Abstract
The potential for nanoscale phosphate amendments to remediate heavy metal contamination has been widely investigated, but the strong tendency of nanoparticles to form aggregates limits the application of this technique in soil. This study synthesized a composite of biochar-supported iron phosphate nanoparticle (BC@Fe3(PO4)2) stabilized by a sodium carboxymethyl cellulose to improve the stability and mobility of the amendment in soil. The sedimentation test and column test demonstrated that BC@Fe3(PO4)2 exhibited better stability and mobility than iron phosphate nanoparticles. After 28 days of simulated in situ remediation, the immobilization efficiency of Cd was 60.2 %, and the physiological-based extraction test bioaccessibility was reduced by 53.9 %. The results of sequential extraction procedures indicated that the transformation from exchangeable (EX) Cd to organic matter (OM) and residue (RS) was responsible for the decrease in Cd leachability in soil. Accordingly, the pot test indicated that Cd uptake by cabbage mustard was suppressed by 86.8 %. Compared to tests using iron phosphate nanoparticles, the addition of BC@Fe3(PO4)2 to soil could reduce the Fe uptake of cabbage mustard. Overall, this study revealed that BC@Fe3(PO4)2 could provide effective in situ remediation of Cd in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In China, more than 200,000 ha of farmland soil has been reported to be contaminated by Cd. Every year, the soil produces 1.46 million tons of agricultural products that exceed the national food standards for Cd (Liu et al. 2007). As a result, the remediation of Cd-polluted soil is an urgent problem. In recent years, in situ solidification/stabilization technology has elicited a great deal of attention due to its high efficiency for reducing the toxicity and bioaccessibility of Cd in soil in the short term. An important remediation technology, phosphate-based amendments have been proven to significantly reduce the dissolution and translocation of heavy metal in soil (Dong et al. 2010; Hodson et al., 2000; Xu et al. 2016). For instance, Liu et al. found that iron phosphate nanoparticles could effectively reduce the TCLP leachabilities of Pb and Cu in soil by 85–95 % and 63–87 %, respectively (Liu and Zhao 2007a, b). Because of the fairly low solubility product of iron phosphate nanoparticles, the secondary pollution caused by too much available phosphorus was avoided (Ding et al. 2012; Zhou and Xu, 2007), and the remediation was sped up at the same time.
However, if iron phosphate nanoparticles are used in situ remediation, the remediation efficiency will decrease due to the nanosize effect, which weakens the stability of nanoparticles. Moreover, the fertility of the soil will be influenced because Fe2+ will substitute for heavy metal cations in the process of remediation, which will lead to a high Fe content in the soil. Some published papers have shown that too much Fe in soil was detrimental to plants and was disadvantageous for replanting (Mengel 1994; Chen et al. 2012; El-Temsah and Joner 2012; Ma et al. 2013). In order to overcome these problems in iron phosphate nanoparticle soil remediation, the use of biochar was proposed in this study. Biochar can not only cover the shortage of iron phosphate nanoparticles, but can also improve soil fertility. Due to the rich oxygen functional groups and the negative charge on its surface (Xu and Fang 2015; Yang and Fang 2014), biochar can firmly adsorb metal cations, thus mitigating the secondary pollution brought about by the release of iron. At the same time, the high content of organic matter and the porous structure of biochar are also beneficial in retaining soil nutrients and moisture, which can increase crop yields (Tang et al. 2013; Asai et al. 2009; Uzoma et al. 2011).
On the basis of a small preliminary laboratory test conducted in shaking bottles, each sample, which contained 500 g of soil in a pot, was tested by simulating in situ remediation. The stability and mobility of loaded iron phosphate nanoparticles (BC@Fe3(PO4)2) were studied, and the remediation efficiency and mechanism were also investigated. In order to evaluate the heavy metal toxicity to plants after the soil was amended by BC@Fe3(PO4)2, a cabbage mustard seedling growth experiment was conducted.
Materials and methods
Materials
Fe3(PO4)2 nanoparticles
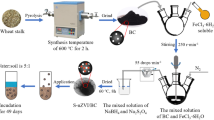
Iron (II) sulfate heptahydrate and sodium phosphate dodecahydrate were dissolved in deionized (DI) water that had been purged with nitrogen gas (N2). All of the following operations were conducted under the N2 condition to avoid oxidation of Fe2+ by air: a Na3PO4 solution containing 0.6 g L−1 PO4 3− was added to the same volume of FeSO4 solution containing 0.53 g L−1 Fe2+ drop by drop with continuous stirring. After 30 min of stirring, Fe3(PO4)2·8H2O was formed.
Biochar
After being air-dried at room temperature, a Chinese herb medicine residue was ground to pass through a 10-mesh sieve. Then, the ground medicine residue was placed in ceramic crucibles, covered with a lid, and pyrolyzed under an oxygen-limited condition in a muffle furnace. The sample was heated to 600 °C at a rate of approximately 20 °C/min, and the final temperature was maintained for at least 2 h to allow sufficient time for complete pyrolysis (Dong et al. 2011; Ding et al. 2014). The biochar was passed through different sieves (24, 60 and 120 meshes) to create particles with different sizes.
BC@Fe3(PO4)2 composite
Ten grams of biochar particles was evenly dispersed in 50 mL 0.25 % NaCMC solution, and 25 mL of a FeSO4 solution containing 1.05 g L−1 Fe2+ was added drop by drop with continuous stirring. After stirring for 30 min, another 25 mL Na3PO4 solution containing 1.19 g L−1 PO4 3− was added dropwise to the mixture. All operations were conducted under the N2 condition.
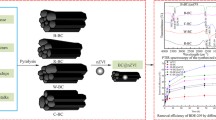
The pH of the biochar was 10.11, which was measured by a pH meter (PHS-3C, Shanghai) in water at a 1:5 (w/v) ratio. The specific surface area of the biochar was 9.4 m2/g, which was measured by using the N2-Brunauer-Emmett-Teller (BET) method (ASAP2020M, USA). Scanning electronic microscopy images (ZEISS Ultra 55, Germany) shows the smooth surface and obvious honeycomb structure of the biochar, whereas images shows numerous particles on the surface of the composite, demonstrating that iron phosphate was distributed on the surface of the biochar. The spectrum of BC@Fe3(PO4)2 from Fourier transform infrared spectrometer (Nicolet 6700, USA) shows that there were C–O and C=O bands at around 1400 and 1020 cm−1(Cao and Harris, 2010; Yao et al., 2011), which were beneficial to its combination with heavy metal (shown in Fig. 1). However, the characteristic bands of BC@Fe3(PO4)2 at around 1400 cm−1 was obviously weakened when compared with biochar. This might due to the C–O combination with Fe, revealing the Fe3(PO4)2 and biochar were combined though chemical bond.
Sedimentation test
BC@Fe3(PO4)2 was synthesized by the biochar, which was passed through sieves with three different sizes (24, 60, and 120 meshes) following the process described in “Materials” section. At the same time, different mass ratios of biochar and iron phosphate nanoparticles (BC:Fe3(PO4)2 = 10:1, 20:1, 30:1) were prepared. In order to compare the stabilities of these various materials, the sedimentation rates of their suspensions were determined. In brief, the suspensions were sonicated for 5 min, while the absorbance was held continuously at 508 nm with a UV-Vis spectrophotometer (Liang et al. 2014).
Column test
The transport behavior of the Fe3(PO4)2 and the BC@Fe3(PO4)2 composite was investigated in silica sand that was packed in a vertical glass column. The following operations were performed following He et al.’s procedure (He et al. 2009): Briefly, the column (1.5 cm i.d.; 10.0 cm length) was packed with silica sand (30–50 meshes; 0.345 porosity) and gently vibrated to ensure uniform packing. Then, eight pore volumes (PVs) of the material suspensions were pumped downwards through the column with a double channel peristaltic pump at a certain velocity. To prevent the sedimentation of the suspensions, the reservoir containing materials was continuously mixed at 250 rpm. The effluent samples were collected at selected time intervals, and the total iron concentration was measured. An aliquot of the material effluent was digested with 1 M HCl for 2 h. Afterwards, the total iron concentrations were determined by atomic absorption spectroscopy (AAS, Perkin Elmer PinAAcle 900 T, USA).
Simulation of in situ remediation of Cd in soil
Topsoil (0 ~ 10 cm) was collected at random from Centre Lake in Guangzhou Higher Education Mega Center, China (23°03′12.59″ N,113°23′25.81″ E). The soil was air-dried and passed through a 60-mesh sieve. There was no Cd detected in the soil. To prepare Cd-polluted soil, the air-dried soil was mixed with a solution containing 5 mg L−1 Cd and 10−3 M CaCl2 at a solution-to-solid ratio of 1:1 (mL/g) and then stirred for 24 h to reach equilibrium conditions. After it was air-dried to a constant weight, the soil was ground to pass through a 10-mesh sieve and was kept in a dryer. The resultant Cd content in the soil was 5 mg/kg.
Five hundred grams of the Cd-polluted soil was mixed with Fe3(PO4)2 at a 1:1 ratio (Fe3(PO4)2/soil, mL/g), biochar at a 0.1:1 ratio (biochar/soil, g/g), and BC@Fe3(PO4)2 at a 1:1 ratio (BC@Fe3(PO4)2/soil, mL/g) in a plastic pot (20 cm diameter; 25 cm height) for 28 days under the intermittent stirring condition. Two control treatments, unpolluted and polluted soil, were also prepared following the same procedure but without adding the amendment. Three replicates were performed for each treatment. During the remediation, the sample was mixed uniformly once daily. After 28 days, 3 g of soil was collected randomly from each sample and divided into three equal parts. Each 1 g portion of treated soil was extracted with diethylene triamine pentaacetate acid (DTPA) solution, and then centrifuged and filtered. The Cd concentration was analyzed by AAS (GB/T 23739-2009). The remediation efficiency was showed by the immobilization rate, which was quantified via Eq. (1).
where M0 and M were the Cd concentrations extracted by DTPA solution before and after treatment.
To evaluate the effect of the composite materials on amending Cd-polluted soil, a physiologically based extraction test (PBET) was used in the investigation of the in vitro bioaccessibility of Cd in soil. The operations followed the procedures described by Kelley et al. (Kelley et al. 2002). In brief, 0.25 g of original or amended soil was mixed with 25 mL PBET extraction solution, which contained 30 g L−1 glycine (0.4 M) with pH 2.3. The mixture was rotated end-to-end at 37 ± 2 °C. After 1 h, the mixture was centrifuged, filtered, and analyzed for Cd.
An SEP was used to analyze the Cd speciation in soils before and after amendment with the composite. Cd speciation in the soil, including the exchangeable fraction, carbonate fraction, Fe-Mn oxides fraction, organic fraction, and residual fraction, was determined by a modified sequential extraction procedure (Tessier et al. 1979; Reddy et al. 2001). After each extraction, the separation was centrifuged and filtered. Subsequently, the filtrate was stored for Cd analysis, and the remaining soil was retained for chemical analysis.
Pot test
A pot experiment was conducted to investigate the influences of unpolluted soil (S0), Cd-polluted soil (S1), Fe3(PO4)2 nanoparticle-amended soil (S2), biochar-amended soil (S3), and BC@Fe3(PO4)2-amended soil (S4) on the development of cabbage mustard and the translocation and accumulation of Cd and Fe in the plant. A plastic pot was filled with 500 g of treated or untreated soil, and deionized water was added to keep the moisture at 60–80 % of the water holding capacity (WHC). Seeds used in the test were disinfected with 0.5 % sodium hypochlorite solution for 10 min and then washed five times with deionized water. Twenty disinfected cabbage mustard seeds were placed in each pot at a certain spacing. The pots were put outdoors and irrigated twice daily to keep the moisture constant. After 30 days of growth, the shoots were harvested and cleaned with deionized water for further use.
The stem lengths of the seedlings were accurately measured. The roots and stems were separated, and dried in an oven at 80 °C for 48 h to measure the dry mass of each part. The dry parts of the seedlings were put into the muffle furnace to calcinate for 5 h at 550 °C. Then, 5 mL of HNO3 was added to each calcinated sample. Samples were then placed on an electric heating furnace to digest to the remaining 1 mL of solution. The 1 % HCl solution was then added to a volume of 5 mL, and the concentrations of Cd and Fe were analyzed by AAS.
Results and discussion
Assessment of BC@ Fe3(PO4)2 composite stability
The sedimentation curves of Fe3(PO4)2 and BC@Fe3(PO4)2 are shown in Fig. 2. For Fe3(PO4)2, the absorbance at 508 nm was reduced by 58.5 % in 30 min, whereas the absorbance for BC@Fe3(PO4)2 was only reduced by 8.8 %. The sedimentation of these two kinds of materials after an hour is shown in Fig. 3. The difference in sedimentation occurred mainly because Fe3(PO4)2 aggregated rapidly to form critical-sized particles, leading to gravity settling. When Fe3(PO4)2 was loaded on the biochar, flocculation would be effectively prevented due to the steric repulsion among the nanoparticles provided by biochar. Moreover, the nanoparticles were encapsulated by a thin layer of negative charge from NaCMC to keep the system stable (Si et al. 2004; He et al. 2007). It was demonstrated that a loading technique could improve the stability of nanoparticles. Greater stability would be advantageous when applying this material to the remediation of heavy metals in soil.
The biochar was passed through three kinds of sieves with 24, 60, and 120 meshes, respectively, and the resultant particle sizes of biochar were 0.8, 0.25, and 0.125 mm. It was shown in Fig. 4 that the absorbance of the composites prepared with 0.8 and 0.25 mm biochar reduced by 40 % after 1 h of sedimentation, whereas the absorbance of the composite prepared with 0.125 nm biochar only reduced by 15 %. These findings suggest that the smaller the biochar particle size, the more stable the composite materials. This is because the mass of composite is mainly determined by biochar. The mass of the composite increases with the increasing size of the biochar, thus increasing the gravity of the composite and resulting in rapid sedimentation. Therefore, the stability of the composite prepared with 0.125 nm biochar was best.
The content of Fe3(PO4)2 in the composite suspension was 0.5 g/L. According to the ratio of biochar and Fe3(PO4)2, the biochar contents were 5, 10, and 15 g/L, respectively. Figure 5 shows that the most stable material was the composite prepared with the ratio of 20:1. This figure illustrates that too low or too high of a biochar content in the composite suspension reduced the stability of the system. As a result, the selected ratio of biochar and Fe3(PO4)2 was 20:1, i.e., adding 1.0 g of biochar to 100 mL of composite suspension.
Assessment the mobility of BC@ Fe3(PO4)2 composite
As indicated in Fig. 6, for Fe3(PO4)2, the relative concentration reached 97.8 % at 0.5 PV; the maximum relative concentration (100 %) was achieved at 1.5 PV. To study the elution behavior of the retained Fe3(PO4)2 nanoparticles after complete breakthrough at 3.5 PV, deionized water was directed upwards into the column, and the effluent Fe concentration was followed until it became undetectable. The curve of Fe3(PO4)2 shows that the relative concentration dropped rapidly to near zero at four PVs, suggesting that Fe3(PO4)2 had good mobility, and no Fe was retained in the silica sand. This is because the constant stirring in the sampling process makes nanoparticles difficult to flocculate, thus leading to smaller sizes and better mobility. Due to the large size of the biochar, the composite was suffocated when BC@Fe3(PO4)2 broke through the low-porosity silica sand. As a result, the relative concentration of BC@Fe3(PO4)2 reached 82.6 % at 0.5 PV, and increased to 100 % at two PVs. Because of the lubrication of NaCMC, the relative concentration also decreased to zero when eluted with deionized water at five PVs, suggesting that no residual composite remained in the silica sand. This proved that BC@Fe3(PO4)2 composite can be applied to remediate soil due to the composite’s good mobility.
In situ remediation of Cd in soil
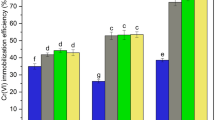
As shown in Fig. 7, all three kinds of material were effective in remediating the Cd-polluted soil with intermittent stirring. The immobilization rates of Fe3(PO4)2 (S2), BC (S3), and BC@Fe3(PO4)2 (S4) were 17.4, 51.2, and 60.2 %, respectively. Our previous work indicated that the immobilization rates of these three kinds of materials were 31.9, 62.9, and 81.3 % under the shaking condition, illustrating that the polluted soil reacted more totally with the amendment when being shaken. However, the immobilization rate of the BC@Fe3(PO4)2 composite was more than 60 %, suggesting that it is a potential amendment for use in soil remediation.
Figure 8 shows reductions after the soil was treated with the amendments. The bioaccessibility of Cd reduced from an original value of 67.3 to 52.3, 43.3, and 31.0 % for the Fe3(PO4)2, BC, and BC@Fe3(PO4)2 treatments, respectively, i.e., reductions of 22.3, 35.7, and 53.9 %. This proved that both Fe3(PO4)2 and BC could effectively immobilize Cd in soil and prevent Cd from being absorbed by organisms. When Fe3(PO4)2 was loaded to biochar, the bioaccessibility reduced by more than half, thus realizing the immobilization of heavy metals in the soil.
In the soil before treatment (S1), Cd existed in the first two fractions. EX and CB accounted for 46.9 and 42.0 %, respectively. Because the contents of the OM and RS fractions were lower than the limits of detection, the sum of these two fractions was calculated by subtracting the amount of the first three fractions from the total amount of Cd in soil. Figure 9 shows the EX fraction obviously decreased after being treated with the amendments, whereas the OM and RS fractions increased. This demonstrated that the composite could effectively reduce the more bioavailable Cd speciation and change it to a much less bioavailable speciation, which helped to account for the reduction in leachability and bioaccessibility.
Compared to the control sample, after being treated with Fe3(PO4)2, the fractions of EX converted to OM and RS. According to the principle of using Fe3(PO4)2 to remediate Cd, it could be speculated that Cd could interact with PO4 3− to generate phosphorus compounds with very low solubilities, thus causing the Cd to turn into an RS fraction (Liu and Zhao, 2007a, b). When treated with biochar, the last three fractions of Cd increased more significantly. This might be because the addition of biochar can increase the pH value of soil, thereby promoting the generation of hydroxide precipitation and increasing the OX fraction (Wang et al. 2014). Moreover, the rich oxygen-containing functional groups on the surface of the biochar could form complexes with Cd to increase the OM fraction (Méndez et al. 2013). These are two of the main mechanisms used to immobilize heavy metal in soil (Tang et al. 2013). For the composite treatment, the more stable fractions (OM + RS) increased more significantly, by more than 20 %. This might be a result of the biochar in the composite adsorbing Cd to its surface first, thus playing a gathering role, followed by the Fe3(PO4)2 exchanging ions with the Cd to generate Cd3(PO4)2 precipitation, resulting in greater immobilization of the Cd in the soil. Compared with Wu et al.’s research, which used four phosphates to immobilize heavy metal in contaminated soil and the result showed that Cd percentage in EX fraction reduced from 3 to 2 %, and the OM and RS fractions nearly did not increase after being treated with K2HPO4, Ca3(PO4)2, Ca5(PO4)3OH, and Ca(H2PO4)2·2H2O at a P application rate equivalent to 2283 mg p/kg-soil (Wu et al. 2013). It implied that the BC@Fe3(PO4)2 composite had the technical advantage.
Plant growth and metal bioavailability
Impact of composite material on plant growth
It is necessary to test the effect of amendment on plant growth and development occurring during the process of immobilizing Cd in soil. Figure 10 shows the stem length and dry biomass of cabbage mustard.
The stem length of the seedlings in S1 was shorter than that in S0, with lengths of 32.8 and 46.0 mm, respectively. The stem lengths of the seedlings in S2 was 26.0 mm, i.e., a 43.5 % reduction compared with S0, suggesting that the overly high Fe content did not benefit root and stem growth and development. The stem length of the cabbage mustard seedlings in S4 was longer than that of S0, reaching 57.0 mm, which was an increase of 23.9 % compared to S0. This showed that the composite could not only inhibit the toxic effects of the heavy metal Cd on the growth of cabbage mustard, but also could help to increase soil nutrition as demonstrated by its positive influence on cabbage mustard growth and development.
For the dry biomass of cabbage mustard, the aboveground part and belowground part showed similar trends. The dry biomass of seedlings planted in S1 and S2 decreased, whereas the biomass of those planted in S3 and S4 increased compared with S0. Due to the Cd toxicity, the biomass of the aboveground and belowground parts in S1 decreased from 4.75 and 0.33 mg to 2.50 and 0.16 mg, respectively, when compared to S0. For S2, the biomass of the aboveground part was 3.10 mg, larger than that of S1 because of the remediation of Fe3(PO4)2. However, the biomass of the belowground part of S2 was the same as that of S1, suggesting that the toxicity of Cd to the plant root was relatively strong, and that Fe3(PO4)2 could not lower the risk completely. For S4, the biomass of each tissue increased, reaching 12.7 and 0.79 mg for the aboveground and belowground parts, respectively. These values were 4.08 times and 3.93 times greater than those at S1, and even 1.67 times and 1.39 times greater than those at S0. These findings were consistent with the apparent phenomenon of cabbage mustard and the measurement results of the length of the stem. The research by Houben et al. found that the biomass of shoot increased from 1 to 2 g at the first harvest (4 weeks after sowing) with 10 % biochar treatment, which was twice as much as those untreated (Houben et al. 2013). Therefore, using the composite to remediate Cd-polluted soil could reduce the toxicity of Cd on plants, thus increasing vegetable yield.
Concentration of Cd and Fe in plants
The purpose of remediating Cd-polluted soil is to reduce the possibility of Cd accumulation and translocation in plants. To verify the remediation effect of Cd in the soil after treatment, the Cd concentrations in plants grown in Cd-polluted soils (shown in Table 1) were measured. In soil samples without remediation (S1), the Cd concentrations of the belowground part was 299.8 mg/kg, showing the strong ability of the cabbage mustard root to take up Cd from the soil. After treatment with an amendment, the Cd concentrations of S2, S3, and S4 plants were reduced to 141.2, 56.6, and 8.2 mg/kg. For the aboveground part, the Cd concentration of S1 was 121.2 mg/kg, and it was reduced to 54.1, 8.7, and 4.5 mg/kg in S2, S3, and S4, respectively. For the whole plant, the Cd concentration reduced by 53.6 % after being treated with Fe3(PO4)2, whereas the Cd concentration reduced by 86.8 % after being treated with BC@Fe3(PO4)2. Previously, Zhang et al. reported that with 5 % oil mallee biochar addition, Cd accumulation in plants reduced from 235 to 163 μg/pot, which only decreased by 30.6 % after 9 weeks of plant growth. (Zhang et al. 2013) This showed that the BC@Fe3(PO4)2 composite could further reduce the uptake of Cd in both the aboveground and belowground parts of cabbage mustard when compared with the single iron phosphate nanoparticle remediation. Therefore, under the intermittent stirring condition, BC@Fe3(PO4)2 could effectively reduce the Cd concentration of the aboveground part of cabbage mustard to a lower level, demonstrating that this composite has potential applications to practical farmland pollution remediation.
To further investigate the effect of amendment on the Cd translocation capability of cabbage mustard, concentration factors (CF) and transfer factors (TF) were calculated. The CF and TF were defined as the ratio of the Cd amount in plant leaves to that in soil, and the Cd amount in leaves to that in roots, respectively (Han et al. 2004).
As Table 2 shows, the CF value of Cd for cabbage mustard was relatively large, suggesting that the cabbage mustard had a strong trend to uptake Cd from soil. After remediation, all the CF values declined. The CF value of S4 decreased most significantly, from 24.23 to less than 1, illustrating that the BC@Fe3(PO4)2 composite could effectively prevent Cd from being uptaken by plant. The significant decline of the TF value of S4 demonstrated that the BC@Fe3(PO4)2 composite could greatly weaken the upward translocation capacity of Cd in the plant, leaving most of Cd in the root and lowering the risk posed by heavy metal pollution to human health. However, some previous researches indicated that the TF value would increase after being treated with biochar. For instance, Zhang et al. reported that TF increased from 0.608 to 1.09 with 5 % wheat chaff biochar addition (Zhang et al. 2013). These results indicate that BC@Fe3(PO4)2 remediation can significantly decrease the bioavailability and bioaccumulation of Cd in plants, indicating that it is technologically feasible from a detoxification perspective.
Iron is an important nutritional ingredient for plant growth and development because it is involved in many vital enzymatic reactions for nitrogen fixation, DNA synthesis (ribonucleotide reductase), and hormone synthesis (lipoxygenase and ACC oxidase) (Briat and Lobréaux 1997; Yamauchi and Peng 1995; Mengel 1994). However, some studies have shown that high levels of Fe in soil might be toxic to organisms (Wang et al. 2014). Therefore, it is necessary to examine the absorption of Fe by plants when adding soil amendments containing Fe. As Table 3 shows, the Fe concentration of the belowground part of the cabbage mustard was higher than that of aboveground part, and the trends of Fe absorption by different tissues were similar for the whole plant. For ease of comparison, the Fe content analysis discussed below is based on the content of the whole plant. The Fe concentration of S0 was 6814.2 mg/kg. In Cd-polluted soil, the absorption of Fe was not influenced by Cd. When Fe3(PO4)2 nanoparticles were added to the soil (S2), the Fe content increased to 9491.7 mg/kg, i.e., 1.39 times the Fe content of S0. In contrast, in the soil treated with the composite (S4), the Fe content decreased rapidly to 7950.0 mg/kg, almost the same concentration as in S0. Overall, the single use of Fe3(PO4)2 nanoparticles appeared to significantly promote the absorption of Fe by the cabbage mustard, whereas the composite inhibited the absorption of Fe released by Fe3(PO4)2. Because of the high Fe content in the soil in South China, decreasing the absorption of Fe by plants is a problem that requires a solution.
Conclusion
The BC@Fe3(PO4)2 composite, based on biochar derived from Chinese herb medicine residue and dispersed with NaCMC, was synthesized to improve the stability and mobility of the treatment in soil. The results showed that when the biochar size was 0.125 mm and the mass ratio of biochar and iron phosphate nanoparticle was 20:1, the stability of the composite was best, and its mobility in a porous medium was enhanced. The remediation test showed that the composite could significantly immobilize Cd in soil, thus reducing Cd leachability and bioaccessibility. Meanwhile, the composite could effectively transform the more bioavailable Cd speciation to a much less bioavailable speciation. When the composite was added to soil, it is not only reduced the bioaccumulation of Cd by the cabbage mustard plants, but also promoted the growth and development of the plants. Therefore, BC@Fe3(PO4)2 is a type of soil remediation material with good application prospects.
References
Asai H, Samson B-K, Stephan H-M, Songyikhangsuthor K, Homma K, Kiyono Y, Inoue Y, Shiraiwa T, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos 1. Field Crop Res 111:81–84
Briat J-F, Lobréaux S (1997) Iron transport and storage in plants. Trend Plan Sci 2:187–193
Cao X, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101:5222–5228
Chen P-J, Tan S-W, Wu W-L (2012) Stabilization or oxidation of nanoscale zerovalent iron at environmentally relevant exposure changes bioavailability and toxicity in Medaka fish. Environ Sci Technol 46:8431–8439
Ding W-C, Dong X-L, Ime I-M (2014) Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 105:68–74
Ding S-F, Xie Z-M, Wu W-H (2012) Research progress on chemical remediation of heavy metal-contaminated soils using phosphorous-containing materials. J Anhui Agric Sci 40:17093–17099
Dong S-H, Li J, Zhao M (2010) Influence of phosphate application on rice absorbing and accumulation of Cd in Cd polluted paddy soil. J Northeast Agric Univ 41:39–48
Dong X-L, Ma L-Q, Li Y-C (2011) Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J Hazard Mater 190:909–915
El-Temsah Y-S, Joner E-J (2012) Ecotoxicological effects on earthworms of fresh and aged nano-sized zero-valent iron (nZVI) in soil. Chemosphere 89:76–82
GB/T 23739 (2009) Soil quality-Analysis of available lead and cadmium contents in soils-Atomic absorption spectrometry
Han F, Sridhar X-B, Monts D-L, Su Y (2004) Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea. New Phytol 162:489–499
He F, Zhao D, Liu J, Roberts C-B (2007) Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for soil and groundwater. Ind Eng Chem Res 46:29–34
He F, Zhang M, Qian T-W, Zhao D-Y (2009) Transport of carboxymethyl cellulose stabilized iron nanoparticles in porous media: column experiments and modelling. J Colloid Interf Sci 334:96–102
Houben D, Evrard L, Sonnet P (2013) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Hodson M-E, Valsami-Jones E, Cottet-Howells J-D (2000) Bone-meal additions as a remediation treatment for metal contaminated soil. Environ Sci Technol 34:3501–3510
Kelley M-E, Brauning S-E, Schoof R-A, Ruby M-V (2002) Assessing oral bioavailability of metals in soil. Battelle Press, USA
Liang B, Xie Y-Y, Fang Z-Q, Tsang E-P (2014) Assessment of the transport of polyvinylpyrrolidone-stabilised zero-valent iron nanoparticles in a silica sand medium. J Nanopart Res 16:2485–2492
Liu X, Fan Z-X, Zhang B, Bi Y-P (2007) Research of cadmium contamination and remediation in soil. Shangdong Agric Sci 6:94–97
Liu R-Q, Zhao D-Y (2007a) Reducing leachability and bioaccessibility of lead in soils using a new class of stabilized iron phosphate nanoparticles. Water Res 41:2491–2502
Liu R-Q, Zhao D-Y (2007b) In situ immobilization of Cu (II) in soils using a new class of iron phosphate nanoparticles. Chemosphere 68:1867–1876
Ma X, Gurung A, Deng Y (2013) Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci Total Environ 443:844–849
Méndez A, Terradillos M, Gascó G (2013) Physicochemical and agronomic properties of biochar from sewage sludge pyrolysed at different temperatures. J Anal Appl Pyrolysis 102:124–130
Mengel K (1994) Iron availability in plant tissues-iron chlorosis on calcareous soils. Plant Soil 165:275–283
Reddy K-R, Xu C-Y, Chinthamreddy S (2001) Assessment of electrokinetic removal of heavy metals from soils by sequential extraction analysis. J Hazard Mater 84:279–296
Si S, Kotal A, Mandal T-K, Giri S, Nakamura H, Kohara T (2004) Size-controlled synthesis of magnetite nanoparticles in the presence of polyelectrolytes. Chem Mater 16:3489–3496
Tang J-C, Zhu W-Y, Kookana R, Katayama A (2013) Characteristics of biochar and its application in remediation of contaminated soil. J Biosci Bioeng 116:653–659
Tessier A, Campbell P-G-C, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Uzoma K-C, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011) Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manage 27:205–212
Wu W-H, Xie Z-M, Xu J-M, Wang F, Shi J-C, Zhou R-B, Jin Z-F (2013) Immobilizeation of trace metals by phosphate in contaminated soil near lead/zinc mine tailings evaluated by sequential extraction and TCLP. J Soil Sediments 13:1386–1395
Wang Y, Fang Z-Q, Liang B, Tsang E-P (2014) Remediation of hexavalent chromium contaminated soil by stabilized nanoscale zero-valent iron prepared from steel pickling waste liquor. Chem Eng J 247:283–290
Xu Y-Z, Fang Z-Q (2015) Advances on remediation of heavy metal in the soil by biochar. Environ Eng 02:156–159
Xu Y-Z, Yan X-M, Fang L, Fang Z-Q (2016) Remediation of Cd (II)-contaminated soil by three kinds of ferrous phosphate nanoparticles. RSC Adv. doi:10.1039/c5ra23299f
Yamauchi M, Peng X (1995) Iron toxicity and stress-induced ethylene production in rice leaves. Plant Soil 173:21–28
Yao Y, Guo B, Inyang M, Zimmerman A-R, Cao X, Pullammanappallil P, Yang L (2011) Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential. Bioresour Technol 102:6273–6278
Yang Z-M, Fang Z-Q (2014) Advances on remediation of Cd, Pb contaminated-soil by biochar. Environ Protec Chem Indus 06:525–531
Zhang Z-H, Solaiman Z-M, Meney K, Murphy D-V, Rengel Z (2013) Biochars immob ilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil. J Soil Sediments 13:140–151
Zhou S-W, Xu M-G (2007) The progress in phosphate remediation of heavy metal-contaminated soils. Acta Ecol Sin 27:3043–3050
Acknowledgments
This research was supported by the Guangdong Technology Research Centre for Ecological Management and Remediation of Urban Water Systems (2012gczxA005) and the Guangzhou External Science and Technology Cooperation Project (2016201604030002).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Xu, Y., Fang, Z. & Tsang, E.P. In situ immobilization of cadmium in soil by stabilized biochar-supported iron phosphate nanoparticles. Environ Sci Pollut Res 23, 19164–19172 (2016). https://doi.org/10.1007/s11356-016-7117-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7117-z