Abstract
Rainwater chemistry was investigated at a semi-rural site in Ya’an, Sichuan basin with rain samples collected from May 2013 to July 2014. The rainwater pH values ranged from 3.25 to 6.86, with an annual volume-weighted mean (VWM) of 4.38, and the acid rain frequency was 74 %. Such severe acidification, 15 % of the total events showed a pH below 4.0, attributed to the deficiency of Ca2+, significant anthropogenic pollution contribution, and rainy pattern to this area. The annual VWM of total ions concentration was 477.19 μeq/L. NH4 + was the most abundant ionic species, followed by SO4 2−, NO3 −, Ca2+, Cl−, Na+, K+, Mg2+, and F− in a descending order. The total ionic concentrations presented a seasonal trend of lower values in autumn and summer but higher ones in winter and spring. Based on enrichment factor, correlation analysis and principle component analysis, three factors were identified: factor 1 (NH4 +, SO4 2−, NO3 −, K+, and Cl−, 47.45 % of the total variance) related to anthropogenic sources (coal/fuel combustion, biomass burning and agriculture), factor 2 (Ca2+, Mg2+, Na+, and Cl−, 34.01 % of the total variance) associated with natural sources, and factor 3 (H+, 11.78 % of the total variance) related to free acidity. Back trajectory analysis indicates that the rainwater chemistry in Ya’an was mainly affected by regional air masses from Sichuan basin. Long-range transported air masses from southwest with heavy anthropogenic pollution increased the total ion concentration and acidity of rainwater. Considering its special topography, anthropogenic emissions from regional and long-range transport (especially from southwest) must be controlled effectively to improve the acid rain condition of non-urban areas in Sichuan basin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain has been becoming a serious regional and even global environmental issue since the past decades. Acid precipitation has negative impacts on aquatic as well as terrestrial ecosystems, such as acidification to surface water and soil, declination of aquatic organisms, forests and crops, and devastation of outdoor constructions including buildings, statues, and roads (Larssen et al. 1999). More critically, acid rain can cause direct and indirect harms to human health (Goyer et al. 1985). China was the third largest region suffered from heavy acid deposition followed by North Europe and North America, where have been being controlled by the strict reduction regulations of SO2 and NOx emissions. Comparing the 3-year average annual non-sea-salt sulfate deposition values between 2000–2002 and 2005–2007, it was found that >60 % of North American sites had a decrease of 0–42 % while >82 % of the European sites had a decrease of 0–58 % (Vet et al. 2014). However, the situations in China are still serious because of rapidly growth of economies, large energy consumption, great agricultural production, and industrialization (Liu et al. 2013; Lynch et al. 2000; Tørseth et al. 2012; Yang et al. 2010). Since the 1990s, Chinese SO2 emissions contributed about one fourth of the global anthropogenic emissions, in particular, the combination of NOx-N and NH3-N emissions fluxes in eastern China was revealed the highest in 2011 (Lu et al. 2010; Vet et al. 2014). In the CHINA-MAP program, Streets and Waldhoff (2000) reported that SO2 and NOx emissions are projected to increase from 25.2 to 12.0 mt, respectively, in 1995 to 30.6 mt and 26.6–29.7 mt, respectively, in 2020. Even though emission controls are implemented to some extent, the emissions of the two species are still concentrated in the populated and industrialized areas of China, including Sichuan basin.
Sichuan basin is located in southwest China, where is one of the four major acid rain regions, polluted by acid rain in the earliest times, and ranked the first place of acid deposition flux in China (Huang et al. 2014; Lu et al. 2013). According to recent assessment on the global S wet deposition (Vet et al. 2014), the highest global values were measured at the IMPACTS (Integrated Monitoring Programmer on Acidification of Chinese Terrestrial Systems) Chongqing-Tie Shan Ping site in Sichuan basin in 2000–2002, and the EANET (Acid Deposition Monitoring Network in East Asia) Chongqing-Jinyunshan site in Sichuan basin in 2005–2007. However, most previous studies conducted in Sichuan basin were mainly focused on megacities, such as Chongqing and Chengdu (Lu et al. 2013; Wang and Han 2011). Limited researches on acid rain were reported in semi-rural and suburban sites. Ya’an, a typical semi-rural area, is famous as a rain city lined on the rain-belt and within the transition zone between the Chengdu plain and the Qinghai-Tibetan plateau showing high terrain of north, west, and south but low-lying east. Due to its unique topography, there is a large amount of regionally transported pollutants are stuck in Ya’an by high mountains, washed out by the frequent rain, and deposited in to this area. Chemical characterization of rainwater not only supplies the blank knowledge on the non-megacity but also provides a full understanding on the regional deposition pattern of this region. Furthermore, the data are valuable for the further researches on the ecosystem and offering scientific evidence on establishment of emission reduction policies in the Sichuan basin.
There are two aims of this study, including: (1) to measure the pH and concentrations of inorganic anions and cations in rainwater collected in Ya’an from May 2013 to July 2014; and (2) to determine the seasonal variations and perform source apportionment with calculation of enrichment factor (EF), principle component analysis (PCA), and back trajectory analysis.
Experimental section
Sample collection and analysis
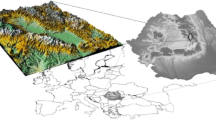
Ya’an (102°51′–103°12′E, 29°40′–30°14′N) is located in the west margin of Sichuan basin, which is within the transition zone between modern urban centers and natural ecological areas. Figure 1 presents the map of Sichuan basin and sampling location at Ya’an. It is a special terrain where is suffered from abundant rainfall (i.e., ∼60 % of the rainy days in a year). The air masses passing through Ya’an are raised up by the Qionglai range with a high elevation. As the air masses rise, moisture gets saturated and then forms rain. Therefore, Ya’an is known as a “Rainy Area of West China” and “Rain city.” The average annual rainfall is ca. 1800 mm. It has characteristics of high relative humidity (RH), less sunshine, and low annual average temperature (14.1∼17.9 °C).
The sampling site was on the roof of a seven-story building on the campus of Sichuan Agricultural University. There were no obvious pollution sources nearby except a campus cafeteria located 100 m away from the site. The rainwater sample was collected into a 1000-mL polyethylene bottle with a funnel, which was placed approximately 120 cm above the floor. The bottle was soaked with acid (2–3 mol/L HCl), rinsed with deionized water, and dried before use. To avoid contamination from dry deposition or particle fallout, the bottle was initially closed and immediately opened once rainfall commenced. The pH of rainwater samples was measured at once after each rainfall event. The pH meter (PHS-320, Shanghai Leici Instruments Co., China) was calibrated by standard pH 4.00 and 6.86 buffer solutions before each measurement. In the laboratory, the samples were filtered through a 0.45-μm Millipore membrane filter and stored in a refrigerator at 4 °C before further chemical analysis conducted within a month. A total of 54 rainwater samples were collected from May 2013 to July 2014. It should be noted that no samples were collected in October 2013, January and February 2014 due to personnel availability, holidays, and insufficient precipitation. Ions were analyzed by an ion chromatography system (IC, Dionex 600, Sunnyvale, CA) equipped with an electrochemical detector. The anions (SO4 2−, NO3 −, Cl− and F−) were determined by an ASI4A column and a gradient elution solution consists of a mixed solution of Na2CO3 and NaHCO3. The cations (NH4 +, Ca2+, Mg2+ and K+) were determined by a CSI2A column, and an isocratic elution solution consists of methanesulfonic acid. The concentrations of NOx, SO2, PM10, and amount of rainfall were obtained by automatic monitoring from government departments.
Data quality
In this study, the sampling and analysis procedures were followed with the technical specifications recommended by acid deposition monitoring in China (State Environmental Protection Administration of China 2004). The quality assurance was routinely conducted using standard reference materials purchased from the National Research Center for Certified Reference Materials, China. The quality procedures included the routine running of blanks and duplicate samples. All of the reported data were deducted from the blank values. Standard deviation of two repeated samples and analytical precision were both less than ±5 %. Ion balance was used to assess the data quality of the individual rainwater samples, reflecting the reliability of analysis data as well as the possibility of any missing ion (Lu et al. 2011). Out of the total collected samples, 13 % (7 samples) has been discarded from the final analysis due to poor ion balance and another 3.7 % (2 samples) has been discarded due to insufficient volume (2–4 mL which is short for complete chemical analysis). Therefore, a total of 46 samples, 85.2 % of the collected samples, were valid and used for discussion. The ion balance between total anions (F−, Cl−, NO3 −, and SO4 2−) and total cations (K+, Na+, Ca2+, Mg2+, and NH4 +) was evaluated through linear regression analysis (Fig. 2). High correlation coefficient (R 2 = 0.90) indicates an acceptable data quality. The slope (0.9) less than 1 and positive intercept (12.04 μeq/L) both illustrate the anion deficiency. This can be ascribed to the exclusion of the HCOO−, CH3COO−, and HCO3 − from the measurements in this study. For those pH value >5.0, chemical formula of 5.1 × 10−6/[H+] represents the concentration of bicarbonate in equilibrium with suburban/industrial measurements (Vet et al. 2014). Considering annual volume-weighted mean (VWM) pH was only 4.38 in this study (discuss later), the concentration of HCO3 − should not be higher than 0.12 μeq/L. It is reported that higher concentrations of organic ions in rainwater are generally observed in forested tropical areas near the equator with a gradual drop toward the poles (Vet et al. 2014). The total concentrations of formic acid and acetic acid were in the range of 3.3–10.0 μeq/L according to several studies conducted in local sites in Thailand (Vet et al. 2014) and at Shenzhen in China (Huang et al. 2010). As the low pH values and the relation of geography position among Ya’an, Thailand and Shenzhen, the intercept value (12.04 μeq/L) approximately covers the non-analyzed organic acids and HCO3 − in this study.
Results and discussion
pH variation
The pH value of samples is a fundamental and extensive standard to judge the acidity of rainwater. The frequency distribution of the rainwater pH is shown in Fig. 3. Table 1 summarizes the VWM pH and ionic concentrations in rainwater as well as the concentrations of PM10, SO2, and NOx in Ya’an. The pH of cloud water in equilibrium with atmospheric CO2 was usually used as a criterion for acid rain (Charlson and Rodhe 1982). Rainwater samples with pH values >5.6 may suggest inputs from alkaline substances into precipitation. The pH of the rainwater samples ranged from 3.25 to 6.86, with an annual VWM pH of 4.38. Approximately 74 % of pH values of the rainwater samples was <5.6. The frequency of acid rain in Ya’an was in the medium level of south China, where had the range of 71–89 % reported from various studies (Huang et al. 2008a, b, 2009, 2010; Wang and Han 2011; Zhao et al. 2013). Fifteen percent of the events recorded a pH below 4.0, indicating severe acidification caused by strong impact of anthropogenic pollution in this area.
Table 2 compares the pH and ionic concentrations in Ya’an with other regions. Our value showed a slightly increase from the VWM of pH 4.03 measured in Ya’an during 2010–2011 (Zhao et al. 2013). Even though the ranges of pH (3.25–6.86 in the study vs 3.36–6.68 in Zhao et al. (2013)) were very close between these two periods, there was an obvious diversity on the frequency of distributions. Up to 68 % of samples was within the range of 3.0–4.5 in 2010–2011, while only 43.5 % in 2013–2014. The percentages of samples having a pH value below 5.6 were 79 % in 2010–2011, but 74 % in this study. All of these differences could be explained by the variety of sampling programs and calculation of weighed mean method. In comparison of the two sampling programs (Table S1, Table S# and Fig. S# denotes materials provided as supporting information), there were less winter samples (8.7 %) and more summer samples (43.5 %) in 2013–2014 study, but they were more even distributed of 21.4 % (winter) and 25 % (summer) in 2010–2011. Furthermore, the lowest pH values occurred in winter and relatively high pH values occurred in summer in both of the studies (discussed later). Based on the calculation of pH with weighted mean method, both frequency and rainfall amount had significant impacts on the average value. Even though the factor of rainfall could be ignored, the relatively low frequency of low pH values in the winter samples and high frequency of high pH values in the summer samples could result in higher annual VWM of pH in this study.
Compared with the values in northern cities (e.g., Beijing and Xi’an), lower pH was measured in Ya’an due to the lower contents of alkaline matters in soil in south China, which contains only 0.1 and 0.5 % of calcium and magnesium, respectively, but up to 3 and 1.5 %, respectively in north China (Fan 2002; Tang et al. 2005). Many researches proved that the concentration of Ca2+ had a remarkably positive correlation with pH, and Ca2+ from mineral aerosol played an important role in buffering rainwater acidity (Larssen et al. 1999; Qiao et al. 2015; Tang et al. 2005; Wang and Han 2011; Xu et al. 2015). In general, rainwater dominated by Ca2+ has a high pH, while dominated by SO4 2− has a low pH (Table 2). Furthermore, it is interesting to find that the VWM of pH in Ya’an was the lowest among all south China cities, such as Chongqing and Chengdu in Sichuan basin. This may be attributed to its characteristic climate, a high frequency of continuous rains, and of 60 % rainy days throughout a year. These phenomena agree with the observation of that the acidity of rainwater increased after continuous rains in Ghore EI-Safi area (Al-Khashman 2009). Jiuzhaigou National Nature Reserve (JNNR) also experienced acid rain mainly caused by SO4 2− in wet seasons, while the ambient alkaline dust concentration was low (Qiao et al. 2015). Semi-continuous washing is efficient in diluting the concentrations of alkaline particles, causing a decrease in concentrations of base cations in rainwater and a consequent reduction in acid-neutralizing capacity (Zhang et al. 2012). In addition, the rainwater pH in Ya’an was lower than those of most abroad cities and countries (e.g., India (5.9), Jordan (6.91), Spain (5.55), Korea (4.7), Japan (4.52), and Newark (4.6)) except Singapore (4.2). It is worth noting that NH4 + was the most abundant ion in Ya’an but did not show significant buffering function due to its lower ability of than Ca2+. Conclusively, the lack of alkaline substance Ca2+, significant anthropogenic influences, and rainy pattern were potential causes for very low pH in rainwater in Ya’an.
The samples were grouped in four seasons, including spring (May 2013, and March to May 2014, n = 16), summer (June to August 2013, and June to July 2014, n = 20), autumn (September and November 2013, n = 6), and winter (December 2013, n = 4). Figure 4 shows the seasonal variation of acidity in precipitation. It is obvious that the lowest pH (3.72) was seen in winter, and higher values were found in summer (4.44) and spring (4.47). Precipitation can incorporate both particles and gases, and then rainwater would wash out the components in the air while it falls on the ground (Huo et al. 2012). High concentrations of acid gases (SO2 and NOx) and particulate ions (sulfate and nitrate) were scavenged, leading to raise the acidity of rainwater. The lowest VWM pH in winter might be explained by the largest emissions of SO2 (0.03 mg/m3) and NOx (0.03 mg/m3), as precursor gases of SO4 2− and NO3 −, respectively, from coal burning and cold start of vehicles, as well as the highest concentration of PM10 (0.11 mg/m3). In addition, the lowest rainfall amount (69 mm) can be contributed to the lowest pH. The relative high pH values of rainwater in summer were mainly attributed to the dilution effect with the highest rainfall in record (961 mm). At a similar rainfall level in autumn, higher VWM pH value was measured in spring due to the stronger buffering ability with higher concentrations of NH4 + and Ca2+ (354.92 and 70.55 μeq/L, respectively) caused by spring plowing than those in autumn (91.93 and 42.74 μeq/L, respectively).
Chemical composition
The total ionic concentrations of rainwater ranged from 61.51 to 4145.85 μeq/L, with an annual VWM value of 477.19 μeq/L, in Ya’an during May 2013–July 2014. The annual contributions of measured species in rainwater were NH4 + > SO4 2− > NO3 − > Ca2+ > Cl− > Na+ > K+ ≈ Mg2+ > F− in a descending order (Fig. 5). SO4 2− was the most dominant anion and its concentration ranged from 7.91 to 1043.27 μeq/L with an annual VWM of 138.37 μeq/L, accounting for approximately 29 % of the total quantified ions and 59 % of the total anions, respectively. This was consistent with the typical characteristic of rainwater across China because coal burning is the major energy carrier (Huang et al. 2010; Tu et al. 2005). The second most abundant anion was NO3 − with an annual VWM of 71.38 μeq/L. The sum of SO4 2− and NO3 − accounted for 90 % of the total quantified anions. As Table 2, it could be seen that the VWM concentrations of SO4 2− were in the range of 138.4–489.7 μeq/L in most urban cities of China (e.g., Beijing, Xi’an, Chengdu, Chongqing and Guangzhou), which were much higher than those in USA (38.1 μeq/L), Japan (50.2 μeq/L), Spain (72.5 μeq/L) and Korea (70.9 μeq/L). This obvious difference was due to the abatement strategies for SO2 anthropogenic emissions and control measures that were implemented in these countries (Aas et al. 2007; Mouli et al. 2005; Sopauskiene and Jasineviciene 2006). The small cities such as Ya’an, Puding, and Lijiang typically have dissimilar composition profiles with the megacities (Beijing, Xi’an, Chongqing, and Chengdu), where SO4 2− and NO3 − were the dominant species from urbanization and industrialization. In Ya’an, the most abundant cation was NH4 + with the concentrations ranging from 8.54 to 1797.42 μeq/L, which accounted for approximately 36 % of the quantified total ions and 70 % of the total cations. NH4 + was also the most abundant cation in Dalian (Zhang et al. 2012). The relative high level coincides with the fact that the emission of ammonia from agricultural activities is enormous in Asian regions, especially in southwest China (Vet et al. 2014; Yang et al. 2010). Additionally, the unique meteorological characteristic with high RH (78 %) promoted the transformation of gaseous NH3 into aerosol NH4 +, which was then scavenged by rainwater. The second most abundant cation was Ca2+ with an annual VWM of 37.91 μeq/L, accounting for approximately 8 % of the total quantified ions. Such level was relatively low among most cities in China (e.g., 9.29 % in Shenzhen (Huang et al. 2010), and 42.73 % in Beijing (Xu and Han 2009)). The low contribution of Ca2+ in Ya’an not only related to the low contents of alkaline matters in soil in south China but also correlated with the serious acidification soil due to the accumulation of acid deposition and high rainfall in past decades. According to the global assessment of wet deposition which was defined as the product of an ion or species concentration in precipitation times the precipitation depth, the highest S values of 34.90 and 49.82 kg S ha−1 a−1, respectively, were measured at Chongqing-Tie Shan Ping site during 2000–2002 and Chongqing-Jinyunshan site during 2005–2007, while the highest N value of 27.07 kg N ha−1 a−1 was also measured at Chongqing-Jinyunshan site in 2005–2007 (Vet et al. 2014). Considered much higher annual average rainfall (1800 mm) in Ya’an, the estimated wet deposition of sulfur and nitrogen could reach 39.85 and 60.72 kg N ha−1 a−1, respectively, which were almost equal to or even higher than those of Chongqing. It should be noted that the deposition of NH4 +-N (42.73 kg S ha−1 a−1) was more than twice of the NO3 −-N deposition (17.99 kg S ha−1 a−1) in Ya’an, implying that the benefit of nitrogen deposition (e.g., enhancing the biological activities and providing nutrients by NH4 +) was higher than the damage to ecosystem caused by NO3 −.
Sulfate and nitrate were the two main anions in precipitation. Table 2 compares SO4 2−/NO3 − ratios measured at various locations in the world. On the whole, the SO4 2−/NO3 − ratios were larger than 1, representing that sulfuric acid was still the dominant factor on the control of acidity of rainwater. In China, with the increasing number of motor vehicles and the transformation of energy structure, the type of acid rain gradually shifts from “sulfuric acid” to “mixture.” Compared to the ratio of 2.52 in 2010–2011 (Zhao et al. 2013), our value (1.94) suggests that the emission of SO2 had been controlled to some extent, and the traffic emission, NOx, has become more enormous with the increasing number of private cars. The traffic emissions control measures should be taken urgently. The lowest SO4 2−/NO3 − ratios occurred in winter, consistent with the findings reported in Zhao et al.’s study (2013), which could be attributed to the increasing of NO3 − in winter due to the cold start of vehicles and favorable gas-to-particle phase inversion from HNO3 to NO3 −. Compared with the data obtained in other cities, the SO4 2−/NO3 − ratio in Ya’an was lower than those of megacities and background areas in China, while was very close to most abroad cities. Ya’an locates in western China with less industrialization, but, as an ecotourism city, more and more tourists travel with self-driving private cars. Even though the number of private cars in other megacities is much higher than that in Ya’an, the contribution from heavy industry is much more crucial to those. In contrast, the lower ratio in the abroad cities was mainly due to the stringent control of sulfuric production (e.g., the reduction of sulfur contents in oil by-products) but less control of NOx emissions (Al-Khashman 2009; Okuda et al. 2005).
Seasonal variation of components in rainwater
Figure 4 presents the seasonal variation of measured ions in precipitation. It could be seen that the highest value of total ionic concentrations occurred in winter (1315.16 μeq/L), followed by spring (910.00 μeq/L), autumn (350.15 μeq/L) and summer (315.32 μeq/L). The seasonal trend should be mainly affected by the meteorological factors and source strengths. Rainfall is a primary meteorological factor. The highest total ionic concentration was accompanied with the lowest rainfall in winter (69 mm), while the lowest one was accompanied with the highest rainfall in summer (961 mm). The remarkable decrease in the event-average ionic concentrations was found when the precipitation amount increased in many other studies (Huang et al. 2008a, b, 2009, 2010; Zhao et al. 2013). In addition, for the crustal species, the long residence time of floating particles in the air during the spring and winter could result in the accumulation of ions, giving rise to high ionic concentrations in rainwater (Chen and Mulder 2007), while wet soils meaning that little surface dust is incorporated into the air during summer and autumn (Huang et al. 2008b). In comparison with north China, higher concentrations of NH4 +, SO4 2−, and NO3 − in Ya’an were mainly caused by the meteorological conditions instead of a large quantity of coal burned for heating in the cold seasons (spring and winter). Furthermore, the high frequency of haze and smog and the formation of inversion layer in winter would inhibit the diffusion of airborne pollutants and lead to their accumulation, causing exceedingly high ionic concentrations in rainwater. The high concentration of NH4 + in spring could be explained by frequent agricultural activities in semi-rural area. The lowest concentrations of NH4 + and K+ did not occur in summer, which was mainly due to the decomposition of potash and nitrogen fertilizers at a high temperature (Coelho et al. 2011; Gioda et al. 2013). The highest contributions of Cl− and Na+ to the total quantified ions (5.39 and 4.18 %, respectively) occurred in summer, probably causing by long-range transport with the influence of subtropical monsoon climate.
Origins of major ionic species
Enrichment factors
Enrichment factors have been normally applied to identify the origin of an element in precipitation (Gioda et al. 2013; Han et al. 2010; Jawad Al Obaidy and Joshi 2006). EF calculation is based on an elemental ratio between ions found in the precipitation as compared to that for a reference material, and can provide useful clues about the sources of elements. Commonly, Na is usually taken as a good reference element for sea salt since it is assumed to be exclusively of marine origin (Huang et al. 2010; Zhang et al. 2007). Al and Ca are two typical lithospheric elements and are normally used as reference elements for continental crust since their compositions in soil, the only natural source for lithospheric elements, are hardly changed (Lu et al. 2011). In order to estimate the marine and terrestrial contributions to rainwater, the EF values for rainwater compositions were calculated by using Ca as a reference element for continental origin and Na as a reference element for marine origin as follows (Han et al. 2010):
where X is the concentration of the desired ion and [X/Na+] seawater is from the seawater composition and [X/Ca2+]soil is from crustal composition. Table 3 lists the EFseawater and EFsoil values for rainwater components in Ya’an. As NO3 − and NH4 + are not significant components neither in sea salt nor in crustal material (Zhang et al. 2007), their EFseawater and EF soil values were not calculated. An EF value much less than 1 or much higher than 1 is considered to dilute or enrich relative to the reference source (Fan 2002). EFsoil value of Na+ was 0.763, which indicates that Na+ was also derived from terrestrial source except from marine source as expected. EFsoil and EFseawater values of Cl− were 183.7 and 1.087, respectively, which suggest that the main sources of Cl− were from marine and anthropogenic activities instead of soil. EFsoil and EFseawater values of SO4 2− were 192.1 and 67.23, respectively, which also indicate that SO4 2− was mainly from anthropogenic sources instead of soil and marine sources. For Mg2+ and K+, EFseawater values were larger than 1, suggesting that marine source contributed a little fraction; while EFsoil values less than 1 demonstrate the contribution from terrestrial source.
Principle component analysis
PCA is used to identify the sources of ions in rainwater. In PCA, the data are subjected to a varimax rotation, which maximizes the variance to obtain a pattern of loadings on each factor that is diverse as possible, lending itself to easier interpretation. The division of information into different groups (common factor) suggests that the variables listed within the same factor might have the same origin (Wang et al. 2011). Table 4 shows the varimax-rotated principal component patterns, and it was found that three major factors with an eigenvalue of >1 accounted for 93.23 % of the total variance. The first factor (F1) accounted for 47.45 % of the total variance with high loadings of NH4 +, SO4 2−, NO3 −, K+, and Cl−, is identified as anthropogenic sources. The secondary origin species of SO4 2−, NO3 −, and NH4 + had strong inter-correlations as expected (Table S1), because they were all formed by conversion of gaseous precursors (Li et al. 2010). SO2, a known precursor of SO4 2−, is mainly from combustion of fuel, fertilizer application, and emissions thermal power plant and oil refinery (Laouali et al. 2012). NO3 − is directly associated with NOx emission, which comes mostly from fuel combustion, including all kinds of industrial furnaces and motor vehicles (Chen and Mulder 2007; Laouali et al. 2012). Cattle breeding and fertilizer application could emit a mass of NH3, which is converted to aerosol NH4 + or directly scavenged by rainwater. K+ is a typical lithospheric element and tracer of biomass burning. However, K+ in F1 could be better explained by biomass burning in this study. Smoke emitted from biomass burning is mainly in the form of submicrometer and accumulation mode, which could be entrained into clouds and increase the rates of chemical reactions responsible for secondary aerosol formed (Balasubramanian et al. 1999). Although the EFseawater of Cl− was only 1.078, the anthropogenic effect should be significant. The severe depletion of Cl− can be due to the HCl-released reaction between sea salt (NaCl) and H2SO4, especially in acidic particle (Yao and Zhang 2012). The potential anthropogenic sources of Cl− include vehicle exhaust, production and application of organochlorine compounds (e.g., dry clean agent), and biomass burning (Li et al. 2010). The high correlation between Cl− and K+ (R 2 = 0.80, Table S1) proves that biomass burning was the main source of Cl−, consistent with the previous result in Ya’an reported by Zhao et al. (2013). Considered that Ya’an is a semi-rural and less industrialized city, the high contribution of anthropogenic sources (F1) may be attributed to regional and/or long-range transport influences, which will be discussed later.
The second factor (F2) with high loadings of Ca2+, Mg2+, Na+, and Cl−, accounting for 34.01 % of the total variance, is identified as the combination of natural crustal and marine sources. Ca2+ and Mg2+ could be reasonably explained by the crustal source. High correlation between Na+ and Cl− (R 2 = 0.73, Table S1) indicates that Na+ is mainly from marine source in Ya’an. Na+ also have fair correlation with Ca2+ (R 2 = 0.63) and Mg2+ (R 2 = 0.65) (Table S1), showing that it was partly derived from rock dust. The similar results were also reported in previous studies (Chen and Mulder 2007; Niu et al. 2013, 2014). The third factors (F3) accounted for 11.78 % of the total variance. High loading of pH with extremely low loadings of other ions in F3 implies that the free acidity could be attributable to the comprehensive effect of all acidifying and neutralizing ions, rather than any single ion in precipitation (Zhang et al. 2007).
Back trajectory analysis
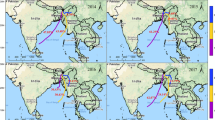
To evaluate the influence of air masses from different directions on the chemical composition of precipitation in Ya’an, air mass backward trajectories were computed using NOAA HYSPLIT 4 Model (Zhang et al. 2007). For each precipitation event, 72-h back trajectories were initiated at the recorded onset of precipitation from 1000, 1500, and 3000 m above ground level over the sampling site. The predominant direction was decided by the 1500 m level considering that cloud formation and wet deposition frequently occurred around this height (Shen et al. 2012). Based on the acquired trajectories, the samples were divided into five sectors, their typical trajectories are presented in Fig. S1. The air masses restricted within the Sichuan basin was firstly defined as regional transport (Fig. S1. a). Other sectors were classified according to the predominant direction outside of Sichuan basin. Sector S-SE means that predominant direction was south-southeast (Fig. S1. b), passing through Hunan, Guangxi, Guizhou, and east of Chongqing in China, which occurred mostly during summer and autumn. The air mass of sector west-northwest (W-NW, Fig. S1. c) was mainly the upper air wind and passed through Xingjiang and west of Gansu in China. The air mass of sector southwest (SW, Fig. S1. d) was mainly from India, Bangladesh, and the Kingdom of Bhutan and occurring prevailingly during winter and spring. Sector north-northeast (N-NE, Fig. S1. e) passed through Inner Mongolia, Xi’an, and the east of Gansu.
The VWM of pH and concentrations of major ions in their respective sectors are shown in Table 5. Obviously, the rainfall events in Ya’an, with a frequency of 37 %, were mainly affected by regional air mass, which was in agreement with the special topography of Sichuan basin. The lowest frequency (8.7 %) of rainfall events was from southwest. The contributions of other sectors were in the medium levels. The VWM total concentration of each sector was in an order of SW (1738 μeq/L) > N-NE (708 μeq/L) > regional (606 μeq/L) > W-NW (432 μeq/L) > S-SE (218 μeq/L). The highest concentration of total ions from SW could be explained by the lowest rainfall amount (30 mm) in winter and spring and the severe anthropogenic pollutions from India, Bangladesh, and the Kingdom of Bhutan. Few researches demonstrated that the air quality in India was extremely poor and the concentrations of PM10 and PM2.5 were extremely high, almost 5–10 times higher than the levels in European cities (Chhabra et al. 2001; Sharma and Maloo 2005). The highest concentrations of NH4 +, SO4 2−, NO3 −, K+, and Cl− (high loading species in F1) and the lowest VWM of pH (4.27) were in the sector of SW, further confirmed that the anthropogenic sources from southwest long-range transport did have significant effects on the chemical composition and acidity of rainwater in Ya’an. Compared with the regional transport, relative higher concentrations of Na+, Mg2+, and Ca2+ (high loading species in F2) were in sectors of N-NE and SW, indicating that natural sources of rainwater were significantly affected by long-range transport, especially from north-northeast and southwest. The highest concentrations of Na+ and Ca2+ occurred in N-NE sector, which were related to sea salt and dust storm, respectively. The dust storm, originated from the western part of Mongolia, moved along the northern boundary of Inner Mongolia, and passed through Gansu Province, was identified as a serious sandstorm affecting large areas of northern China (Zhang et al. 2007). The highest concentration of Ca2+ in N-NE sector indicates that the influence of north sandstorm had extended to Sichuan basin. The VWM of pH of rainwater affected by N-NE air mass was close to that of regional but higher than that of SW sector. In comparison, higher pH in N-NE than SW was mainly caused by the decrease of SO4 2−. The rainwater affected by W-NW had the highest pH (4.60), which could be explained by its relatively good air quality and dominated natural sources.
Conclusion
In this study, the rainwater chemistry was studied at the semi-rural city of Ya’an in Sichuan basin. The severe acidification was mainly due to the lack of Ca2+, significant anthropogenic influences, and rainy pattern on this area. The highest contribution of NH4 + measured in the busy farming season suggests the characteristic of the semi-rural site with considerably frequent agricultural activities. In comparison with most cities in China, a relatively low contribution of Ca2+ was observed in Ya’an. The abundant anions of SO4 2− and NO3 − and the low SO4 2−/NO3 − ratio can be explained by the complex pollution sources from high coal/fuel consumption in urbanization and the rapid increasing number of motor vehicles. The total ionic concentrations had a characteristic of lower in autumn and summer but higher in winter and spring, due to both of meteorological factors and source strengths. Combined with EF, correlation analysis, and PCA, NH4 +, SO4 2−, NO3 −, K+, and Cl− were well related with anthropogenic sources. Other ions such as Ca2+, Mg2+, Na+, and Cl− were associated with natural crustal and marine sources. The free acidity could be attributable to the comprehensive effects of all acidifying and neutralizing ions, rather than any single ion in precipitation. According to back trajectory analysis, the rainfall events in Ya’an were mainly affected by regional air mass. Both heavy anthropogenic and natural crustal sources from SW long-range transport had significant effects on the chemical composition and acidity of rainwater in Ya’an. Natural crustal and marine sources were mainly affected by long-range transport from N-NE. Air masses from W-NW dominated by natural crustal sources had relative high neutralization ability. Considering that the special topography, the controls of anthropogenic emissions from regional transport (especially from megacities, e.g., Chengdu and Chongqing) and long-range transport (especially from southwest) should be taken in order to reduce the acidity in the precipitation of rural and semi-rural areas in Sichuan basin.
References
Aas W, Shao M, Jin L, Larssen T, Zhao D, Xiang R, Zhang J, Xiao J, Duan L (2007) Air concentrations and wet deposition of major inorganic ions at five non-urban sites in China, 2001–2003. Atmos Environ 41:1706–1716
Al-Khashman OA (2009) Chemical characteristics of rainwater collected at a western site of Jordan. Atmos Res 91:53–61
Baez A, Belmont R, García R, Padilla H, Torres MC (2007) Chemical composition of rainwater collected at a southwest site of Mexico City, Mexico. Atmos Res 86:61–75
Balasubramanian R, Victor T, Begum R (1999) Impact of biomass burning on rainwater acidity and composition in Singapore. J Geophys Res 104(26):881–890
Budhavant KB, Rao PSP, Safai PD, Ali K (2011) Influence of local sources on rainwater chemistry over Pune region, India. Atmos Res 100:121–131
Celle-Jeanton H, Travi Y, Loÿe-Pilot MD, Huneau F, Bertrand G (2009) Rainwater chemistry at a Mediterranean inland station (Avignon, France): local contribution versus long-range supply. Atmos Res 91:118–126
Charlson RJ, Rodhe H (1982) Factors controlling the acidity of natural rainwater. Nature 295:683–685
Chen XY, Mulder J (2007) Atmospheric deposition of nitrogen at five subtropical forested sites in South China. Sci Total Environ 378:317–330
Chhabra SK, Chhabra P, Rajpal S, Gupta RK (2001) Ambient air pollution and chronic respiratory morbidity in Delhi. Arch Environ Health Int J 56(1):58–64
Coelho CH, Allen AG, Fornaro A, Orlando EA, Grigoletto TL, Campos MLA (2011) Wet deposition of major ions in a rural area impacted by biomass burning emissions. Atmos Environ 45:5260–5265
Fan H (2002) On worldwide acid rain research. J Fujian Coll For 22:71–375 (in Chinese)
Feng ZW, Huang YZ, Feng YW, Ogura N, Zhang FZ (2001) Chemical composition of precipitation in Beijing area, northern China. Water Air Soil Pollut 125:345–356
Gioda A, Mayol-Bracero OL, Scatena FN, Weathers KC, Mateus VL, McDowell WH (2013) Chemical constituents in clouds and rainwater in the Puerto Rican rainforest: potential sources and seasonal drivers. Atmos Environ 68:208–220
Goyer RA, Bachmann J, Clarkson TW, Ferris BG Jr, Graham J, Mushak P, Perl DP, Rall DP, Schlesinger R, Sharpe W, Wood JM (1985) Potential human health effects of acid rain: report of a workshop. Environ Health Perspect 60:355–368
Han G, Tang Y, Wu Q, Tan Q (2010) Chemical and strontium isotope characterization of rainwater in karst virgin forest, Southwest China. Atmos Environ 44:174–181
Hu GP, Balasubramanian R, Wu CD (2003) Chemical characterization of rainwater at Singapore. Chemosphere 51:747–755
Huang Y, Wang YL, Zhang LP (2008a) Long-term trend of chemical composition of wet atmospheric precipitation during 1986–2006 at Shenzhen City, China. Atmos Environ 42:3740–3750
Huang K, Zhuang G, Xu C, Wang Y, Tang A (2008b) The chemistry of the severe acidic precipitation in Shanghai, China. Atmos Res 89:149–160
Huang DY, Xu YG, Peng PA, Zhang HH, Lan JB (2009) Chemical composition and seasonal variation of acid deposition in Guangzhou, South China: comparison with precipitation in other major Chinese cities. Environ Pollut 157:35–41
Huang XF, Li X, He LY, Feng N, Hu M, Niu YW, Zeng LW (2010) 5-Year study of rainwater chemistry in a coastal mega-city in South China. Atmos Res 97:185–193
Huang J, Sun S, Xue Y, Li J, Zhang J (2014) Spatial and temporal variability of precipitation and dryness/wetness during 1961–2008 in Sichuan province, west China. Water Resour Manag 28:1655–1670
Huo M, Sun Q, Bai Y, Li J, Xie P, Liu Z, Wang X (2012) Influence of airborne particles on the acidity of rainwater during wash-out process. Atmos Environ 59:192–201
Jawad Al Obaidy AHM, Joshi H (2006) Chemical composition of rainwater in a tropical urban area of northern India. Atmos Environ 40:6886–6891
Laouali D, Galy-Lacaux C, Diop B, Delon C, Orange D, Lacaux JP, Akpo A, Lavenu F, Gardrat E, Castera P (2012) Long term monitoring of the chemical composition of precipitation and wet deposition fluxes over three Sahelian savannas. Atmos Environ 50:314–327
Larssen T, Seip HM, Semb A, Mulder J, Muniz IP, Vogt RD, Lydersen E, Angell V, Tang D, Eilertsen O (1999) Acid deposition and its effects in China: an overview. Environ Sci Pol 2:9–24
Lee BK, Hong SH, Lee DS (2000) Chemical composition of precipitation and wet deposition of major ions on the Korean peninsula. Atmos Environ 34:563–575
Lei HC, Tanner PA, Huang MY, Shen ZL, Wu YX (1997) The acidification process under the cloud in southwest China: observation results and simulation. Atmos Environ 31:851–861
Li Y, Yu X, Cheng H, Lin W, Tang J, Wang S (2010) Chemical characteristics of precipitation at three Chinese regional background stations from 2006 to 2007. Atmos Res 96:173–183
Liu X, Zhang Y, Han W, Tang A, Shen J, Cui Z, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang F (2013) Enhanced nitrogen deposition over China. Nature 494:459–462
Lu Z, Streets DG, Zhang Q, Wang S, Carmichael GR, Cheng YF, Wei C, Chin M, Diehl T, Tan Q (2010) Sulfur dioxide emissions in China and sulfur trends in East Asia since 2000. Atmos Chem Phys 10:6311–6331
Lu X, Li LY, Li N, Yang G, Luo D, Chen J (2011) Chemical characteristics of spring rainwater of Xi’an city, NW China. Atmos Environ 45:5058–5063
Lu QM, Zhao L, Li L, Yang FM, Yang QL, Wei SQ, He KB, Cheng GC (2013) Chemical composition of precipitation and its spatiotemporal variations in the Three Gorges Reservoir Region. Acta Sci Circum 33(6):1682–1689 (in Chinese)
Lynch JA, Bowersox VC, Grimm JW (2000) Changes in sulfate deposition in eastern USA following implementation of Phase I of Title IV of the Clean Air Act Amendments of 1990. Atmos Environ 34:1665–1680
Moreda-Piñeiro J, Alonso-Rodríguez E, Moscoso-Pérez C, Blanco-Heras G, Turnes-Carou I, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D (2014) Influence of marine, terrestrial and anthropogenic sources on ionic and metallic composition of rainwater at a suburban site (northwest coast of Spain). Atmos Environ 88:30–38
Mouli PC, Mohan SV, Reddy SJ (2005) Rainwater chemistry at a regional representative urban site: influence of terrestrial sources on ionic composition. Atmos Environ 39:999–1008
Niu H, He Y, Zhu G, Xin H, Du J, Pu T, Lu X, Zhao G (2013) Environmental implications of the snow chemistry from Mt. Yulong, southeastern Tibetan Plateau. Quatern Int 313:168–178
Niu H, He Y, Lu XX, Shen J, Du J, Zhang T, Tao P, Xin H, Chang L (2014) Chemical composition of rainwater in the Yulong Snow Mountain region, Southwestern China. Atmos Res 144:195–206
Okuda T, Iwase T, Ueda H, Suda Y, Tanaka S, Dokiya Y, Fushimi K, Hosoe M (2005) Long-term trend of chemical constituents in precipitation in Tokyo metropolitan area, Japan, from 1990 to 2002. Sci Total Environ 339:127–141
Qiao X, Xiao W, Jaffe D, Kota SH, Ying Q, Tang Y (2015) Atmospheric wet deposition of sulfur and nitrogen in Jiuzhaigou National Nature Reserve, Sichuan Province, China. Sci Total Environ 511:28–36
Sharma M, Maloo S (2005) Assessment of ambient air PM10 and PM2.5 and characterization of PM10 in the city of Kanpur, India. Atmos Environ 39:6015–6026
Shen Z, Zhang L, Cao J, Tian J, Liu L, Wang G, Zhao Z, Wang X, Zhang R, Liu S (2012) Chemical composition, sources, and deposition fluxes of water-soluble inorganic ions obtained from precipitation chemistry measurements collected at an urban site in northwest China. J Environ Monitor 14:3000–3008
Song F, Gao Y (2009) Chemical characteristics of precipitation at metropolitan Newark in the US East Coast. Atmos Environ 43:4903–4913
Sopauskiene D, Jasineviciene D (2006) Changes in precipitation chemistry in Lithuania for 1981–2004. J Environ Monitor 8:347–352
State Environmental Protection Administration of China (2004) Technical specifications for acid deposition monitoring. Beijing (In Chinese)
Streets DG, Waldhoff ST (2000) Present and future emissions of air pollutants in China: SO2, NOx, and CO. Atmos Environ 34:363–374
Tang A, Zhuang G, Wang Y, Yuan H, Sun Y (2005) The chemistry of precipitation and its relation to aerosol in Beijing. Atmos Environ 39:3397–3406
Tørseth K, Aas W, Breivik K, Fjæraa AM, Fiebig M, Hjellbrekke AG, Myhre L, Solberg S, Yttri KE (2012) Introduction to the European Monitoring and Evaluation Programme (EMEP) and observed atmospheric composition change during 1972–2009. Atmos Chem Phys 12:5447–5481
Tu J, Wang H, Zhang Z, Jin X, Li W (2005) Trends in chemical composition of precipitation in Nanjing, China, during 1992–2003. Atmos Res 73:283–298
Vet R, Artz RS, Carou S, Shaw M, Ro CU, Aas W, Baker A, Bowersox VC, Dentener F, Galy-Lacaux C, Hou A, Pienaar JJ, Gillett R, Forti CM, Gromov S, Hara H, Khodzherm T, Mahowald NM, Nickovic S, Rao PSP, Reid NW (2014) A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos Environ 93:3–100
Wang H, Han G (2011) Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos Res 99:190–196
Wang G, Li J, Cheng C, Hu S, Xie M, Gao S, Zhou B, Dai W, Cao J, An Z (2011) Observation of atmospheric aerosols at Mt. Hua and Mt. Tai in central and east China during spring 2009–Part 1: EC, OC and inorganic ions. Atmos Chem Phys 11:4221–4235
Wu Q, Han G, Tao F, Tang Y (2012) Chemical composition of rainwater in a karstic agricultural area, Southwest China: the impact of urbanization. Atmos Res 111:71–78
Xu Z, Han G (2009) Chemical and strontium isotope characterization of rainwater in Beijing, China. Atmos Environ 43:1954–1961
Xu Z, Tang Y, Ji J (2012) Chemical and strontium isotope characterization of rainwater in Beijing during the 2008 Olympic year. Atmos Environ 107:115–125
Xu Z, Wu Y, Liu WJ, Liang CS, Ji J, Zhao T, Zhang X (2015) Chemical composition of rainwater and the acid neutralizing effect at Beijing and Chizhou city, China. Atmos Res 164–165:278–285
Yang FM, He KB, Lei Y, Ma YL, Yu XC, Tanaka S, Okuda T, Iwase T (2004) Chemical characters of atmospheric precipitation in Beijing in years of 2001–2003. China Environ Sci 24(5):538–541 (in Chinese)
Yang R, Hayashi K, Zhu B, Li F, Yan X (2010) Atmospheric NH3 and NO2 concentration and nitrogen deposition in an agricultural catchment of Eastern China. Sci Total Environ 408:4624–4632
Yao X, Zhang L (2012) Chemical processes in sea-salt chloride depletion observed at a Canadian rural coastal site. Atmos Environ 46:189–194
Zhang M, Wang S, Wu F, Yuan X, Zhang Y (2007) Chemical compositions of wet precipitation and anthropogenic influences at a developing urban site in southeastern China. Atmos Res 84:311–322
Zhang X, Jiang H, Zhang Q, Zhang X (2012) Chemical characteristics of rainwater in northeast China, a case study of Dalian. Atmos Res 116:151–160
Zhao D, Xiong J, Xu Y, Chan W (1988) Acid rain in southwestern China. Atmos Environ 22:349–358
Zhao M, Li L, Liu Z, Chen B, Huang J, Cai J, Deng S (2013) Chemical composition and sources of rainwater collected at a semi-rural site in Ya’an, Southwestern China. Atmos Clim Sci 3:486–496
Zhou ZY, Chen DR, Ying J, Zhang WL (2003) The chemical composition of precipitation analysis in Chongqing city. Chongqing Environ Sci 25(11):112–114 (in Chinese)
Acknowledgments
This study was supported by the National Natural Science Foundation (41205095) and (21305097) of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Yun-Chun Li and Meng Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 579 kb)
Rights and permissions
About this article
Cite this article
Li, YC., Zhang, M., Shu, M. et al. Chemical characteristics of rainwater in Sichuan basin, a case study of Ya’an. Environ Sci Pollut Res 23, 13088–13099 (2016). https://doi.org/10.1007/s11356-016-6363-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6363-4