Abstract
The origin and evolution of brine and saline groundwater have always been a challenged work for geochemists and hydrogeologists. Chemical and isotopic data of brine and saline waters were used to trace the sources of salinity and therefore to understand the transport mechanisms of groundwater in Xishanzui, Inner Mongolia. Both Cl/Br (molar) versus Na/Br (molar) and Cl (meq/L) versus Na (meq/L) indicated that salinity was from halite dissolution or at least a significant impact by halite dissolution. The logarithmic plot of the concentration trends of Cl (mg/L) versus Br (mg/L) for the evaporation of seawater and the Qinghai Salt Lake showed that the terrestrial halite dissolution was the dominated contribution for the salinity of this brine. The stable isotope ratios of hydrogen and oxygen suggested that the origin of brine was from paleorecharge water which experienced mixing of modern water in shallow aquifer. δ37Cl values ranged from −0.02 to 3.43 ‰ (SMOC), and reflecting mixing of different sources. The Cl isotopic compositions suggest that the dissolution of halite by paleometeoric water had a great contribution to the salinity of brine, and the contributions of the residual seawater and the dissolution of halite by the Yellow River water could be excluded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater salinization is one of the most conspicuous phenomena of water quality degradation, and it is more serious in arid and semiarid regions where groundwater is the main source for water supply in the local area (e.g., Ghassemi et al. 1995; Vengosh 2003; Carmelita et al. 2010; Al-Harbi et al. 2014; Yamanaka et al. 2014; Kwong et al. 2015). The increasing demands for water have created tremendous pressures on water resources that have resulted in lowering water level and increasing salinization. Concerns for the sustainable management of groundwater resources require sufficient knowledge on the distribution of fresh and saline groundwater and the processes affecting groundwater salinization (Langman 2008). However, the origin of water and solutes of brines in sedimentary basin remains a controversial aspect of sedimentary and hydrogeochemistry (Stueber and Walter 1991) due to the various sources of groundwater salinity such as natural saline groundwater; halite dissolution; seawater intrusion; oil and gas field brine; domestic, agricultural, and industrial effluents; and road salts (Park et al. 2005; Salem and El-horiny 2014). To successfully describe groundwater salinization processes, methods detailed characterization and identification of the source of salinity and the origin of waters is required (Kura et al. 2014; Bouzourra et al. 2015).
Chemical and isotopic compositions of different kinds of waters such as brine, lake water, river water, and spring water exposed in the surrounding salt sediments are good indicators for understanding the origin of brine and saline groundwater. For instance, Cl and Br ions can be applied as indicators of water sources and rock interactions because they are commonly considered to be conservative tracers (Carpenter 1978; Edmunds 1996; Stotler et al. 2010). Oxygen and hydrogen isotopic ratios can provide information regarding the source of water molecules and effects of water-rock interactions (Moldovanyi et al. 1993; Grobe et al. 2000). The fundamental relationships between δ18O, δ2H, and δ18O and salinity can be used to identify different salinization pathways (Gaye 2001). Chlorine stable isotopes are mainly used to define salinity sources, mixing of different waters, and water-rock interaction (Kaufmann 1984; Kaufmann et al. 1992; Eastoe and Guilbert 1992; Eggenkamp and Coleman 1998; Eastoe et al. 2001; Zhang et al. 2007; Bagheri et al. 2014). However, many geochemical processes, such as water-rock interaction, ion exchange, and mineral alteration, may influence the concentration of chemical compositions in the process in groundwater salinization. It is often ambiguous to differentiate the sources of salinity from major ion chemistry (Tellam and Lloyd 1986) without information from isotopes. Multiple isotope approach can provide unique information on sources of groundwater salinity (Gaye 2001). Previous studies suggested that the stable Cl isotope ratio together with other geochemical parameters was a useful tool to differentiate the sources of solute and to trace the transport mechanisms of solute (e.g., Eggenkamp 1994; Eastoe et al. 1999; Hendry et al. 2000; Godon et al. 2004; Zhang et al. 2007).
The terrestrial brine with total dissolved solid (TDS) up to 90 g/L was found at the depth of 5∼50 m in Xishanzui area, Inner Mongolia. Some studies (Liu 2009; Yu et al. 2010; Xiao et al. 2012) on geochemistry suggested that brine originated from deep aquifer flowing upward through fractures. But, they did not give any discussions on the occurrence and genesis of brine in deep aquifer because of the absence of isotopic data. The results of geochemical modeling by PHREEQC (Liu 2009) suggested the salinity originated not only from halite dissolution, but also from other sources. Unluckily, the sources have not been well demonstrated yet. The origin of shallow brine is still an unanswered question. The main objectives of this study are to differentiate the sources of salinity and trace the origin and movement of brine by using Cl, H, and O stable isotopes and chemical data.
Study area
Regional geological and hydrogeological setting
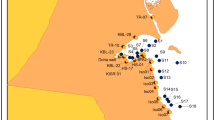
The study area is located at the eastern part of Hetao plain, which lies in the western part of Inner Mongolia. The Hetao plain, covering a total area of 13,040 km2 with total population about one million, is bounded by the Langshan Mountains in the north and the Yellow River in the south (Figs. 1 and 2). Geographically, the mean altitude of Hetao plain ranges from 1025 to 1040 m a.s.l., and the terrain is high in the SW and low in the NE. The plain consists of alluvial plain and alluvial-lacustrine plain. The alluvial fans occur in the piedmont plain and extend from the mountain front to the flat plain. The shallow saline waters and brine deposits are located at alluvial-lacustrine plain in Xishanzui, which is surrounded by Yellow River to the south and Ulansuhai Lake to the east (Fig. 1). The climate is continental and semiarid, with a low annual mean precipitation of 130–220 mm and high evaporation of 2000–2500 mm (Zhu et al. 2015). Precipitation mainly concentrates in July and August, accounting for 56.3 % of the total rainfall (Tong et al. 2014). The annual mean temperature ranges from 5.6 to 7.8 °C, and the relative humidity is about 48.5 %. The Yellow River flows across the plain from west to east.
Geological cross section along the line A–A’ of quaternary aquifers in Hetao basin (modified from Chen. 2002)
The Langshan Mountains are mainly composed of a metamorphic complex (slate, gneiss, and marble), generally of Jurassic to Cretaceous age, which is folded and fractured (Li and Li 1994). Hetao basin is also a Cenozoic rift basin which is controlled by series of faults (F1–F7) striking NE–SW and NW–SE (Fig. 2). The bedrock is Precambrian granitic gneiss overlying Cretaceous, Tertiary, and Quaternary formations. The overlying Tertiary formation is fluvio-lacustrine sediments. It consists of red sandstone and mudstone with scattered gypsum or evaporate. The Tertiary sediments often occur in the conditions with great amounts of salinity. The Quaternary formation is a thick lacustrine-fluvial deposit consisting of gravel, sand, and silt. The thickness ranges from 500 to 1500 m in the southeast and from 1000 to 2400 m in the northwest. Mirabilite deposits were found within the middle Pleistocene formation in the south of Baotou (Fig. 2).
There are three uplifts and depressions from west to east, which were successively named Langshan uplift, Linhe depression, Xishanzui uplifted, Baiyanhua depression, Baotou uplifted, and Huhe depression (Fig. 2). The Xishanzui uplifted zone (area of brine deposits) is bordered by faults in the north and south and extends about 100 km from west to east and 12 km from north to south, covering an area of 1200 km2. The depth to the bedrock is 1–2 km in the eastern and increases to 2–4 km toward the western part. The geological formations from Cretaceous, Tertiary, and Quaternary were overlain the bedrock (Fig. 3).
The regional aquifer system consists of Quaternary and Neogene aquifers (Fig. 3). The Quaternary aquifer system is composed of alluvial and lacustrine deposits and changes from a thick single layer with coarse deposits in the piedmont to a multi-layer confined aquifer system in the central plain. The single-layer aquifer consists of sandy gravel with pebble and coarse-medium sand with gravel. The thickness of the aquifer is about 50∼80 m in the upper part of alluvial fan and 20∼40 m in the distal fan. Groundwater changes from HCO3-Ca.Na (Ca.Mg) with the TDS of 0.5 g/L to HCO3.Cl-Na.Ca or Cl.HCO3-Na.Mg with the TDS of 3.0 g/L The multi-layer aquifers in the alluvial lacustrine plain are mainly composed of medium-fine sand, fine sand, and silt-fine sand. Medium-coarse sand with gravel occasionally occurs in some areas. The depth to the roof of aquifer is less than 20 m. And, the floor of aquifer is 80∼100 m in the eastern part, 150∼300 m in the western part, 30∼80 m in the southern part, and 100∼200 m in the northern part. Accordingly, the thickness of aquifers increases from east (60–80 m) to west (150–240 m) and from south (20–60 m) to north (100–200 m). The water type is mainly Cl-Na with the TDS of 0.4∼60.5 g/L. The maximum value of TDS was found in the south Xishanzui uplift zone where the area of the present study has brine deposits. The Neogene aquifer is mainly composed of fine sandstone with a thickness up to 300 m. A well-penetrated aquifer produced geothermal water of Cl-Na type with the TDS of 47.0 g/L and the temperature of 57.0 °C.
The contributions of recharge to aquifers are successively as follows: the seepage of river and irrigation ditch, the infiltration of irrigation water from the Yellow River, the infiltration of local precipitation, and the lateral flows from mountain area. Naturally, groundwater flows from the piedmont to the lowland. And, the regional groundwater flows from the west to east and discharges into the Ulansuhai Lake. Groundwater for potable use in the rural areas is abstracted mainly from the shallow aquifer with depths less than 30 m (Deng et al. 2009).
Shallow saline waters and brine deposits in Xishanzui
The shallow saline water and brine deposits occupy an area of 32 km long and 3–12 km wide, covering a total area of about 238.8 km2. It is surrounded by Yellow River to the south and Ulansuhai Lake to the east (Fig. 1). The aquifer contained saline water, and brine is the Upper Pleistocene formation which is mainly composed of medium to fine sand. The overlying Holocene formation with the thickness of 3∼13 m consists of silt and clay. The thickness of aquifer increases from east (10∼30 m) to west (60∼75 m) and from south (10∼30 m) to north (30∼60 m). Groundwater flows slowly from the west to east (Fig. 1). And, the depth to water table is often less than 5 m. Groundwater is NaCl type with TDS >10.0 g/L, and the maximum value is found nearby the south Xishanzui uplifted zone. The area of TDS >50.0 g/L occurs in the middle part of the study area with a total area of 27.0 km2 (Fig. 1).
Methods
Groundwater samples were collected for the measurement of chemistry and isotopes from pumping wells in August 2011 along the flow path. Sampling sites were also considered to cover the different area of TDS concentrations and different well depths. The sample sites were shown in Fig. 1. At least three borehole casing volumes of groundwater were extracted before a water sample was taken. Samples for stable O, H, and Cl isotope analyses were collected in 50- and 250-mL glass bottles, respectively. Samples for tritium measurement (3H) were collected in 500-mL glass bottles. Samples for chemical analysis were filtered through a 0.45-μm filter paper and acidified to a pH value of less than 2 for cation analysis. Parameters such as temperature, pH, electrical conductance, and alkalinity were measured at the sampling sites. Sample measurements were performed at the Institute of Hydrogeology and Environmental Geology (IHEG), Chinese Academy of Geological Sciences. The measurements for the stable isotope ratio of oxygen and hydrogen were analyzed with a Finnigan MAT 253. The isotope compositions were reported in standard δ-notation represented as per mil deviation from the Vienna Standard Mean Ocean Water (V-SMOW) standard. The precision of the δ2H and δ18O values is ±1.0 and ±0.1 ‰, respectively. Tritium values were electrolytically enriched and measured using the liquid scintillation counting method (1220 Quantulus), and the results are reported in tritium units (TU), with a typical error of ±0.5 TU. Sample measurements for Cl isotope were performed at the Salt Lake Analytical and Test Department, Qinghai Institute of Salt Lakes, Chinese Academy of Sciences. The method used for Cl isotope analysis was first proposed by Xiao and Zhang (1992) and modified by Xiao et al. (1995). The water sample was run successively through Ba-form resin and H-form resin to remove sulfates and cation and then run through Cs-form resin to produce a solution of Cs2Cl+. The Cl isotope ratio was measured with Cs2Cl+ ions on a thermal ionization mass spectrometer (Triton). The reference NaCl ISL354 was analyzed with each sample set and has a mean value with 0.319078 ± 0.000054 ‰ (n = 9, 2σ).
Results
Water chemistry
The chemical compositions of samples in the study area are given in Table 1. The samples can be divided into two groups: one group is fresh and brackish water, and the other is saline and brine water. TDS concentrations of two groups are 0.7–2.9 g/L with a mean value of 1.7 g/L for fresh and brackish group and 19.1–90.8 g/L with a mean value of 46.5 g/L for saline and brine group. The pH of the saline and brine water samples varies from 6.64 to 8.36. The dominated ions are Na+ (5500.00–26,599.90 mg/L) and Cl− (9573.40 –26,513.66 mg/L) in the saline and brine water and Na+ (80.81–984.6 mg/L) and HCO3 − (203.20 –654.40 mg/L) in the fresh and brackish water. The piper plot of the major ions shows that samples of saline and brine water (○ and ●) are predominantly Na-Cl-type water, and the samples of fresh and brackish water (△ and □) are mainly Na-Cl·HCO3-type water (Fig. 4).
Hydrogen and oxygen isotope ratios of groundwater
The δ2H and δ18O values of samples ranged from −82 to −52 ‰ and from −10.5 to −5.5 ‰, respectively (Table 1). All groundwater samples lie to the right of the local meteoric water line in the plot of δ2H vs. δ18O (Fig. 5). Fresh groundwater samples are plotted at the left end of LMWL within a narrow range of δ18O (δ2H) values between −10.5 ‰ (−85 ‰) and −8.9 ‰ (−73 ‰). The mean value of δ18O and δ2H (−9.7 and −77 ‰) for these samples was more negative than the mean value of precipitation in Baotou (−8.1 and −57 ‰), but close to the mean value of Yellow River water. This suggested that recharge from Yellow River has a significant contribution to the fresh groundwater. The samples of brine and saline waters are scattered along a fitted line δ2H = 6.5δ18O −18.4. They had a wide range of δ18O (−7.6 ‰ < δ18O < −1.7 ‰) and δD (−56 ‰ < δD < −41 ‰) values, which was more negative than the mean value of precipitation in Baotou and less negative than the mean value of Yellow River water.
Stable isotopic compositions of hydrogen and oxygen of brine and saline water samples in Xin’an town (notes: isotopic values (mean value −10.5 and −78.4 ‰) of Yellow River water were from ten samples from Toudaoguai in Tuoketuo, inner Mongolia (Gao et al., 2011); precipitation and its average vale were from IAEA’s GNIP data in Baotou station)
Tritium contents of groundwater
The tritium content of fresh groundwater samples (TDS <3.0 g/L) which were sampled from the wells with depth less than 20 m ranged from 2 up to 40 TU. The high tritium contents (28∼40 TU) were found for the groundwaters (Nos. wd3, wd5, wd6) emerging along the NW boundary (Xixiaozhao) of brine and saline water area, while the low (<5 TU) tritium contents (Nos. wd1, wd4, wd7, wd8) were found for the groundwaters along the north boundary except wd8 which was located in the uplift zone of Xishanzui-Wulashan. The tritium content in brine and saline water samples (TDS >19.0 g/L), which were sampled from the depth of 20∼44 m, ranged from <1 up to 29 TU. The high tritium contents (>5 TU) were found in wells located at the southern part near the river, which was attributed to admixture of modern river water with high tritium content. The low (<5 TU) tritium contents were found for the groundwaters located far away from river.
Chlorine isotope ratios of groundwater
δ37Cl values for brine and saline water samples range from −0.02 to 3.43 ‰. Only sample (wx1) shows a negative value. Most samples fell in the range of 0.00∼2.00 ‰ with the high frequency range of 0.50∼1.00 ‰ (Fig. 6).
Discussion
Sources of recharge to aquifer
The δ2H and δ18O are good conservative tracers to identify the sources of recharge since they are part of the water molecule itself, which can reflect its history and origin before infiltration (Clark and Fritz 1997).
As Fig. 5 shown, all groundwater samples lie to the right of the local meteoric water line in the plot of δ2H vs. δ18O. The delta values of fresh groundwater samples vary in a narrow range, and most of them fall on the left of local meteoric water line (except for very few samples). Their values are more negative than the average value of local precipitation and close to the mean value of the Yellow River water. These features show that the Yellow River water is the dominated source of recharge to fresh groundwater in the study area. Whereas δ18O and δ2H values of brine are apparently higher than those of fresh groundwater, indicating the different sources of recharge from the fresh groundwater, the δ2H and δ18O values of brine samples are lower than the mean value of local precipitation, but higher than the mean value of the Yellow River water. This indicates that the source of recharge to brine should not be the Yellow River water and local precipitation. The big range of δ18O and δ2H values (or the scatter of brine samples in Fig. 5) suggests that brine was recharged by multi-sources or mixed by different groundwater when brine moved in aquifers. The δ18O values of brine are less than the mean value of modern precipitation, indicating a cold recharge period. However, the brine samples which contain the considerable content of tritium fall near the line fitted by the data of samples of Yellow River. This fact suggests that these brine samples have been mixed with water from the Yellow River, whereas those samples free of tritium should not be affected by modern recharge.
Sources of salinity
Indications from hydrogeochemical data
The ratios of chemical compositions are a useful tool for tracing the sources of salinity of brine and its evolution. The ratios of Cl/Br (molar) and Na/Br (molar) are usually used to identify salinity source of brine. When seawater experienced evaporation and crystallization, Br is not easy to enter into the mineral lattice of halite and would concentrate in the remained seawater, which lead to a significant decrease of Na/Br (molar) and Cl/Br (molar) ratio in the residual seawater. In contrast, the brine, which formed from the re-dissolution of precipitated halite by fresh water, has much higher Na/Br (molar) and Cl/Br (molar) ratios than seawater. Walter et al (1993) proposed that the plot of Cl/Br-Na/Br can differentiate the sources of salinity. This diagram can be used to identify source of salt and determine dilution effect in depleted Br water (such as meteoric water) and halite dissolution. The plot of Cl/Br (molar) vs. Na/Br (molar) is shown in Fig. 7a. Seawater falls on the line of slope 1, and all brine samples plot along the line. The Na/Br and Cl/Br ratios are bigger than those of seawater except two samples. These suggest that the source of salt may predominantly originate from the dissolution of halite.
The ratio of Cl/Na (meq/L) can be used as an indicator for tracing sources of salinity of brine. Figure 7b presents the variation of Cl/Na (meq/L) ratio in the study area. Water whose salinity originates from the dissolution of halite would lie along a line with the slope of 1. However, brine samples in the study area plot along the line of 1:1.3 slightly shift to the high concentration of chloride. This suggests that the source of salt was dominated by the dissolution of halite, and some other geochemical processes should be responsible for the deviation from the 1:1 line. The possible processes involve with (1) cation exchange and adsorption, (2) mixture of Ca-Cl-type water, and (3) dissolution of other Cl-bearing minerals following the dissolution of halite, such as epsomite (KCl), carnallite (KMgCl3·6H2O), and bischofite (MgCl2·6H2O). Cation exchange and adsorption would remove K and Na from solution and increase concentration of Ca and Mg. The poor correlation between Na + K and Ca + Mg (Fig. 7c) indicates that this process could not be responsible for the elevated Cl/Na ratio in the brine. Mixing with Ca-Cl-type water would result in the elevation of Cl concentration in groundwater. However, the Ca-Cl-type water was not found in the study area, and therefore, this process should be excluded. The dissolution of Cl-bearing minerals would elevate the Cl concentration and give a Cl/(K + Na + Mg) ratio of about 1 in groundwater. The samples of brine were plotted on a fitted line of slope 0.94 in the diagram of K + Na + Mg vs. Cl (Fig.7d). This indicates that the sources of salts of brine were from the dissolution of halite and other Cl-bearing minerals.
The ratio of Cl/Br (mg/L) is also used for tracing sources of salinity of brine. Bromine usually occurs in solution, and the Br-bearing minerals are rarely found in the nature. Zherebtsova and Volkova (1966) suggested that the bromine content would increase until the precipitation of potash salts (e.g., sylvite and carnallite) when the seawater evaporated. And, Li and Br would remain in the solution during the formation of potash deposition. Figure 8 illustrates the logarithmic relationship between Cl (mg/L) and Br (mg/L), in which the evaporation lines of Qinghai Salt Lake and the sequence of mineral precipitation were from the experiment results given by Sun et al. (2002), and the evaporation line of seawater and the sequence of mineral precipitation are from the results given by Matray’s study after 1984 (Stash 2008). The concentration of Br in brine samples is less than that in initial seawater and the residual seawater and plots above the evaporation line of seawater. Normally, the sources of salinity of brine could be from the residual seawater and dissolution of evaporites. Those samples with Cl concentrations less than those of initial seawater may indicate the dilution of fresh water if the salinity originated from the residual seawater. But, this inference was not true because the occurrence of residual seawater has been excluded in the above discussion.
The dissolution of evaporites by precipitation also could be the source of salinity. The Br concentration in marine evaporites, such as halite and sylvite, ranges from 68 to 260 ppm (Valyaskho 1956; Holser 1979). The typical saturated concentration of bromine is 24 –93 mg/L in salt water which originated from the dissolution of marine evaporates by precipitation and 90 –150 mg/L in the salt water which originated from the dissolution of marine evaporates by seawater (Rittenhouse 1967). The Br concentration of brine samples in the study area varies from 4 to 80 mg/L, which is close to the range of halite dissolution by precipitation. Unluckily, no marine evaporites were found in the study area. In contrast, many terrestrial evaporates were found in the study area. Thus, the possible source of salinity is from the dissolution of terrestrial evaporites because the dissolution and recrystallization of halite would increase the Br concentration in water. In Fig. 8, three samples fall on the evaporation line of Qinghai Salt Lake and other samples deviate from and plot below the line; i.e., the Br concentration of most samples is higher than that of evaporated water in Qinghai Lake. This phenomenon suggests that solute may originate from the evaporation of salt lake or dissolution of terrestrial halite by precipitation. When the dissolution of halite is re-equilibrium with respect of brine, the Br will be moved into the liquid phase from the halite, resulting in elevated Br content in brine.
Indications from chloride stable isotope
Kaufmann and Lon (1988) stated that the Cl isotopic composition, especially the relationship between δ37C1 and the Cl− concentrations, can be used to determine the sources of Cl− and mechanisms responsible for fractionation of Cl isotopes. Generally, δ37C1 values in natural material vary from −8.00∼ to +8.00 ‰, and most of them vary from −2.00 to +2.00 ‰. There are various δ37C1 values for different sources: δ37C1 values are +0.42∼+2.53 ‰ for aerosol, +0.74∼+2.85 ‰ for river water, −2.06∼+1.01 ‰ for salt lake brine, −0.50∼+0.69 ‰ for groundwater, and −0.60∼+1.20 ‰ for halite.
δ37Cl values of samples show a larger variation range, which suggests that the salinity is from multi-sources. The high-frequency samples give a δ37Cl range of 0.00∼2.00 ‰, which overlaps with the range of halite. This fact indicates that the chloride of samples with δ37Cl <1.00 ‰ was predominately from the dissolution of halite. Those samples of δ37Cl values >1.00 ‰ (the maximum value up to 3.40 ‰) may indicate the mixing of different water body or the dissolution of different evaporites. The possible reason for 37Cl enrichment in some samples is the dissolution of terrestrial carnallite. Generally, 37Cl would preferentially move from the solution into the salt minerals during the salt crystallization and precipitation, which result in 37Cl depletion in solution and enrichment in salt deposits. For example, Vengosh et al. (1989) reported that a sample of carnallite collected from Chaidan basin in Qinghai, China, has a δ37Cl value 24.70 ± 2.90 ‰. On the contrary, the 37Cl will be enriched in solution as salt mineral dissolution. Thus, the leaching of evaporites by groundwater which may recharge from precipitation or surface water could be responsible for the high δ37Cl value of those samples. This contribution to the source of salinity has been differentiated by the chemical indicators and discussed in the section of sources of salinity.
Conceptual model
Synthesizing the previous results inferred from multiple chemical and isotopic methods with the geologic structure and hydrogeological conditions, a conceptual model for the origin of saline and brine groundwater in shallow aquifer can be inferred (Fig. 9). For saline and brine water, faults can be considered as an effective channel for salt transport and the movement of saline and brine water. The uplifting of basement plays an important role for the migration of brine in the deep aquifers which consists of red sandstone and mudstone with scattered gypsum or evaporite in the overlying Tertiary formation. Another possible channel is the aquitards (mainly composed of silty sand) between the shallow aquifer and deep aquifer due to the difference of water pressure, which makes the saline and brine water in shallow aquifers recharged by the saline and brine water in deep aquifers by leakage. The alternation of salt-enriched strata and the lakes in the basin results in elevated salinity groundwater under the influence of paleoclimate and sedimentary environment in the study area. So, the third possible origin is the salt accumulation from halite dissolution by precipitation or surface water followed by strong evaporation due to a closed basin and shallow groundwater table.
Conclusion
Identifying the origin of saline and brine groundwater can provide a better understanding of groundwater salinization processes. Multiple chemical and isotopic methods have been used in this study to analyze the sources of water and salt of saline and brine water in Xishanzui area. Evidences from chloride isotope and hydrogen and oxygen stable isotope indicate that not only there is salt origin in this region but also it is multiple. Its source has no obvious relationship with the Yellow River, and it may originate from paleometeoric water but not from residual seawater. The ratio of Cl/Br presents that salinity mainly from salt lake evaporation or continental halite dissolved by precipitation. The ratios of Cl/Br (molar) and Na/Br (molar) indicate that the source of salinity is not from residual seawater but dissolved halite or has had a strong impact from halite dissolution. The ratio of Cl/Na (molar) shows that salinity originated from halite dissolution and there are other salts containing Cl− concentration dissolved during this processes. According to geological and hydrogeological conditions, fracture structure plays a significant controlling role in the formation and distribution of brine and salt transport and leakage of saline and brine water in deep aquifers through the intermediate aquitards may be another possible channel. In consideration of paleoclimatic condition and paleosedimentary environment in the study area, the salt accumulation from halite dissolution by precipitation or surface water may be another possible origin of saline and brine water in shallow aquifers.
References
Al-Harbi M, Al-Ruwaih FM, Alsulaili A (2014) Statistical and analytical evaluation of groundwater quality in Al-Rawdhatain field. Environ Prog Sustain Energ 33(3):895–904
Bagheri R, Nadri A, Raeisi E, Eggenkamp HGM, Kazemi GA, Montaseri A (2014) Hydrochemical and isotopic (δ18O, δ2H, 87Sr/86Sr, δ37Cl and δ81Br) evidence for the origin of saline formation water in a gas reservoir. Chem Geol 384:62–75
Bouzourra H, Bouhlila R, Slama F, Bouhlila R, Slama F, Elango L, Ouslati N (2015) Characterization of mechanisms and processes of groundwater salinization in irrigated coastal area using statistics, GIS and hydrogeochemical investigations. Environ Sci Pollut Res 22:2643–2660
Carmelita S, Thushyanthy M, Barathithasan T, Saravanan T (2010) Irrigation water quality based on hydro chemical analysis, Jaffna, Sri Lanka. Am-Euras J Agr Environ Sci 7:100–102
Carpenter AB (1978) Origin and chemical evolution of brines in sedimentary basins. Okl Geol Surv Circ 79:60–77
Chen LC (2002) Paleoearthquakes, the law of strong earthquake recurrence and potential sites for the occurrence of future strong earthquakes in the Hetao fault-depression zone (Master’s thesis).
Clark I, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press, Boca Raton, p 328
Deng YM, Wang YX, Ma T (2009) Isotope and minor element geochemistry of high arsenic groundwater from Hangjinhouqi, the Hetao Plain, Inner Mongolia. Appl Geochem 24:587–599
Eastoe CJ, Guilbert JM (1992) Stable chlorine isotopes in hydrothermal processes. Geochim Cosmochim Acta 56:4247–4255
Eastoe CJ, Long A, Knauth LP (1999) Stable chlorine isotopes in the Palo Duro Basin, Texas: evidence for preservation of Permian evaporate brines. Geochim Cosmochim Acta 63(9):1375–1382
Eastoe CJ, Long AL, Land LS, Kyle JR (2001) Stable chlorine isotopes in halite and brine from the Gulf Coast Basin: brine genesis. Chem Geol 176:343–360
Edmunds WM (1996) Bromine geochemistry of British groundwaters. Mineral Mag 60:275–284
Eggenkamp HGM (1994) δ37Cl: the geochemistry of chlorine isotopes. Utrecht University, Utrecht (Ph.D. Thesis)
Eggenkamp HGM, Coleman ML (1998) Heterogeneity of formation waters within and between oil fields by halogen isotopes. Proc. 9th Int. Symp. Water–Rock Interaction, 309–312.
Gao JF, Ding TP, Luo XR, Tian SH, Wang HB, Li M (2011) δD and δ18O variations of water in the Yellow River and its environmental significance. Acta Geol Sin-Engl 85(4):596–602
Gaye CB (2001) Isotope techniques for monitoring groundwater salinization. First International Conference on Saltwater Intrusion and Coastal Aquifers—monitoring, modeling, and management. Essaouira, Morocco.
Ghassemi F, Jakeman AJ, Nix HA (1995) Salinization of land and water resources: human causes, extent, management and case studies. University of New South Wales Press, Sydney
Godon A, Jendrzejewski N, Eggenkamp HGM, Banks DA, Ader M, Coleman ML, Pineau F (2004) A cross-calibration of chlorine isotopic measurements and suitability of seawater as the international reference material. Chem Geol 207:1–12
Grobe M, Machel HG, Heuser H (2000) Origin and evolution of saline groundwater in the Münsterland Cretaceous Basin, Germany: oxygen, hydrogen, and strontium isotope evidence. J Geochem Explor 69–70:5–9
Hendry MJ, Wassenaar LI, Kotzer T (2000) Chloride and chlorine isotopes (36Cl and 37Cl) as tracers of solute migration in a thick, clay-rich aquitard system. Water Resour Res 36:285–296
Holser WT (1979) Trace elements and isotopes in evaporites. In: Burns RG (ed) Reviews in mineralogy, In: Marine minerals, 6. Mineralogical Society of America, Washington, pp 295–346
Kaufmann RS (1984) Chlorine in ground water: stable isotope distribution. University of Arizona, Tucson (PhD Thesis)
Kaufmann R, Lon CDJ (1988) Chlorine isotope distribution in formation waters, Texas and Louisiana. Am Asso Pet Geol Bull 72:839–844
Kaufmann RS, McNutt R, Frape SK, Eastoe C (1992) Chlorine stable isotope distribution of Michigan Basin and Canadian Shield formation waters. In: Kharaka, Y.K. and Maest, A.S. (eds., International Symposium on); 7th International Symposium on Water-Rock Interaction, Park City, Utah, USA. Balkema, Rotterdam, Brookfield. 2, 943-946.
Kura NU, Ramli MF, Ibrahim S, Sulaiman WNA, Aris AZ (2014) An integrated assessment of seawater intrusion in a small tropical island using geophysical, geochemical, and geostatistical techniques. Environ Sci Pollut Res 21:7047–7064
Kwong HT, Jiao JJ, Liu K, Guo HP, Yang SY (2015) Geochemical signature of pore water from core samples and its implications on the origin of saline pore water in Cangzhou, North China Plain. J Geochem Explor 157:143–152
Langman JB (2008) A multi-tracer study of saltwater origin, cross-formational flow, and the geochemical evolution of groundwater in the southern high plains aquifer along the Western Caprock Escarpment, east-central New Mexico (PhD Thesis).
Li SF, Li HJ (1994) Study on characteristics and the origin of geological environment in endemic arseniasis area, Hetao, Inner Mongolia. Chinese J Geol Hazard Control 5:213–219
Liu JF (2009) Evaluation of shallow saline and brine in the south of Xin’an Town, Urad Front Banner, Inner Mongolia Autonomous Region (Ph.D. Thesis).
Moldovanyi EP, Walter LM, Land LS (1993) Strontium, boron, oxygen, and hydrogen isotope geochemistry of brines from basal stata of the Gulf Coast sedimentary basin, USA. Geochim Cosmochim Acta 57:2083–2099
Park SC, Yun ST, Chae GT, Yoo IS, Shin KS, Heo CH, Lee SK (2005) Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J Hydro 313:182–194
Rittenhouse G (1967) Bromine in oil-field waters and its use in determining possibilities of origin of these waters. Am Assoc Petroleum Geol Bull 51:2430–2440
Salem ZE, El-horiny MM (2014) Hydrogeochemical evaluation of calcareous eolianite aquifer with saline soil in a semiarid area. Environ Sci Pollut Res 21:8294–8314
Stash OS (2008) Evaluation of stable chlorine and bromine isotopes in sedimentary formation fluids (Ph.D. Thesis).
Stotler RL, Frape SK, Freifeld BM, Holden B, Onstott TC, Ruskeeniemi T (2010). Hydrogeology, chemical and microbial activity measurement through deep Permafrost. Ground water, 1-17.
Stueber AM, Walter LM (1991) Origin and chemical evolution of formation waters from Silurian-Devonian strata in the Illinois basin, USA. Geochim Cosmochim Acta 55:309–325
Sun DP, Li BX, Ma YH, Liu QZ (2002) An investigation on evaporating experiments for Qinghai Lake Water, China. J Salt Lake Res 10(4):1–12
Tellam JH, Lloyd JW (1986) Problems in the recognition of seawater intrusion by chemical means: an example of apparent chemical equivalence. Q J Eng Geol Hydrogeol 19:389–398
Tong JT, Guo HM, Wei C (2014) Arsenic contamination of the soil-wheat system irrigated with high arsenic groundwater in the Hetao Basin, Inner Mongolia, China. Sci Total Environ 496:479–487
Valyaskho MG (1956) Geochemistry of bromine in the processes of salt deposition and the use of the bromine content as a genetic and prospecting criterion. Geokhimiya 6:570–589
Vengosh A (2003) Salinization and saline environments. Treatise on Geochemistry 9:1–35
Vengosh A, Chivas A, McCulloch MT (1989) Direct determination of boron and chlorine isotopic compositions in geological materials by negative thermal-ionization mass spectrometry. Chem Geol (Isotopic Geoscience Section) 79:333–343
Walter LM, Martini MM, Stueber AM, Moldovanyi EP (1993) Saline formation waters: new constraints on origin and migration from comparisons at the basin scale. Geol Soc Am Abs Prog 25(6):1–23
Xiao YK, Zhang CG (1992) High precision isotopic measurement of chlorine by thermal ionization mass spectrometry of the Cs2Cl+ ion. Int J Mass Spectrometry Ion Process 116:183–192
Xiao YK, Zhou YM, Liu WG (1995) Precise measurement of chlorine isotopes based on Cs2Cl+ by thermal ionization mass spectrometry. Anal Lett 28(7):1295–1304
Xiao CL, Liu JF, Qiu SW, Zhang N (2012) Genesis analysis of shallow brine in the south of Xin’an town, Urad Front Banner, Inner Mongolia Autonomous Region. J Xi’an Shiyou Univ (Natural Science Edition) 27(4):13–18
Yamanaka M, Bottrell SH, Wu JH, Kumagai Y, Mori K, Satake H (2014) Chlorine stable isotope evidence for salinization processes of confined groundwater in southwestern Nobi Plain aquifer system, central Japan. J Hydro 519:295–306
Yu J, Zhang YL, Li ZH, Wang WZ, Wang LJ, Cao WG (2010) Migration and origin of brines in the south of Xin-an township of Inner Mongolia. South-to-North Water Transfers Water Sci Technol 8(6):33–35, 49
Zhang M, Frape SK, Love AJ, Herczeg AL, Lehmann BE, Beyerle U (2007) Chlorine stable isotope studies of old groundwater, southwestern Great Artesian Basin, Australia. Appli Geochem 22:557–574
Zherebtsova IK, Volkova NN (1966) Experimental study of behavior of trace elements in the process of natural solar evaporation of Black Sea water and Sasyk-Sivash brine. Geochem Int 3:656–670
Zhu YC, Zhao XY, Chen M, Luo YQ, Zhou X (2015) Characteristics of high arsenic groundwater in Hetao Basin, Inner Mongolia, northern China. Sci Cold Arid Regions 7(1):104–110
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC grant No. 41272252 and No. 41502250).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Kenneth Mei Yee Leung
Rights and permissions
About this article
Cite this article
Liu, J., Chen, Z., Wang, L. et al. Chemical and isotopic constrains on the origin of brine and saline groundwater in Hetao plain, Inner Mongolia. Environ Sci Pollut Res 23, 15003–15014 (2016). https://doi.org/10.1007/s11356-016-6617-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6617-1