspended solids into the aquatic system could have contributed to the higher levels detected and recorded in the sediment during the wet season.
References
Adewuyi GO, Adeleye AO (2013) Evaluation of polybrominated diphenyl ethers in sediment of Lagos Lagoon, Nigeria. Afric J Environ Sci Tech 7(7):686–693
Allchin C, de Boer J (2001) Results of comprehensive survey for PBDEs in the River Tees, UK. Organohalogen Compd 52:30–34
Arinaitwe K, Muir DCG, Kiremire BT, Fellin P, Li H, Teixeira C (2014) Polybrominated diphenyl ethers and alternative flame retardants in air and precipitation samples from the Northern Lake Victoria Region, East Africa. Environ Sci Tech 48:1458–1466
Asante KA, Sudaryanto A, Devanathan G, Bello M, Takahashi S, Isobe T, Tanabe S (2010) Polybrominated diphenyl ethers and polychlorinated biphenyls in cow milk samples from Ghana. Interdisciplinary Studies on Environmental Chemistry-Environmental Specimen Bank, 191–198.
Bodin N, N’Gom Ka R, Le Loc’h F, Raffray J, Budzinski H, Peluhet L, Tito de Morais L (2011) Are exploited mangrove molluscs exposed to persistent organic pollutant contamination in Senegal, West Africa? Chemosphere 84:318–327
Bodin N, N’Gom Ka R, Le Loc’h F, Raffray J, Budzinski H, Peluhet L, Tito de Bouwman H, Booyens P, Govender D, Pienaar D (2014) Chlorinated, brominated and fluorinated organic pollutants in Nile crocodile eggs from the Kruger National Park, South Africa. Ecotoxicol Environ Safety 104:393–402
Bouwman H, Booyens P, Govender D, Pienaar D, Polder A (2014) Chlorinated, brominated, and fluorinated organic pollutants in Nile crocodile eggs from Kruger national park, South Africa. Ecotoxicol Environ Saf 104:393–402
Chapman D (1996) Water quality assessment: a guide to the use of biota, sediments and water in environmental monitoring, 2nd edn. University Press, Cambridge
Darnerud PO, Aune M, Larsson L, Lignell S, Mutshatshi T, Okonkwo J, Botha B, Agyei N (2010) Levels of brominated flame retardants and other persistent organic pollutants in breast milk samples from Limpopo Province, South Africa. Sci of the Total Environ 409:4048–4053
Daso AP, Fatoki OS, Odendaal JP (2011a) Development of analytical procedures for the simultaneous determination of tri-to heptabrominated diphenyl ethers and hexabrominated biphenyl (BB153) in sediment samples. Wat South Afric 37(3):331–337
Daso AP, Fatoki OS, Odendaal JP, Olujimi OO (2011b) Occurrence of selected polybrominated diphenyl ethers and 2, 2’,4,4’,5,5’-hexabromobiphenyl (BB-153) in sewage sludge and effluent samples of a wastewater-treatment plant in Cape Town, South Africa. Arch Environ Contam Toxicol 62:391–402
Daso AP, Fatoki OS, Odendaal IP, Olujumu OO (2013a) Polybrominated diphenyl ethers (PBDEs) and 2, 2’, 4,4’,5,5’-hexabromobiphenyl (BB-153) in landfill leachates in Cape Town, South Africa. Environ Monit Assess 185(1):431–439
Daso AP, Fatoki OS, Odendaal JP (2013b) Occurrence of polybrominated diphenyl ethers (PBDEs) and 2,2’,4,4’,5,5’-hexabromobiphenyl (BB-153) in water samples from the Diep River, Cape Town, South Africa. Environ Sci Pollut Res 20:5168–5176
Daso AP, Fatoki OS, Odendaal JP (2013c) Evaluation of extraction methods for the analysis of selected polybrominated diphenyl ethers and hexabromobiphenyl (BB-153)—application to aqueous environmental samples. Inter J Phy Sci 8(29):1506–1514
Daso AP, Fatoki OS, Odendaal JP (2016) polybrominated diphenyl ethers (PBDEs0 and hexabromobiphenyl in sediments of the Diep and Kuils Rivers in South Africa. Int J Sediment Res 31(1):61–70
De Wit CA (2002) An overview of brominated flame retardants in the environment. Chemosphere 46:583–624
Dean WE Jr (1974) Determination of carbonate and organic matter in calcareous sediment and sedimentary rocks by loss of ignition: comparison with other methods. J Sedimentary Petrology 44:242–248
Eljarrat E, de la Cal A, Raldua D, Duran C, Barcelo D (2004) Occurence and bioavailability of polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from the Cinca river, a tributary of the Ebro River (Spain). Environ Sci Technol 38(9):2603–2608
Eljarrat E, Marsh G, Labandeira A, Barcelo D (2008) Effect of sewage sludge contaminated with polybrominated diphenyl ethers on agricultural soils. Chemosphere 71:1079–1086
Environment Canada (2009) State of the science report on the bioaccumulation and transformation of decabromodiphenyl ether. Canada. 134
Forstner U (2004) Sediment dynamics and pollutants mobility in rivers: an interdisciplinary approach. Lakes and reservoirs. Res Manage 9:25–40
FOTE. Friends of the Environment (2006) Assessment of the Lagos lagoon for POPs sources, types and impacts. The International POPs Elimination Project (IPEP), Lagos
Guan Y-F, Wang J-Z, Ni H-G, Luo X-J, Mai B-X, Zeng EY (2007) Riverine inputs of polybrominated diphenyl ethers from pearl River delta (China) to the coastal ocean. Environ Sci Technol 41:6007–6013
Guerra P, Alaee M, Eljarrat E, Barcel D (2011) Introduction to brominated flame retardants: commercially productions, applications and physicochemical properties. In: Eljarrat E, Barcelo D (eds) The handbook of environmental chemistry 16. Brominated flame retardants. Springer Verlag Berlin Heidelberg, New York
Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG (2003) Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int 29:771–779
Hale RC, LaGuardia MI, Harvey EP, Mainor TM, Duff WH, Gaylor MO (2001) Polybrominated diphenyl ether flame retardants in Viginia freshwater fishes (USA). Environ Sci Technol 35:4585–4591
Hassine SB, Ameur WB, Gandoura N, Driss MR (2012) Determination of chlorinated pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human milk from Bizerte (Tunisia) in 2010. Chemosphere 89:369–377
Hu G, Xu Z, Dai J, Mai B, Cao H, Wang J, Shi Z (2005) Distribution of polybrominated diphenyl ethers and decabromo diphenyl ethane in surface sediments from Fuhe River and Baiyangdian lake, North China. J Environ Sci 22(12):1833–1839
Karter MJ Jr (2008) Fire loss in the United States 2007. National Fire Protection Association, USA
Karter MJ Jr (2014) Fire loss in the United States 2013. National Fire Protection Association, USA
Kefeni KK, Okonkwo JO (2012) Analysis of major congeners of polybrominated biphenyls and polybrominated diphenyl ethers in office dust using high resolution gas chromatography-mass spectrometry. Chemosphere 87:1070–1075
Kefeni KK, Okonkwo JO (2014) distribution of polybrominated diphenyl ethers and dust particle size fractions adherent to skin in indoor dust, pretoria, South Africa. Environ Sci Pollut Res 21:4376–4386
Kupper T, Felippe de Alencastro L, Gatsigazi R, Furrer R, Grandjean D, Tarradellas J (2008) Concentrations and specific loads of brominated flame retardants in sewage sludge. Chemosphere 71:1173–1180
Kuriyama SN, Talsness CE, Grote K, Chahoud I (2005) Developmental exposure to low dose PBDE 99. Effect on male fertility and neurobehavior in rat offspring. Environ Health Perspect 113:1173–1180
La Guardia MJ, Hale RC, Newmna B (2013) Brominated flame-retardants in Sub-Saharan Africa: burdens in inland and coastal sediments in the eThekwini Metropolitan Municipality, South Africa. Environ Sci Technol 47:9643–9650
Law K, Halldorson T, Danell R, Stern G, Gewurtz S, Alaee M (2006) Bioaccumulation and trophic transfer of some brominated flame retardants in a Lake Winnipeg (Canada) food web. Environ Toxicol Chem 25(8):2177–2186
Leung A, Cai ZW, Wong MH (2006) Environmental contamination from electronic waste recycling at Guiyu, southern China. J Mater Cycles Waste Manage 8:21–33
Leung AOW, Luksemburg WJ, Wong AS, Wong MH (2007) Spartial distribution of polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in soil and combusted residue at Guiyu an electronic waste recycling site in southeastern China. Env Sci and Tech 4:2730–2737
Linderholm L, Biague A, Manson F, Norrgren H, Bergman A, Jakobsson K (2010) Human exposure to persistent organic pollutants in West Africa- A temporal trend study from Guinea-Bissau. Environ Int 36:675–682
Liu Y, Zheng GJ, Yu H, Martin M, Richardson BJ, Lam MHW, Lam PKS (2005) Polybrominated diphenyl ethers (PBDEs) in sediments and mussel tissues from Hong Kong marine waters. Mar Pollut Bull 50:1173–1184
Mai B, Chen S, Luo X, Chen L, Yang Q, Sheng G, Peng P, Fu J, Zeng EY (2005) Distribution of polybrominated diphenyl ethers in sediments of the Pearl River delta and adjacent South China sea. Environ Sci Technol 39:3521–3527
Moon HB, Kannan K, Lee SJ, Choi M (2007) Atmospheric deposition of polybrominated diphenyl ethers (PBDEs) in coastal area in Korea. Chemosphere 46:745–755
Odusanya DO, Okonkwo JO, Botha B (2009) Polybrominated diphenyl ethers (PBDEs) in leachates from selected landfill in South Africa. Waste Manag 29:96–102
Ogunbiyi O, Nnorom JC, Osibanjo O, Schluep M (2012) Nigeria e-waste country assessment. Basel Convention Coordinating Centre for Africa (BCCC-Nigeria) and Swiss Federal Laboratories for Materials Science and Technology (EMPA), Ibadan, Nigeria and St. Gallen, Switzerland
Ogunfowokan AO, Oyekunle JAO, Olutona GO, Atoyebi AO, Lawal A (2013) Speciation study of heavy metals in water and sediments from Asunle River of the Obafemi Awolowo University, Ile-Ife, Nigeria. Int J Environ Protect 3(3):6–16
Olukunle O, Okonkwo J, Odusaanya O (2011) accelerated solvent extraction of common polybrominated diphenyl ethers from river sediment. Tshwane university of technology, department of Environmental, water and Earth Science, 175, Nelson Mandela Drive, Building 4, pretoria, South Africa, 9pp
Olukunle O, Okonkwo J, Odusanya O (2012a) Accelerated solvent extraction of common polybrominated diphenyl ethers from river sediments. Bull Environ Contam Toxicol 88:461–466
Olukunle O, Okonkwo J, Kefeni K, Lupankwa M (2012b) Concentrations of polybrominated diphenyl ethers in sediments from Jukskei River, Gauteng, South Africa. Bull Environ Contam Toxicol 88(3):461–466
Parolini M, Guazzoni N, Cormolli R, Binelli A, Tremolada P (2013) Background levels of polybrominated diphenyl ethers (PBDEs) in soils from Mount Meru Area, Arusha District (Tanzania). Sci of the Total Environ 452–453:253–261
Polder A, Venter B, Skaare JU, Bouwman H (2008) Polybrominated diphenyl ethers and HBCD in bird eggs of South Africa. Chemosphere 73:148–154
Premium Times (2013) 185 people die in 470 fire incidents in Nigeria- fire service. Premium Times Newspaper, April 29, 2013 page 1
Samara F, Tsai CW, Aga DS (2006) Determination of potential sources of PCBs and PBDEs in sediments of the Niagara River. Environ Pollut 139:489–497
Sellstrom U, Lindberg P, Haggberg L (2001) Higher brominated PBDEs found in eggs of peregrine falcons (Falco peregrinus) breeding in Sweeden. The second International Workshop on Brominated Flame Retardants: BFR 2001 Stoskholm, Sweeden, 159–162
Sindiku O, Babayemi J, Osibanjo O, Schlummer M, Schlucp M, Watson A, Weber R (2014) Polybrominated diphenyl ethers listed as Stockholm Convention POPs, other brominated flame retardants and heavy metals in e-waste polymers in Nigeria. 12th IHPA forum and selected studies on POPs. Environ Sci. Pollut Res. 13
Song M, Chu S, Letcher RJ, Seth B (2006) Fate partitioning, and mass loading of polybrominated diphenyl ethers (PBDEs) during the treatment processing of municipal sewage. Environ Sci Techn 40:6241–6246
Stockholm Convention (2011) Work programmes on new persistent organic pollutants. 5th Conference of parties meeting UNEP/POPS/COP5/15
The Punch Newspaper (2013) Nigeria loses over N50 bn to fire disaster annually- Fire Siervice. The Punch , July 20, 2013
The Vanguard. Lagos State lost N54 bn property to inferno in 3 years—fire service. Tuesday January 20, 2015. www.vanguard.com/2015/..../lagoss..
Troitzch JH (1990) International plastics flammability handbook: principles, regulations, testing and approval. Hanser Publishers, Munich
USEPA (2010) An exposure assessment of poly brominated diphenyl ethers> National Centre for Environmental Assessment, Washington, DC, EPA/600/R-08/086F. 378
Wepener V, Smit N, Covaci A (2012) Seasonal bioaccumulation of organohalogens in tigerfish hydrocynus vittatus Castelnau, from Lake Pongolapoort, South Africa. Bull Environ Contam Toxicol 88:277–282
Yun SH, Addink R, McCabe JM, Ostaszewski A, Mackenzie-Taylor D, Taylor AD (2008) Polybrominated diphenyl ethers and polybrominated biphenyls in sediment and floodplain soils of the Saginaw River watershed, Michigan, USA. Arch Environ Contam Toxicol 55:1–10
Zhang M, Buekens A, Li X (2016) Brominated flame retardants and the formation of dioxins and furans in fires and combustion. J Hazard Mater 304:26–39
Zhao YX, Qin XF, Li Y, Liu PY, Tian M, Yan SS, Qin ZF, Xu XB, Yang YJ (2009) Diffusion of polybrominated diphenyl ether (PBDE) from an e-waste recycling area to the surrounding regions in Southeast China. Chemosphere 76:1470–1476
Acknowledgments
The authors appreciate the three anonymous reviewers for their objective criticisms and suggestions which helped to improve the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Olutona, G.O., Oyekunle, J.A.O., Ogunfowokan, A.O. et al. Assessment of polybrominated diphenyl ethers in sediment of Asunle stream of the Obafemi Awolowo University, Ile-Ife, Nigeria. Environ Sci Pollut Res 23, 21195–21205 (2016). https://doi.org/10.1007/s11356-016-7270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7270-4