Abstract
In this study, a titanium plate was impregnated with SnO2 and Sb (Ti/SnO2–Sb2O4) for the electrocatalytic removal of phenol from wastewater, and the chemical degradation pathway was presented. The effects of various parameters such as pH, current density, supporting electrolyte, and initial phenol concentration were studied. At optimum conditions, it was found that phenol was quickly oxidized into benzoquinone because of the formation of various strong radicals during electrolysis by the Ti/SnO2–Sb2O4 anode from 100 to <1 mg/L over 1 h. The results of GC/MS analysis showed the presence of some esters of organic acid such as oxalic acid and formic acid. HPLC analysis showed only trace amounts of benzoquinone remaining in the solution. The efficiency of TOC removal at the Ti/SnO2–Sb2O4 anode surface showed a degradation rate of 49 % over 2 h. Results showed that the molecular oxygen potential at the electrode was 1.7 V. The phenol removal mechanism at the surface of the Ti/SnO2–Sb2O4 anode was influenced by the pH. Under acidic conditions, the mechanism of electron transfer occurred directly, whereas under alkaline conditions, the mechanism can be indirect. This research shows that the proposed electrolyte can significantly influence the efficiency of phenol removal. It can be concluded that the treatment using an appropriate Ti/SnO2–Sb2O4 electrode surface can result in the rapid oxidation of organic pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic hydrocarbons are among the most common pollutants found in industrial wastewaters from numerous industries such as petrochemical, chemical material production, plastic production, paper production, textile, detergent production, pesticide, and pharmaceutical industries. These materials are resistant to biological degradation and are often toxic to biological constituents. In natural waters, phenol and its compounds have a toxic effect on humans, animals, and plants, and even at low concentrations, they can cause undesirable taste and odor in drinking water. Thus, many phenolic compounds are included in environmental legislation (Babuponnusami and Muthukumar 2013). Most phenolic compounds are resistant to traditional physicochemical and biological treatments (Xiaoyue et al. 2013). Moreover, some methods for removing phenol are not cost effective and biological processes for low concentrations are slow (Weiss et al. 2008). Therefore, further techniques need to be developed for the efficient removal of phenol. Some advanced oxidation methods such as photochemical reaction, Fenton oxidation, and ozonation are used (Lia et al. 2005). Another attractive method is the electrochemical process, in which toxic organic pollutants are effectively oxidized by electrochemical reactions (An et al. 2012). The efficiency of an electrochemical process is highly dependent on the type of anode used. Dimensionally Stable Anodes (DSA), which are prepared by coating a metal-oxide layer on a base metal like titanium, have been used successfully in electrochemical treatments (Jianrui et al. 2012). It has been observed that some oxides such as RuO2 and IrO2 do not have the reactivity required to oxidize organic compounds. Other oxide coatings such as PbO2 and SnO2 have been applied to modify the performance of DSA. PbO2 highly influences organic compound degradation and has been used as a high-efficiency anode for the oxidation of toxic organics (Orozco et al. 2013), although the possible leakage of a toxic metal such as lead has limited its reliable usage (An et al. 2011). Coatings with a SnO2 base show activity similar to PbO2, and with the advantage of its non-toxicity, this oxide layer has frequently been used in the electrochemical oxidation of organic pollutants (Ding et al. 2007). Antimony oxide is used in various oxide compounds and has an important role in the catalytic process known as electron donation (Batzill and Diebold 2005). It has been used in the preparation of numerous commercial catalysts for the oxidation of hydrocarbons and shows good performance as a catalyst in combination with other metal oxides (Huang and Ruiz 2005). In the present study, phenol, being the base unit of aromatic compounds, was used as the pollutant model for degradation by electrochemical oxidation. A titanium anode coated with SnO2 and modified with Sb (Ti/SnO2–Sb2O4) was used for the electrochemical oxidation of phenol. The effects of various operational parameters such as pH, current density, type of supporting electrolyte, and initial phenol concentration were also studied. Some by-products of the treatment were determined to investigate the degradation pathway of phenol and its related mechanism.
Material and methods
Electrode fabrication
To study the electrochemical oxidation of phenol, a Ti/SnO2–Sb2O4 electrode, which displays appropriate electrocatalytic properties for organic compound oxidation, was used as the anode. A titanium rectangular plate (99.5 % purity) 2 × 3 cm2 in area and 0.5 mm thick was selected. The surface was polished using sand paper (320 grade) then degreased in 40 % NaOH at a temperature of 80 °C for 2 h. The substrate was etched in 15 % oxalic acid at a temperature of 98 °C for 2 h then completely washed with distilled water. This treated plate was then used to prepare the Ti/SnO2–Sb2O4 electrode using electrical deposition to provide the internal layer and dip coating for the external coating. This method has also been used by other researchers. For the electrical deposition phase, the previously treated titanium plate was placed, as the cathode, into a solution containing 100 ml propanol (99 %), 17.5 g of SnCl4·5H2O (Aldrich), and 0.73 g (Aldrich) of Sb2O3; furthermore, 2 ml of concentrated HCl (37 %) was added. A constant DC current of 0.12 A was applied for 25 min. During this phase, the platinum plate was employed as the anode. The Ti-coated plate was then placed in an oven at 400 °C for 2 h. For the external coating, the treated Ti plate was placed in a solution containing 30 g SnCl4·5H2O, 0.8 g of Sb2O3, and 2.5 ml of concentrated (37 %) HCl in 50 ml of propanol. After five dipping–drying cycles at room temperature, the Ti plate was annealed in a furnace at 550 °C for 2 h. The annealing temperature plays an important role in the dispersion of antimony oxide (Sb2O4) throughout the alloy (Fujda et al. 2007). This preparation method was repeated five times to provide a proper coating on Ti, which can be ensured by measuring the weight of the substrate for oxide loading. The internal coating is essential as it increases the stability of the coating.
Chemical analysis
The morphology of the electrode surface was determined by SEM-EDS using the VEGA software. The crystalline structure of the electrode was determined using an XRD (X’Pert MPD) instrument with a Cu Kα lamp (1.78897 A) with 40 kV voltage and 30 mA current (Netherland Phillips Company). The electrochemical properties of the electrode were measured by the three-electrode system. In this system, the prepared electrode was used as the working electrode, and a platinum wire was used as the counterelectrode. The reference electrode was an Ag/AgCl electrode saturated in KCl. Electrochemical measurements included cyclic voltammetry, performed using an EGSG galvanostat/potentiostat instrument (model 273A) with a scan speed of 100 mvs−1 in a 0.25-M solution of Na2SO4 at ambient temperature. TOC measurement was conducted using a Rosemount Analytical Dohrmann DC-190 with the standard 5310B method. High-performance liquid chromatography (HPLC) was used for the analysis of the electrochemical oxidation of the aromatic by-products of phenol degradation, including para-benzoquinone and hydroquinone. The HPLC 1100 Series Agilent was equipped with a Zorbax column (Eclipse XDB-C8, ID = 4.6 mm, length = 150 mm, with ChemStation software and a UV detector at wavelengths of 290 and 245 nm). A methanol/water mix with a ratio of 80 %/20 % (v/v) was used as the mobile phase with a flow rate of 0.9 ml/min. Before each analysis, samples were filtered through a 0.2-μm membrane. Gas chromatography/mass spectrometry (GC/MS) was used to identify any possible organic acids produced during the phenol electrolysis. A GC (Agillent7890A) HP5 column with a length of 30 cm and detectors MS (5975C) was used. The GC temperature program include the following: injector 250 °C, temperature interface = 280 °C, program column temperature, and 2 min at 50 °C with ramp 5 °C per minute to 280 °C. For MS, a thermocouple temperature of 230 °C and a 150 °C ion source were used and the scan range of 30 m/z was set to 500. Sample preparation for injection into the GC system was based on the methods described in other studies (Lia et al. 2005).
Phenol electrocatalysis
Electrochemical degradation of phenol was performed in a 100-ml electrolysis batch cell. The container had a lid with two slits to accommodate the electrodes. In this system, the Ti/SnO2–Sb2O4 electrode and a steel stainless plate with the same dimensions (2 × 3 cm2) were used as the anode and cathode, respectively. The electrodes were placed 1 cm from each other. A DC potentiometer (power supply–TEK 8051 DC) with an output voltage of 30 V was used as the power supply. For the electrolysis, the phenol solution (100 mg/l) was poured into the cell containing 0.25 M Na2SO4. A constant DC current of 0.12 A was passed through the cell. This current is equal to a current density of 20 mA/cm2. The cell was placed on a magnetic stirrer. The solution temperature remained at about 25 °C without any special control. At specific time intervals, samples were collected for chemical experiments (residual phenol concentration, TOC, and pH). Using a UV–Vis 9200 spectrophotometer, the absorption intensity of the residual phenol solution was measured using the 4-aminopyridine method mentioned in the standard method.
Results and discussion
Characterization of Ti/SnO2–Sb2O4 electrode
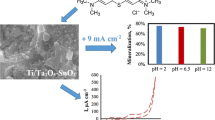
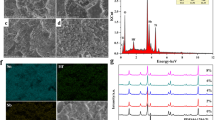
Figure 1 shows the SEM image of the surface of the Ti/SnO2–Sb2O4 electrode. The surface of titanium electrode was internally and externally covered with SnO2–Sb2O4 in several stages. The formation of this coating on the electrode can prevent the rapid diffusion of the electrolyte into the surface, the formation of the TiO2 layer, and the deactivation of the electrode. This finding is in line with XRD pattern of the electrode. This result concurs with other studies (Cheng et al. 2013; Xu et al. 2011). Energy-dispersive spectrometry (Fig. 2) shows the presence of Sn and Sb in the coating. EDS analysis indicated enrichment of Sb atoms on the electrode surface (16.6 at.%). The electrical conductivity of SnO2 is modified by doping with Sb. Figure 3 shows the XRD pattern of the Ti/SnO2–Sb2O4 electrode, indicating tetragonal crystalline SnO2 coated on the electrode surface. There was no peak corresponding to TiO2. In the XRD pattern of the Ti/SnO2–Sb2O4 electrode, peaks at 31(110), 31.5(101), and 60.9(211) show the crystalline structure of SnO2 on the electrode surface. The average crystallinity of SnO2 was calculated using the Scherrer equation. The average crystal plane size (110) was calculated as 9 nm, smaller than in other studies (Hammad and Hejazy 2012). The smaller crystallite size increases the ability of the electrode during the electrochemical process (Hao et al. 2014). The XRD spectrum shows that the phases were completely combined. Loading rate is an effective way to improve the performance of electrodes in electrochemical processes (Zhao et al. 2009).
The oxide loading is the weight difference of the electrode in the coating experiments. The oxide loading was 17 mg/cm2 in this study, whereas in other studies, oxide loading of 16 mg/cm2 has been reported(Tang et al. 2014). The use of both electrical deposition and dip coating methods in the preparation of the coating can effectively improve the load.
Cyclic voltammetry
Cyclic voltammetry studies of Ti/SnO2–Sb2O4 show that the fabricated electrode had an O2 production potential (oxygen evolution) of 1.7 V (vs. the standard Ag/AgCl) (Fig. 4). In oxidation reactions where the oxygen atoms transfer indirectly, the oxygen radicals, particularly OH radicals produced through water electrolysis, play an important and critical role in the electrochemical oxidation of organic compounds (Feng et al. 2010; Shao et al. 2014). The formation of OH radicals on the anode surface can be explained by Eq. 1, in which A represents the anode (Lia et al. 2005).
The OH radicals quickly react with organic compounds diffused onto or adjacent to the anode and result in oxidation, as shown in Eq. 2.
The OH radicals also react with each other to form O2 molecules in order to complete the electrolysis of water molecules, as shown in Eq. 3.
In an organic wastewater treatment, the production of O2 molecules at the anode results in energy loss and reduces the current efficiency for both direct and indirect oxidation of organic compounds. Therefore, using an anode with a higher O2 production overpotential is preferred (Lia et al. 2005). A Ti/SnO2–Sb2O4 electrode with an O2 production overpotential of 1.7 V (vs. the standard Ag/AgCl) is appropriate for an organic pollutant treatment by OH radicals. The high overpotential of the Ti/SnO2–Sb2O4 anode can convey that oxygen production is delayed according to Eq. 3. Such a delay is appropriate for oxidation of phenol by hydroxyl radicals. The high oxygen production overpotential of the electrode results in an increased life span for the hydroxyl radicals on the anode and enables transfer of more oxygen from the radicals to the organic compounds for oxidation (Equation 2). In the presence of phenol, the density of the electrode current was reduced. This indicates that phenol has a strong effect on the oxidation at the electrode surface. Studies have shown that an anodic peak of 1.35 V and cathodic peak of −0.3 V are consistent with the hydroquinone/benzoquinone pair (Zhang et al. 2010). An oxidation peak of 0.5 V is consistent with the oxidative potential of phenolic compounds, including various polymers, because some yellowish products were observed on the electrode surface.
Performance of the Ti/SnO2–Sb2O4 electrode
Phenol was oxidized by the Ti/SnO2–Sb2O4 anode, decreasing its concentration from 100 ppm to less than 1 ppm over 10 h. In terms of TOC removal efficiency, the Ti/SnO2–Sb2O4 anode showed a degradation rate of 49 % over 2 h. In the Ti/SnO2–Sb2O4 system, the pH changes during the oxidation process. During the electrochemical oxidation of phenol, it was noted that the initial pH value (10.5) decreased. However, after 4 h, the pH value started increasing to its initial value. The decrease in pH is apparently due to the formation of organic acids, which are one of the main by-products of phenol degradation (Feng et al. 2008).
Effect of pH
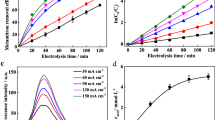
Figure 5 shows the effect of pH on the electrochemical degradation of phenol at the Ti/SnO2–Sb2O4 anode. Phenol removal was studied at pH 3, 7, and 10.5. The results show that the initial pH of the solution influences the efficiency of phenol removal. An alkaline pH of 10.5 was determined to be optimum for phenol removal. These studies demonstrate that increasing pH has an important effect on OH radical production. An alkaline pH causes increased production of radicals and enhances oxidation of pollutants. These results are consistent with other studies (Rasalingam et al. 2014). It was observed that phenol was efficiently removed on the Ti/SnO2–Sb2O4 electrode under acidic pH. Oxidation of phenol begins with one electron transfer, and this results in the reaction of phenoxy radicals (Feng et al. 2010; Lia et al. 2005). It can be concluded that pH has a significant impact on the mechanism of electron transfer in the solution. Electron transfer may directly occur through phenol absorption at the electrode surface. The release of one electron and the formation of phenoxy radicals indicate that oxidation proceeds well in an acidic medium. Considering that absorption of the organic compounds in their molecular form is improved here, it may be assumed that phenol absorption is conducted efficiently at the electrode surface in an acidic pH (weak acid with pKa = 10) (Chiou et al. 2008). Therefore, it can be noted that the electron transfer mechanism is through a direct transfer. Phenol is absorbed onto the anode surface and donates its electrons to the anode. Oxidation may indirectly occur through the production of hydroxyl radicals on the anode surface and radical reaction with the organic compounds absorbed on or near the anode surface. The oxidation of organic substances is enhanced in an alkaline medium. Since the oxidation strength of hydroxyl radicals is very high, the increasing removal of phenol in the alkaline medium on the Ti/SnO2–Sb2O4 surface (%R = 96 at pH = 10.5) is acceptable compared with the percent of phenol removal in the acidic medium (%R = 93 at pH = 3). In the following experiments, pH = 10.5 was selected as the optimum.
Effect of phenol concentration
Figure 6 shows the relationship between the percentage of phenol removal and its initial concentration. As phenol concentration increased from 50 to 200 ppm, the percentage of phenol removal gradually decreased from 96 to 72 %. This can be attributed to the fact that the production of strong oxidizing agents of OH at the electrode surface in a constant current density does not increase. Therefore, increasing the pollutant concentration in a constant current density decreases the rate of electrooxidation degradation.
Effect of current density
Current density plays an important role in the oxidation process. Increasing the current density had a positive influence on the phenol removal in the range of 10–40 mA/cm2 (cell potential was in the range of 3.2–4.8 V) (Fig. 7). The main reason for this increase was that the high current density resulted in the production of more hydroxyl radicals (OH) due to the oxidation of water (Un et al. 2008).
Effect of supporting electrolyte
The present study shows that electrolytes can largely influence phenol removal efficiency (Fig. 8). Sodium sulfate and sodium chloride solutions (0.25 M) were used in this study. After 10 h, the removal efficiency approached 98.5 % using the sodium sulfate solution, whereas the efficiency increased to 99 % after only 1 h using the sodium chloride solution. The studies show that the short life span of the hydroxyl radicals results in them reacting only with those organic molecules adjacent to the electrode surface. In this case, the oxidation rate can be modified by increasing the number of some ions, generally chloride (Bonfatti et al. 2005; Li et al. 2009). Chloride ions can be oxidized and participate in the oxidation of organic compounds as a mediator. Chloride is oxidized at the surface of the anode and produces dissolved chlorine gas in the solution (Eq. 4) (Zhang et al. 2010).
According to Eq. 5, chlorine hydrolyzes into hypochlorous acid.
In alkaline and neutral media, hypochlorite is the dominant species (Eq. 6).
Chloro-species react with hydroxyl radicals to form stronger oxidants at the electrode surface and also produce perchlorate (Eq. 7) (Feng et al. 2008).
The Ti/SnO2–Sb2O4 electrode, due to high overpotential, can also produce other, more stable oxidants. The type of oxidant is dependent on the type of supporting electrolyte (Un et al. 2008). In a medium containing sulfate, disulfide oxidants are formed. The studies have shown that electrooxidation is more sensitive to the presence of chloride ion than the other oxidation process.
Phenol degradation mechanism
Several studies have investigated the electrochemical oxidation mechanism of phenol and its derivatives. (Lia et al. 2005; Duan et al. 2013; Fierro et al. 2010; Wang et al. 2010; Xiupei et al. 2009) It is generally accepted that phenol oxidation begins with an electron transfer, which leads to phenoxy radical reactions (Lund and Baizer 1991). Phenoxy radical reactions produce benzoquinone, which is assumed to be an intermediate compound in phenol oxidation. Later, the benzoquinone can break the ring to yield different carboxylic acids, namely, maleic acid, succinic acid, and acetic acid, and eventually carbon dioxide and water. Furthermore, studies reveal that hydroquinone and benzoquinone, in the absence of strong oxidizing radicals, accumulate in the solution and yield oxidation-resistant polymers (Tahar and Savall 1999; Iniesta et al. 2001). Kondru et al. reported complete and rapid mineralization of phenol in alkaline pH. Phenol polymerization-induced blocking of the surface of the electrode was not reported at this pH. Furthermore, Wu et al. proved that complete elimination of phenol occurs at pH 11 due to formation of more radicals as compared to acidic pH. The positive effect of the alkaline environment containing chloride ion on accelerating phenol degradation has been reported in several studies (Kondru et al. 2009; Wu et al. 2007). Tasic et al. assumed that different radical forms of chlorine, namely, ClO2, HClO, and Cl2, can oxidize organic materials in presence of hydroxyl radicals (Tasic et al. 2014).
During the electrolysis of phenol in the presence of a sodium sulfate electrolyte, color changes in the solution were observed. During the initial 6 h of phenol oxidation, the color of the solution changed to yellowish-brown. When the phenol concentration reached its minimum, this color practically disappeared to such an extent that at the end of the 10-h electrolysis, the solution was mostly transparent. According to previous studies, formation of a 1:1 benzoquinone–hydroquinone complex results in yellow color (Suresh et al. 2012), and this is produced by polymeric compounds generated during the electrochemical oxidation of phenol. These include benzoquinone–hydroquinone monomer units, which are formed through radical reaction by phenate anions. Association of benzoquinone–hydroquinone in the presence of a sodium sulfate electrolyte has been observed in previous studies (Lia et al. 2005). However, electrochemical oxidation of phenol in the presence of a sodium chloride electrolyte did not show color change in the solution during the oxidation process. There was no hydroquinone in the solution (hydroquinone has a very low vapor pressure of 0.000019 mmHg and log Kow = 0.59). It is thought that during electrolysis in the presence of a sodium chloride electrolyte, phenol is rapidly oxidized into benzoquinone due to the formation of various strong radicals at the electrode surface. Moreover, the higher rate of electrolysis in the presence of sodium chloride provides no opportunity for the polymeric products that produce the yellow-brown color to form. The results of GC/MS analysis indicated the presence of some organic acids (Table 1). Therefore, as a higher current density and low phenol concentration, oxidation can occur through a straight path of mineralization and CO2 production (Suresh et al. 2012); the presence of electrolytes producing strong oxidants in the solution may also be effective in shortening the phenol oxidation pathway and preventing the formation of polymeric films at the anode surface and precipitation on its surface. The results of the present experiment pertaining phenol removal and by-product formation are completely consistent with the proposed pathway of phenol degradation (Lia et al. 2005). This pathway can be proposed for phenol oxidation at a Ti/SnO2–Sb2O4 anode in the presence of a sodium chloride electrolyte with some modifications in the formation of aromatic side products, the colored polymeric compounds, and the pathway (Fig. 9).
Conclusions
Based on the results, we reached the following conclusions:
Phenol can be rapidly oxidized on the fabricated Ti/SnO2–Sb2O4 surface anode in the presence of a sodium chloride electrolyte. Some by-products such as benzoquinone and organic acids such as oxalic acid and formic acid were identified. During phenol electrolysis at the Ti/SnO2–Sb2O4 anode in the presence of a sodium chloride electrolyte, phenol rapidly oxidizes into benzoquinone, and this oxidation probably continues due to the formation of various strong radicals on the surface of the electrode. The anode surface and the presence of electrolytes producing strong oxidizing agents in the solution shorten the phenol oxidation pathway, preventing the formation of polymeric films in the solution and at the anode surface. pH has a significant impact on the mechanism of electron transfer in the solution. Increasing the phenol concentration from 50 to 200 mg/l resulted in a decrease in the percentage removed from 96 to 72 %.
References
An H, Li Q, Tao D, Cui H, Xu X, Ding L, Sun L, Zhai J (2011) The synthesis and characterization of Ti/SnO2–Sb2O3/PbO2 electrodes: the influence of morphology caused by different electrochemical deposition time. Appl Surface Sci 25:218–224
An H, Cui H, Zhang W, Zhai J, Qian Y, Xie X, Li Q (2012) Fabrication and electrochemical treatment application of a microstructured TiO2-NTs/Sb–SnO2/PbO2 anode in the degradation of C.I. Reactive Blue 194 (RB 194). J Chem Eng 209:86–93
Babuponnusami A, Muthukumar K (2013) Treatment of phenol-containing wastewater by photoelectro-Fenton method using supported nanoscale zero-valent iron. Environ Sci Pollut Res 20:1596–1605
Batzill M, Diebold U (2005) The surface and materials science of tin oxide. Prog Surf Sci 79:47–154
Bonfatti F, Ferro S, Lavezzo F, Malacarne M, Lodi G, De Battisti A (2005) Electrochemical incineration of glucose as a model organic substrate. II. Role of active chlorine mediation. J Electrochem Soc 147:592–596
Cheng W, Yang M, Xie Y, Liang B, Fang Z, Pokeung TE (2013) Enhancement of mineralization of metronidazole by the electro-Fenton process with a Ce/SnO2–Sb coated titanium anode. J Chem Eng 220:214–220
Chiou CH, Wu CY, Juang RS (2008) Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2 process. Chem Eng J 139:322–329
Ding H, Feng Y, Liu J (2007) Preparation and properties of Ti/SnO2–Sb2O5 electrodes by electro deposition. Material Lett 61:4920–4923
Duan X, Ma F, Yuan Z, Chang L, Jin X (2013) Electrochemical degradation of phenol in aqueous solution using PbO2 anode. J Taiwan Inst Chem Eng 44:95–102
Feng Y, Cui T, Logan B (2008) Liu Z (2008) Performance of Gd-doped Ti-based Sb-SnO2 anodes for electrochemical destruction of phenol. Chemosphere 70:1629–1636
Feng Y, Cuia YH, Liu J, Logana BE (2010) Factors affecting the electro-catalytic characteristics of Eu doped SnO2/Sb electrode. J Hazard Mater 178:29–34
Fierro S, Nagel T, Baltruschat H, Comninellis C (2010) Comparison between IrO2 prepared by thermal treatment of iridium metal and IrO2 prepared by thermal decomposition of H2IrCl6 solution. Electrochem Commun 12:172–174
Fujda M, Misicko R, Rusnakova L, Sojko M (2007) Effect of solution annealing temperature on structure and mechanical properties of EN AW 2024 aluminium alloy. J Metals Materials Minerals 17:35–40
Hammad TM, Hejazy NK (2012) Structural, electrical, and optical properties of ATO thin films fabricated by dip coating method. Int Nano Letters 2:7–11
Hao X, Dan S, Qian Z, Honghui Y, Yan W (2014) A Composite Sb-doped SnO2 electrode based on the TiO2 nanotubes prepared by hydrothermal synthesis. RSC Adv 4:25011–25017
Huang Y, Ruiz P (2005) Antimony dispersion and phase evolution in the Sb2O3 − Fe2O3 system. J Phys Chem B 109:22420–22425
Iniesta J, Michaud PA, Panizza M, Cerisola G, Aldaz A, Comninellis C (2001) Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim Acta 46:3573-3578
Jianrui S, Haiyan L, Haibo L, Lili D, Weimin H, Hongdong L, Tian C (2012) Electrochemical oxidation of aqueous phenol at low concentration using Ti/BDD electrode. Se. Purif Technol 88:116–120
Kondru AK, Kumar P, Chand S (2009) Catalytic wet peroxide oxidation of azo dye (Congo red) using modified Y zeolite as catalyst. J Hazard Mater 166:342–347
Li P, Zhao G, Cui X, Zhang Y, Tang Y (2009) Constructing stake structured TiO2-NTs/Sb-doped SnO2 electrode simultaneously with high electrocatalytic and photocatalytic performance for complete mineralization of refractory aromatic acid. J Phys Chem C 113:2375–2383
Lia X, Cuib Y, Fengb Y, Xiea Z, Guc J (2005) Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes. Water Res 39:1972–1981
Lund H, Baizer MM (1991) Organic Chemistry. Marcel Dekker, New York, p 616
Orozco SI, Blanco LM, Garza MA, González VA, Borrás C, Sharifker B (2013) Phenol degradation using glassy carbon electrodes modified with particles of Co-Mo alloy. Int J Electrochem Sci 8:5698–5709
Rasalingam S, Peng R, Koodali RT (2014) Removal of hazardous pollutants from wastewaters: applications of TiO2-SiO2 mixed oxide materials. Journal of Nanomaterials ID 617405:1–42
Shao D, Yan W, Cao L, Li X, Xu H (2014) High-performance Ti/Sb–SnO2/Pb3O4 electrodes for chlorine evolution: preparation and characteristics. J Hazard Mater 267:238–244
Suresh S, Srivastava VC, Mishra IM (2012) Adsorption of catechol, resorcinol, hydroquinone and their derivatives: a review. Int J Energy Environ Eng 3:32–49
Tahar NB, Savall A (1999) Electrochemical degradation of phenol in aqueous solution on bismuth doped lead dioxide: a comparison of various electrode formulations. J Appl Electrochem 29:277–283
Tang X, Xiao L, Yang C, Lu J, Zhuang L (2014) Noble fabrication of Ni-Mo cathode for alkaline water electrolysis and alkaline polymer electrolyte water electrolysis. Int J Hydrogen Energy 39:3055–3060
Tasic Z, Gupta VK, Antonijevic MM (2014) The mechanism and kinetics of degradation of phenolic in wastewaters using electrochemical oxidation. Int J Electrochem Sci 9:3473–3490
Un UT, Altay U, Koparal AS, Ogutveren UB (2008) Complete treatment of olive mill wastewaters by electrooxidation. Chem Eng J 139:445–452
Wang Y, Shen Z, Li Y, Niu J (2010) Electrochemical properties of the erbium–chitosan–fluorine–modified PbO2 electrode for the degradation of 2,4-dichlorophenol in aqueous solution. Chemosphere 79:987–996
Weiss E, Groenenserrano K, Savall A (2008) A comparison of electrochemical degradation of phenol on boron doped diamond and lead dioxide anodes. J Appl Chem 38:329–337
Wu D, Liu M, Dong D, Zhou X (2007) Effects of some factors during electrochemical degradation of phenol by hydroxyl radical. Microchem J 85(2):250–256
Xiaoyue D, Fang M, Zhongxin Y, Limin C, Xintong J (2013) Electrochemical degradation of phenol in aqueous solution using PbO2 anode. J Taiwan Institute Chem Eng 44:95–102
Xu H, Li A, Cheng X (2011) Electrochemical performance of doped SnO2 coating on Ti base as electro oxidation anode. Int J Electrochem Sci 6:5114–5124
Xiupei Y, Ruyi Z, Feng H, Duochang C, Dan X (2009) Preparation and characterization of Ti/SnO2–Sb2O3–Nb2O5/PbO2 thin film as electrode material for the degradation of phenol. J Hazard Mater 164:367–373
Zhang W, Rosendahl SM, Burgess IJ (2010) Coupled electron/proton transfer studies of benzoquinone-modified monolayers. J Phys Chem C 114:2738–2745
Zhao G, Cui X, Liu M, Li P, Zhang Y, Cao T, Li H, Lei Y, Liu L, Li D (2009) Electrochemical degradation of refractory pollutant using a novel microstructured TiO2 nanotubes/Sb-doped SnO2 electrode. Environ Sci Technol 43:1480–1486
Acknowledgments
The authors gratefully acknowledge the financial support from the Tarbiat Modares University. Moreover, the authors thank Dr. Golmohammadi, Mr Sadeghi, Ms. Fardindoost, and Mr. Akhgar for their assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Rights and permissions
About this article
Cite this article
Loloi, M., Rezaee, A., Aliofkhazraei, M. et al. Electrocatalytic oxidation of phenol from wastewater using Ti/SnO2–Sb2O4 electrode: chemical reaction pathway study. Environ Sci Pollut Res 23, 19735–19743 (2016). https://doi.org/10.1007/s11356-016-7110-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7110-6