Abstract
From April 2008 to November 2009, a field decomposition experiment was conducted to investigate the effects of sediment burial on macro (C, N) and microelement (Pb, Cr, Cu, Zn, Ni, and Mn) variations in decomposing litter of Phragmites australis in the coastal marsh of the Yellow River estuary. Three one-off sediment burial treatments [no sediment burial (0 mm year−1, S0), current sediment burial (100 mm year−1, S10), and strong sediment burial (200 mm year−1, S20)] were laid in different decomposition sites. Results showed that sediment burials showed significant influence on the decomposition rate of P. australis, in the order of S10 (0.001990 day−1) ≈ S20 (0.001710 day−1) > S0 (0.000768 day−1) (p < 0.05). The macro and microelement in decomposing litters of the three burial depths exhibited different temporal variations except for Cu, Zn, and Ni. No significant differences in C, N, Pb, Cr, Zn, and Mn concentrations were observed among the three burial treatments except for Cu and Ni (p > 0.05). With increasing burial depth, N, Cr, Cu, Ni, and Mn concentrations generally increased, while C, Pb, and Zn concentrations varied insignificantly. Sediment burial was favorable for C and N release from P. australis, and, with increasing burial depth, the C release from litter significantly increased, and the N in litter shifted from accumulation to release. With a few exceptions, Pb, Cr, Zn, and Mn stocks in P. australis in the three treatments evidenced the export of metals from litter to environment, and, with increasing burial depth, the export amounts increased greatly. Stocks of Cu and Ni in P. australis in the S10 and S20 treatments were generally positive, evidencing incorporation of the two metals in most sampling times. Except for Ni, the variations of C, N, Pb, Cr, Cu, Zn, and Mn stocks in P. australis in the S10 and S20 treatments were approximated, indicating that the strong burial episodes (S20) occurred in P. australis marsh in the future would have little influence on the stocks of these elements. With increasing burial depths, the P. australis was particularly efficient in binding Cu and Ni and releasing C, N, Pb, Cr, Zn, and Mn, implying that the potential eco-toxic risk of Pb, Cr, Zn, and Mn exposure might be very serious. This study emphasized the effects of different burials on nutrient and metal cycling and mass balance in the P. australis marsh of the Yellow River estuary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal marshes have long been recognized by their remarkable rates of primary productivity (Bouchard and Lefeuvre 2000), and a substantial part of the annual plant production becomes litter (Foote and Reynolds 1997). The decomposition rates of detrital material in coastal marshes not only affect the accumulation rates of organic matter and the transfer of nutrients and chemical elements between trophic levels (Sun et al. 2012a) but also influence the potential export of material from coastal marshes and estuaries to coastal waters (Hodson et al. 1984; White and Howes 1994). A number of extreme conditions such as high potential evapotranspiration, frequent ebb and flow of tide, inundation by seawater, and burial in sediment exist in intertidal zone (Baldwin and Maun 1983; Maun 1998; Deng et al. 2008). Among them, sediment burial has been recognized as a major factor influencing the decomposition rates of detrital material in coastal marshes (Vargo et al. 1998). Different sediment disturbances can result in the litters being directly buried within the sediment to different extent, which may greatly affect the processes of material cycling (Sun et al. 2015).
Coastal marshes generally act as a geochemical trap for heavy metals bonded in the sediments (Williams et al. 1994), and various hydrological processes and sediment physicochemical properties significantly influence the biochemical processes of heavy metals in marshes (Bai et al. 2014). With the rapid industrialization and economic development in coastal zone, heavy metal has become one of the most serious pollutants in coastal regions due to their toxicity, persistence in natural conditions, and ability to be incorporated into food chains (Armitage et al. 2007; Sakan et al. 2009; Wang et al. 2013; Xiao et al. 2015). Most of heavy metals imported into coastal marshes are rapidly fixed onto the solid phase, where a number of physical and chemical properties will determine the strength of metal retention. Only a small proportion of the metals dissolves and becomes available for plant uptake (Du Laing et al. 2006). Plant uptake directly reduces the input of metals into adjacent waters (Chen et al. 2000; Vandecasteele et al. 2005). Plant detritus can provide a sink if, during decomposition, metals could be bound to the litter by passive sorption on organic surfaces or by physiological mechanisms of microbial colonizers. Plant detritus can also act as a metal source when microbial activity mobilizes metals or when it becomes available to deposit feeders (Gadd 1993; Ledin 2000; Weis and Weis 2004). Particularly, the consumption of plant detritus that contained metals could cause metal accumulation and toxic effects in higher trophic levels (Dorgelo et al. 1995; Weis et al. 2002; Du Laing et al. 2002; Zhang et al. 2010). Considerable efforts have been conducted in the past two decades to study the litter decomposition in different coastal marshes (Mendelssohn et al. 1999; Anesio et al. 2003; Du Laing et al. 2006; Menéndez and Sanmartí 2007; Quintino et al. 2009; Simões et al. 2011) and mangrove swamps (Robertson 1988; Tam et al. 1998; Dick and Osunkoya 2000; Nielsen and Andersen 2003; Ramos e Silva et al. 2007; Sánchez-Andrés et al. 2010; Keuskamp et al. 2015). Most of these studies focused on exploring the decomposition rates and nutrient dynamics of different litters and the roles of abiotic (temperature, nutrient enrichment, salinity gradient, and tidal flooding, duration, etc.) and biotic factors (fungi, meiofauna, free and attached microorganisms, etc.) on decomposition, whereas information on the effects of sediment burial on litter decomposition is still very limited. In addition, only few studies discussed the variation or accumulation of heavy metals in plant detritus in different coastal marshes (Larsen and Schierup 1981; Zawislanski et al. 2001; Weis and Weis 2004; Du Laing et al. 2006; Pereira et al. 2007), while information on the influences of sediment burial on heavy metal dynamics in decomposing litters remains scarce. In China, studies on litter decomposition of mangrove swamps in the tropical and subtropical regions have been carried out in the early 1990s (Zhuang and Lin 1993; Zhang and Lin 1998; Huang et al. 2001; Sheng 2009; Chen 2013; Li and Ye 2014). In contrast with that, the research in coastal marshes or estuarine marshes start quite late, and current studies also focus on the tropical and subtropical regions of China, such as the coastal marshes in the Hangzhou Bay (Shao et al. 2014), and the estuarine marshes in the Yangtze River estuary (Zhou et al. 2006; Chen 2008; Guan 2013) and the Min River estuary (Tong et al. 2011; Zhang et al. 2014), while information on the coastal marshes in the warm temperate regions of China (such as Liao River estuary and Yellow River estuary) remains scarce. Moreover, present studies mainly focus on litter decomposition characteristics and its related affecting factors, while information on heavy metal dynamics in decomposing litters is still very limited.
The Yellow River is well known as a sediment-laden river. Every year, approximately 1.05 × 107 tons of sediment is carried to the estuary and deposited in the slow flowing landform, resulting in vast floodplain and special marsh landscape (Xu et al. 2002). Sediment deposition is an important process in the formation and development of coastal marsh in the Yellow River Delta. The deposition rate of sediment in the Yellow River not only affects the formation rate of coastal marsh but also influences the water or salinity gradients and the succession of plants from the land to the sea. Phragmites australis is a prevalent plant in the coastal marsh of the Yellow River estuary, which is often affected by the sediment of tide physical disturbance and river flooding. It was reported that the annual runoff of the Yellow River showed great interannual changes since the 1980s. The runoff reached the maximum value of 49.1 billion m3 in 1983 and then decreased and fluctuated at 20.0 billion m3 in the following several years. From 1997 to 2002, the annual runoff was below 10.0 billion m3 (Cui et al. 2009). The low flows of the Yellow River led to a significant decrease in freshwater supply to the estuary, and the P. australis marshes near the estuary exhibited seriously degraded status. In order to restore degraded marshes, the “flow-sediment regulation project” (FSRP) was initiated by the nation in 2002. The purpose of the FSRP was to increase the supply of freshwater and sediment for the Yellow River estuary by discharging the water in the Xiaolangdi Reservoir and scouring the sediment in the reservoir and riverbed (Cui et al. 2009). Although the FSRP increased the runoff and sediment for the Yellow River estuary, it could also produce some negative effects. Before the enforcement of FSRP, lower concentrations of As and heavy metals were reported in marsh soils of the Yellow River estuary (Rui et al. 2008). However, the concentrations of some heavy metals (such as As, Cr, Pb, and Cd) in the restored marsh were much higher than those in the degraded marsh, indicating that the heavy metal pollution in the restored marsh might become more serious after the regulation (Bai et al. 2012, 2015). In addition, during the FSRP (from June to July every year), the river water flooded the P. australis marshes near the estuary and resulted in the litters being directly buried within the sediment to considerable thickness (approximately 50∼60 mm), which might significantly influence litter decomposition rate and element variations in decomposing litter (Mou 2010). However, little is known about the impacts of one-off burial on decomposition rate and heavy metal stocks of P. australis in the coastal marsh of the Yellow River estuary.
In this paper, the effects of one-off burial disturbance on macro (C, N) and microelement (Pb, Cr, Cu, Zn, Ni and Mn) variations of P. australis were investigated by litterbag technique. It is hypothesized that the decomposition rates and macro/microelement dynamics differ among different burial disturbances, which will have great influences on the functioning of P. australis marsh in the Yellow River estuary. The primary objectives of this study were (i) to examine whether sediment burials caused by one-off burial episodes would have great impacts on decomposition of P. australis, (ii) to determine the dynamics of C, N, and metals in P. australis during decomposition as affected by different sediment burials, and (iii) to determine the influences of sediment burials on C, N, and metal stocks in P. australis during decomposition.
Materials and methods
Study site
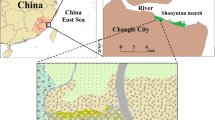
This study was conducted in the coastal marsh of the northern Yellow River estuary (Fig. 1), located in the Nature Reserve of Yellow River Delta (37° 35′ N∼38° 12′ N, 118° 33′ E∼119° 20′ E) in Dongying City, Shandong Province, China. The nature reserve is of typical continental monsoon climate with distinctive seasons. The average temperatures in spring, summer, autumn, and winter are 10.7, 27.3, 13.1, and −5.2 °C, respectively. Annual evaporation is 1962 mm, and annual precipitation is 551.6 mm, with about 70 % of precipitation occurring between June and August. The soils in the study area are dominated by intrazonal tide soil and salt soil, and the main vegetations include P. australis, Suaeda salsa, Triarrhena sacchariflora, Myriophyllum spicatum, and Tamarix chinensis (Sun et al. 2012b). Coastal marsh is the main marsh type, with an area of 964.8 km2, accounting for 63.06 % of the total area of the Yellow River Delta (Cui et al. 2009). The tide in the intertidal zone of the Yellow River estuary is irregular semidiurnal tide, and the mean tidal range is 0.73∼1.77 m (Li et al. 1991). The sequence of geomorphic units is complete in intertidal zone of the Yellow River estuary due to the protection of Nature Reserve, which generally comprises three areas in a seaward direction: high marsh (river bank), middle marsh, and low marsh. In the river bank, the most common species is P. australis, and to a less extent by Calamagrostis pseudophragmites and Imperata cylindrica (in similar proportions (from 5 to 10 % ground cover)). As a prevalent plant in high marsh, P. australis is often affected by the sediment deposition of tidal disturbance, bioturbation, and Yellow River flooding during FSRP. The sedimentary rate in P. australis marsh is about 90∼100 mm year−1, and approximately 60∼70 mm occurs from June to July due to the significant effects of both tidally induced sediment and FSRP (Mou 2010). One experimental plot was laid in the abovementioned high marsh, and three subplots were laid in it along the river bank (Fig. 1).
Experimental design
Litter decomposition was studied by litterbag technique at the experimental plot from April 2008 to November 2009. On 20 March 2008, the litters were collected from P. australis community. In order to weaken the fragmentation impact of snowfalls and strong winds in winter, the standing litter was selected for use in this study. Each 20 × 20 cm litterbag was made of nylon netting (0.5 mm mesh) and filled with 15 g litter (dried weight). In order to investigate the effects of one-off burial disturbance on decomposition and macro/microelement concentrations of P. australis, three one-off sediment burial treatments (no sediment burial (0 mm year−1, S0), current sediment burial (100 mm year−1, S10), and strong sediment burial (200 mm year−1, S20)) were laid in different decomposition subplots. On 21 April 2008, the litterbags were artificially buried to depths of 0, 100, and 200 mm to simulate the S0, S10, and S20 treatments, respectively. In order to prevent the litterbags from being affected by sediment burial disturbance during decomposition, each subplot was tightly enclosed by nylon netting (0.25 mm mesh, 1.5 m height). The bulk density of sediment used in this experiment is 1.28 ± 0.08 g cm−3 and presents silty clay texture, with 7.83 ± 2.52 % of clay, 76.84 ± 2.39 % of silt, and 15.33 ± 0.13 % of sand (fine sand). The sediment shows low organic matter and total nitrogen contents, with the values of 1.10 ± 0.14 % and 0.63 ± 0.03 mg g−1, respectively. The pH and electrical conductivity of the sediment are 7.90 ± 0.05 and 3.58 ± 1.48 mS cm−1, respectively. The concentrations of Pb, Cr, Cu, Zn, Ni, and Mn in sediment are 48.01 ± 0.94, 47.17 ± 10.80, 29.65 ± 1.48, 66.43 ± 3.01, 26.95 ± 1.36, and 579.46 ± 14.84 mg kg−1, respectively.
The experiment included nine sampling times with different intervals (11 July 2008 (80 days), 09 August 2008 (109 days), 20 September 2008 (151 days), 20 October 2008 (181 days), 15 November 2008 (207 days), 26 April 2009 (371 days), 25 June 2009 (431 days), 25 August 2009 (492 days), and 12 November 2009 (571 days)), and on each sampling date, three or four litterbags were retrieved from each subplot. After retrieval, these litterbags were immediately taken back to the laboratory, and the plant roots, lichen, sediment, and macroinvertebrates were removed from the remaining litter. All litterbags were further cleaned gently in deionized water and weighed after being dried to a constant weight.
In situ measurements
Sediment temperatures (0, 10, and 20 cm) were measured in the three subplots on sampling date. Sediment moisture and electrical conductivity (EC) in 0, 10, and 20 cm depths were determined in situ by high-precision moisture measuring instrument (AZS-2) and soil and solution EC meter (Field Scout), respectively. Sediment pH in 0, 10, and 20 cm depths was measured by portable pH meter (IQ150).
Sample analysis
The samples of decomposing material and sediment were ground (<0.25 mm) using a Wiley mill and analyzed for total carbon (TC) and total nitrogen (TN) concentrations by an element analyzer (Elementar Vario Micro, German). The organic matter and grain size for the sediment used in this experiment were determined by K2Cr2O7 oxidation method (The Committee of Agro-chemistry of the Chinese Society of Soil Science 1983) and the Coulter Laser granulometer, respectively. A 0.1000-g homogenized sediment subsample was digested with 2 mL HNO3, 1 mL HClO4, and 5 mL HF at 160∼190 °C for 16 h. The residue was dissolved in 2 mL of 4 mol L−1 HCl and then diluted to 10 mL with deionized water for heavy metal analysis. A 0.2000-g plant subsample was digested in a mixture of 65 % HNO3 (2 mL) and 30 % H2O2 (1 mL). The residue was diluted with deionized water to 10 mL for analyzing heavy metal concentrations. The concentrations of heavy metals (Pb, Cr, Cu, Zn, Ni, and Mn) in all samples were determined by Agilent 7500 ICP-MS (Agilent Company, America). Quality assurance and quality control were assessed using duplicates, method blanks, and standard reference materials (GBW07401 and GBW08513) from the National Research Center for Standards in China with each batch of samples (one blank and one standard for each 20 samples).
Parameter calculation
Litter mass loss (R, %) and decomposition rate (day−1) were calculated by the following equations (Olson 1963):
where W 0 (g) is the original dry mass and W t (g) is the dry mass at time “t”; k is the decay constant and t (day) is decomposition time in days.
The accumulation index of the “i” macro/microelement (C, N, Pb, Cr, Cu, Zn, Ni, and Mn) (AI i ) was used to express its accumulation or release status during litter decomposition, which could be calculated by the following equation (Sun et al. 2012a):
where M 0 is the original dry mass, C 0 is the original element concentration in litter, M j is the dry mass at time “j”, and C j is the element concentration in litter at time “j”. AI > 100 % indicated net element accumulation, whereas AI < 100 % indicated net element release.
Statistical analysis
The samples were presented as means over the replications, with standard deviation (SD). The analysis of variance (ANOVA) tests (SPSS for windows 11.0) was employed to determine if treatments differed significantly (p < 0.05). If ANOVA showed significant differences, multiple comparison of means was undertaken by Tukey’s test with a significance level of p = 0.05.
Results
Mass loss and decomposition rate
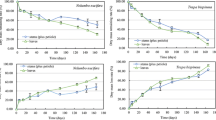
The mass losses of P. australis in the three burial treatments generally increased and the values presented S10 ≈ S20 > S0 (p < 0.05) (Fig. 2). Over 571 days of decomposition, the percent of dry mass remaining in the S0, S10, and S20 treatments were 56.67, 26.25, and 33.08 %, respectively. Sediment burials showed great influence on the decomposition rate of P. australis, in the order of S10 (0.001990 day−1) ≈ S20 (0.001710 day−1) > S0 (0.000768 day−1) (p < 0.05) (Table 1). Significantly higher t 0.95 (time needed for 95 % of dry mass decomposed) were observed for S0 treatment (10.49 years) compared to the S10 (3.98 years) and S20 (4.55 years) treatments.
Macro and microelement concentrations
During decomposition, the C concentrations in P. australis fluctuated greatly and varied between 39.11 and 42.26 % for S0, from 31.59 to 41.77 % for S10, and from 38.23 to 42.62 % for S20, respectively (Fig. 3a), while the N concentrations in the three treatments generally demonstrated increasing tendency (Fig. 3b). The trace metal concentrations in the three burial treatments exhibited different temporal variations except for Cu, Zn, and Ni. With a few exceptions, the Cu and Ni concentrations in the S10 and S20 treatments and the Zn concentrations in the three burials generally showed increasing tendency. Compared to the S10 and S20 treatments, the variations of Pb, Cr, Cu, and Ni concentrations in the S0 treatment were less pronounced. With increasing burial depth, Cr, Cu, Ni, and Mn concentrations generally increased, while Pb and Zn concentrations varied insignificantly (Fig. 4). Except for Cu and Ni, no significant differences in C, N, Pb, Cr, Zn, and Mn concentrations were observed among the three burial treatments (p > 0.05).
Macro and microelement stocks
The AI c of P. australis in the three burial treatments presented S10 ≈ S20 > S0 (p < 0.05), indicating that sediment burial was favorable for C release (Fig. 5a). The AI N variations of P. australis in the three treatments were significantly different (p < 0.05), and, with increasing burial depth, the N in litters generally shifted from accumulation to release during decomposition, indicating that sediment burial was also favorable for N release (Fig. 5b). With a few exceptions, Pb, Cr, and Mn stocks in P. australis in the three treatments evidenced the export of metals from litters to environment and, with increasing burial depth, the export amounts increased greatly (Fig. 6). Stocks of Cu and Ni in P. australis in the S10 and S20 treatments were generally positive, evidencing incorporation of the two metals in most sampling times. With increasing burial depth (particularly in the S20 treatment), stocks of Zn in P. australis shifted from accumulation to release in most periods (Fig. 6).
Discussion
Effects of sediment burial on decomposition rate and macroelement dynamics
Previous studies have indicated that sediment burials significantly inhibited the decomposition of detrital materials via a series of direct and indirect mechanisms, such as compaction of detritus, reduction of gas (O2 and CO2) exchange between the detrital layer and the surrounding, and suppression of bacterial and fungal breakdown of detritus (Vargo et al. 1998; Nielsen and Andersen 2003). However, we have drawn a different result. Although there was little difference in decomposition rates of the S10 and S20 treatments, the values were much higher than that in the S0 treatment, indicating that sediment burials stimulated the decomposition rate of P. australis. There were three possible reasons. Firstly, the difference in decomposition rates in the three treatments was closely correlated with litter quality. Present studies have indicated that litter quality clearly affected decomposition rate, and the C/N ratio was often used as predictors of decomposition rates since it reflected the ratio of carbohydrate and lignin to protein in litter; a high ratio generally induced a slow decomposition rate (Harmon et al. 1990; Hobbic 1996; Chen 1999; Alicia and Roberto 2003). In this study, the C/N ratios of P. australis in the S10 and S20 treatments during decomposition were generally lower than those in the S0 treatment (Fig. 7), which, to some extent, could better explain the higher decomposition rates in burial treatments. Secondly, the difference in decomposition rates in the three treatments might be affected by the key environmental variables in different burial depths. Ming-Yi et al. (1993) indicated that the anoxic degradation was independence of temperature, and this was tested in our study. In the three treatments, although the average temperatures differed by up to 4.10 and 5.98 °C between 0 and 10 cm depths and between 10 and 20 cm depths, respectively, the difference in sediment temperatures among the three treatments were not large enough to contribute to the observed difference in decomposition rates as evidenced by the absence of significant correlations between temperatures and decomposition rates (p > 0.05). Moreover, no significant differences in sediment pH and EC were observed among the three burial depths (Table 2), indicating that these variables might be less important in inducing the difference of decomposition rates. In contrast with them, sediment moisture was significantly different among the three burial treatments (p < 0.05), and, with increasing burial depth, the moisture significantly increased (Table 2). Previous studies have indicated that, with increasing of moisture, the O2 in litter would be depleted rapidly, and the metabolism of decomposition microbes would be restrained (Cai 2000; Laiho et al. 2004). As the devoid of O2, the enzymes such as phenol oxidase, which required O2 for their activity, were rarely active, and thus inhibited the decomposition of organic matter (Freeman et al. 2004). However, Zhao et al. (2015) found that, in the long-flooding coastal wetland, the mass loss of P. australis was the highest. Webster and Benfield (1986), reviewing decomposition in aquatic environments, noted that the effects of anaerobiosis on decomposition rate were not always inhibitory. Particularly, after the organic matter was buried in sediment, the anaerobic conditions formed in different burial depths might have either positive (Ming-Yi et al. 1993), negative (Benner et al. 1984), or even no effect at all (Kristensen and Blackburn 1987) on the decomposition of organic matter. In this study, the positive effect of sediment burials (S10 and S20 treatments) on decomposition was probably ascribed to the maintenance of adequate sediment moisture for microbial/fungal colonization and activity (Neckles and Neill 1994; Mendelssohn et al. 1999), and this might be verified by Wang et al. (2009) who found that, in the P. australis marsh of the Yellow River estuary, although the amounts of bacteria, fungi, and actinomycete in sediment of 10 and 20 cm depths were slightly lower than the surface sediment, the activities of microorganisms in 5∼15 cm depth were still very high (Chen and Shi 2010). The high activities of microbes in 10∼20 cm depth also could be tested by the lower C/N ratios of P. australis in the S10 and S20 treatments compared to the S0 treatment (Fig. 7). Finally, the activities of macrobenthos such as Macrophthalmus japonicus and Helice wuan in P. australis marsh might also affect the difference in decomposition rates of P. australis in the three burial treatments. It was reported that the vertical depth of macroinvertebrates (crabs) caves in P. australis marsh (high marsh) varied from 10 to 20 cm, and in these depths, the horizontal or slop habitats were generally established (Mou 2010, Sun et al. 2015). Li (2011) also indicated that the biomass and habitat density of macrobenthos in high marsh of the Yellow River Delta were very high, and the values reached 92.58, 192.37 g m−2, and 168.66, 690.26 ind m−2 during spring and autumn. Thus, the great disturbances of macrobenthos in P. australis marsh, to some extent, might improve the gas exchange between the detritus in 10∼20 cm depth and the surrounding, which probably stimulated the decomposition of P. australis in the S10 and S20 burial treatments. This paper also found that there was little difference in decomposition rates between S10 and S20 treatments, and the reason might be related to the insignificant differences in sediment temperature, moisture, EC, and pH between them (Table 2). Compared to the S10 treatment, the decomposition rate in the S20 treatment was slightly lower, which might be dependent on the weak inhibitory on decomposition caused by the relative deficiency of O2 in 20 cm depth. Moreover, the amounts and activities of microorganisms in sediment of the S20 treatment were slightly lower than those of the S10 treatment (Wang et al. 2009), which could better explain the lower decomposition rate.
It was anticipated that the dynamics of macroelement (C and N) in decomposing litter differed among the three burial treatments, and this was also tested in this study. Previous studies have indicated that the P. australis marsh in the Yellow River estuary was very limited by N (Sun 2015; Cao et al. 2015). Thus, over all sampling periods, the increase of N concentration in P. australis in the three burial treatments might be attributed to the N immobilization by microbes from sediment or river-water/seawater in decomposition environments (Sun et al. 2015). The N concentrations in P. australis in the S10 and S20 treatments were generally higher than those in the S0 treatment (Fig. 3b), and there were three possible reasons. Firstly, although the litterbags in the S0 treatment were placed in close contact with sediment layer, the chance of infiltration of sediment particles into the litterbags were much lower compared to the S10 and S20 treatments. Secondly, as mentioned above, although the amounts of microorganisms in sediments of the S10 and S20 treatments were slightly lower than those in the S0 treatment, the activities of microorganisms in the two burial depths were still very high. Thirdly, significantly higher sediment moisture was observed in the S10 and S20 treatments compared to the S0 treatment during decomposition (Table 2), which might increase the chance of N in sediment water for immobilization by microbes. Similarly, Gessner (2000) found that the N concentrations tended to increase in the leaf, culm, and sheath of P. australis, and the reasons were mainly related to the external biological immobilization from decomposition environment (lake water). Sun et al. (2012a) also indicated that the increase of N concentrations in Calamagrostis angustifolia during decomposition was dependent on the biological immobilization from sediment and marsh water. Another study by Gessner (2001) indicated that microbial immobilization was a very important process controlling the nutrient dynamics in litter, which was mainly regulated by the C/N ratios in litter and the N availability in decomposition environment (Berg 1986; Köchy and Wilson 1997). Pearson correlation analyses indicated that significantly negative correlations were observed between C/N ratios and N concentrations in the S0 (r = −0.934), S10 (r = −0.882), and S20 (r = −0.921) treatments (p < 0.01), but no significant correlations occurred between C/N ratios and C concentrations (p > 0.05), indicating that C/N ratios might control the N dynamics in the three burial treatments, while the C variations in litters might be more subjected to the sediment moisture and the activities of microorganisms and macrobenthos as mentioned previously.
This study indicated that sediment burial was favorable for C release from P. australis. With increasing burial depth, the release amounts after 571 days of decomposition enhanced 23.67∼32.09 % (Fig. 5a), which could be better explained by both appropriate sediment moisture and high activities of microbes and macrobenthos in the S10 and S20 treatments as mentioned above. This paper also found that, with increasing burial depth, the N in P. australis generally shifted from accumulation to release (Fig. 5b). The difference in N release patterns of the three burial treatments was mainly dependent on the variations of C/N ratios during decomposition. As mentioned previously, the C/N ratios of P. australis in the S10 and S20 treatments were generally lower than those in the S0 treatments (Fig. 7), indicating that, compared to the S0 treatment, the N in current (S10) and strong (S20) burial treatments might not be very limited for microorganism during decomposition. Thus, the superfluous N in P. australis could be greatly released to the decomposition environments (Fig. 5b). Similar with decomposition rate, the C and N release from P. australis in the S10 and S20 treatments were approximated; implying that the strong one-off burial episodes (S20) occurred in P. australis marsh in the future would have little influence on the C and N release from litters.
Effects of sediment burial on microelement concentrations and stocks
This study found that, with a few exceptions, the Cu and Ni concentrations in the S10 and S20 treatments and the Zn concentrations in the three burial treatments generally showed increasing tendency (Fig. 4). Similar results were reported by other studies. Du Laing et al. (2006) indicated increasing metal concentrations (Cu, Cr, Ni, Pb, and Zn) in leaf blades, sheaths, and stems of P. australis during decomposition. Windham et al. (2004) found increasing Cr, Cu, Pb, and Zn concentrations in decomposing leaves and stems of plants in reed marshes. Increases of the metal concentrations could be attributed to different factors, such as contamination by sediment particles, passive sorption onto recalcitrant organic fractions, and active accumulation by microbial colonizers (Breteler et al. 1981; Gadd 1993; Zawislanski et al. 2001; Kovacova and Sturdik 2002; Du Laing et al. 2006). Although the marsh sediment in the S0 treatment could be easily resuspended by river flooding or tidal wave action, the risk of infiltration of fine particles into the litterbags were much lower compared to the S10 and S20 burial treatments. Accompanying with river flooding or tidal wave action, the metals in river water or seawater also increased the chance of sorption by P. australis in different burial treatments. It was reported that C/N ratio was an effective index in representing decomposition rate and microbial activity since it reflected the ratio of carbohydrate and recalcitrant organic fractions (lignin, cellulose, and hemicellulose etc.) to protein and available nitrogen in litter (Harmon et al. 1990; Hobbic 1996; Cai 2000; Sun et al. 2012a). Pearson correlation analyses showed that significantly negative correlations were observed between Cu concentrations and C/N ratios in the three burial treatments (p < 0.01). Significantly negative correlations also occurred between Zn concentrations and C/N ratios in the S0 treatment (p < 0.05) and between Ni concentrations and C/N ratios in the S10 and S20 treatments (p < 0.01) (Table 3). In this study, the C/N ratios of P. australis in the three treatments during decomposition generally decreased (Fig. 7), indicating that the activities of microbes in litters might be greatly enhanced, and this might significantly increase the active accumulation of Cu, Zn, and Ni by microorganisms. This conclusion was tested by some related studies. Windham et al. (2004) found that adsorption and accumulation of fine sediment could not be the major cause of increasing metal concentrations in litter, and microbial action was likely one of the major mechanisms responsible for the metal enrichment. Du Laing et al. (2006) also indicated that fungal biomass showed significantly positive correlations with metal concentrations in stem tissue, suggesting an involvement of fungal activity in metal accumulation. Moreover, with the process of decomposition, the proportions of recalcitrant organic fractions significantly increased, which might enhance the chance of physicochemical sorption of Cu, Zn, and Ni onto the remaining recalcitrant organic fractions.
Except for Cu, Zn, and Ni, Pb, Cr, and Mn concentrations in the three burial treatments exhibited different temporal variations during decomposition. On the one hand, the fluctuations of metals in P. australis in different burials might be dependent on the complex interactions of the abovementioned factors such as infiltration of fine particles into litters, passive sorption onto recalcitrant organic fractions, and active accumulation by microorganisms. On the other hand, the metal variations in the three burial treatments might be rested with carbon/metal (C/M) and C/N ratios. Once carbon becomes the major constituent of litter, metal concentration could be normalized to carbon content to better interpret the variation of metal concentrations as organic matter decomposed (Pereira et al. 2007). In this study, significantly negative correlations were observed between metal concentrations (Pb, Cr, Cu, Zn, Ni, and Mn) and C/M ratios in the three burial treatments (p < 0.01 or p < 0.05) (Table 3), indicating that C/M ratios, to a great extent, might control the metal dynamics in P. australis in different burial treatments. Except for Cu, Zn, and Ni, significantly negative correlations also occurred between Mn concentrations and C/N ratios in the S10 treatment (p < 0.05), implying that the Mn variation might be greatly influenced by both active accumulation by microorganisms and physicochemical sorption of dissolved metals onto recalcitrant organic fractions. Although the correlations between Pb concentrations and C/N ratios in the three burial treatments were positive, significant correlation only occurred in the S0 treatment (p < 0.05) (Table 3). Previous studies have indicated that the Pb behavior was greatly influenced by iron cycling and organic matter degradation. The oxidation of organic matter might lead to the use of Fe oxide as electron acceptor, and the reduced Fe form might leach from litter (Sundby et al. 2005). Particularly, Pb could be included in formed Fe sulfides as a tracer, and as Fe oxides were reduced, the Pb mobilization occurred (Pereira et al. 2007). In the Yellow River estuary, the Fe concentrations in sediments of coastal marsh were very high, and the values ranged from 16.49 to 33.11 g kg−1 (Sun et al. 2013), implying that the Pb mobilization might be enhanced as the Fe oxides were substantially reduced. This paper also found that Cr, Cu, Ni, and Mn concentrations generally increased with increasing burial depth (Fig. 4). There were three probable reasons. Firstly, as mentioned above, the chance of infiltration of fine particles into the litters in the S10 and S20 burial treatments was much higher than that in the S0 treatment, which might greatly increase metal concentrations in P. australis. Secondly, significantly higher sediment moisture were observed in the S10 and S20 treatments compared to the S0 treatment (Table 2), which might increase the chance of metals in sediment water for immobilization by microbes. Finally, the C/N ratios of P. australis in the S10 and S20 treatments were generally lower than those in the S0 treatment (Fig. 7), implying that the activities of microbes in litters might be greatly enhanced, and this might significantly increase the active accumulation of metals by microorganisms.
This paper showed that, in most periods, Pb, Cr, Zn, and Mn stocks in P. australis in the three burial treatments were always lower than the initial ones, indicating that release during the 571-day experiment always exceeded incorporation (Fig. 6). However, the variations of metal stocks between sampling times meant that export was not uniform. Stocks of Cu and Ni in P. australis in the S10 and S20 treatments were generally positive, evidencing incorporation of the two metals in most sampling times. It was hypothesized that the release of Cu and Ni from P. australis in the S10 and S20 treatments was not counterbalanced by sorption due to the strong reducing conditions in sediments (Pereira et al., 2007), as proved by the high concentrations of acid volatile sulfides (AVS) in sediments of the Yellow River estuary (Wu et al. 2007). Particularly, the incorporation of Cu occurred in current (S10) and strong (S20) burial treatments at all times (Fig. 6). Similar results were reported by Windham et al. (2004) and Pereira et al. (2007) who also found Cu enrichment in litters as decomposition proceeded. Therefore, the P. australis in the three burial treatments acted as cation exchanger absorbing Cu from sediments or sediment water, and the strong affinity of Cu to organic matter might promote this sorption (Stumm and Morgan 1996). Except for Ni, the variations of Pb, Cr, Cu, Zn, and Mn stocks in P. australis in the S10 and S20 treatments were approximated, indicating that the strong one-off burial episodes (S20) occurred in P. australis marsh in the future would have little influence on the stocks of the five metals. With increasing burial depths, the P. australis was particularly efficient in binding Cu and Ni and releasing C, N, Pb, Cr, Zn, and Mn, implying that the potential ecotoxic risk of Pb, Cr, Zn, and Mn exposure might be very serious. This study emphasized the effects of different one-off burial episodes on nutrient and metal cycling and mass balance in the P. australis marsh of the Yellow River estuary.
References
Alicia SM, Roberto AD (2003) Decomposition of and nutrient dynamics in leaf litter and roots of Poa ligularis and Stipa gyneriode. J Arid Environ 55:503–514
Anesio AM, Abreu PC, Biddanda BA (2003) The role of free and attached microorganisms in the decomposition of estuarine macrophyte detritus. Estuar Coast Shelf S 56:197–201
Armitage PD, Bowes MJ, Vincent HM (2007) Long-term changes in macroinvertebrate communities of a heavy metal polluted stream: the river Nent (Cumbria, UK) after 28 years. River Res Appl 23(9):997–1015
Bai JH, Xiao R, Zhang KJ, Gao HF (2012) Arsenic and heavy metal pollution in wetland soils from tidal freshwater and salt marshes before and after the flow-sediment regulation regime in the Yellow River Delta, China. J Hydrol 450–451:244–253
Bai JH, Xiao R, Zhao QQ, Lu QQ, Wang JJ, Reddy KR (2014) Seasonal dynamics of trace elements in tidal salt marsh soils as affected by the flow-sediment regulation regime. PLOS ONE 9(9):e107738
Bai JH, Zhao QQ, Lu QQ, Gao ZQ, Wang JJ, Reddy KR (2015) Effects of freshwater input on trace element pollution in salt marsh soils of a typical coastal estuary, China. J Hydrol 520:186–192
Baldwin KA, Maun MA (1983) Microenvironment of Lake Huron sand dunes. Can J Bot 61:241–255
Benner R, Maccubbin AE, Hodson RE (1984) Anaerobic biodegradation of the lignin and polysaccharide components of lignocellulose and synthetic lignin by sediment microflora. Appl Environ Microbiol 47:998–1004
Berg B (1986) Nutrient release from litter anlhumus in coniferous forest soils—a mini review. Scand J Forest Res 1:359–369
Bouchard V, Lefeuvre JC (2000) Primary production and macro-detritus dynamics in a European salt marsh: carbon and nitrogen budgets. Aquat Bot 67:23–42
Breteler RJ, Teal JM, Giblin AE, Valiela I (1981) Trace element enrichments in decomposing litter of Spartina alterniflora. Aquat Bot 11:111–120
Cai XM (2000) Ecosystem Ecology. Science Press, Beijing
Cao L, Song JM, Li XG, Yuan HM, Li N, Duan LQ, Wang QD (2015) Biogeochemical characteristics of soil C, N, P in the tidal wetlands of the Yellow River Delta. Mar Sci 39(1):84–92
Chen H (1999) Root decomposition in three coniferous forest: effects of substrate quality, temperature, and moisture. Ph. D. Dissertation, Oregon State University
Chen HL (2008) Effect of Spartina alterniflora invasions on nematode communities in salt marshes of the Yangtze River estuary: patterns and mechanisms. Ph. D. Dissertation, Fudan University
Chen H (2013) Carbon sequestration, litter decomposition and consumption in two subtropical mangrove ecosystems of China. Ph. D. Dissertation, Xiamen University
Chen WF, Shi YX (2010) Distribution characteristics of microbes in new-born wetlands of the Yellow River Delta. Acta Agrest Sin 18(6):859–864
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234
Cui BS, Yang QC, Yang ZF, Zhang KJ (2009) Evaluating the ecological performance of wetland restoration in the Yellow River Delta, China. Ecol Eng 35:1090–1103
Deng ZF, An SQ, Zhao CJ, Chen L, Zhou CF, Zhi YB, Li HL (2008) Sediment burial stimulates the growth and propagule production of Spartina alterniflora Loisel. Estuar Coast Shelf S 76:818–826
Dick TM, Osunkoya OO (2000) Influence of tidal restriction floodgates on decomposition of mangrove litter. Aquat Bot 68:273–280
Dorgelo J, Meester H, Vanvelzen C (1995) Effects of diet and heavy metals on growth rate and fertility in the deposit-feeding snail Potamopyrgus jenkinsi (Smith) (Gastropoda: Hydrobiidae). Hydrobiologia 316:199–210
Du Laing G, Bogaert N, Tack FMG, Verloo MG, Hendrickx F (2002) Heavy metal contents (Cd, Cu, Zn) in spiders (Pirata piraticus) living in intertidal sediments of the river Scheldt estuary (Belgium) as affected by substrate characteristics. Sci Total Environ 289:71–81
Du Laing G, Van Ryckegem G, Tack FMG, Verloo MG (2006) Metal accumulation in intertidal litter through decomposition leaf blades, sheaths and stems of Phragmites australis. Chemosphere 63:1815–1823
Foote AL, Reynolds KA (1997) Decomposition of salt meadow cordgrass (Spartina patens) in Louisiana coastal marshes. Estuaries 20:579–588
Freeman C, Ostle NJ, Fenner N, Kang H (2004) A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol Biochem 36:1663–1667
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Gessner MO (2000) Breakdown and nutrient dynamics of submerged Phragmites shoots in the littoral zone of a temperate hardwater lake. Aquat Bot 66(1):9–20
Gessner MO (2001) Mass loss, fungal colonization and nutrient dynamics of Phragmites australis leaves during senescence and early aerial decay. Aquat Bot 69(2–4):325–339
Guan YZ (2013) Responses of decomposition of Phragmites australis litter to simulated temperature enhancement in coastal wetland. Master degree dissertation, East China Normal University
Harmon ME, Baker GA, Spycher G, Greene SE (1990) Leaf litter decomposition in the Picea-Tsuga forest of Olympic National Park, Washington, USA. Eur J Soil Biol 31:55–66
Hobbic SH (1996) Temperature and plant species control over litter decomposition in Alaskan Tundra. Ecol Monogr 66:503–522
Hodson RE, Christian RR, Maccubbin AE (1984) Lignocellulose and lignin in the salt marsh grass Spartina alterniflora: initial concentrations and short-term, post-depositional changes in detrital matter. Mar Biol 81:1–7
Huang LN, Lan CY, Shu WS (2001) Leaf decomposition of two species in a mangrove community in Futian of Shenzhen. Chin J Appl Ecol 12(1):35–38
Keuskamp JA, Hefting MM, Dingemans BJJ, Verhoeven JTA, Feller I (2015) Effects of nutrient enrichment on mangrove leaf litter decomposition. Sci Total Environ 508:402–410
Köchy M, Wilson SD (1997) Litter decomposition and nitrogen dynamics in Aspen forest and mixed-grass prairie. Ecology 78(3):732–739
Kovacova S, Sturdik E (2002) Interactions between microorganisms and heavy metals including radionuclides. Biologia 57:651–663
Kristensen E, Blackburn T (1987) The fate of organic carbon and nitrogen in experimental marine sediment systems: influence of bioturbation and anoxia. J Mar Res 45:231–257
Laiho R, Laine J, Trettin CC, Finer L (2004) Scots pine litter decomposition along drainage succession and soil nutrient gradients in peatland forests, and the effects of inter-annual weather variation. Soil Biol Biochem 36:1095–1109
Larsen VJ, Schierup H (1981) Macrophyte cycling of zinc, copper, lead, and cadmium in the littoral zone of a polluted area and a non-polluted lake: II. Seasonal changes in heavy metal content of above-ground biomass and decomposing leaves of Phragmites australis (Cav.) Trin. Aquat Bot 11:211–230
Ledin M (2000) Accumulation of metals by microorganisms processes and importance for soil systems. Earth-Sci Rev 51:1–31
Li JR (2011) Macrobenthic ecology of the intertidal zones of Yellow River Delta. Master degree dissertation, Ocean University of China
Li T, Ye Y (2014) Dynamics of decomposition and nutrient release of leaf litter in Kandelia obovata mangrove forests with different ages in Jiulongjiang estuary, China. Ecol Eng 73:454–460
Li YF, Huang YL, Li SK (1991) A primarily analysis on the coastal physiognomy and deposition of the modern Yellow River Delta. Acta Oceanol Sin 13(5):662–671
Maun MA (1998) Adaptations of plants to burial in coastal sand dunes (1997 George Lawson medal review). Can J Bot 76:713–738
Mendelssohn IA, Sorrell BK, Brix H, Schierup HH, Lorenzen B, Maltby E (1999) Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquat Bot 64:381–398
Menéndez M, Sanmartí N (2007) Geratology and decomposition of Spartina versicolor in a brackish Mediterranean marsh. Estuar Coast Shelf S 74:320–330
Ming-Yi S, Lee C, Aller RC (1993) Laboratory studies of oxic and anoxic degradation of chlorophyll-a in Long Island Sound sediments. Geochim Cosmochim Acta 57:147–157
Mou XJ (2010) Study on the nitrogen biological cycling characteristics and cycling model of tidal wetland ecosystem in Yellow River estuary. Master degree dissertation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai
Neckles HA, Neill C (1994) Hydrologic control of litter decomposition in seasonally flooded prairie marshes. Hydrobiologia 286:155–165
Nielsen T, Andersen FØ (2003) Phosphorus dynamics during decomposition of mangrove (Rhizophora apiculata) leaves in sediments. J Exp Mar Biol Ecol 293:73–88
Olson JS (1963) Energy storage and the balance of products and decomposers in ecological systems. Ecology 44(2):322–331
Pereira P, Caçador I, Vale C, Caetano M, Costa AL (2007) Decomposition of belowground litter and metal dynamics in salt marshes (Tagus Estuary, Portugal). Sci Total Environ 380:93–101
Quintino V, Sangiorgio F, Ricardo F, Mamede R, Pires A, Freitas R, Rodrigues AM, Basset A (2009) In situ experimental study of reed leaf decomposition along a full salinity gradient. Estuar Coast Shelf S 85:497–506
Ramos e Silva CA, Oliveira SR, Rêgo RDP, Mozeto AA (2007) Dynamics of phosphorus and nitrogen through litter fall and decomposition in a tropical mangrove forest. Mar Environ Res 64:524–534
Robertson AI (1988) Decomposition of mangrove leaf litter in tropical Australia. J Exp Mar Biol Ecol 116(3):235–247
Rui YK, Qu LC, Kong XB (2008) Effects of soil use along Yellow River basin on the pollution of soil by heavy metals. Spectrosc Spect Anal 28:934–936
Sakan S, Dordević DS, Manojlović DD, Predrag PS (2009) Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J Environ Manage 90(11):3382–3390
Sánchez-Andrés R, Sánchez-Carrillo S, Alatorre LC, Cirujano S, Álvarez-Cobelas M (2010) Litterfall dynamics and nutrient decomposition of arid mangroves in the Gulf of California: their role sustaining ecosystem heterotrophy. Estuar Coast Shelf S 89:191–199
Shao XX, Liang XQ, Wu M, Ye XQ, Jiang KY (2014) Decomposition and phosphorus dynamics of the litters in standing and litterbag of the Hangzhou Bay coastal wetland. Environ Sci 35(9):3381–3388
Sheng HX (2009) Studies on dynamics of heavy metal with decomposition of litter fall in mangrove wetland at Jiulongjiang River estuary. Master degree dissertation, Xiamen University
Simões MP, Calado ML, Madeira M, Gazarini LC (2011) Decomposition and nutrient release in halophytes of a Mediterranean salt marsh. Aquat Bot 94:119–126
Stumm W, Morgan JJ (1996) Aquatic chemistry—chemical equilibria and rates in natural waters, 3rd edn. Wiley, USA
Sun WG (2015) Effects of ecological restoration projects on the key nitrogen cycling processes in wetlands of the Yellow River estuary. China. Master degree dissertation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai
Sun ZG, Mou XJ, Liu JS (2012a) Effects of flooding regimes on the decomposition and nutrient dynamics of Calamagrostis angustifolia litter in the Sanjiang Plain of China. Environ Earth Sci 66: 2235–2246
Sun Z.G, Mou XJ, Sun JK, Song HL, Yu X, Wang LL, Jiang HH, Sun WL, Sun WG (2012b) Nitrogen biological cycle characteristics of seepweed (Suaeda salsa) wetland in intertidal zone of Huanghe (Yellow) River estuary. Chin Geogra Sci 22(1):15–28
Sun WG, Gan ZT, Sun ZG, Li LL, Sun JK, Sun WL, Mou XJ, Wang LL (2013) Spatial distribution characteristics of Fe and Mn contents in the new-born coastal marshes in the Yellow River estuary. Environ Sci 34(11):275–282
Sun ZG, Mou XJ, Wang LL, Sun WL, Sun WG (2015) Effects of sedimentation intensity on decomposition and nitrogen dynamics of Suaeda salsa litters in salt marshes in tidal bank of the Yellow River estuary. Wetland Sci 13(2):135–144
Sundby B, Caetano M, Vale C, Gobeil C, Luther G, Nuzzio D (2005) Root-induced cycling of lead in salt marsh sediments. Environ Sci Technol 39(7):2080–2086
Tam NFY, Wong YS, Lan CY, Wang LN (1998) Litter production and decomposition in a subtropical mangrove swamp receiving wastewater. J Exp Mar Biol Ecol 226:1–18
The Committee of Agro-chemistry of the Chinese Society of Soil Science (1983) The conventional analysis methods in soil agro-chemistry. Science Press, Beijing
Tong C, Zhang LH, Wang WQ, Gauci V, Marrs R, Liu BG, Jia RX, Zeng CS (2011) Contrasting nutrient stocks and litter decomposition in stands of native and invasive species in a sub-tropical estuarine marsh. Environ Res 111:909–916
Vandecasteele B, Meers M, Vervaeke P, De Vos B, Quataert P, Tack FMG (2005) Growth and trace metal accumulation of two Salix clones on sediment-derived soils with increasing contamination levels. Chemosphere 58:995–1002
Vargo SM, Neely RK, Kirkwood SM (1998) Emergent plant decomposition and sedimentation: response to sediments varying in texture, phosphorus content and frequency of deposition. Environ Exp Bot 40:43–58
Wang ZY, Xin YZ, Li FM, Gao DM (2009) Microbial community characteristics in a degraded wetland of the Yellow River Delta. Period Ocean Univ Chin 39(5):1005–1012
Wang Y, Liu RH, Fang DJ, Yu P, Wang JY, Tang AK (2013) Distribution and accumulation characteristics of heavy metals in sediments in southern sea area of Huludao City, China. Chin Geogra Sci 23(2):194–202
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. An Re Eco System 17:567–594
Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Envion Int 30:685–700
Weis JS, Windham L, Santiago-Bass C, Weis P (2002) Growth, survival, and metal content of marsh invertebrates fed diets of detritus from Spartina alterniflora Loisel. and Phragmites australis Cav. Trin. Ex Steud. From metal-contaminated and clean sites. Wetlands Ecol Manag 10:71–84
White DS, Howes BL (1994) Nitrogen incorporation into decomposition litter of Spartina alterniflora. Limnol Oceanogr 39:133–140
Williams TP, Bubb JM, Lester JN (1994) Metal accumulation within salt marsh environment: a review. Mar Pollut Bull 28:277–290
Windham L, Weis JS, Weis P (2004) Metal dynamics of plant litter of Spartina alterniflora and Phragmites australis in metal-contaminated salt marshes. Part 1: patterns of decomposition and metal uptake. Environ Toxicol Chem 23:1520–1528
Wu QQ, Ma QM, Wang JG, Jiang ZH, Wang X (2007) The AVS in surface sediment of near sea area of Huanghe estuary. Mar Environ Sci 26(2):126–129
Xiao R, Bai JH, Lu QQ, Zhao QQ, Gao ZQ, Wen XJ, Liu XH (2015) Fractions, transfer, and ecological risks of heavy metals in riparian and ditch wetlands across a 100-year chronosequence of reclamation in an estuary of China. Sci Total Environ 517:66–75
Xu XG, Guo HH, Chen XL, Lin HP, Du QL (2002) A multi-scale study on land use and land cover quality change: the case of the Yellow River Delta in China. GeoJournal 56(3):177–183
Zawislanski PT, Chau S, Mountford H, Wong HC, Sears TC (2001) Accumulation of selenium and trace metals on plant litter in a tidal marsh. Estuar Coast Shelf S 52:589–603
Zhang YL, Lin P (1998) Changes of mass and energy in decomposing course of Kandelia candel root in Jiulongjiang River estuary. J Nanjing Forest Univ 22(4):47–50
Zhang HG, Cui BS, Xiao R, Zhao H (2010) Heavy metals in water, soils and plants in riparian wetlands in the Pearl River Estuary, South China. Procedia Environ Sci 2:1344–1354
Zhang LH, Tong C, Marrs R, Wang TE, Zhang WJ, Zeng CS (2014) Comparing litter dynamics of Phragmites australis and Spartina alterniflora in a sub-tropical Chinese estuary: contrasts in early and late decomposition. Aquat Bot 117:1–11
Zhao QQ, Bai JH, Liu PP, Gao HF, Wang JJ (2015) Decomposition and carbon and nitrogen dynamics of Phragmites australis litter affected by flooding periods in coastal wetland. Clean-Soil Air Water 43(3):441–445
Zhou JL, Wu Y, Zhang J, Sun CX (2006) Study on putrefaction and decomposition process of Scirpus triqueter on the Changjiang estuary tidal flat. Adv Mar Sci 24(1):44–50
Zhuang TC, Lin P (1993) Soil microbial amount variations of mangroves (Kandelia candel) in process of natural decomposition of litter leaves. J Xiamen Univ (Nat Sci) 32(3):365–370
Acknowledgments
This study was financially supported by the National Nature Science Foundation of China (No. 41171424, 41371104), the Award Program for Min River Scholar in Fujian Province, and the Program for New Century Excellent Talents in Fujian Province University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sun, Z., Mou, X. Effects of sediment burial disturbance on macro and microelement dynamics in decomposing litter of Phragmites australis in the coastal marsh of the Yellow River estuary, China. Environ Sci Pollut Res 23, 5189–5202 (2016). https://doi.org/10.1007/s11356-015-5756-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5756-0