Abstract
Water and sediment samples were collected from up to 17 sites in waterways entering the Corner Inlet Marine National Park monthly between November 2009 and April 2010, with the Chemcatcher passive sampler system deployed at these sites in November 2009 and March 2010. Trace metal concentrations were low, with none occurring at concentrations with the potential for adverse ecological effects. The agrochemical residues data showed the presence of a small number of pesticides at very low concentration (ng/L) in the surface waters of streams entering the Corner Inlet, and as widespread, but still limited contamination of sediments. Concentrations of pesticides detected were relatively low and several orders of magnitude below reported ecotoxicological effect and hazardous concentration values. The low levels of pesticides detected in this study indicate that agricultural industries were responsible agrochemical users. This research project is a rarity in aligning both agrochemical usage data obtained from chemical resellers in the target catchment with residue analysis of environmental samples. Based on frequency of detection and concentrations, prometryn is the priority chemical of concern for both the water and sediments studied, but this chemical was not listed in reseller data. Consequently, the risks may be greater than the field data would suggest, and priorities for monitoring different since some commonly used herbicides (such as glyphosate, phenoxy acid herbicides, and sulfonyl urea herbicides) were not screened. Therefore, researchers, academia, industry, and government need to identify ways to achieve a more coordinated land use approach for obtaining information on the use of chemicals in a catchment, their presence in waterways, and the longer term performance of chemicals, particularly where they are used multiple times in a year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are widely used for the suppression of unwanted plants (weeds) and removal of nuisance insects and fungi in domestic, municipal, and business environments as well agricultural production systems. In monetary and volume terms, herbicides are the top ranked category of pesticides sold in Australia (based on final aggregated 2012–2013 data: 2866 products, A$1262 million), more than three times that of insecticides (1308 products; A$351 million) and seven times the sales of fungicides (799 products; A$170 million; APVMA 2014a). This data includes pesticides registered and sold for domestic and commercial use as well as agricultural uses. There are over 300 pesticides (active ingredients) registered for use in Victoria (not including repeat registrations and registrations of manufacturing concentrates of an otherwise registered chemical; APVMA 2014b), of which some 115 are herbicides, 64 insecticides, 63 fungicides, and about 60 “other” chemicals (including acaricides, molluscicides, nematicides, rodenticides, and plant growth regulators). However, the actual number and amount of chemicals used in the state in any year is unknown because, while farmers and other commercial pesticide users must keep written records of pesticide use, there is currently no requirement for any user, whether farmer, licensed chemical user, business, or householder, to report pesticide use to either local or centralised authority.
Grab (or spot) samples are commonly used to characterise pesticide residues in surface waters, although integrative sampling with passive samplers (or passive sampling) is becoming a more commonly used alternative. A “passive sampler” can be defined as a device that is able to acquire a sample from discrete location without the active media transport induced by pumping or purge techniques (ITRC 2006). Hence, most passive samplers consist of a receiving phase with high affinity for organic contaminants, separated from the aquatic environment by a diffusion-limiting membrane. Some of the most commonly used devices that rely on diffusion and sorption to accumulate analytes in the sampler are semi-permeable membrane devices (SPMDs) and passive in situ samplers (such as the Chemcatcher™ system (CC)). Passive samplers had been little utilised on natural water samples in Australia at the inception of this study. In Australia, a number of relatively recent studies have used passive samplers to detect residues of atrazine, diuron, hexazinone, and simazine in rivers and estuaries on the eastern seaboard of Australia (Escher et al. 2006; Lewis et al. 2009; Muller et al. 2008; O’Brien et al. 2011; Shaw et al. 2009, 2010; Stephens et al. 2009). In Victoria, the most detailed study involving passive sampling to date was conducted in the Yarra Valley in 2008–2009 using SPMD and CC passive samplers to screen for a wide range of fungicides and herbicides (e.g., see Schäfer et al. 2011; Wightwick et al. 2012; Allinson et al. 2014). However, prior to this study, there had been no significant studies of pesticides in surface waters in regional and rural Victoria in this century and none investigating contamination of waters entering the Corner Inlet Marine National Park.
Today, more than ever, consumers demand “clean” wholesome food, and their concerns about chemical residues must be addressed to protect Australia’s valuable domestic and international markets, while ensuring the protection of the natural environment. To mitigate the risk of unacceptable pesticide side effects and costs to the economy through increased human health problems, trade disruption, reduced farm productivity, and environmental degradation, at the time of this study (2009–2010), the Victorian Government had placed the responsibility of establishing standards for chemical use in Victoria with its then Chemical Standards Branch (CSB). In part, CSB achieved this through the provisions of the Agricultural and Veterinary Chemicals (Control of Use) Act 1992 (AgVet 1992) and through partnerships with industry programs designed to protect and enhance the reputation of Victoria’s agricultural industries. In 2009, CSB funded this pilot project to assess approaches for measuring the number and levels of a subset of the AgVet (1992)-specified pesticides in Victorian surface waters. Corner Inlet (Fig. 1) was chosen for the pilot study because it is an important livestock and dairy farming region and its catchments drain into the Corner Inlet Marine National Park, which supports healthy populations of coastal marine animals and plants that are rare or absent elsewhere in Victoria, including the only extensive beds of Poisidonia seagrass in Victoria (DNRE 2002).
In recognition of the potential risks that chemicals pose to aquatic ecosystems and the lack of robust information on the levels of such compounds in Victoria, water and sediment samples were collected from up to 17 sites in the drainages entering Corner Inlet between November 2009 and April 2010, with the CC system deployed in November 2009 and March 2010. Samples were prepared for a number of chemical tests, including measurement of more than 50 pesticides using liquid chromatography-tandem mass spectrometry (LC-MS/MS) techniques, with, uniquely for Australia, the residue data obtained compared with usage information obtained from a concurrent survey of local agrochemical suppliers. The benefits to the government for implementing such a project were that it would assist in determining risk mitigation strategies (industry action plans developed by CSB to effect change where it has been determined that the use of chemicals has had an impact from use practices) and, furthermore, in Victoria to assist in determining the risks posed by the presence of specified chemicals (which, in turn, would allow CSB to provide information and advice to the Commonwealth and other agencies of the hazards and mitigation strategies). Finally, the project would provide sound evidence when informing the community of the sustainable practices or otherwise of primary producers, which will ensure the long-term protection of the natural resource base.
Materials and methods
Site selection and sampling
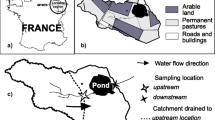
The Corner Inlet catchment is located ∼200 km southeast of Melbourne to the north and east of Wilsons Promontory and comprises the catchments feeding into Corner Inlet and Nooramunga Marine and Wildlife Reserve (Fig. 1). Corner Inlet is the most easterly, and consequently the warmest, of Victoria’s large bays, with a complex network of mangroves, salt marsh, mud banks, seagrass beds, and rocky islands amid deeper channels; much of this embayment is a designated Ramsar wetland. The Corner Inlet Ramsar Site Strategic Management Plan (DNRE 2002) noted that the condition of water quality flowing into Corner Inlet is dependent on surrounding land use practices and identified pollution from agriculture as a priority risk that may reduce the Ramsar site’s amenity and biodiversity value.
Seventeen sites on 11 streams in the catchment were investigated in this study (Fig. 1): Two reference sites in the Wilsons Promontory National Park (sites #1 and 2, Barry Ck and Darby River) were sampled on the assumption that there would be minimal pesticide usage within this almost undeveloped wilderness area; seven sites were selected in the Franklin River catchment, of which four were on the Franklin River itself (sites #3, 4, 6, and 8), one site on the Little Franklin River (site #7), and two sites on Deep Creek (sites #5 and 12); four sites were selected in the Agnes River watershed, of which three were on the Agnes River itself (sites #9, 10, and 11) with a single site on Dingo Creek, an upstream tributary (site #17). Four sites were selected around Foster, two sites on Stockyard Creek upstream and downstream of the township (sites #13 and 14, respectively), and one site each on Bennison and Old Hat Creeks (sites #15 and 16, respectively). The sites were selected based on their relative positions within the Corner Inlet catchment or reference locations, e.g., head of catchment, mid catchment, and lower catchment, in known agricultural areas, or in forestry or national parks and were considered to be a broad representation of the wide range of waterways found in the catchment, across the major soil types and land use in the region.
Spot (or grab) water and sediment samples were collected from up to 13 from the original 17 sites on five occasions in 2009–2010, namely 18–26 November 2009, 15–16 December 2009, 2–3 March 2010, 29–30 March 2010, and 27–28 April 2010. These sampling events are referred to as the NOV, DEC, e-MAR (early March), l-MAR (late March), and APR sampling rounds, respectively. Not all sites were sampled on all occasions for a mixture of practical and pragmatic reasons (see Table SI 1), e.g., sites #7 and 8 in the upper Franklin River and Little Franklin River catchments, respectively, were deemed too dangerous to sample due to major forestry (logging) operations in the area, and despite stakeholder suggestions to the contrary, sites #3 and 16 turned out to be estuarine, and so, these sites were dropped from this freshwater program. For full details of spot water and sediment sampling methods, readers are directed to the Supplementary Information.
Passive sampling
The CC fitted with an Empore™ SDB-XC disk (47 mm; 3 M, MN, USA) as the receiving phase and a polyethersulfone (PES) membrane (Sterlitech Corp, WA, USA) as the diffusion-limiting membrane was deployed to target polar chemicals with log Kow < ∼3.0. Duplicate CCs were deployed for 28 days at 11 sites in November 2009 and 10 sites in March 2010 for time-integrated monitoring to allow firstly a qualitative assessment (i.e., presence/absence) and then, where possible, a semi-quantitative assessment (i.e., based on estimated time-weighted average water concentrations) of herbicides in the catchment. For full details of the passive sampling methods, readers are directed to the Supplementary Information.
Analytical testing methods: pesticides
Measurement of pesticides in aquatic ecosystems can be expensive in Australia, which inhibits monitoring by water authorities (as providers of water), catchment management authorities (as custodians of the natural environment), and consumers (e.g., irrigators, industry, and household and domestic water users), potentially resulting in increased risk to the natural environment. Strategies and/or tools are therefore required to focus monitoring and risk assessment programs in a cost-effective manner. The provisions of the AgVet 1992 act place restrictions on a number of high-risk chemicals (“restricted use” chemicals) in terms of who can use them and how they can be used. The restrictions were placed on those chemicals because they are considered to potentially have higher risk of adversely affecting the users’ health, the environment, and/or trade. Five herbicides have been listed in s25A of the Control of Use Act 1992 (atrazine, 2,4-D ester, 2-methyl-4-chlorophenoxyacetic acid (MCPA) ester, paraquat, and methyl bromide), and in this context, the initial approach was to prioritise testing by linking the s25A herbicides with known CC passive sampling approaches within a study region. The chemical properties of the restricted pesticides listed in s25A of AgVet 1992 meant that detection work with CC passive samplers and the broad spectrum residue screens then available to the project was limited to atrazine. However, the analytical methods were also appropriate for measuring the levels of a number of Schedule 7 Poisons (Dangerous Poisons): azinphos-methyl, dichlorvos, fenamiphos, methiocarb, and methomyl.
The final extracts from the SPE cleanups of water and sediment samples, and CC disk eluates were screened using two different analysis programs: triazines with LC-MS/MS and a range of other polar pesticides using a multi-residue LC-MS/MS screen (see Supplementary Information for the chemicals determined with each program and details of extraction methods and instrument parameters). The results were not corrected for recovery, which was determined by spiking randomly selected samples of each analytical batch of water and sediment samples (typically the 13 sites) with each reported pesticide.
Analytical testing methods: trace metals
In this survey, sediments were analysed for nine metals (cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), molybdenum (Mo), nickel (Ni), and zinc (Zn), hereafter referred to simply as trace metals, by a commercial analytical laboratory). Trace metals were determined for the sediment phase only since we were primarily interested in the toxicity of sediments as the major vector for metal transport, and metal concentrations in sediment are typically orders of magnitude higher and much less variable than in water (Horowitz 1991). Total metals were determined by inductively coupled plasma atomic emission spectroscopy following appropriate acid digestion of the sediment. Quantification was by comparing absorbance against a calibration curve.
Results and discussion
Field water quality
Field testing of water temperature, pH, and electrical conductivity was undertaken at time of sampling. For the most part, water temperatures were higher in the late spring (NOV, DEC) than in autumn (e-MAR and l-MAR; Table SI 2), which perhaps reflects the cooler and wetter than average summer conditions experienced by Victoria in 2009–2010. In general, almost all of the surveyed sites on the northern coast of Corner Inlet were generally circumneutral in pH across the four sampling events (average pH 7.2, range 6.5–7.9), with the exception of site #15 (∼pH 6.7), although the streams at some sites became more acidic during the summer (e.g., sites #10 and 11). The pH of water samples collected from agricultural areas were within the lower and upper pH limits set for lowland rivers in eastern Victoria (pH 6.5–8.0; ANZECC and ARMCANZ 2000), in contrast to the pH at the two reference sites on Wilson’s Promontory (sites #1 and 2) which were consistently acidic (average pH 6.1, range 5.4–6.9; Table SI 2). There was no difference in the salinity (as measured by electrical conductivity (EC)) of the reference site waters (average EC of reference sites 460 μS/cm, range 400–540 μS/cm) compared to the other sites (average EC 310 μS/cm, range 110–730 μS/cm; Table SI 2). The observed EC is well within the range of default trigger values for conductivity set by ANZECC and ARMCANZ (2000). There was also no difference in total nitrogen (TN) concentrations of the reference site waters (average TN of reference sites 0.7 mg/L, range 0.5–0.9 mg/L) compared to the other sites (average TN 0.8 mg/L, range 0.4–2.5 mg/L); however, in this case, all sites exceeded the default trigger values of 0.35 mg/L TN set by ANZECC and ARMCANZ (2000) for rivers in eastern Victoria.
Pesticide residues in water
Eight different pesticide residues were observed in one or more water samples from the Corner Inlet sites (Table 1); chemicals observed at more than 20 % of sites were prometryn (33 %) and simazine (23 %). Of the selected Schedule 7 Poisons targeted, only dichlorvos was observed, on two occasions at concentrations at limits of reporting (LOR; 0.01 μg/L). Sediment samples were contaminated with eight different pesticides; no chemicals were observed at more than 20 % of sites, with the most commonly reported chemical being prometryn (18 % samples; Table 2). Most of the other chemicals observed in the sediments were fungicides, and of which, metalaxyl was the only chemical also observed in the water samples. Only two sites (sites #9 and 13) had two pesticide residues in a water sample (Table SI 5), and only three sites (sites #4, 11, and 12) had two or more residues in a sediment sample (Table SI 6). That some pesticide residues were observed more often in water samples than in sediment samples is likely a reflection of the higher analytical LORs for agrochemicals in sediments rather than lack of contamination (in this study, pesticide LORs were ≥1 μg/kg for sediments compared to ≥1 ng/L for the same chemicals in water). The highest number of combined (sediment + water) detections of pesticide residues (4) was observed at site #4 in e-MAR.
Prometryn was reported in almost one third of the study’s water samples, albeit at very low concentrations (average concentration 0.002 μg/L; max 0.01 μg/L; Table 1). Simazine is the current use triazine herbicide most often reported in environmental water samples collected in Victoria (e.g., see Schäfer et al. 2011; Allinson et al. 2014), and this chemical was observed in 23 % of samples, albeit usually at low concentrations (average concentration, 0.004 μg/L; max 0.16 μg/L; Table 1). Nationally and internationally, the most commonly reported herbicides in water samples are triazine/triazinone chemicals, and in this context, our prometryn data are much lower than that observed in rivers elsewhere in Australia (e.g., up to 4 μg/L; Muschal and Warne 2003), whereas similar levels of simazine have been observed in Queensland (e.g., up to 0.050 μg/L, Duke et al. 2005; 0.043 μg/L, Bengtson Nash et al. 2006). The simazine concentrations were, however, two orders of magnitude lower than those observed in Victoria’s Yarra Valley by Allinson et al. (2014). There are many fewer international reports of prometryn detections than simazine residues, and in that context, the prometryn data is lower than reports from North America (e.g., <0.05–3.73 μg/L, Coupe et al. 2005; up to 1.8 μg/L, Schuler and Rand 2008) but similar to those from Europe (e.g., <0.030–0.43 μg/L, Claver et al. 2006; <0.01–0.050 μg/L, Vryzas et al. 2009; 0.031–0.478 μg/L, Sraka et al. 2007); simazine concentrations have been reported in the range 0.045–0.047 μg/L in Brazil (Dores et al. 2008), 0.001–0.896 μg/L in Canada (Woudneh et al. 2009), 0.052–0.580 μg/L in Croatia (Sraka et al. 2007), and <0.001–0.60 μg/L in Greece (Vryzas et al. 2009). Interestingly, simazine has recently been observed in French storm water samples despite the ban on its use in the EU (e.g., <0.01–0.15 μg/L simazine, Zgheib et al. 2012). The presence of these relatively polar herbicides in the autumnal samples perhaps reflects the resurgence in agricultural applications associated with increased soil water concentrations and plant growth with the change to winter rainfall patterns beginning in the Victorian Autumn (March to May). We did not target specific rain events or spray applications, so it is not possible to elucidate whether or not the prometryn and simazine were entering the waterways diffusively or via episodic pulse events or to identify point sources of contamination but that these chemicals were detected in such a high proportion of the collected samples is a clear indication that these chemicals are migrating off-site after application in the studied catchments. This is not to say that farmers and commercial operators are using these chemicals inappropriately, rather the results from this study suggest that the regular application of prometryn and simazine can result in loading of these compounds in catchments and make herbicides a significant non-agricultural chemical input to Corner Inlet.
Pesticide residues in sediments
There are fewer reports of the levels of herbicides in sediment compared to many other chemicals, despite the potential effects they may have on benthic primary producers. Noppe et al. (2007) were the first to report on the occurrence of triazine herbicides in suspended solids and sediment, perhaps because, as Noppe et al. contend, up to that point that it had generally been assumed that these chemicals are mainly in the dissolved phases based on their physico-chemical characteristics. For instance, as a group, the triazines are relatively polar, with the logarithm of their octanol-water partition coefficients (Kow) typically <2.5 and with good water solubility (up to 30 mg/L). In that context, prometryn was observed in 18 % of sediments (Table 2); unfortunately, there are no other studies with which to compare our data. The absence of simazine in sediments is perhaps surprising given that Allinson et al. (2014) reported considerable levels of simazine in other Victorian sediments but may simply be a reflection of very low water concentrations (mean simazine concentration 0.03 μg/L; Table 1) resulting in concentrations of this herbicide that did not accumulate above quantifiable limits in sediments.
Only five chemicals were observed on one or more CC receiving disk, namely atrazine, carbaryl, hexazinone, metribuzin, and simazine (Table SI 7). Sampling rates (R s) for carbaryl are not available for the SDB-XC disk use in this study, but R s for atrazine, hexazinone, metribuzin, and simazine in the CCs were determined experimentally in a flow through system by Allinson et al. (2014). The R s were atrazine 12.0, metribuzin 6.8, prometryn 10.6, and simazine 8.3 mL/day. These rates are similar to those reported by Tran et al. (2007) in a laboratory continuous flow system (21–26 mL/day). The sampling rates of chemicals into passive samplers are dependent on a range of factors, both intrinsic to the passive samplers themselves, and extrinsic factors (Leonard et al. 2002). Intrinsic factors that may affect uptake include the polarity of the contaminant (as measured by its log10Kow), the diffusivity of the molecules that have to pass through the aqueous boundary layer, sampler design, exposure time, and concentrations of chemicals in the surrounding water. In the laboratory, the exposure media is typically controlled (temperature, agitation, contaminant concentrations, and physico-chemical parameters). All of these factors may change in the field, which perhaps explains why the time weighted average water concentrations (TWAWCs) generated for simazine using experimentally determined sampling rates of Allinson et al. (2014) are about two times higher than concentrations observed in water samples, with hexazinone TWAWCs derived in the same manner an order of magnitude higher than measured hexazinone concentrations.
Trace metal concentrations in sediments
For the most part, trace metal concentrations in the sediment samples were low (Table 3). For instance, of the eight trace metals screened, one (Cd) was below reporting limits in all of the samples. All other trace metals were observed at concentrations above LOR at all sites. Zinc concentrations were noticeably higher than the other toxicologically important essential elements (e.g., Zn average 52 mg/kg; max 83 mg/kg cf. Cu average 10 mg/kg; max 16 mg/kg; Table 3). Both Zn and Cu concentrations were lower than those reported in Melbourne’s Dandenong Creek for the same sediment size fraction (e.g., Zn average 372 mg/kg; max 890 mg/kg; Cu average 29 mg/kg; max 59 mg/kg (Marshall et al. 2010).
Chemical risk assessment: pesticides
The ecological risks of chemicals detected in the environment can be assessed by comparisons with environmental quality values and reported ecotoxicological effect values for key sentinel aquatic species. In Australia, these are collated in the ANZECC and ARMCANZ (2000) water quality guidelines at four species protection levels (99, 95, 90, and 80 %, respectively). The default value used in Australia is the 95 % species protection value, which is designed to allow for 95 % species protection in “slightly moderately disturbed ecosystems.” In that context, none of the pesticide residues observed in this study exceeded their respective 95 % species protection guideline values. For instance, despite the detection of simazine in 23 % water samples, the simazine 95 % species protection threshold value was not exceeded (Table SI 9).
When the risk quotients for reported pesticide residues (RQmed and RQmax) were calculated from median and maximum measured environmental concentration (MEC) and published no effect or EC50 concentrations, no RQmed was exceeded (Table SI 9), although the RQmax was exceeded for three pesticide residues (simazine, hexazinone, carbaryl).
A third way to assess potential toxic impacts from chemical data is to use the toxic unit (TU) concept, which compares the detected concentration of chemical with the respective toxicity of the substance (Liess and Von der Ohe 2005). We calculated the TU for each chemical in each water sample for fish and zooplankton and for the six photosystem II (PSII) herbicides detected, also for aquatic pants and phytoplankton (Table SI 10); a log10TU of −3 and higher is considered to pose some risk to community structure. In that context, the maximum measured environmental concentration of carbaryl had a log10TUf > −3, with carbaryl, simazine, and tetraconazole having a log10TUzp > −3, respectively, and are considered to have been posing a possible short-term risk to fish. Hexazinone and simazine, both PSII-inhibiting herbicides, had log10TUalg above −3 and so may also have posed a risk to primary producers in the waterways at the time of sampling.
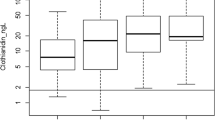
The presence of multiple pesticides in surface water is common in monitoring programs (Gilliom et al. 2006; Gregoire et al. 2010). These mixtures of different chemicals have the potential for additive, synergistic, or antagonistic effects on toxicity (ANZECC and ARMCANZ 2000). So, for all the samples with pesticide detections above the LOR, the sum of toxic units (ΣTU) was calculated for each site following the method reported by Bundschuh et al. (2014)) for fish, zooplankton, aquatic plants, and algae. The Σlog10TU values were then compared to the European Commission’s unified principles (UP) guideline values for fish and aquatic invertebrates and algae (Σlog10TU 0.01 and 0.1, respectively; European Commission 2011). In that context, no sites exceeded the UP for fish, invertebrates, or algae (Fig. 2).
Summary of ∑TU for fish, zooplankton, aquatic plants, and algae calculated on the basis of all measured pesticides in the grab water samples from the Corner Inlet sites (all four surveys combined). Note: The lower the log ΣTU, the higher the toxicity;  , arithmetic mean; dividing line within data boxes, data median; upper and lower boundaries of boxes, 75th and 25th percentile of data; error bars represent the range. The dotted line represents the Uniform Principles (UP_of the European Union for daphnids and fish (−log 0.01), while the dashed line represents that for algae (−log 0.1)
, arithmetic mean; dividing line within data boxes, data median; upper and lower boundaries of boxes, 75th and 25th percentile of data; error bars represent the range. The dotted line represents the Uniform Principles (UP_of the European Union for daphnids and fish (−log 0.01), while the dashed line represents that for algae (−log 0.1)
The potential impact of the pesticides in the sediments can also be assessed using the toxicity unit concept. Measured sediment concentrations were converted to an estimated “pore water” concentration using an equilibrium partitioning approach (Wetzel et al. 2013). This method usually compares whole sediment concentrations (normalised to 1 % organic carbon (OC)) with effect data (also normalised to 1 % OC). However, because this study used only the <63-μm fraction and normalising data from this sediment fraction to 1 % OC is considered inappropriate by ANZECC and ARMCANZ (2000), our calculations took the very conservative approach of considering measured concentrations as being the concentrations normalised to 1 % OC. In that context, Wetzel et al. (2013) suggest that using the maximum TU (TUmax) in the sediment sample accounts for the minimum expected effect of any mixture, with the toxicity thresholds set at log10TU of 0.01 for Daphnia magna, with TUs of 0.1 for the fish Pimephales promelas, and the algae Pseudokirchneriella subcapitata; none of these values exceed when assessing the potential impact of sediment pesticides using this method (Table SI 11), suggesting little potential impact of pesticide mixtures on sediment dwelling organisms.
Chemical risk assessment: trace metals
There are only ten trace metals listed in the ANZECC and ARMCANZ (2000) sediment quality guidelines; the Interim Sediment Quality Guidelines (ISQG) low and high values described therein correspond to the low and median effect range in the database reported by Long et al. (1995) designed to rank coastal and estuarine sediments. This database was adopted by ANZECC and ARMCANZ (2000) due to the lack of appropriate Australian sediment toxicity data in the late 1990s. The values represent a statistical probability of adverse effects (10 or 50 %) when tested against only one or two species, principally amphipods. When comparing this study’s data with ANZECC and ARMCANZ (2000) trigger values, specifically the relevant ISQG low value (the lowest concentration that toxic effects may become apparent), none of the measured trace metals had average sediment concentrations above their respective ISQG low (Table 3). As noted by ANZECC and ARMCANZ (2000), the ecological relevance of the ISQG low and high is questionable, but in general, one can assume that if the ISQG low for a metal does not exceed in the <63-μm fraction, it is unlikely that the concentrations observed in the whole sediment will result in any biological disturbance for organisms inhabiting that sediment.
The potential impact of trace metals in sediments can also be assessed using the threshold and probable effect concentration quotient (TECQ and PECQ) method (MacDonald et al. 2000). For individual contaminants, Kemble et al. (2013) suggest that there is higher likelihood of toxicity for samples with TECQMi and PECQMi values >1 and a higher likelihood of combined toxicity effects where the mean TECQMi,site and PECQMi,site is >0.5 and >0.2, respectively. In that context, when TECQMi and PECQMi were calculated for each metal at each site and then averaged, little toxicity was predicted (Tables SI 12(a,b) and 13(a,b)); no sites had TECQMi values >1, and no of sites had an average TECQMi,site > 0.5. No sites exceeded the PECQMi,site > 0.2 threshold, again suggesting that it is unlikely that the concentrations observed in the whole sediment will result in any biological disturbance for organisms inhabiting that sediment
Chemical use in the Corner Inlet catchment
Off-site pesticide movement is potentially a risk from all agricultural land uses. The presence of some fungicides in the water and sediment samples was, however, unexpected, since much of Corner Inlet’s agricultural land is utilised as pasture/grazing to support a number of animal-based industries, including beef and dairy, and few fungicides are used in animal fodder production. Given the very low concentrations of fungicides observed, one possibility is that those detections were artifacts of the analytical process, although examination of the QA/QC data in our then NATA-accredited laboratory strongly suggests that there was no cross-contamination within the laboratory and that the residues observed were in fact the result of chemical use within the catchments. Consequently, it is a legitimate question to ask where the fungicides were coming from. This, in turn, requires information on the amounts of individual pesticides used within the study area.
In Australia, there is currently no detailed and publicly available information on the usage of individual pesticides either in terms of active ingredient or in terms of formulated products (Radcliffe 2002). Information on pesticides (herbicides, insecticides, fungicides) registered for use in Victoria on pasture systems was therefore extracted from the PUBCRIS database (Table SI 14). The pesticide use regime of a farmer is influenced by the pest, disease, and weed threats facing her or his crops. For example, broad-leaved and other weeds reduce the quality of pasture, and so, there are a greater number of herbicides registered for use on pastures in Victoria (47) than fungicides (7); Table SI 14). This is consistent with findings reported in Connecticut, USA, that where land use is predominately pasture, herbicides account for ∼60 % of the total volume of chemicals used. Plantation forests are established in the high rainfall areas of the Corner Inlet catchments. In that context, the main chemicals observed in water samples (prometryn and simazine) are both registered for use in pasture systems; only two of the fungicides observed in sediments are registered for use on pasture systems (metalaxyl and trifloxystrobin), with no fungicides registered for use in forestry.
Other options for obtaining the information required to target pesticide residues screening include an indirect approach such as surveying agricultural experts and the very direct method of surveying farmers. Both approaches were tried by Bendor et al. (2008) to collect information about pesticide use in Tasmanian river catchment—31 priority pesticides were nominated by a committee of agricultural experts, whereas the “grower” survey identified some 128 pesticides in use across 20 different crops. More than 30 herbicides were reported by the growers that were not prioritised by the expert committee method. That the expert committee method returned only ∼25 % of the herbicides used by growers perhaps suggests that listening to agricultural “experts” is no substitute for collecting data. In that context, in this study, we adopted an indirect approach to obtaining pesticide use information by surveying agrochemical resellers in the three largest towns near Corner Inlet (Leongatha, Foster, and Yarram) in conjunction with the then CSB staff, with chemical sales information provided voluntarily by the manager of each reseller outlet from annual sales estimates (see Table SI 16). Resellers in Leongatha and Yarram suggested that the vast majority of the products they sold were sold to farmers outside our study area, whereas resellers in Foster suggested that most of their sales would have been made to properties in the study area (in 2010, 43 chemicals with approximately 80 % sold to farmers in Corner Inlet river catchments). The raw sales data provided suggested that most purchases were of herbicide products (Table SI 14), with lesser amounts of insecticides and very small amounts of home garden and termite control chemicals. No fungicides were sold locally to farmers.
Using the sales data, an estimated maximum of ∼300 km2 (Table SI 17) of the Corner Inlet catchment will have been treated by herbicides in 2010. The actual area is likely to be considerably smaller, since some of the herbicides identified will be applied in mixtures, and those applications might occur more than once per year. In that context, robust approaches are needed to obtain more accurate pesticide usage data for defined catchment areas in Australia to ensure that current pesticide usage is reflected in monitoring programs and risk assessments. Such pesticide use data needs to be kept up to date since pesticide usage can vary over time due to changes in land use, pest and disease pressures, climatic conditions, development of herbicidal resistance, new chemicals entering the market, and old chemicals becoming deregistered. In other countries, such as the USA, pesticide use reporting systems are in place (Radcliffe 2002). These systems require farmers to submit monthly reports on chemical use and have enabled pesticide risk to be more accurately predicted, although considerable resources are required to keep such pesticide usage databases up to date. The conclusion that can be drawn from this information is that in the future, Victoria should consider creating a pesticide use reporting function within the current regulatory framework, while ensuring that the benefits exceed the costs of doing so.
Conclusions
The study has shown the presence of residues of a small number of pesticides at very low concentration in the surface waters of streams entering the Corner Inlet, and as widespread, but still limited contamination of sediments. Concentrations of pesticides detected were relatively low and mostly several orders of magnitude below reported ecotoxicological effect and hazardous concentration values. Based on frequency of detection and concentrations, prometryn is the priority chemical of concern for both the water and sediments studied. Trace metal concentrations were also low, with none occurring at concentrations with the potential for adverse ecological effects. However, the risks may be greater than the data would suggest since some commonly used herbicides (such as glyphosate, phenoxy acid herbicides such as 2,4-D and MCPA, and sulfonyl urea herbicidess) were not screened. To progress toward a more thorough assessment of the ecological risks posed, future research should focus on gaining a better understanding of the ecotoxicological effects of priority chemicals, particularly to the lower trophic levels of aquatic ecosystems.
References
Allinson G, Bui A, Zhang P, Rose G, Wightwick AM, Allinson M, Pettigrove V (2014) Investigation of 10 herbicides in surface waters of a horticultural production catchment in south-eastern Australia. Arch Environ Contam Toxicol 67(3):358–73
ANZECC and ARMCANZ (2000) Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra, Australia
APVMA (2014a). Final pesticide and veterinary medicines product sales 2012-13 Financial Year. Commonwealth of Australia Gazette No APVMA 4, Tuesday 25 February 2014
APVMA (2014b). PUBCRIS - Public chemical registration information system. Sourced from the Australian Pesticides and Veterinary Medicines Authority at https://portal.apvma.gov.au/pubcris Last accessed 21 May 2014
Bendor M, Parr I, Goninon C (2008) The Tasmanian River Catchment Water Quality Initiative: the development and evaluation of a methodology for identifying the nature and extent of chemical pesticide usage in Tasmanian river catchments. Department of Primary Industries and Water, Hobart, Tasmania
Bengtson Nash SM, Goddard J, Müller JF (2006) Phytotoxicity of surface waters of the Thames and Brisbane River Estuaries: a combined chemical analysis and bioassay approach for the comparison of two systems. Biosens Bioelectron 21:2086–93
Bundschuh M, Goedkoop W, Kreuger J (2014) Evaluation of pesticide monitoring strategies in agricultural streams based on the toxic-unit concept - Experiences from long-term measurements. Sci Total Environ 484:84–91
Claver A, Ormad P, Rodriguez L, Ovellerio J (2006) Study of the presence of pesticides in surface waters in the Ebro river basin (Spain). Chemosphere 64:1437–43
Coupe RH, Welch HL, Pell AB, Thurman EM (2005) Herbicide and degradate flux in the Yazoo River Basin. Int J Environ Anal Chem 85(15):1127–40
Dores EFGC, Carbo L, Ribeiro ML, De-Lamonica-Freire EM (2008) Pesticide levels in ground and surface waters of Primavera do Leste region, Mato Grosso, Brazil. J Chromatog Sci 46:585–90
DNRE (2002). Corner Inlet Ramsar Site Strategic Management Plan. Parks Victoria, Department of Natural Resources and Environment, East Melbourne, Victoria 3002. Available online: http://www.depi.vic.gov.au/__data/assets/pdf_file/0005/241394/Corner-Inlet-Ramsar-Site-Strategic-Management-Plan.pdf Last accessed: 31 December 2014
Duke NC, Bell AM, Pederson DK, Roelfsema CM, Bengtson Nash S (2005) Herbicides implicated as the cause of severe mangrove dieback in the Mackay region, NE Australia: consequences for marine plant habitats of the GBRWorld Heritage Area. Mar Pollut Bull 51:308–24
Escher BI, Quayle P, Muller R, Schreiber U, Mueller JF (2006) Passive sampling of herbicides combined with effect analysis in algae using a novel high-throughput phytotoxicity assay (Maxi-Imaging-PAM). J Environ Monit 8:456–64
European Commission (2011) Implementing regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. Off J Eur L155:127–75
Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scot, JC, Stackelberg PE, Thelin GP, Wolock DM (2006). The quality of our nation’s waters—pesticides in the nation's streams and groundwater, 1992—2001: U.S Geological Survey Circular 1291. U.S Geological Survey, Reston, Virginia, USA
Gregoire C, Payraudeau S, Domange N (2010) Use and fate of 17 pesticides applied on a vineyard catchment. Int J Environ Anal Chem 90:406–20
Horowitz AJ (1991) A primer on sediment-trace element chemistry, 2nd edn. Lewis Publishers, Chelsea, MI, USA
ITRC (2006). Technology overview of passive sampler technologies. DSP-4. Washington, D.C.: Interstate Technology and Regulatory Council, Authoring Team. Available on-line: www.itrcweb.org/Guidance/ListDocuments?TopicID=17&SubTopicID=27 Last accessed 12 April 2014
Kemble NE, Hardesty DK, Ingersoll CG, Kunz JL, Sibley PK, Calhoun DL, Gilliom RJ, Kuivila KM, Nowell LH, Moran PW (2013). Contaminants in stream sediments from seven United States metropolitan areas: Part II—Sediment toxicity to the amphipod Hyalella azteca and the midge Chironomus dilutes. Arch Environ Contam Toxicol 64:52–64
Lewis SE, Brodie JE, Bainbridge ZT, Rohde KW, Davis AM, Masters BL, Maughan M, Devlin MJ, Mueller JF, Schaffelke B (2009) Herbicides: a new threat to the Great Barrier Reef. Environ Pollut 157:2470–84
Leonard AW, Hyne RV, Pablo F (2002) Trimethylpentane-containing passive samplers for predicting time-integrated concentrations of pesticides in water: laboratory and field studies. Environ Toxicol Chem 21(12):2591–9
Liess M, Von der Ohe PC (2005) Analyzing effects of pesticides on invertebrate communities in streams. Environ Toxicol Chem 24(4):954–65
Long ER, MacDonald DD, Smith SL, Calder ED (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Marshall S, Pettigrove V, Carew M, Hoffmann A (2010). Isolating the impact of sediment toxicity in urban streams. Environ Poll 158:1716–1725
Muller R, Schreiber U, Escher BI, Quayle P, Bengston Nash SM, Mueller JF (2008) Rapid exposure assessment of PSII herbicides in surface water using a novel chlorophyll a fluorescence imaging assay. Sci Total Environ 401:51–9
Muschal M, Warne MSJ (2003) Risk posed by pesticides to aquatic organisms in rivers of northern inland New South Wales, Australia. Hum Ecol Risk Assess 9:1765–87
Noppe H, Ghekiere A, Verslycke T, De Wulf E, Verheyden K, Monteyne E, Polfliet K, van Caeter P, Janssen CR, De Brabander HF (2007) Distribution and ecotoxicity of chlorotriazines in the Scheldt Estuary (B-Nl). Environ Pollut 147:668–76
O’Brien D, Bartkow M, Mueller JF (2011) Determination of deployment specific chemical uptake rates for SDB-RPD Empore disk using a passive flow monitor (PFM). Chemosphere 83:1290–5
Radcliffe JC (2002) Pesticide use in Australia: a review undertaken by the Australian Academy of Technological Sciences and Engineering. Australian Academy of Technological Sciences and Engineering, Victoria Australia
Schäfer RB, Pettigrove V, Rose G, Allinson G, Wightwick A, von der Ohe PC, Shimeta J, Kefford BJ (2011) Effects of pesticides monitored with three sampling methods in 24 sites on macroinvertebrates and microorganisms. Environ Sci Technol 45(4):1665–72
Shaw M, Eaglesham G, Mueller JF (2009) Uptake and release of polar compounds in SDB-RPS Empore™ disks; implications for their use as passive samplers. Chemosphere 75:1–7
Shaw M, Furnas MJ, Fabricius K, Haynes D, Carter S, Eaglesham G, Mueller JF (2010) Monitoring pesticides in the Great Barrier Reef. Mar Pollut Bull 60:113–22
Schuler LJ, Rand GM (2008) Aquatic risk assessment of herbicides in freshwater ecosystems of south Florida. Arch Environ Contam Toxicol 54:571–83
Sraka M, Vidacek Z, Smit Z, Bensa A, Vrhovec D (2007) Herbicides in the soil and waters of River Drava catchment area. Cereal Res Commun 35(2):1089–92
Stephens BS, Kapernick AP, Eaglesham G, Mueller JF (2009) Event monitoring of herbicides with naked and membrane-covered Empore disk integrative passive sampling devices. Mar Pollut Bull 58:1116–22
Tran ATK, Hyne RV, Doble P (2007) Calibration of a passive sampling device for time-integrated sampling of hydrophilic herbicides in aquatic environments. Environ Toxicol Chem 26(3):435–43
Vryzas Z, Vassiliou G, Alexoudis C, Papadopoulou-Mourkidou E (2009) Spatial and temporal distribution of pesticide residues in surface waters in northeastern Greece. Water Res 43:1–10
Wetzel MA, Wahrendorf D-S, von der Ohe PC (2013) Sediment pollution in the Elbe estuary and its potential toxicity at different trophic levels. Sci Total Environ 449:199–207
Wightwick AM, Allinson G (2007) Pesticide contamination of waterways within agricultural areas of Victoria: a review. Aus J Ecotoxicol 13:91–112
Wightwick A, Bui A, Zhang P, Rose G, Allinson M, Myers J, Reichman SM, Menzies NW, Pettigrove V, Allinson G (2012) Environmental fate of fungicides in surface waters of a horticultural production catchment in southeastern Australia. Arch Environ Contam Toxicol 62:380–90
Woudneh MB, Ou Z, Sekela M, Tuominen T, Gledhill M (2009) Pesticide multiresidues in waters of the Lower Fraser Valley, British Columbia. Canada. Part II. Surface water. J Environ Qual 38:940–7
Zgheib S, Moilleron R, Chebbo G (2012) Priority pollutants in urban stormwater: part 1—case of separate storm sewers. Water Res 46:6683–92
Acknowledgments
This project was funded by the then Department of Primary Industries (DPI) Chemical Standards Branch and DPI Future Farming Systems Research Key Project FF104 Accountable Agriculture (Project 06889).
The authors would like to thank the Agrochemicals Research team for “putting in the hard yards” to make this project a success under short notice and at times trying circumstances, despite the difficulties posed by such a complex, field-based analytical program, including Simon Phelan, Karen Young, Ron Walsh, and John Cauduro. The authors would also like to thank Mahendrra Raj and CSB’s Michael Laity for their help with the chemical resellers survey
The project team wishes to express its thanks to the members of South Gippsland Water, West Gippsland CMA, Parks Victoria, and the Centre for Aquatic Pollution Identification and Management (CAPIM) for their assistance with site selection and sampling.
This work was conducted under National Parks Act 1975 Research Permit # 10005174, issued by the Department of Sustainability and Environment, November 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 522 kb)
Rights and permissions
About this article
Cite this article
Allinson, G., Allinson, M., Bui, A. et al. Pesticide and trace metals in surface waters and sediments of rivers entering the Corner Inlet Marine National Park, Victoria, Australia. Environ Sci Pollut Res 23, 5881–5891 (2016). https://doi.org/10.1007/s11356-015-5795-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5795-6