Abstract
Drinking water (DW) disinfection represents a milestone of the past century, thanks to its efficacy in the reduction of risks of epidemic forms by water micro-organisms. Nevertheless, such process generates disinfection by-products (DBPs), some of which are genotoxic both in animals and in humans and carcinogenic in animals. At present, chlorination is one of the most employed strategies but the toxicological effects of several classes of DBPs are unknown. In this investigation, a multidisciplinary approach foreseeing the chemical analysis of chlorinated DW samples and the study of its effects on mixed function oxidases (MFOs) belonging to the superfamily of cytochrome P450-linked monooxygenases of Cyprinus carpio hepatopancreas, was employed. The experimental samples derived from aquifers of two Italian towns (plant 1, river water and plant 2, spring water) were obtained immediately after the disinfection (A) and along the network (R1). Animals treated with plant 1 DW-processed fractions showed a general CYP-associated MFO induction. By contrast, in plant 2, a complex modulation pattern was achieved, with a general up-regulation for the point A and a marked MFO inactivation in the R1 group, particularly for the testosterone metabolism. Together, the toxicity and co-carcinogenicity (i.e. unremitting over-generation of free radicals and increased bioactivation capability) of DW linked to the recorded metabolic manipulation, suggests that a prolonged exposure to chlorine-derived disinfectants may produce adverse health effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
Disinfection by-products are generated during drinking water disinfection.
Xenobiotic metabolism modulation in Cyprinus carpio fish treated with samples from two distribution networks is investigated.
Significant modulation of carcinogens metabolising enzymes is reported.
Results seem to support the increased cancer risk observed in drinking water consumers.
Introduction
Water-related diseases still represent a worldwide alarming problem. Inadequate drinking water (DW) sanitation and hygiene are estimated to cause 842,000 diarrheal disease deaths per year (World Health Organization and UNICEF 2014). In this contest, DW disinfection represents a milestone of the past century, thanks to its efficacy in the reduction of risks of epidemic forms by water micro-organisms (i.e. bacteria, protozoa and viruses). From 1990 to 2012, the percentage of global population that has had access to improved DW is enlarged from 76 to 89 % (World Health Organization and UNICEF 2014). Nevertheless, if on one hand water disinfection has enormously decreased both morbidity and mortality of waterborne diseases (Richardson 1998; Ashbolt 2004), on the other hand, the generation of unintended disinfection by-products (DBPs), chemicals formed through the reaction between disinfectants and organic and inorganic matter in the source water, represents an important public health concern (Richardson et al. 2007). At present, chlorination is one of the most employed strategies worldwide, and it produces several classes of DBPs, such as halogenated trihalomethanes (THMs; e.g. chloroform, dibromochloromethane and bromoform) and haloacetic acids (HAAs; e.g. dichloroacetic acid, trichloroacetic acid and bromate) (Mitch 2010). Among such chemicals, some have already shown genotoxic properties in various experimental biological systems (Richardson et al. 2007; Ohe et al. 2004; Singer 2008; Krasner 2009; Mitch 2010; Zhang et al. 2010) and in humans (Leavens et al. 2007; Spivey 2009; Kogevinas et al. 2010). In addition, in 2006, the US Environmental and Protection Agency (EPA), after a rigorous evaluation, concluded that ‘new cancer data strengthen the evidence of a potential association of chlorinated water with bladder cancer’ and suggested ‘an association for colon and rectal cancer’ (US Environmental Protection Agency 2006). Such sentences have been confirmed by further epidemiological and genotyping studies (Villanueva et al. 2006, 2007; Cantor et al. 2010; Regli et al. 2015). Actually, the precise by-products responsible for human enhanced cancer risk has yet to be identified, because of the lack of knowledge about the potential synergistic/antogonistic interactions of these molecules and their role in the multiphasic mechanism of carcinogenesis. In absence of such kind of information, an evaluation of human toxicological risk is at least necessary, through a multidisciplinary investigation.
Biotransformation reactions involved in xenobiotic metabolism have several effects on their substrates, being able to produce inactive metabolites or, in some instance, more or comparable bioactive compounds with respect to the parental one. The most dangerous situation obviously concerns the conversion of pre-mutagens and pre-carcinogens to final detrimental toxins. The increased reactivity of the metabolites results in a large capacity to react with the endogenous cellular macromolecules. One of the main consequences of the up-regulation of CYP superfamily of monooxygenases (MFO), the main actor in carcinogen metabolism, is the increased generation of reactive oxygen species (ROS) yielding an oxidative stress when cellular mechanisms involved in ROS quenching fail; this condition is further complicated by the enhanced, or, in some instances reduced, catalytic activity, with a consequent ‘personal predisposition’ to the deleterious biological epigenetic effects closely related with MFO alterations (i.e. co-toxicity, co-mutagenicity/co-carcinogenicity and promotion) (Paolini et al. 2004).
A large number of toxicological researches, focused on the link between chlorination DBPs and carcinogenesis, are reachable in the current scientific literature (King et al. 2000; Villanueva et al. 2004; Villanueva et al. 2007; Nieuwenhuijsen et al. 2009; Cantor 2010; Chowdhury et al. 2011), while very few data about the effects of DBD mixtures on MFO apparatus are available (Canistro et al. 2012; Sapone et al. 2007, 2015). Since some oxidative and post-oxidative metabolising enzymes (i.e. CYP2E1, glutathione S-transferase theta-1 and glutathione S-transferase zeta-1) are responsible for the oxidation and the activation of THMs, dichloro- and α-haloacids, and aliphatic hydrocarbons, to mutagenic and carcinogenic compounds, and several chemical species in the DBPs are putative CYP substrates, any modulation of their activity could result in a gain of toxic metabolite production (Cantor et al. 2010).
The aim of this work was to propose a methodological approach to evaluate DW quality, by means of its chemical analysis and the effects on MFOs in hepatopancreas of C. carpio. C. carpio represents an interesting and vastly used specimen in such type of investigations thanks to its capacity in bioaccumulation, wide distribution and great adaptability (Buschini et al. 2004; Canistro et al. 2012). Fishes were treated with concentrated DW samples collected from two Italian plants; the first (plant 1) is located near the mouth of the River Po and purifies water from the river; the second (plant 2) is located in the sub-Alpine area (comes from a well). For the first time, the effects of DW derived from two completely different sources on C. carpio MFOs were investigated. The possible implications for public health are here discussed.
Materials and methods
Chemicals
Nicotinamide adenine dinucleotide phosphate in its oxidised and reduced forms (NADP+ and NADPH), pentoxyresorufin, methoxyresorufin, 7-ethoxyresorufin, p-nitrophenol, aminopyrine, glutathione, 16-hydroxytestosterone, corticosterone and androste-4-ene-3,17-dione were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glucose 6-phosphate, glucose 6-phosphate dehydrogenase and cytochrome c were from Boehringer-Mannheim (Germany). High-performance liquid chromatography (HPLC)-grade methanol, tetrahydrofuran and dichloromethane were acquired from Labscan Ltd. Co. (Dublin, Ireland); 7α-, 6β- and 16β-hydroxytestosterone were from Sterlaloid (Wilton, NH, USA); and 6α-, 2α- and 2β-hydroxytestosterone were a generous gift from Dr. P. Gervasi (CNR, Pisa, Italy). All other chemicals were of the highest purity commercially available. C18 cartridges (Sep-Pak Plus tC18 Environmental Cartridges) were from Waters Chromatography (Milford, MA, USA).
Water chemical analysis
DW sampled along the distribution system was analysed to determine total organic carbon (TOC), total trihalomethane (TTHM) and HAA concentrations (APHA 1998). The main by-products of the use of chlorine dioxide, chlorite (ClO− 2) and chlorate (ClO− 3) were analysed (APHA 1998). THM formation potential (THMFP) was also determined as this parameter estimates the expected concentration of THM in water samples treated with an excess of free chlorine (APHA 1998). Chemical analysis were conducted by Maffei et al. (2009) and Marabini et al. (2007) in previous studies belonging to the same Ministerial project of the present one. Chemical values were clearly different in relation to the plant from which the water was derived. Briefly, the most significant data regarded the values of TOC, THMFP and ultraviolet (UV) absorbance, which are clearly higher in plant 1 than plant 2 (Maffei et al. 2009; Marabini et al. 2007).
Water sampling and concentration
The water sampling was carried out at two Italian treatment/distribution networks supplying DW: the first is located near the mouth of the River Po (plant 1), and the second in the north of Italy, in a sub-Alpine area (plant 2). Chlorine dioxide was used as a disinfectant in both plants. The stage of treatment was similar in both plants: water sedimentation, flocculation, rapid sand filtration, pre-disinfection with ozone, granular activated carbon filtration and post-disinfection with ClO2 (Maffei et al. 2009).
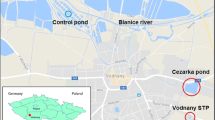
As previously reported (Fig. 1), the DW was sampled in different pipeline stations of each distribution network: before the distribution system (W), immediately after the treatment with disinfectants (A) and in one point of the piping system (R1); toxic compounds could indeed be produced not only from the treatment with disinfectants but also from the water distribution system (Maffei et al. 2009; Marabini et al. 2007). DW samples were taken weekly (100 L) for 5 weeks at each sampling point (500 L/point per plant), immediately acidified with hydrochloric acid (pH 2–2.5) and then concentrated by solid-phase adsorption with 10 g trifunctional silica gel C18 cartridges (Sep-Pak Plus tC18 Environmental Cartridges; Waters Chromatography, Milford, MA). Cartridge activation was performed with ethyl acetate, dichloromethane, methanol and distilled water in sequence (40 mL of each solvent). The DW was sucked through the cartridge (20 L/cartridge) with a pump in a multisample concentration system (VAC ELUT SPS 24, Varian, Leini, Italy) (Buschini et al. 2004; Guzzella et al. 2004). The elution of the adsorbed material from the cartridges was done with ethyl acetate, dichloromethane and methanol (40 mL/solvent) (Monarca et al. 2002). The eluates were pooled for each point, dried with a rotary evaporator, re-dissolved in dimethyl sulfoxide (DMSO) and used for biological assays.
Schematic representation of water sampling and carps treatment. For conducting chemical analysis, water was sampled at point W (river water for plant 1, spring water for plant 2). Carps (6/group) were treated intraperitoneally with water sampling at points A and R1. A third group was used as negative control and treated intraperitoneally with DMSO (vehicle)
Fish acclimatisation and treatment
Adult individuals of C. carpio were supplied by the ‘Centro Ittiogenico di Sant’Arcangelo’ (Perugia, Italy). Thirty-six male samples, weighing between 320 and 420 g, were divided into three groups (12 fishes per group). After 20 days of acclimatisation in stainless steel tanks supplied with DW after the transition from a purifier, each group was placed in a different tank. Fishes derived from the first group were treated daily, for three consecutive days, intraperitoneally with concentrated DW from the distribution networks of plant 1, at 3 L/eq dosage: group A (n = six fishes) was treated with DW immediately derived from the disinfection process, while DW sampled in a precise point along the distribution system was injected in group R1 (n = six fishes).
Fishes derived from the second group were treated as the previous one, but with concentrated DW derived from plant 2. The third group was used as negative control and treated intraperitoneally with DMSO (vehicle).
A schematic representation of the DW sampling and the animal treatment is shown in Fig. 1.
Preparation of subcellular fractions
After the treatment period, the hepatopancreas was rapidly removed from each fish, homogenised and then centrifuged at 9000×g. The post-mitochondrial supernatant was then centrifuged for 60 min at 105,000×g, after which the pellet was resuspended in 0.1 M K2P2 O7, 1 mmol/L ethylenediaminetetraacetic acid (EDTA; pH7.4) and centrifuged again for 60 min at 105,000×g to give the final microsomal fraction. Washed microsomes were then suspended with a hand-driven potter Elvehjem homogeniser in a 10-mmol/L Tris-HCl buffer (pH 7.4), containing 1 mmol/L EDTA and 20 % (v/v) glycerol. The fractions were immediately frozen in liquid nitrogen, stored at −80 °C and used within a week for enzymatic analyses (Bauer et al. 1994; Canistro et al. 2012).
Protein concentration
Protein concentration was determined according to the method described by Lowry (Lowry et al. 1951) as revised by Bailey (1967), using bovine serum albumin as standard and diluting microsomes 200 times and cytosol 1000 times to provide a suitable protein concentration. Such method was based on the formation of complex between ion Cu2+ and four peptide nitrogen atoms of proteins. In alkaline condition, Cu2+ was reduced to Cu+, which catalysed in turn the reduction of phosphotungstate and phosphomolybdate presents in the Folin-Ciocalteu reagent. Such reaction produces a blue coloration that was measured at 700–750 nm.
Blank (B), standard (S) and test (T) were prepared in test-tubes, respectively, with 1 mL of distilled water, 1 mL of bovine serum albumin and 1 mL of diluting sample. In each test-tube, 2 mL of cuprum reagent in alkaline solution (0.5 mL of CuSO4 0.5 % in sodium citrate + 25 mL of Na2CO3 in NaOH 0.1 N) was added. After 10 min, add 0.200 mL of diluting Folin-Ciocalteu reagent (2 mL of Folin-Ciocalteu reagent + 3 mL of distilled water). After 30 min of incubation in the dark, the blue colour developed by the reaction of Cu+, and the Folin-Ciocalteu reagent was read at 750 nm.
Aminopyrine N-demethylase
Demethylation of aminopyrine generates formaldehyde (CH2O). In presence of acetylacetone and ammonium salts (Nash reagent), CH2O produces 3,5-diacetyl-1,4-dihydrolutidine (DDL) (Mazel 1971). The total incubation volume was 3 mL, composed of 0.5 mL of a buffer solution of 50 mM aminopyrine and 25 mM MgCl2, 1.48 mL of 0.60 mM NADP+, 3.33 mM glucose 6-phosphate in 50 mM Tris-HCl buffer (pH 7.4), 0.02 mL glucose 6-phosphate dehydrogenase (0.93 U/mL) and 0.125 mL of sample (0.5 mg of protein); under these conditions, the method was linear up to 50 nmol mg−1 min−1. After 5 min of incubation at 37 °C, the yellow colour of DDL developed by the reaction of released CH2O with the Nash reagent being read at 412 nm, and the molar extinction coefficient of 8 mM−1 cm−1 was used for calculation (Nash 1953; Canistro et al. 2012).
Ethoxycoumarin O-deethylase
Activity was determined by quantification of umbelliferone formation, according to Aitio (1978). This product is generated by dealkylation of 7-ethoxycoumarin by various CYPs isoforms. Incubation mixture consisted 2.6 mL, composed of 1 mM ethoxycoumarin, 5 mM MgCl2, NADPH-generating system (see aminopyrine assay) and 0.25 mL of sample. After 5 min of incubation at 37 °C, the reaction was stopped by addiction of 0.85 mL of trichloroacetic acid (TCA) at 0.31 M. The pH of the mixture is brought to about 10 by adding 0.65 mL of 1.6 M NaOH-glycine buffer (pH 10.3); amount of umbelliferone was measured fluorimetrically (excitation, 390 nm; emission, 440 nm).
p-Nitrophenol hydroxylase
The incubation medium consisted of 2 mM p-nitrophenol in 50 mM Tris-HCl buffer (pH 7.4), 5 mM MgCl2 and a NADPH-generating system consisting of 0.4 mM NADP+, 30 mM isocitrate, 0.2 U of isocitrate dehydrogenase and 1.5 mg of proteins in a final volume of 2 mL, as previously described (Reinke and Mayer 1985). After 10 min at 37 °C, the reaction was stopped by adding 0.5 mL of 0.6 N perchloric acid. Under these conditions, the reaction was linear up to ∼5.5 nmol mg−1 min−1. Precipitated proteins were removed by centrifugation, and 1 mL of the supernatant was mixed with 1 mL 10 N NaOH. Absorbance at 546 nm was immediately measured, and the concentration of 4-nitrocatechol determined (ɛ = 10.28 mM−1 cm−1) (Canistro et al. 2012).
Pentoxyresorufin O-dealkylase, ethoxyresorufin O-deethylase and methoxyresorufin O-demethylase
Pentoxyresorufin O-dealkylase (PROD) reaction mixture consisted of 0.025 mM MgCl2, 200 mM penthoxyresorufin, 0.32 mg of proteins and 130 mM NADPH in 2.0 mL of 0.05 M Tris-HCl buffer (pH 7.4). Resorufin formation at 37 °C was calculated by comparing the rate of increase in relative fluorescence with the fluorescence of known amounts of resorufin (excitation 563 nm, emission 586 nm) (Lubet et al. 1985). Ethoxyresorufin O-deethylase (EROD) and methoxyresorufin O-demethylase (MROD) activities were measured exactly in the same manner as described for the penthoxyresorufin assay, except that substrate concentration is 1.7 mM for ethoxyresorufin and 5 mM for methoxyresorufin (Burke et al. 1985).
NADPH cytochrome (P450) c-reductase (CYP-red)
The analytical method is based on the determination of the reduction rate of cytochrome c at 550 nm (ɛ = 19.1 mM−1 cm−1), according to a previously defined procedure (Bruce 1976). The incubation mixture contained 1.6 mL of 0.05 M Tris-HCl buffer (pH 7.7) with 0.1 mM EDTA, 0.5 mg cytochrome c and 0.2 mL of microsomes.
Testosterone hydroxylase
An incubation mixture was prepared containing hepatopancreas microsomes (equivalent to 1–2 mg protein), 0.6 mM NADP+, 8 mM glucose 6-phosphate, 1.4 U glucose 6-phosphate dehydrogenase and 1 mM MgCl2, in a final volume of 2 mL of 0.1 M phosphate Na+/K+ buffer (pH 7.4). The mixture was pre-incubated for 5 min at 37 °C. The reaction was performed at 37 °C by shaking and started by the addition of 80 mM testosterone (dissolved in methanol). After 10 min, the reaction was stopped with 5 mL ice-cold dichloromethane and 9 nmol corticosterone (internal standard) in methanol. After 1 min of mixing, phases were separated by centrifugation at 2000×g for 10 min, and the aqueous phase was extracted once more with 2 mL dichloromethane. The organic phase was extracted with 2 mL 0.02 N NaOH, in order to remove lipid constituents, dried over anhydrous sodium sulphate and transferred to a small tube. Dichloromethane was evaporated at 37 °C under nitrogen and the dried samples stored at -20 °C. The samples were dissolved in 100 μL methanol and analysed by HPLC (Van der Hoeven 1984).
Chromatographic separations were performed through the use of a system consisting of a high-pressure pump (Waters Model 600E, Multisolvent Delivery System), a sample injection valve (Rheodyne Model 7121, CA, USA) with a 20-μL sample loop and an UV detector (254 nm, Waters Model 486, Tunable Absorbance Detector) connected to an integrator (Millennium 2010, Chromatography Manager). For reversed-phase separation of metabolites, a NOVA-PAK C18 analytical column (60 Å, 4 mm, 3.9 × 150 mm, Waters) was employed for the stationary phase. The mobile phase was a mixture of solvent A (7.5 % (v/v) tetrahydrofuran in water) and solvent B (7.5 % (v/v) tetrahydrofuran and 60 % (v/v) methanol in water) at 1 mL/min flow rate. Metabolite separation was performed by a gradient from 30 to 100 % (v/v) of solvent B over 30 min. The eluent was monitored at 254 nm, and the area under the absorption band was integrated. The concentration of metabolites was determined by the ratio between respective metabolite peak areas and corticosterone (the internal standard), and the calibration curves were obtained with synthetic testosterone derivatives (Van der Hoeven 1984).
Statistical and computer analysis
Statistical analysis for the enzymatic activities was performed with t test method. In order to express a rigorous statistical evaluation, the probability p of casual event was determined, and limits of significance were fixed as p = 0.05 (5 %) and p = 0.01 (1 %). Differences were considered significant when p < 5 % and p < 1 %.
Results
MFOs in hepatopancreas of carps treated with plant 1 DW
A general increase of phase I metabolic modulation in fishes treated with DW samples derived from plant 1, both in points A and R1, is appreciable from Fig. 2a and Table 1. Particularly, animals treated with DW sampled immediately after the disinfection process (A) showed a significant (p < 0.01) up-regulation of several MFOs: aminopyrine N-demethylase (APND), up to 101 %; MROD, up to 441 %; PROD, up to 17 %; and EROD, up to 41 %. On the other hand, for ethoxycoumarin O-deethylase (ECOD)- and p-nitrophenol hydroxylase (pNPH)-supported monooxygenases, a significant inactivation (19 and 55 % loss, respectively; p < 0.01) was observed. Carps treated with DW samples derived from point R1 showed significant (p < 0.01) increases of almost all the tested MFOs: APND (145 %), pNPH (330 %), MROD (123 %), PROD (88 %) and EROD (396 %).
CYP-linked and testosterone hydroxylase enzymatic activity in hepatopancreas microsomes of Cyprinus carpio treated with plant 1 water. The enzymatic activity is expressed as percentage variation of treated groups (A and R1) with respect to the control (fixed as 100). a Enzymes activity by single probe assays; b testosterone hydroxylase activity. *p < 0.05, significant differences between treated groups and their respective controls, using t test method; **p < 0.01, significant differences between treated groups and their respective controls, using t test method
Figure 2b and Table 2 show the metabolism of testosterone by C. carpio treated with plant 1 DW samples. A significant (p < 0.01) increment of testosterone 16α-hydroxylase (TH; 37 %) and 2α-hydroxylase (2α-TH; 46 %) was recorded in animals treated with point A DW with respect to the control. On the contrary, 16β-hydroxylase (16β-TH) showed a significant inactivation (18 % loss, p < 0.01). Moreover, an induction (from 30 for 2α-TH to 87 % for 6β-TH, with the exception of a 34 % decrement for testosterone 2β-TH, p < 0.01) was observed in fishes treated with DW sampled at point R1.
MFOs in hepatopancreas of carps treated with plant 2 DW
MFO changes in hepatopancreas microsomes of carps treated with plant 2 DW is shown in Fig. 3a and Table 3. The MFOs of group A increased in a significant way (p < 0.01) for APND, pNPH and MROD (from 20 to 87 %), and it was reduced for PROD (25 % loss, p < 0.05). On the other hand, a significant inactivation (p < 0.01) was evident in three of the evaluated MFOs of carps treated with DW sampled at point R1, with the maximum recorded for EROD, 40 % loss, while APND and MROD were markedly induced (respectively up to 45 and 109 %, p < 0.01).
CYP-linked and testosterone hydroxylase enzymatic activity in hepatopancreas microsomes of Cyprinus carpio treated with plant 2 water. The enzymatic activity is expressed as percentage variation of treated groups (A and R1) with respect to the control (fixed as 100). a Enzymes activity by single probe assays; b testosterone hydroxylase activity. *p < 0.05, significant differences between treated groups and their respective controls, using t test method; **p < 0.01, significant differences between treated groups and their respective controls, using t test method
Finally, in Fig. 3b and Table 4, the metabolism of testosterone in C. carpio hepatopancreas, treated with DW derived from plant 2, is reported. Treatment with DW sampled in point A caused a marked increase of testosterone 6α-hydroxylase (6α-TH), 16α-hydroxylase (16α-TH), 16β-hydroxylase (16β-TH), 2β-hydroxylase (2β-TH), and androst-4-ene-3,17-dione (17-OT)-linked monooxygenases, from a minimum for 17-OT (18 % increase, p < 0.01) to the maximum for 16β-TH (149 % increase, p < 0.01). Only 6β-TH and 2α-TH were significantly (p < 0.01) reduced (19 and 34 % loss, respectively). By contrast, carps treated with R1 DW showed an almost complete MFO inactivation (from 16 for 17-OT, to 80 % loss for 2β-OHT, p < 0.01), with the exception of 16α-TH, which was induced up to 26 % (p < 0.01).
Discussion
To the author’s knowledge, this is the first work investigating the carcinogen metabolising enzyme perturbation in C. carpio exposed to DW samples derived from river or spring water.
The physico-chemical parameters of plant 2 were evidently more suitable than plant 1. Such dissimilarity was probably due to the different human geography of the two plants. On one hand, the groundwater of the Po Valley (plant 1) collects a large amount of industrial, urban and agricultural waste, and the location is characterised by a wide industrial, agricultural and stock-farming practice (agriculture is one of the most important sector of the provincial economy) as well as high population density. The situation is very different for plant 2, whose groundwater pollutant concentrations were predominantly derived from industrial and chemical activities. This scenario is reflected in a different water composition between the two plants, and perhaps it justifies the higher TOC value in raw water of plant 1 than plant 2. More than once, it was confirmed that both natural process (i.e. seawater intrusion and dissolution of geologic sources) and anthropologic activities, such as seawater desalination, generation of mining tailings, chemical production, production of sewage and industrial effluents, may contribute, for example, to bromide concentration in DW sources (Magazinovic et al. 2004; Richardson et al. 2007; Valero et al. 2010; von Gunten et al. 1995).
Here, chlorine dioxide was used as disinfectant in both plants 1 and 2. As known, the reactions between such type of compound with fulvic, humic acids and other organic matter produce a range of disinfection by-products, many of which have been reported to be genotoxic in several biological systems (Richardson et al. 2007; Ohe et al. 2004; Singer 2008; Krasner 2009; Mitch 2010; Zhang et al. 2010). Moreover, recent studies suggest a role of DBPs in improving the cancer risk incidence (Villanueva et al. 2006, 2007; Cantor et al. 2010; Regli et al. 2015), even if the putative mechanisms of actions remain still unclear. In that context, an evaluation of xenobiotic metabolism in presence of DBPs appears to be appropriate. As mentioned before, indeed, the chemical-mediated induction of phase I biotrasformation reactions, is able to produce, in some instances, more or comparable reactive metabolites with respect to the initial ones. In consequence of this phenomenon, active metabolites can increase their capacity to react with endogenous molecules. CYP up-regulation can thus affect the biotransformation of ubiquitous pre-toxics (co-toxicity), pre-mutagens (co-mutagenicity) and pre-carcinogens (co-carcinogenicity). Moreover, oxidative stress generated in consequence of the increased MFO makes the individuals more susceptible to some biological alterations (i.e. membrane lipid peroxidation, oxidative modification in proteins and DNA) and pathological conditions such as cancer, atherosclerosis and ageing.
In plant 1, a general trend of MFO up-regulation was recorded, both for single probe and the multibioprobe testosterone. However, while MFOs measured with specific probes showed inductions of stronger entity, those supported by the hydroxylations of testosterone affected almost all the investigated isoforms and were constantly more marked in fishes treated with point R1 DW. The difference between A (milder modulations) and R1 points of the same network might be due to the chlorites and chlorates amount, which was almost the double in R1 than A.
By contrast, in plant 2, the MFO up-regulations found in point A was clearly reduced at point R1. Such phenomenon was maybe due to an immediate effect of the depuration system, whose strength decreased along the distribution network (as Table 1 reports). In accordance with this interpretation, the hazardous role of chlorine derived seems to have been confirmed by several epidemiological studies, which suggested a link between the consumption of chlorinate DW and reproductive and developmental outcomes, such as increased spontaneous abortions and intrauterine growth retardation (Nieuwenhuijsen et al. 2000; Graves et al. 2001; Tardiff et al. 2006; Savitz et al. 2006; Howards and Hertz-Piciotto 2006) and bladder and gastrointestinal tract cancer (King et al. 2000; Villanueva et al. 2004, 2007; Nieuwenhuijsen et al. 2009; Cantor 2010; Chowdhury et al. 2011). To date, the International Agency for Researce of Cancer (IARC) has published two monographs about the association between cancer and some DBPs (i.e. chloramines, chloral, chloral hydrate, dichloroacetic acid and trichloroacetic acid [IARC 2004] and bromoacetic acid, dibromoacetic acid and dibromoacetonitrile [IARC 2013]).
As DBPs are generated in consequence of the reaction between chemical disinfectants and the naturally occurring inorganic and organic material in the source water, the natural organic matter (NOM) present in source water represents the major precursor to the DBP formation. TOC, dissolved organic carbon (DOC), UV absorbance and specific ultraviolet absorbance (SUVA) are the parameters currently employed to estimate NOM reactivity toward DBP formation (Edzwald et al. 1985; Karanfil et al. 2002; Tan et al. 2005; Matilainen et al. 2011). In accordance with these premises, TOC, but especially, THM potential formation (TTHMFP) was evidently higher in plant 1 than plant 2 (Maffei et al. 2009; Marabini et al. 2007), whose collected DW produced the greatest recorded changes on our in vivo model. These results hold particular interest, especially, if considering that in a prospective cohort study of a representative sample of the US population, a significant correlation between the baseline blood THM species and total cancer mortality was found; this supports the existing data for the possible carcinogenic effects of THMs, and, more in general, brings us back once more to the critical issue that DW chlorination could represent a growing health hazard (Min and Min 2016).
Biotransformation of xenobiotics seems to be involved in several pathological human conditions. Many of such pathologies have mutations as the triggering event. Indeed, xenobiotics can directly or indirectly produce genetic mutations, chromosomal aberrations and aneuploidy/polyploidy. Mutations at somatic level have an important role in aetiology of many degenerative diseases, such as cancer, atherosclerosis and ageing. Even if the inherited genetic change may be not be sufficient enough to increase the likelihood of developing cancer, it makes the individual more susceptible to biotransformation of the wide range of carcinogenic and pre-carcinogenic substances to which an individual is daily exposed. Such vulnerability is conferred not only by mutational events but also by a sort of ‘bioactivated’ state of the cells, due to the MFO manipulation. The MFO boost indeed, makes constantly the organism ready to better ‘bioactivate’ exogenous compounds, with the manifestation of the consequences described so far. Thus, even if mutagenesis and carcinogenesis are stochastic events, the MFO modulation toward the bioactivation, increases human vulnerability to xenobiotic toxicity, and to the onset of degenerative pathologies.
Although more than 600 DBPs have been investigated and reported in current literature, they represent less than half of all possible environmental DBPs. These observations suggest that an integrated approach (i.e. chemical and biological analysis) should be ever applied and promoted in DW analysis. Various research groups have already employed this approach, and they have obtained encouraging results on MFO changes as well as on cytotoxicity and genotoxicity of DW from lake water supply (Sapone et al. 2015; Canistro et al. 2012; Maffei et al. 2009; Marabini et al. 2007). Specifically, the citotoxic and/or genotoxic evaluation of different mixtures of compounds in DW samples derived from the same plants of this work, by means of comet assay and micronuclei test, was previously investigated; for plant 1, the disinfection process generally reduced the toxicity of water, but the presence of potential direct DNA-damaging compounds was detectable after the treatment with chlorine disinfectants (Maffei et al. 2009); a general homogeneity of water quality for the three sampling points of plant 2, with a low level of both genotoxicity and cytotoxicity was also found (Marabini et al. 2007). These works are consistent with our findings and, once again, it seems that the best water quality (plant 2) reflects in a general modest toxicity. These investigations suggest that the same compounds contained in DW may initiate different biological mechanisms of toxicity and they can act independently or synergistically, with a consequent production of a wide variety of unhealthy effects. For example, the increase of MFOs caused by DBPs in water, may intensify the bioactivation of DBPs themselves, as well as the ubiquitous pre-toxic, pre-mutagens and pre-carcinogens (i.e. co-toxicity, co-carcinogenicity) to which individuals are simultaneously exposed. These results find support in data reported in previous studies, where investigating the effects of lake DW (treated with chlorine and alternative disinfectants) on MFOs in different experimental models (i.e. Dreissenia polymorpha and C. carpio), a complex pattern of CYP modulation in terms of both induction and suppression was found (Sapone et al. 2007, 2015; Canistro et al. 2012). Another toxicological consequences of MFO up-regulation is the generation of an excess of ROSs yielding an unremitting oxidative stress, which may act at all levels of multistep carcinogenesis (Perocco et al. 2006). The role of genetic polymorphisms, responsible for the presence in the population of high and low metabolisers, which were coupled with CYP-dependent induction/inhibition has been linked to an increase in cancer risk, may also constitute a complicating factor (Paolini et al. 1997, 2003). Furthermore, MFOs not only metabolise exogenous substances but also have a pivotal role in cellular and systemic functions, such as steroid hormones and cholesterol. Phase I enzymes are involved in the maintenance of non-peptide metabolites involved in cell growth, differentiation, apoptosis, homeostasis and neuro-endocrine functions (Nebert 1994). Such cellular activities are conserved with the participation in several transcription pathways and thus with the synthesis of enzymes involved, for example, in arachidonic acid pathway, bile acid biosynthesis, steroid hormones, androgen and oestrogen syntheses and vitamin D3 metabolism. Finally, MFO manipulation may also alter the metabolism of co-administered drug, particularly after repeated use. Thus, extrapolating to human, if DW is responsible for MFO manipulation, it has to be considered that such product is daily assumed and in large quantity. In this contest, it must be highlighted that the present study showed the acute effects of chlorinated drinking water on drug metabolism (i.e. 3-day of exposition), so it would be interesting to investigate such events on a longer exposition time.
Conclusions
Our data suggest that, to a better health safeguard, major attention to DW disinfection process should be paid, especially in the cases in which ground waters are influenced by strong industrial and agricultural activities. Indeed, in this study, the difference between two groundwater influenced both by divergent geographical location (Po valley for plant 1, sub-alpine area for plant 2) and anthropological activities (industrial, agricultural, urban waste for plant 1, chemical and urban waste for plant 2) is deeply evident. Such characteristics, as well as the specific population density (higher for plant 1), are reflected in a dissimilar water composition between the two groundwater examined in this investigation and may justify the better suitability for human use of plant 2 water than that of plant 1 for human use. DW is one of the elements that humans deliberately assume regularly. For this reason, disinfection processes have certainly represented an important milestone reached in the last century. However, research, constantly evolving, is responsible to evaluate the risk assessment of such processes, in order to safeguard humans and environment. In this view, the evidence that emerged from the present investigation was that chlorine-derived disinfectants might have, in particular instances, a significant role in the perturbation of xenobiotic metabolism. Such manipulation, along with the potential genotoxicity previously reported, regardless of DW origin, suggests that a regular exposure of individuals to chlorine-derived disinfectants may produce adverse health effects. Further studies of subacute or chronic toxicity of DW are necessary in order to confirm and reinforce, or alternatively contrast, the evidences of the present research. Even if the disinfection derived from is the most widely employed strategy, efficacy in purification coupled with low-cost, alternative, non-toxic methods of water treatment is necessary. Strategies for minimising DBP formation already exist such as DBP precursor removal, optimising disinfection to minimise DBP formation and DBP removal prior to water distribution (Wu and Wu 2009). Since all the disinfection processes produce their suite of DBPs, such strategies, in particular the ones that act in a non-specific manner (i.e. DBP precursor removal), seem to be effective in improving DW property and in increasing the trust in the quality of water produced (Watson et al. 2012).
References
APHA (1998) Standards method for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, 5-18-5-68
Aitio A (1978) A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal Biochem 85:488–91
Ashbolt NJ (2004) Risk analysis of drinking water microbial contamination versus disinfection by-products (DBPs). Toxicology 198:255–262
Bailey YL (1967) Techniques in protein chemistry. Elsevir Amsterdam
Bauer C, Corsi C, Paolini M (1994) Stability of microsomal monooxygenases in murine liver S9 fractions derived from phenobarbital and b-naphthoflavone induced animals under various long term condition of storage. Teratog Carcinog Mutgen 14:13–22
Bruce M (1976) Microsomal NADPH cytochrome c-reductase. Methods Enzymol 10:551–557
Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparan T, Meyer RT (1985) Ethoxy-, pentoxy- and benzyloxy-phenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P450. Biochem Pharmacol 34:3337–3345
Buschini A, Martino A, Gustavino B, Monfrinotti M, Poli P, Rossi C, Santoro M, Dörr AJ, Rizzoni M (2004) Comet assay and micronucleus test in circulating erythrocytes of Cyprinus carpio specimens exposed in situ to lake waters treated with disinfectants for potabilization. Mutat Res 557:119–129
Canistro D, Melega S, Ranieri D, Sapone A, Gustavino B, Monfrinotti M, Rizzoni M, Paolini M (2012) Modulation of cytochrome P450 and induction of DNA damage in Cyprinus carpio exposed in situ to surface water treated with chlorine or alternative disinfectants in different seasons. Mutat Res 729:81–89
Cantor KP (2010) Carcinogens in drinking water: the epidemiologic evidence. Rev Environ Health 25:9–16
Cantor KP, Villanueva CM, Silverman DT, Figueroa JD, Real FX, Garcia Closas M, Maltas N, Chanock S, Yager M, Tardon A, Garcia Closas R, Serra C, Carrato A, Castaño-Vinylas G, Samanic C, Rothman N, Kogevinas M (2010) Polymorphism in GSTT1, GSTZ1, and CYP2E1, disinfection by-products, and risk of bladder cancer in Spain. Environ Health Perspect 118:1545–1550
Chowdhury S, Rodriguez MJ, Sadiq R (2011) Disinfection byproducts in Canadian provinces: associated cancer risk and medical expenses. J Azar Mater 187:574–584
Edzwald JK, Becker WC, Wattier KL (1985) Surrogate parameter for monitoring organic matter and THM precursors. J Am Water Works Assoc 77(4):122–132
Graves GC, Matanoski GM, Tardiff RG (2001) Weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection by-products: a critical review. Regul Toxicol Pharmacol 34:103–124
Guzzella L, Monarca S, Zani C, Ferretti D, Zarbini I, Buschini A, Poli P, Rossi C, Richardson S (2004) In vitro potential genotoxic effects on surface drinking water treated with chlorine and alternative disinfectants. Mutat Res 564:341–347
Howards PP, Hertz-Piciotto I (2006) Invited commentary: disinfection by-products and pregnancy loss-lessons. Am J Epidemiol 164:1052–1055
IARC (2004) Some drinking water disinfectants and contaminants, including arsenic. In: IARC monographs on the evaluation of carcinogenic risks to humans, vol 84. IARC Scientific Publications, International Agency for Research on Cancer Press, Lyon, France
IARC (2013) Some chemicals in industrial and consumer products, some food contaminants and flavourings, and water by-products. In: IARC monographs on the evaluation of carcinogenic risks to humans, vol 101. IARC Scientific Publications, International Agency for Research on CancerPress, Lyon, France
Karanfil T, Schlautman MA, Erdogan I (2002) Survey of DOC and UV measurement practices with implications for SUVA determination. J Am Water Works Assoc 94(12):68–80
King WD, Marret LD, Woolcott GC (2000) Case-control study of colon and rectal cancers and chlorination by-products in treated water. Cancer Epidemiol Biomarkers Prev 9:813–818
Kogevinas M, Villanueva CM, Font-Ribera L, Liviac D, Bustamante M, Espinoza F, Nieuwenhuijsen MJ, Espinosa A, Fernandez P, DeMarini DM, Grimalt JO, Grummt T, Marcos R (2010) Genotoxic effects in swimmers exposed to disinfection by-products in indoor swimming pools. Environ Health Perspect 118:1531–1537
Krasner SW (2009) The formation and control of emerging disinfection by-products of health concern. Philos Trans A Math Phys Eng Sci 367:4077–4095
Leavens TL, Blount BC, DeMarini DM, Madden MC, Valentine JL, Case MW, Silva LK, Warren SH, Hanley NM, Pegram RA (2007) Disposition of bromodichloromethane in humans following oral and dermal exposure. Toxicol Sci 99:432–445
Lowry OH, Rosembrough HJ, Farr AL, Randall RJ (1951) Protein mesurement with Folin phenol reagent. J Biol Chem 193:265–275
Lubet RA, Mayer MJ, Cameron JW, Raymond WN, Burke M, Wolf FT, Guengerich FP (1985) Dealkylation of pentoxyresorufin. A rapid and sensitive assay for measuring induction of cytochrome(s) P450 by phenobarbital and other xenobiotics in rat. Arch Biochem Biophys 238:43–48
Maffei F, Carbone F, Cantelli Forti G, Buschini A, Poli P, Rossi C, Marabini L, Radice S, Chiesara E, Hrelia P (2009) Drinking water quality: an in vitro approach for the assessment of cytotoxic and genotoxic load in water sampled along distribution system. Environ Int 35:1053–1061
Magazinovic RS, Nicholson BC, Mulcahy DE, Davey DE (2004) Bromide levels in natural waters: its relationship to levels of both chloride and total dissolved solids and the implications for water treatment. Chemosphere 57:329–335
Marabini L, Frigerio S, Chiesara E, Maffei F, Cantelli Forti G, Hrelia P, Buschini A, Martino A, Poli P, Rossi C, Radice S (2007) In vitro citotoxicity and genotoxicity of chlorinated drinking water sampled along the distribution system of two municipal networks. Mutat Res 634:1–13
Matilainen A, Gjessing ET, Lahtinen T, Hed L, Bhatnagar A, Sillanpaa M (2011) An overview of the methods used in the characterization of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 83:1431–1442
Mazel P (1971) Fundamentals of drug metabolism and drug disposition. William and Wilkins, Baltimore, pp 546–550
Min JY, Min KB (2016) Blood trihalomethane levels and the risk of total cancer mortality in US adults. Environ Pollut 312:90–96
Mitch WA (2010) Comparison of byproduct formation in waters treated with chlorine and iodine: relevance to point-of-use treatment. Environ Sci Technol 44:8446–8452
Monarca S, Richardson SD, Ferretti D, Grottolo M, Thruston AD, Zani C, Navazio G, Ragazzo P, Zerbini I, Alberti A (2002) Mutagenicity and disinfection by-ptoducts in surface drinking water disinfected with paracetic acid. Environ Toxicol Chem 21:309–318
Nash T (1953) Colorimetric estimation of formaldehyde by means of Hantzsch reaction. Biochem J 55:416–421
Nebert DW (1994) Drug-metabolizing enzymes in ligand-modulated transcription. Biochem Pharmacol 47:25–37
Nieuwenhuijsen MJ, Toledano MB, Eaton NE, Fawell J, Elliot P (2000) Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup Environ Med 57:73–85
Nieuwenhuijsen MJ, Smith R, Golfinopoulos S, Best N, Bennet J, Aggazzotti G, Righi E, Fantuzzi G, Bucchini L, Cordier S, Villanueva CM, Moreno V, La Vecchia C, Bosetti C, Vartiainen T, Rautiu R, Toledano M, Iszatt N, Grazuleviciene R, Kogevinas M (2009) Health impact of long-term exposure to disinfection by-products in drinking water in Europe: HIWATE. J Water Health 7:185–207
Ohe T, Watanabe T, Wakabayashi K (2004) Mutagens in surface waters: a review. Mutat Res 567:109–149
Paolini M, Pozzetti L, Sapone A, Mesirca R, Perocco P, Mazzullo M, Cantelli-Forti G (1997) Molecular non genetic biomarkers of effect related to acephate cocarcinogenesis. Sex- and tissue-dependent induction or suppression of murine CYPs. Cancer Lett 117:7–15
Paolini M, Sapone A, Canistro D, Antonelli MA, Chieco P (2003) Diet and risk of cancer. Lancet 361:257–258
Paolini M, Sapone A, Gonzalez FJ (2004) Parkinson’s disease, pesticides and individual vulnerability. Trends Pharmacol 25:124–129
Perocco P, Bronzetti G, Canistro D, Valgimigli L, Sapone A, Affatato A, Pedulli GF, Pozzetti L, Broccoli M, Iori R, Barillari J, Sblendorio V, Legator MS, Paolini M, Abdel-Rahman SZ (2006) Glucoraphanin, the bio-precursor of the widely extolled chemopreventive agent sulforaphane found in broccoli, induces phase-I xenobiotic metabolizing enzymes and increases free radical generation in rat liver. Mutat Res 595:125–136
Regli S, Chen J, Messner M, Elovitz MS, Letkiewicz FJ, Pegram RA, Pepping TJ, Richardson SD, Wright JM (2015) Estimating potential increased bladder cancer risk due to increased bromide concentrations in sources of disinfected drinking waters. Environ Sci Technol 49:13094–13102
Reinke LA, Mayer MJ (1985) p-Nitrophenol hydroxylation: a microsomal oxidation which is highly inducible in ethanol. Drugs Metab Dispos 13:548–552
Richardson SD (1998) Drinking water disinfection byproducts. In: Meyers RA (ed) The encyclopedia of environmental analysis and remediation. John Wiley & Sons, New York, pp 1398–1421
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM (2007) Occurrence, genotoxicity and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636:178–242
Sapone A, Gustavino B, Monfrinotti M, Canistro D, Broccoli M, Pozzetti L, Affatato A, Valgimigli L, Forti GC, Pedulli GF, Biagi GL, Abdel-Rahman SZ, Paolini M (2007) Perturbation of cytochrome P450, generation of oxidative stress and induction of DNA damage in Cyprinus carpio exposed in situ to potable surface water. Mutat Res 626:143–54
Sapone A, Canistro D, Vivarelli F, Paolini M (2015) Perturbation of xenobiotic metabolism in Dreissena polymorpha model exposed in situ to surface (Lake Trasimene) purified with various disinfectants. Chemosphere 144:548–554
Savitz DA, Singer PC, Herring AH et al (2006) Exposure to drinking water disinfection by-products and pregnancy loss-lesson. Am J Epidemiol 164:1043–1051
Singer PC (2008) Development and interpretation of disinfection byproduct formation models using the Information Collection Rule database. Environ Sci Technol 42:5654–5660
Spivey A (2009) Drinking water quality: better biomarker of DBP exposure. Environ Health Perspect 117:A487
Tan Y, Kilduff JE, Kitis M, Karafil T (2005) Dissolved organic matter removal and disinfection byproduct formation control using ion exchange. Desalination 176:189–200
Tardiff RG, Carson ML, Ginevan ME (2006) Updated weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection by-products. Regul Toxicol Pharmacol 45:185–205
US Environmental Protection Agency (2006) National primary drinking water regulations: stage 2 disinfectant and disinfection byproduct rule. Environmental Protection Agency 40 CFR, part 9, 141, 142. Fed Reg 71(2):388–493
Valero F, Barcelo A, Arbós R (2010) Electrodialysis technology: theory and applications. In: M Schorr (ed) Desalination, trends and technologies. InTech Open Access
Van der Hoeven TH (1984) Assay of hepatic microsomal testosterone hydroxylases by high-performance liquid chromatography. Anal Biochem 138:57–65
Villanueva CM, Cantor KP, Cordiler S, Jaakkola JJ, King WD, Lynch CF, Porru S, Kogevinas M (2004) Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology 15:357–367
Villanueva CM, Cantor KP, Grimalt JO, Castano-Vinylas G, Maltas N, Silverman D, Kogevinas M (2006) Assessment of lifetime exposure to trihalomethanes through different routes. Occup Environ Med 63:273–277
Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Garcia-Closas R, Serra A, Carrato G, Castaño-Vinylas G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M (2007) Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165:148–156
von Gunten U, Hoigne J, Bruchet A (1995) Bromate formation during ozonation of bromide-containing waters. Water Supply 13:45–50
Watson K, Farré MJ, Knight N (2012) Strategies for the removal of halides from drinking water sources and their applicability in disinfection by-products minimization: a critical review. J Environ Manage 110:276–298
World Health Organization and UNICEF (2014) Progress on drinking water and sanitation. ISBN 978 92 4 150724 0
Wu F, Wu S (2009) Removal of Trihalomethanes from Drinking Water by Air Stripping. Proceedings of the 2009 International Conference on Energy and Environment Technology 02:695–698
Zhang SH, Miao DY, Liu AL, Zhang L, Wei W, Xie H, Lu WQ (2010) Assessment of the cytotoxicity and genotoxicity of haloacetic acids using microplate-based cytotoxicity test and CHO/HGPRT gene mutation assay. Mutat Res 703:174–179
Acknowledgement
Financial support from MIUR (Rome) is gratefully acknowledged.
Funding
All sources of financial and material support were provided by a grant from the Italian Ministry of Education, University and Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None
Additional information
Responsible editor: Henner Hollert
Silvia Cirillo and Donatella Canistro contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cirillo, S., Canistro, D., Vivarelli, F. et al. Effects of chlorinated drinking water on the xenobiotic metabolism in Cyprinus carpio treated with samples from two Italian municipal networks. Environ Sci Pollut Res 23, 18777–18788 (2016). https://doi.org/10.1007/s11356-016-7091-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7091-5