Abstract
Significant knowledge gaps exist regarding the fate and transport of persistent organic pollutants like dichlorodiphenyltrichloroethane (DDT) in tropical environments. In Brazil, indoor residual spraying with DDT to combat malaria and leishmaniasis began in the 1950s and was banned in 1998. Nonetheless, high concentrations of DDT and its metabolites were recently detected in human breast milk in the community of Lake Puruzinho in the Brazilian Amazon. In this work, we couple analysis of soils and sediments from 2005 to 2014 at Puruzinho with a novel dynamic floodplain model to investigate the movement and distribution of DDT and its transformation products (dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD)) and implications for human exposure. The model results are in good agreement with the accumulation pattern observed in the measurements, in which DDT, DDE, and DDD (collectively, DDX) accumulate primarily in upland soils and sediments. However, a significant increase was observed in DDX concentrations in soil samples from 2005 to 2014, coupled with a decrease of DDT/DDE ratios, which do not agree with model results assuming a post-ban regime. These observations strongly suggest recent use. We used the model to investigate possible re-emissions after the ban through two scenarios: one assuming DDT use for IRS and the other assuming use against termites and leishmaniasis. Median DDX concentrations and p,p′-DDT/p,p′-DDE ratios from both of these scenarios agreed with measurements in soils, suggesting that the soil parameterization in our model was appropriate. Measured DDX concentrations in sediments were between the two re-emission scenarios. Therefore, both soil and sediment comparisons suggest re-emissions indeed occurred between 2005 and 2014, but additional measurements would be needed to better understand the actual re-emission patterns. Monte Carlo analysis revealed model predictions for sediments were very sensitive to highly uncertain parameters associated with DDT degradation and partitioning. With this model as a tool for understanding inter-media cycling, additional research to refine these parameters would improve our understanding of DDX fate and transport in tropical sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the original persistent organic pollutants (POPs) listed under the Stockholm Convention, DDT (dichlorodiphenyltrichloroethane) use is currently restricted to indoor residual spraying (IRS) for malaria vector control in countries where other locally affordable, safe, and effective options are not available or insecticide resistance has developed against alternative pesticides (WHO 2012). In 2014, nearly 3.3 billion people worldwide were at risk of malaria, which is endemic to tropical countries, including Brazil. In Brazil, DDT has been used since 1945 in agriculture and for indoor residual spraying (Torres et al. 2009). Agricultural use in Brazil was banned in 1985, and DDT use for vector control was reduced in the mid-1990s and definitively prohibited by 2009 (Ferreira et al. 2011). However, because of its associated health risks (Bouwman et al. 2011) and persistence, DDT remains an important issue in areas with former use. In a traditional Amazonian settlement around Lake Puruzinho, in the Madeira River Basin, biomonitoring studies in 2004 detected high concentrations of DDT and its transformation products (referred to hereafter as total DDX) in human breast milk (Azeredo 2007). These concentrations were nearly ten times higher than those measured from 2001 to 2003 in 20 other communities in the Madeira River Basin with a similar history of IRS, but within the range of values found for women living in houses with ongoing IRS treatment in the tropics worldwide (Ritter et al. 2011; WHO 2011). When this DDT content is transformed into infant daily intakes as described by Mariën and Laflamme (1995), the mean intake at Puruzinho is 40 μg kg−1 day−1 (range 14–53 μg kg−1 day−1), which is four times higher than the WHO acceptable daily intake for DDT (FAO and WHO 2000). Mothers in Puruzinho traditionally breastfeed for up to 2 years, increasing infant exposure to DDT. This is worrisome given the growing body of literature documenting the potential adverse health effects of DDT and its metabolites, which include the disruption of neural and physical development of children, breast cancer, diabetes, and spontaneous abortion (Bouwman and Kylin 2009; Eskenazi et al. 2009; Longnecker 2005).
Human exposure to DDT used for IRS can occur through multiple pathways: through inhalation and dermal exposure to the indoor air and dust and also through direct and indirect interactions with DDT that has reached the outdoor environment, such as dietary items grown in contaminated soils. Indigenous riverine communities in the Amazon subsist on manioc and fruit cultivated around the houses and gathered from the surrounding forest and fish from local water bodies (Azeredo et al. 2008; Oliveira et al. 2010). Thus, it is important to characterize the level of contamination in the soils, water, air, and sediments in the environment around the IRS-treated houses and to understand the fate and transport of DDT in this local environment. In Brazil, only a few studies are available with DDT measurements in multiple media (soils and sediments) in areas with a history of IRS. However, usually only one data point is given at a single location (Torres et al. 2002), or multiple data points are available for only one time point (Saldanha et al. 2010) or for time points that are too close to observe trends in DDX concentrations and the effects of changes in emissions on environmental concentrations (Vieira et al. 2001). This scarce information results in an incomplete understanding of the fate and transport of DDT used in IRS for malaria vector control in rural communities in Brazil. In Puruzinho, only one study assessing DDX contamination was carried out in 2005, which found DDX residues in soils, sediments, and fish (Saldanha et al. 2010). Therefore, here, we develop a novel multimedia model for the Puruzinho environment and combine it with additional DDX measurements to gain insight into the pathways that have led to DDT accumulation in different environmental media in Puruzinho and have potentially contributed to high DDT levels in humans.
Although several multimedia models have been developed to describe the fate of persistent organic pollutants in lakes and lagoons (Hollander et al. 2006; Sommerfreund et al. 2010; Whelan 2013), most of these models describe temperate environments and are at steady state. Those that are dynamic neglect the seasonality of the hydrological cycle (Wania et al. 2006; Xia et al. 2011) or do not explicitly model the water body involved in flooding (Camenzuli et al. 2012). We developed a dynamic multimedia environmental model that includes floodplain and water compartments whose volumes change continuously throughout the year. The goal of this model is to capture the highly variable environmental conditions, particularly rainfall and dramatic changes in water levels, that characterize tropical floodplain lakes and to assess their effects on the distribution and mobilization of DDT and its metabolites. The model describes the degradation of DDT into DDE (dichlorodiphenyldichloroethylene) and DDD (dichlorodiphenyldichloroethane) and tracks the fate of these transformation products. We measured DDX concentrations in soil samples collected in 2005, 2011, and 2014 and sediment samples from 2011 to 2012 and compared them to model predictions. By coupling the multimedia environmental model with field measurements, our aim is to describe DDT accumulation in different media and identify the most important processes governing the fate and transport of DDX. This work will also add to a small but growing body of literature that seeks to improve our understanding of the fate and transport of persistent organic pollutants in tropical ecosystems.
Methods
Study site
Puruzinho is a remote, rural village located in the municipality of Humaitá, in the southern part of the State of Amazonas, Brazil. The area of study is around 8 km long and is inhabited by 170 people living in 20 houses scattered around a lake of approximately 3.5 km2. Lake Puruzinho is fed by three black-water tributaries; outflow from the lake goes through a small channel to the Madeira River, a primary tributary of the Amazon River. A map of the site is presented in the Supplementary Material (SM), Fig. S1.
As a result of precipitation and interactions with the Madeira River, the water level of Lake Puruzinho varies from 1 m during low water season (June to October) to 12 m during high water season (December to May) (Almeida 2006; Azevedo e Silva 2011). As water levels rise, a floodplain area, consisting of bare soil and low-lying forest, floods. The houses are located on upland (“terra-firme”) soils that are rarely inundated. Lake Puruzinho behaves like a lentic aquatic system during extreme low and high water events and like a lotic system during the transition periods (Saldanha et al. 2010).
Model setup and parameterization
Environmental description
A dynamic multimedia environmental fate model was developed based on the fugacity approach (Mackay 2001). The Puruzinho environment was characterized using six well-mixed compartments: air, water, sediment, flooded floodplain soil, aerated (non-flooded) floodplain soil, and upland terra-firme soil. Chemicals are transported across the compartments through intermedia exchange processes and are lost within each compartment as a result of advective processes and degradation, as depicted in Fig. 1. Intermedia transfer processes and advective removal processes are described by mass transfer coefficients, while degradation processes are described by first-order rate constants. Time-dependent mass balances for each compartment were solved through numerical integration to take into account variability in environmental input parameters and DDT emissions through time. Lake Puruzinho is a floodplain lake whose water levels change dramatically throughout the year, which affects pesticide transport processes such as runoff and aerosol deposition, and results in a dynamic floodplain whose area and composition changes seasonally. Thus, in the model, the floodplain was characterized as two compartments: a flooded floodplain whose area increases as water levels rise and an aerated floodplain whose area increases as water levels fall. It was assumed that, when aerated, the floodplain behaves like a soil compartment and, when flooded, like a sediment compartment. Details on the mathematical description of all processes and mass balances are found in SM section S2.4.
Model input parameters describing the environment, mass transfer processes, and chemical loss processes included values measured or calculated specifically for Puruzinho whenever possible or default values sourced from literature otherwise. Values for all parameters are given in Table S4. It was assumed that runoff from the terra-firme soil went directly to the water compartment, bypassing the floodplain (Camenzuli et al. 2012). The organic carbon content of the entire floodplain was estimated to be between that of the terra-firme soil and that of the sediment. We measured the concentration of organic carbon in soil and sediment as well as the water level of the lake (see “Field measurements” section). An average of water level measurements from October 2011 to June 2013 was used in the model for the time during which data were not available. The concentration of suspended particles in water was available from a previous study carried out in the dry season (Almeida 2006). Seasonal changes in turbidity in floodplain lakes in the Brazilian Amazon have been attributed to changes in suspended solids concentrations (Alcântara et al. 2010; Moreira-Turcq et al. 2004). High turbidity during low and decreasing water levels is attributed to a higher influence of the wind on sediment re-suspension and to bioturbation. As water levels rise, particle settling is less disrupted and turbidity decreases. We correlated turbidity measurements (transparency using a Secchi disk) from Puruzinho in the wet and dry seasons to the concentration of suspended solids in order to represent this seasonal variability. The atmospheric aerosol load in the Brazilian Amazon also depends on the season. Higher aerosol concentrations are observed in the dry season as a result of lower precipitation and biomass burning (Artaxo et al. 1998). As Puruzinho is a remote site in Southern Amazonas, close to the border with the state of Rondônia, aerosol concentrations measured in the wet and dry season in the Jaru Biological Reserve in Rondônia were used in the model (Artaxo et al. 2002). We used 10-day average rainfall data and monthly average wind speed and direction data available from 1940 to 1990 for Humaitá, the nearest large settlement (located approximately 13 km away from lake Puruzinho) (INMET 2012). Daily, intermittent rainfall was simulated by distributing the 10-day averages based on the rain pattern in 2009 in Humaitá, for which daily resolved data were available (INMET 2015). The median monthly temperature in all stations in the state of Amazonas monitored by the Brazilian Meteorological Services from 1961 to 1990 was 25.6 °C, ranging from 24 to 28.5 °C (INMET 2012). A constant temperature of 25 °C was used in the model.
The lake dimensions at minimum water level and the width of the terra-firme soil were based on a map created from NASA Landsat Images for the dry season by the Program for the Estimation of Deforestation in the Brazilian Amazon (Fig. S1). These parameters were combined with water level measurements and local observations regarding the lake geomorphology to define the areas of the floodplain that were aerated and flooded and the changing volume of the lake (SM section S2.1). The volumes of the sediment and terra-firme soil remained constant throughout the model run. Since discharge data were not available for the tributaries feeding lake Puruzinho, the water advection was calculated based on the changes in the water volume, which were characterized by a filling period, when the lake volume increases and there is no advection out of the lake, and an emptying period, when the lake volume decreases and advection out of the lake occurs.
Emissions
Concentrations of DDT in background (remote and rural) sites in Brazil are generally low, as evidenced by monitoring campaigns that yield air concentrations ranging from 0.001 to 10 pg/m3 (Bogdal 2012; Bogdal et al. 2013; Meire et al. 2012; Pozo et al. 2008). Therefore, it was assumed that background DDT inputs into the study site were negligible compared to inputs through in situ emissions from IRS. In Puruzinho, DDT was only used for malaria vector control (Azeredo 2007). Since the Campaign for the Eradication of Malaria began in 1965 (Loiola et al. 2002) and the earliest data available on DDT production and imports in Brazil were from 1959 to 1962 (Almeida et al. 2007; Barra et al. 2006; GEF 2006), we assumed that starting in 1960, 2 g/m2 of active ingredient were applied to the inside walls of the houses twice per year, as recommended by the WHO (WHO 2013). The houses in Puruzinho have a wooden structure, with spaces between the floor boards, which can result in direct contamination of the soil (Azeredo 2007; Loiola et al. 2002). It was assumed that when DDT was sprayed, 10 % of the emissions occurred into the soil and the rest remained on the walls, based on a WHO generic model for exposure during IRS (WHO 2011). DDT was then continuously released from the walls into the air until the next application. In 1992, use of DDT for vector control was restricted to leishmaniasis (Azeredo et al. 2008). Although the final ban of DDT use, production, commercialization, and import occurred in 2009 (Ferreira et al. 2011), DDT use for vector control was prohibited in Brazil between 1997 and 1998 (Almeida et al. 2007; Ferreira et al. 2011; GEF 2006). Thus, in our baseline scenario, we assume that IRS in Puruzinho ceases after 1998.
There is substantial uncertainty regarding the possibility of continued use after 1998. Therefore, we created two additional scenarios to investigate possible DDT re-emissions after a peak in malaria incidence in the Amazon in 2005, with most cases occurring in rural communities and indigenous areas in the state of Amazonas (Oliveira-Ferreira et al. 2010; SVS 2013). The first re-emissions scenario assumes that DDT is applied in the same manner as it was used for IRS (90 % of the emissions occur into the air and 10 % into the soil). The second re-emission scenario assumes greater inputs into the soil as a result of potential spraying of DDT on the outside walls to control leishmaniasis (Vieira et al. 2001) and direct application of DDT on the soil to control termites (Kenneth 2003; Su et al. 1999). In this scenario, it was assumed that 90 % of the emissions occurred into the soil and 10 % into the air. This is a default value used in the Netherlands for registration of pesticides that are sprayed on crops in the soil (Linders et al. 2000), and its use has resulted in good agreement between multimedia model results and measured data (Becker et al. 2011; Linders et al. 2000; Schenker et al. 2008).
To capture the history of DDT use for IRS in Puruzinho, and the fate of DDT and its metabolites in the post-ban era, all model scenarios were run from 1960 to 2014.
Chemical properties
Our model tracks the p,p′-isomer of DDT, which constitutes 75 % of the active ingredient, and its degradation products: p,p′-DDE and p,p′-DDD. Partition coefficients for p,p′-DDT and its metabolites and their degradation half-lives (t 1/2) in soil were obtained from Schenker et al. (2008). Studies on DDT dissipation in the water under tropical conditions show that degradation can be faster (Zayed et al. 1994a, b) or slower (Hussain et al. 1994a; Hussain et al. 1994b) in water than in the soil. A biodegradability assessment of grab sample data from chemicals in the BIODEG and CHEMFATE databases also found inconclusive evidence about the relationship between aerobic degradation rates in freshwater and surface soils and therefore suggests that when no chemical-specific information is available, no assumption can be made as to whether degradation in water is faster or slower than in soil (Boethling et al. 1995). Hence, in the model, it was assumed that the degradation rate of DDT and its metabolites in the water was the same as in the terra-firme soil. In sediments, only scarce measurements spanning large ranges (from 6 months to 21 years) are available for DDT degradation (Kuo-Ching Ma et al. 2006), and none is available for DDE and DDD. In tropical and subtropical environments, enhanced degradation of DDT has been observed in flooded soils and in soils with alternating aerobic-anaerobic conditions, when compared to unflooded soils (Foght et al. 2001; Kenneth 2003; Lawrence Boul 1996; Wang et al. 2007). Based on these studies, we assumed that the degradation of DDT and its transformation products in the sediment was six times faster than in the terra-firme soil (see SM section S2.3 for details). We also assumed that the degradation half-lives in the flooded floodplain were the same as in the sediments, and the degradation half-lives in the aerated floodplain were the same as in the terra-firme soil. Degradation half-lives in the air were estimated using the AOPWin Program from Epi Suite v. 4.11 (USEPA 2014) for reaction with OH radicals and annual zonal average OH radical concentrations for 5°S from Bahm and Khalil (2004). Degradation half-lives and partition coefficients for p,p′-DDT, p,p′-DDE, and p,p′-DDD are given in Table S3.
DDT degradation into DDE and DDD in the different compartments was described in terms of fractions of formation (Fenner et al. 2000). It was assumed that the isomeric structure was conserved during degradation (Nowell 1999). DDT is primarily biotransformed by reductive dechlorination into DDD under anaerobic conditions and to DDE by dehydrochlorination under aerobic conditions (Aislabie et al. 1997; Eggen and Majcherczyk 2006; Foght et al. 2001; Pereira et al. 1996; Ricking and Schwarzbauer 2012). In addition, 14C-DDT dissipation studies in tropical and subtropical soils have found that under field and laboratory conditions, DDE is the main product formed in non-flooded soils exposed to light (Hussain et al. 1994c; Kadir 1988; Lalah et al. 1994; Sjoeib et al. 1994; Stephens et al. 1994; Tayaputch 1988; Zayed et al. 1994b), while DDD is the main metabolite formed under flooded conditions (Lawrence Boul 1996; Samuel and Pillai 1988; Xu et al. 1994). Therefore, we assume that under aerobic conditions, DDT degrades into 70 % DDE and 30 % DDD, while under anaerobic conditions, DDT degrades into 30 % DDE and 70 % DDD. In the model, the terra-firme soils are always aerobic, while sediments and flooded floodplain soils alternate between aerobic and anaerobic states. The onset of aerobic and anaerobic conditions in sediments and flooded floodplains was determined based on dissolved oxygen measurements in the water column available from previous measurement campaigns (Almeida 2006; Azevedo e Silva 2011). In the air, we assumed that 90 % of the DDT degrades into DDE and the rest is mineralized, following Schenker et al. (2007).
Sensitivity and uncertainty analysis
A preliminary one-factor-at-a-time sensitivity analysis was carried out to identify the input parameters with the greatest influence on the model results, so that efforts could then be directed toward quantifying these parameters and their uncertainties, as suggested by Buser et al. (2012). Each input parameter was varied by 10 %, while all other parameters remained constant. Sensitivity factors were calculated as the changes in output concentrations divided by the change in the value of input parameters.
Based on the sensitivity analysis, all parameters with a sensitivity factor greater than 0.5 were chosen for further probabilistic uncertainty analysis. Monte Carlo simulations were performed to quantify the uncertainty in the concentrations of DDX in all media arising from the uncertainty of the selected input parameters. 1000 Monte Carlo simulations were carried out, and it was assumed that uncertainties in input parameters were uncorrelated. Details on the uncertainty distributions used in the simulations can be found in Table S7. The parameters with the greatest contribution to the output variance were identified using Spearman’s rank correlations (MacLeod et al. 2002; Schenker et al. 2009).
Field measurements
Water level measurements
The water levels of Lake Puruzinho were measured from October 2011 to June 2013 using a series of aluminum rulers (1 m height) mounted vertically on wooden boards and placed in the lake during the dry season. Twelve additional rulers were installed at one meter intervals along a cross-sectional transect of the lake.
Soil and sediment analysis
Surface soil samples collected in 2011 (n = 7) and 2014 (n = 8), and surface sediment samples collected in 2011 (n = 6) and 2012 (n = 2) were analyzed for p,p′-/o,p′-DDT and their main metabolites p,p′-/o,p′-DDE, and p,p′-/o,p′-DDD. Soil samples obtained from a previous measurement campaign in 2005 (n = 7, Saldanha et al. 2010) were also analyzed. Soil samples were collected next to the oldest houses in Puruzinho and from forested areas near the houses. Two of the 2011 sediment samples were collected from the flooded floodplain next to forested area and one sediment sample was collected from the center of the lake. The remaining sediment samples were collected from the flooded floodplain at the edge of the lake closest to the houses where the soil samples were taken. The total organic carbon content (TOC) of soil and sediment samples was measured with a TOC analyzer equipped with a solid sample combustion unit.
2011 soil and sediment samples were analyzed in the laboratories at the Federal University of Rio de Janeiro. Samples were Soxhlet extracted, desulfurized, and cleaned up with Florisil chromatography. An internal standard (2,4,5,6-tetrachloro-m-xylene) was added to the purified extracts and target analytes were detected and quantified by gas chromatography with an electron capture detector.
Soil and sediment samples from 2005, 2012, and 2014 were prepared and analyzed in the laboratories at ETH Zurich, following the procedure of Bogdal et al. (2008). In brief, samples were spiked with isotope-labeled 13C12-p,p′-DDT as internal standard, Soxhlet extracted and cleaned up with silica gel chromatography. 13C-hexachlorobenzene was added to the purified extracts as a recovery standard and samples were analyzed for DDX by gas chromatography coupled to electron ionization high-resolution mass spectrometry. Additional details on sample analysis are given in SM section S1.
Statistically significant differences between DDX concentrations in soils from different years were confirmed with the Wilcoxon rank-sum test, a non-parametric test deemed more suitable for the sample size. It was not possible to apply this test for sediments, as only a few measurements were available. The results from all soil and sediment measurements are reported on a dry weight (dw) basis.
Results and discussion
For convenience, the following conventions are used: DDX refers to the sum of all isomers of DDT, DDE, and DDD; p,p′-DDX refers to the sum of p,p′-DDT and its main metabolites p,p′-DDE, and p,p′-DDD; DDT refers to the sum of p,p′- and o,p′-DDT; DDE refers to the sum of p,p′- and o,p′-DDE; and DDD refers to the sum of p,p′- and o,p′-DDD.
Model (baseline scenario)
DDX distribution in the environment
Long-term trend of DDX
The fate and transport of DDX in Puruzinho is highly dependent on the total amount of DDT that reaches the outdoor environment and the environmental media into which DDT is emitted. Concentrations of p,p′-DDX in all compartments follow the emissions trend (2 g DDT/m2 of indoor wall surface were applied from 1960 to 1998 and no emissions occurred after 1998) throughout the model run (Fig. 2). The concentration of p,p′-DDX increases rapidly after the first DDT application. Steady state is reached within a few days in the air and water and after 2 years in sediments, 3 years in floodplain soils, and 15 years in the terra-firme soil. Once steady state is reached and while emissions remain constant, the concentrations of p,p′-DDX in all compartments oscillate seasonally around a constant value. When emissions cease, p,p′-DDX concentrations in all compartments decrease, with the slowest response in the terra-firme soil.
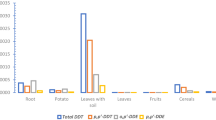
Median p,p′-DDT concentrations in the model compartments (lines) throughout the model run from 1960–2014 and median measured concentrations in the soils (circles) and sediments (squares). The uncertainty bands of the model results show the 95 % confidence intervals, while whiskers show the range in the measurements. 2005 forest soil data is from Saldanha et al. (2010), and its lower whisker represents the LOD. Concentrations in flooded and aerated floodplains were similar, so only the flooded floodplain is shown
Partitioning and distribution of DDT
Even though most of the p,p′-DDT was assumed to be emitted into the air, the model predicts the highest amounts of p,p′-DDT in soil and sediment throughout the modeled time span. When steady state is reached in all compartments, before the ban, 73 % of the p,p′-DDT is in the terra-firme soil and 23 % in the sediment. Two years after the ban, the terra-firme soil remains the main repository of p,p′-DDT, with 97 % of the DDT mass, followed by the sediments with 1.4 % of the mass. p,p′-DDT has a very high octanol-water partition coefficient (K ow = 106.41), resulting in a preferential partitioning into the organic carbon in soil and sediment solids. The organic content of soils in Puruzinho is twice as high as that of sediments; hence, higher accumulation occurs in the soil. In addition, 10 % of the emissions occur directly into the soil, while p,p′-DDT can only be transported to the sediments indirectly through the settling of suspended particles from the water column and to a lesser extent by diffusion from the water into sediment pore water. Much less p,p′-DDT accumulates in the air and the water compared to the soil and sediment, as p,p′-DDT in these compartments is mainly associated with particles (suspended solids in water and aerosols in air), which constitute only a small fraction of the bulk compartment volumes and which are ultimately deposited to the soil and sediments. Furthermore, p,p′-DDT degradation occurs much faster in air (t 1/2 = 1.4 days) and sediments (t 1/2 = 171 days) than in soil (t 1/2 = 1025 days).
We also tested the assumption that 100 % of the DDT applied to the walls is released into the air. Under this assumption, DDT still accumulates to a much larger extent in the terra-firme soil and sediment than in the air and water compartment. However, DDX concentrations in soils and sediments before the ban are nearly equal under this scenario (Fig S4). While no data are available in Puruzinho pre-ban, in other rural communities in Brazil, DDX concentrations in outdoor soils were 1–3 orders of magnitude higher than those in sediments while emissions were potentially ongoing (Torres et al. 2009; Torres et al. 2002). This is in much better agreement with our original assumption that 10 % of the sprayed DDT goes into the soil. Hence, the following discussion will refer to the scenario with 90 % emissions from sprayed walls to air and 10 % emissions into the soil.
Seasonal patterns of DDT
The volume of Lake Puruzinho changes continuously throughout the year, driven by regional rainfall. The p,p′-DDT mass in the air, water, aerated floodplain soil, and flooded floodplain soil follows a cyclic seasonal pattern in response to these dynamics (Fig S5). Inputs of p,p′-DDT into the water increase with increasing rainfall and water levels, as a larger volume and surface area of water can interact with the air and the flooded floodplain, and thus more p,p′-DDT can be transferred between these compartments. In addition, wet gaseous and aerosol deposition from the air to the surface media and soil runoff also increase with rainfall, increasing p,p′-DDT inputs into the water and outputs from the air compartment. As water levels rise, the area of the flooded floodplain increases while the area of the aerated floodplain shrinks, so the mass of DDT in the flooded floodplain increases. The opposite occurs as water levels decrease. Nonetheless, the cyclical pattern of the p,p′-DDT mass is dampened in the media with the highest p,p′-DDT accumulation, given that p,p′-DDT is less mobile in the terra-firme soil and in the sediment due to its high affinity for the organic carbon in the solids. In comparison to the sediment, concentrations in the soil vary less as p,p′-DDT is constantly emitted into this compartment from 1960 to 1998, and it has a longer degradation half-life in the soil.
Input and loss processes of p,p′-DDT
In addition to exhibiting seasonal variability (represented by ranges in the following discussion), fluxes in and out of each compartment also had different contributions pre- and post-ban. Before the ban, the main inputs (>98 %) of p,p′-DDT into the soil and air compartments were from direct emissions. Deposition from the air was the main source of DDT in the water column (45–85 % of inputs) and the aerated floodplain (nearly 100 % of inputs). DDT was transported to the sediments and flooded floodplain primarily through deposition of suspended particles from the water column, accounting for 83–94 % of inputs. In pre-ban years, advection with wind and deposition are the main loss processes in the air (accounting for 50–87 and 12–50 % of losses, respectively), suspended particle deposition is the main loss process in the water (80 % of losses), and degradation is the main loss process in the terra-firme soil (95–97.5 % of losses), aerated floodplain (95–97.5 % of losses), and sediment (63–79 % of losses). Diffusion from the water and degradation alternate as the main loss process in the flooded floodplain, accounting for 71 and 99 % of losses, respectively.
When emissions change, the contributions of input and loss processes to the mass in some compartments also change. As a result, after the ban, there is a transition period during which the mass of p,p′-DDT in each compartment decreases at a different rate, after which concentrations in all compartments decrease at the same rate. The fastest post-ban decrease occurs in the air compartment, as direct p,p′-DDT emissions into this compartment stop and instead diffusion of p,p′-DDT from the terra-firme soil becomes the main source of p,p′-DDT to the air, accounting for 58–98 % of all inputs. After the ban, the main losses of p,p′-DDT in the water and air compartments are still driven by advection and deposition, but the magnitude decreases very sharply in the air compartment and more gradually in the water. Even though much less p,p′-DDT is transferred to the water through deposition from the air post-ban (3–5 % of all inputs 3 years post-ban), DDT inputs into the water still occur through re-suspension and diffusion from sediments (accounting for 60–95 % of all inputs 3 years post-ban), so the mass of DDT in the water does not drop as abruptly as in the air. Afterwards, runoff from the terra-firme soil becomes the main source of p,p′-DDT into the water compartment, representing 50–80 % of yearly inputs. In the sediments and flooded floodplain, the relative contribution of most loss and input processes to the overall mass of p,p′-DDT remains the same as before the ban, but the magnitude of all fluxes decreases gradually, at a similar rate as the water, the main source of p,p′-DDT for these compartments. The only pre- and post-ban difference in fluxes to and from the sediment is that losses through degradation slightly exceed p,p′-DDT inputs from suspended solids in the water in the transition period, while before and after this period, these fluxes are similar. In the terra-firme soil, deposition from the air becomes the main source of p,p′-DDT after the ban, as it is for the aerated floodplain throughout the model run. Degradation is still the main loss process in the terra-firme soil and aerated floodplain post-ban, accounting for 95–98 % of all losses, and runoff accounts for most of the remaining losses. Approximately 6 years after the ban, concentrations in all compartments decrease at the same rate, which is dictated by the main loss process (degradation) in the terra-firme soil, as secondary emissions from this compartment are the main source of DDT for the remaining compartments.
Environmental fate of DDT metabolites
p,p′-DDE and p,p′-DDD have similar physico-chemical properties and thus exhibit a similar partitioning behavior in the environment as their parent compound (Fig. S6). Sediments and soils also represent long-term repositories for these metabolites. The same patterns hold here as were discussed for DDT, only the magnitude of chemical concentrations in the different compartments changes.
Sensitivity and uncertainty analysis
The sensitivity of model outputs to emissions to air and soil was evaluated through scenario testing (as described in the “Environmental description” section). Sensitivity to the remaining model inputs was evaluated using Spearman’s rank correlation coefficients (r). The absolute value of r for a specific parameter is the magnitude of the influence of this parameter on the model output (Schenker et al. 2009; MacLeod et al. 2002), while the sign indicates whether an increase in an input value leads to an increase (+) or decrease (−) in an output value. In sediments, terra-firme soils, and floodplain soils, p,p′-DDX concentrations are sensitive to similar parameters throughout each year. In the air compartment, the influence of some parameters on p,p′-DDX concentrations varies seasonally. In all compartments, differences in model output sensitivity to specific inputs arise between pre- and post-ban years. Overall, the most influential model inputs were the degradation half-lives in soils and sediments, whose importance generally increased as time progressed, the air-water and octantol-air partition coefficients, and the fractions of formations of DDE and DDD.
The five most influential parameters pre- and post-ban are displayed in Fig. 3 for the sediments. We here show the sediments because DDT inputs into this compartment are influenced by interactions between the air, water, and soil (see “DDX distribution in the environment”). The results of the sensitivity analysis for the other compartments are described in SM section S3.3. Before the ban, the degradation half-life in the sediment is the parameter with the highest rank correlation (negative) to concentrations of all compounds in the sediments. K ow also has a moderate (positive) contribution to the variance of DDT concentrations in the sediment, while sediment burial (−r) and the fractions of formation (+r) have a moderate contribution to the variance of DDE and DDD in the sediments. After the ban, although the magnitude of its rank correlation coefficient decreases, the degradation half-life in the sediment still has the highest contribution to the variance of DDE and DDD concentrations in the sediments, followed closely by the degradation half-life in the terra-firme soil. The degradation half-life in the terra-firme soil becomes the most influential parameter for DDT concentrations in sediments, followed by the degradation in the sediments in post-ban years (both with negative correlations).
The most influential parameters identified through the sensitivity analysis were also the parameters with the highest uncertainty (see Table S7). As shown in Fig. 2 for p,p′-DDT and Fig S6 for p,p′-DDE and p,p′-DDD, the uncertainty range of the model predictions increases with time, particularly after DDT was phased out, when highly uncertain parameters associated with degradation have the largest contributions to the variance of DDX concentrations.
Measurements
Total DDX concentrations
Soil
The DDX content of 2005 soil samples (mean = 10, median = 6.8 ng/g dw) measured in this study agrees with concentrations measured in the same samples in a previous study (mean = 16, median = 2.1 ng/g dw) (Saldanha et al. 2010; n = 10), although the studies relied on different analytical methods. Total DDX concentrations in 2005 and 2011 house soil samples are substantially higher than concentrations measured in forest soils (n = 10) in 2005 (Saldanha et al. 2010) and 2011 (this study). This difference is larger for 2011 samples. The low levels of total DDX in background forest soils in both studies supports the assumption that DDT inputs into the Puruzinho environment are from local sources. p,p′-DDX concentrations measured in 2005 house soil samples were significantly lower than those found in 2011 house soil samples (p < 0.05, see Table S8 for results of all Wilcoxon rank-sum tests). The p,p′-DDT and p,p′-DDD content of 2005 house soils was also significantly lower from that of 2014 house soil samples. The median of DDX concentrations measured in 2011 and 2014 is three orders of magnitude larger than those measured in 2005 and the maximum concentrations measured are 3–4 orders of magnitude larger. The highest concentrations measured in 2005 samples are within the lower range of those measured in 2011 and 2014. p,p′-DDX concentrations were not significantly different in 2011 and 2014 samples. These findings are unusual for samples taken in a post-ban era.
Sediment
Among sediments collected in 2011, the highest concentrations of total DDX are found in sediments collected from the flooded floodplain next to the houses (hereafter referred to as floodplain sediments). Total DDX concentrations in sediments collected from the flooded floodplain next to the forested area (forest sediments) and in a sample from the lake center are similar and one order of magnitude lower than those in the floodplain sediments. Based on the few sediment samples available, we can observe that total DDX concentrations in sediments in 2011 and 2012 are within the same range. A temporal trend could not reasonably be established, as no sediment samples were available from 2005 and only two were available from 2012.
Soil vs. sediments
p,p′-DDX concentrations measured in 2011 sediments were significantly lower than those found in 2011 house soil samples. 2011 sediment samples also contained significantly lower concentrations of p,p′-DDT and p,p′-DDD than 2014 soil samples. Overall concentrations of DDT and its metabolites in terra-firme soil samples from 2011 (median 660 ng/g dw) and 2014 (median 403 ng/g dw) are higher than those found in floodplain sediment samples collected next to the houses from 2011 (median 15 ng/g dw) and 2012 (median 13 ng/g dw). In contrast, similar total DDX concentrations were found in floodplain sediments collected next to the forested area and in forest soils samples in 2011. No DDT use was reported in forest soil, which suggests that DDT is mobilized through the air and runoff from the soil surrounding the IRS-treated dwellings to the forest soils and sediments.
Variability in DDX concentrations
For all compounds analyzed, concentrations in soil and sediment samples span ranges of up to three orders of magnitude (Figs. 2 and S6). Large variability in soil samples has also been observed in other tropical rural areas with a similar history of IRS as Puruzinho and has been attributed to spatial variability in soil properties (Herrera-Portugal et al. 2005; Martínez-Salinas et al. 2011; Martínez et al. 2012; Pérez-Maldonado et al. 2010; Van Dyk et al. 2010; Yáñez et al. 2002). In two rural villages in Brazil, a spatial gradient in total DDX concentrations in soil samples was observed, with decreasing concentrations with increasing distance from the IRS-treated dwellings. The lowest sum DDX concentrations (1–2 orders of magnitude lower than those in soil samples collected next to houses) were found in sediments from the closest river (Torres et al. 2002; Vieira et al. 2001) and in house soil samples with low organic matter content (Vieira et al. 2001). In Puruzinho, all of the soil samples were taken next to the sprayed houses and the TOC of the samples ranged from 1 to 7 % (dry weight), but no correlation was found between the TOC and the concentration of total DDX found in the samples. Although based on a small number of samples, a spatial gradient is also observed in the 2011 Puruzinho sediment samples, in which higher DDX concentrations are detected in floodplain sediments collected closer to the houses than in forest sediments and in a mid-lake sediment sample.
Metabolite pattern
Relative amounts of DDT and its transformation products can give some insight into the source and freshness of DDT inputs into environmental media. Low DDE/DDT ratios (smaller than 0.5) can indicate that recent DDT was recently applied, meaning less time was available for DDT degradation, or that the DDT degradation is very slow (Harner et al. 1999). On the other hand, high ratios are associated with aged DDT residues that have been degraded (Harner et al. 1999; Qiu et al. 2004; Zhang et al. 2002). Thus, increasing DDE/DDT ratios through time could mean that degradation is taking place and no new inputs have occurred, if the total amount of DDX remains the same, or that new inputs of DDE have occurred, if the total amount of DDX also increases. A higher percentage of DDD than DDE in samples is usually an indicator that anaerobic degradation is the dominant degradation pathway (Foght et al. 2001; Ge et al. 2013; Lawrence Boul 1996; Vieira et al. 2001; Zhang et al. 2002). However, care must be taken when interpreting the relative amounts of DDX, particularly when comparing data from different locations, as the rate of transformation of DDT in the environment is influenced by environmental properties such as the temperature, moisture, and organic carbon content (Hitch and Day 1992, Vieira et al. 2001), and the relative amounts of metabolites can also depend on mass transport processes.
House soils
In 2005 house soil samples, with one exception, all DDE/DDT ratios were greater than 1. 2011 house soils, on the other hand, total DDX contained 55–90 % DDT, and hence had low DDE/DDT ratios (less than 0.7). Soil samples from 2014 contained even lower DDE/DDT ratios (less than 0.4, except for 1 sample), with the DDT content ranging from 68 to 97 % of total DDX in all samples. The lower DDE/DDT ratios found in 2011 and 2014 samples compared to 2005, coupled with the high amounts of DDT, suggest new inputs of DDT into the soil around the houses after 2005. DDD is the compound found in the smallest relative and absolute amounts in house soils, constituting 6–10 % of the median total DDX. p,p′-DDE/p,p′-DDT ratios were consistent with total DDE/DDT ratios in soils and sediments, as the p,p′- isomers were the most abundant.
Sediments
Sediment samples collected in Puruzinho contain a different metabolite pattern from soils. All floodplain sediment samples have a higher DDD content than soils (median of 18 % compared to a median of 6 % in the soils), as expected given that DDT is degraded to DDD under anaerobic conditions. The DDD and DDT contents of Puruzinho sediments were similar in 2011. However, in 2012, the percentage of DDD decreased from a median of 24 to 10 %, while that of DDT increased from a median of 25 to 36 %, once again suggesting new inputs of DDT. This would be supported if the total amount of DDX in the sediments also increased. However, this is difficult to ascertain based on only two sediment samples available from 2012. Nonetheless, p,p′-DDE was the most abundant compound measured in most sediment samples (except in forest sediments) through all years, accounting for approximately 50 % of the DDX content, with 2–3 times higher concentrations than p,p′-DDD. This is contrary to the findings of Vieira et al. (2001), who found ten times lower p,p′-DDE concentrations compared to p,p′-DDD in stream sediments collected in 1997 in the vicinity of rural houses that had been sprayed from 1960 to 1990 for leishmaniasis control. p,p′-DDD was also the most abundant metabolite found in sediments from lake Sibaya, in KwaZulu-Natal, South Africa, which is located next to villages with a history of IRS spraying from 1950 until the present (Humphries 2013).
The low percentage of DDD measured in the Puruzinho sediments could be caused by the variable flooding patterns of the floodplain soils. As explained in the “Soil and sediment analysis” section, most of the sediment samples were taken from the flooded floodplain. The floodplain soils alternate between flooded and unflooded stages, depending on the water level of the lake. The onset of anoxic conditions in the water column above flooded floodplain soils and bottom lake sediments is also influenced by the water level. Thus, anaerobic conditions that favor the formation of DDD are not permanently maintained on the flooded floodplain and in lake sediments. Another explanation for the high content of DDE compared to DDD in sediments could be that most of the DDE that ultimately accumulates in the floodplain originates from the terra-firme soil, which is in agreement with our model predictions. This has been observed in several other studies on DDX in tropical sediments in a post-ban era. Zhang et al. (2002) reported an increasing amount of total DDX and of the DDE/DDT ratio in sediment cores from the Pearl River Delta, in Southern China, in the 1990s, even though DDT production was banned in 1983. The authors attribute this increase to inputs originating mainly from weathered DDT residues in soil, rather than from recently applied DDT, which would contain a lower concentration of metabolites. Sediments enriched in DDE compared to DDD have also been found in other locations where DDT has been banned for decades, such as in the San Joaquin River in California (Pereira et al. 1996), in Mississippi Delta profiles (Santschi et al. 2001), and in North African wetland profiles (Peters et al. 2001). Finally, it should be noted that in the analysis of 2011 samples from Puruzinho, dieldrin coeluted with DDE in the gas spectrometer, so it is possible that DDE concentrations in 2011 are overestimates.
Forest soils
The metabolite profile in the 2011 forest soils was between that found in house soils and sediments in 2011: the DDD content (6 %) was similar to that of house soils, while the DDE content (53 %) was similar to that of sediments, and the DDT content (41 %) was lower than in house soils, but higher than in sediments. Forest soil samples collected in Puruzinho in 2005 (Saldanha et al. 2010) contained a higher mean and median DDE/DDT ratio (4.4 and 6.9, respectively) than the 2011 forest soil samples that we analyzed (DDE/DDT = 1.3). These differences again point toward new DDT emissions into the forest soils in 2011.
Model versus measurements
The model results for the baseline scenario agree with DDX levels measured in house soil samples from 2005. Most of the 2005 house soil data fall within the uncertainty range of the model predictions for the terra-firme soil, as shown in Fig. 2 (for p,p′-DDT) and Fig. S6 (for the remaining compounds). The medians from 1000 Monte Carlo runs for p,p′-DDT and p,p′-DDE are in close agreement with the geometric means of measured concentrations in house soils, while the modeled median p,p′-DDD is slightly higher than the geometric mean of the measurements. This suggests that the processes and assumptions used to describe the transport of DDT from the house walls to the terra-firme soils and the formation of DDT metabolites in the terra-firme soil were adequate. Also in agreement with the measurements, the model shows a larger concentration of DDX in terra-firme soils than in sediments. However, two important differences can be observed between model results for the baseline scenario and the measurements. First, while the model predicts a continuous decrease in concentrations in all compartments after emissions cease, DDX concentrations measured in Puruzinho house soils increase from 2005 to 2014. For the sediments, not enough measurements are available for a discernible time trend to compare to the model, since samples are only available for 2011 and 2012. Nonetheless, the model results are nearly three orders of magnitude lower than DDX concentrations measured in sediment samples. Second, the model calculates increasing p,p′-DDE/p,p′-DDT ratios during this time period, as expected in aging residues in a post-ban scenario, while soil measurements show a p,p′-DDE/p,p′-DDT ratio that decreases from 2005 to 2014.
These discrepancies between model results and measurements point to a pattern of DDT use that is different from the assumptions in the baseline scenario. Saldanha et al. (2010) reported DDT use against termites that damage the wooden houses of the Puruzinho community after the ban, but no further details regarding this unofficial use were given. Unofficial DDT trade for termiticidal purposes in local markets has also been observed in other countries (van den Berg 2009). Several studies have ascribed patterns in DDX concentrations in environmental media that deviate from those expected in a post-ban era to unauthorized usage or to undocumented usage patterns in a pre-ban era. In their study of organochlorines in the air of Chiapas, Mexico, Alegria et al. (2006) found p,p′-DDE/p,p′-DDT ratios that suggest p,p′-DDX originating from a mixture of fresh and weathered sources. The authors learned that DDT from leftover stockpiles or illegal purchases had been used in agriculture and that it was unclear whether DDT was being used for vector control after an official ban. In addition, studies in rural areas with a history of IRS in Mexico (Martínez-Salinas et al. 2011; Martínez et al. 2012) and Central America (Pérez-Maldonado et al. 2010) have also attributed the unexpected metabolite profiles found in soil samples from a post-ban era to unauthorized use (p,p′-DDE/ p,p′-DDT ratios much smaller than 1). In Brazil, according to an inventory of DDT stocks carried out by the Ministry of Environment (MMA 2015), the DDT stock reported in the states of Amazonas and Rondônia by December 2012 was 2829 kg. However, this amount probably only partially accounts for the existing DDT stocks in these states, as several challenges were faced while compiling the inventory, including the scattered location of farms in this large region as well as underreporting. Several of the public agencies that were interviewed to obtain data did not have any information regarding stocks, as holders of stocks are often reluctant to declare them as they do not want to bear the clean-up costs (MMA 2015). Furthermore, the isomeric composition of all soil and sediment samples collected at Puruzinho (except for one 2011 forest sediment sample), with the p,p′- isomer constituting 68 to 100 % of the total DDT, suggests that the technical DDT formulation used for IRS was the source of DDT in the samples (ASTDR 2002; Ge et al. 2013). This profile, in combination with the high DDT content in 2011 and 2014 house soils in Puruzinho, points toward potential post-ban use of stocks.
In light of the unexpected increase in DDX concentrations and decrease in p,p-DDE/p,p-DDT ratios in Puruzinho house soils from 2005 to 2014, we investigated the possible effect of DDT re-emissions after the ban through additional model scenarios. Re-emissions were simulated starting in 2006, after a peak in malaria incidence in the Amazon, through two scenarios. The first scenario assumed that DDT was applied in the same manner as it was used for IRS (90 % of the emissions occur into the air and 10 % into the soil), while the second scenario assumed greater emissions into the soil (90 %) as a result of potential use against termites or leishmaniasis.
In both reemission scenarios, we scaled the DDT emissions so that model outputs matched the geometric mean of 2011 and 2014 concentrations of p,p′-DDT in house soils. This model-based emissions estimate indicates that 435 kg of DDT would need to be applied from 2006 to 2014 under the first scenario, while 65 kg of DDT would be applied under the second scenario. These amounts correspond to 15.1 % (scenario 1) and 2.2 % (scenario 2) of the reported DDT stock in Amazonas and Rondônia in 2012.
The results of both reemission scenarios are shown in Figs. 4 and 5, along with the field data. As the 95 % confidence intervals for DDX concentrations in the sediments overlapped with DDX concentrations in the floodplain (as shown in Fig. 2), only sediment concentrations are shown. Under both scenarios, adjusting re-emissions to approximate the geometric means of p,p′-DDT concentrations in 2011 and 2014 house soil samples results in a good fit between model results and the geometric means for p,p′-DDE and p,p′-DDD concentrations in 2011 and 2014 house soil samples. This suggests that the degradation half-life of DDT in the soils and the fractions of formation used to describe the formation of metabolites in aerobic soils are reasonable. In addition, p,p′-DDE/p,p′-DDT ratios predicted by the model under both reemission scenarios are in close agreement with the ratios found in the field samples, as shown in Table 1. The only reasonable explanation for decreasing ratios associated with increasing total DDX concentrations through time is a new source of DDT. As explained in the “Metabolite pattern” section, the DDT isomer profile of the soil and sediment samples, which consists mainly of p,p′-DDT and only a small percentage of the o,p′-isomer, suggest that the technical DDT formulation used for IRS is a likely source.
Model results (lines) showing median p,p′-DDX concentrations (ng/g dw) in soils and sediments under reemission scenario 1, in which continuous emissions restart in 2006, 90 % into the air and 10 % into the soil. The shaded area represents the 95th and 5th percentiles from Monte Carlo simulations. Markers indicate p,p′-DDX concentrations (ng/g dw) measured in soils and sediments in Lake Puruzinho
Model results (lines) showing median p,p′-DDX concentrations (ng/g dw) in soils and sediments under reemission scenario 2, in which continuous emissions restart in 2006, 90 % into the soil and the rest into the air. The shaded area represents the 95th and 5th percentiles from Monte Carlo simulations. Markers indicate p,p′-DDX concentrations (ng/g dw) measured in soils and sediments in Lake Puruzinho
In sediments, DDX concentrations measured in field samples are between those predicted by the two reemission scenarios. Model results from scenario 1 agree with the upper range of p,p′-DDE and p,p′-DDD concentrations measured in sediments, which correspond to samples taken from the flooded floodplain, and are higher than measured concentrations of p,p′-DDT. Model results from scenario 2 are within the lower range of DDX concentrations in the sediments, which correspond to mid-lake sediment samples in 2011 and one (for p,p′-DDT and p,p′-DDE) or both (for p,p′-DDD) of the 2012 sediment samples. These observations point to a DDT usage pattern from 2005 to 2014 involving between 10 and 90 % emissions into the soil. However, none of the scenarios accurately represented the metabolite profile observed in field measurements, as both scenarios predict larger concentrations of p,p′-DDT than p,p′-DDE and similar concentrations of p,p′-DDE and p,p′-DDD, while p,p′-DDE was the most abundant metabolite in the field samples. Results of the sensitivity analysis showed that the concentrations of DDX in sediments were very sensitive to the degradation half-lives and the fractions of formation of the transformation products in the sediment. As both of these parameters are very uncertain, several assumptions were made regarding their values (“Chemical properties” section). To improve our description of the sediment compartment in the multimedia model, further research is required to reduce the uncertainty in these key parameters. Additional soil and sediment measurements would also allow us to confirm the 2005 to 2014 time trends observed in the soils and better understand the likely reemission pattern.
Comparison to other tropical countries with a history of IRS
2005 soils
The total DDX content of 2005 house soil samples from Puruzinho is within the range of values found in 2008 in the Limpopo Province of South Africa (Table 2), where emissions are still ongoing and DDT was used since 1945 for IRS only (Bornman et al. 2010), and within the lower range of those in 1997 samples from other remote communities in the Madeira River Basin, where DDT use for IRS had stopped in the 1990s but was still allowed for the control of leishmaniasis (Torres et al. 2002). However, the median p,p′-DDE/p,p′-DDT ratios measured in these South African and Brazilian communities are nearly ten times higher than those measured in Puruzinho. The high ratios could be due to a faster degradation of DDT in the soils from Limpopo and the villages in the Madeira River Basin compared to the Puruzinho soils. In contrast, while the median p,p′-DDE/p,p′-DDT ratio in Puruzinho was higher than in Mexican communities 1–9 years after the ban on DDT in 2000 (Herrera-Portugal et al. 2005; Martínez et al. 2012; Yáñez et al. 2002) and in a Brazilian village in the Tapajós river basin 1 year after DDT use ceased (Torres et al. 2002), the median DDX concentrations in outdoor soils were much lower in Puruzinho.
2011 and 2014 soils
The mean total DDX concentration measured in 2014 soil samples from Puruzinho is higher than that found in soil samples of two high-exposure communities in Chiapas, Southern Mexico: Cigüeña, where DDT was used for agriculture and IRS from 1957 to 2000 (Herrera-Portugal et al. 2005), and Miguel Aleman, with ongoing IRS since 1955 and potential agricultural use (Yáñez et al. 2002). However, one sample in Puruzinho had a very high DDT content, resulting in a standard deviation four times higher than that in the Chiapas samples, which also span a large range. Without this sample, the mean and median DDX content of 2011 and 2014 Puruzinho soils is higher than that found in a lower exposure community in Chiapas, where DDT was used for IRS from 1957 to 1980 (Herrera-Portugal et al. 2005), but lower than in the high-exposure communities in Chiapas and in Oaxaca, Mexico, 2 years after the ban. Soil samples collected after the ban (most likely in 2009, when blood samples were also collected) in Chihuahua, Northern Mexico, where DDT had been used for IRS and agriculture, had a DDX concentrations between those measured in Puruzinho in 2005 and 2014 (Martínez et al. 2012). Median total DDX concentrations in 2011 and 2014 soil samples from Puruzinho were similar to that found in 1994 soil samples from a village in the Tapajós River Basin, in the Brazilian Amazon, sprayed with DDT for IRS until 1993 (Torres et al. 2002).
The mean p,p′-DDE/p,p′-DDT ratio of 2011 and 2014 Puruzinho soil samples is within the range of ratios measured in Mexico, in better agreement with the ratio measured in the high exposure communities in Chiapas, and one order of magnitude higher than the ratio found in the Tapajós River Basin sample.
2011 and 2012 sediments
Total DDX concentrations in Puruzinho 2011 and 2012 sediments are much higher than those found in 1997 sediment samples from the Madeira River Basin, and within the range of those measured in the Tapajós River Basin in 1993. Also, the mean p,p′-DDE/p,p′-DDT ratio in sediments from the Tapajós River Basin is similar to that found in 2011 Puruzinho sediment samples. The DDX content of sediments in Puruzinho was within the lower range of those measured in the western arm of lake Sibaya, in KwaZulu-Natal, located close to villages where DDT use for IRS is ongoing since 1946 (with an interruption between 1996 and 2000) (Humphries 2013). However, the p,p′-DDE/p,p′-DDT ratio of these sediments is 2–3 times higher than that found in Puruzinho samples.
Conclusion
Using a novel a multimedia model that we developed to describe a dynamic tropical floodplain, we explored how variable environmental conditions, particularly seasonal rainfall patterns and changes in water levels, as well as different emissions scenarios associated with IRS, affect the movement and distribution of p,p′-DDT and its degradation products. The model results show that p,p′-DDT accumulation occurred primarily in the soil and sediment compartments, which represent long-term repositories for DDT in which concentrations remain stable as emissions are ongoing and decrease slowly after emissions cease. While the model results agree with 2005 house soil measurements, the significant increase in total DDX concentrations observed in the house soils from 2005 to 2014 and the concomitant decrease of p,p′-DDE/p,p′-DDT ratios does not agree with the model results in a post-ban era. This observation strongly suggests that the usage pattern of DDT in Puruzinho was different from the documented IRS pattern, in which DDT use ceased after a ban in 1998. When re-emissions after the 2005 peak in malaria incidence are simulated, the p,p′-DDE/p,p′-DDT ratio of the model results agrees with ratios measured in 2011 and 2014 soils. Through the use of uncertainty analysis, we identified important knowledge gaps regarding key parameters such as the fractions of formation of the transformation products, the degradation half-lives of DDX in soils and sediments, and partition coefficients.
Given that 2004 DDX concentrations in breast milk were already cause for concern, and the results of this study show that concentrations in the soil have increased significantly from 2005 to 2011 and 2014, there is a concern of higher human exposure to DDT. Several studies have shown a strong association between blood and serum levels of p,p′-DDT and sum DDX in children and adults and DDT concentrations in soils (Pérez-Maldonado et al. 2010; Van Dyk et al. 2010). Highly contaminated soil represents a particular risk to children, who play outside for long periods, have frequent hand-to-mouth contact, and ingest non-dietary items (Van Dyk et al. 2010; Yáñez et al. 2002). A strong association has also been found between DDX concentrations in outdoor soil samples and food items such as chicken and fish (Pérez-Maldonado et al. 2010; Van Dyk et al. 2010). This is particularly relevant for the Puruzinho community, which lives in close ties with its environment and still relies on local sources of water and food, including fruits and legumes grown around the houses, fish, and free-ranging poultry (Oliveira et al. 2010). Thus, breastfeeding infants represent a high-risk group, as they can be exposed to DDX through direct contact with contaminated media, breastfeeding, and food. Newborn infants of mothers living in IRS-treated dwellings have been found to have blood concentrations of DDT and DDE that are approximately 40 % of those found in maternal blood (WHO 2011).
Through the insights provided by a mechanistic multimedia model and field measurements, we have contributed to a greater understanding of the dynamics of DDT around IRS-treated dwellings in the tropics. Such understanding is crucial to identify suitable precautionary actions that minimize unnecessary exposure and contamination of the environment arising from indoor residual spraying (Bouwman et al. 2011).
References
Aislabie JM, Richards NK, Boul HL (1997) Microbial degradation of DDT and its residues—a review. N Z J Agric Res 40:269–282. doi:10.1080/00288233.1997.9513247
Alcântara E, Novo E, Stech J, Lorenzzetti J, Barbosa C, Assireu A, Souza A (2010) A contribution to understanding the turbidity behaviour in an Amazon floodplain. Hydrol Earth Syst Sci 14:351–364. doi:10.5194/hess-14-351-2010
Alegria H, Bidleman TF, Figueroa MS (2006) Organochlorine pesticides in the ambient air of Chiapas, Mexico. Environ Pollut 140:483–491. doi:10.1016/j.envpol.2005.08.007
Almeida R (2006) Geostatistic analysis of mercury concentrations in Puruzinho Lake-Western Amazon. Dissertation, Universidade Federal de Rondônia (in Portuguese)
Almeida FV, Centeno AJ, Bisinoti MC, Jardim WF (2007) Persistent toxic substances in Brazil. Química Nov. 30:1976–1985. (in Portuguese)
Artaxo P, Gerab F, Yamasoe M (1998) Long term atmospheric aerosol characterization in the Amazon Basin. In: Wasserman J, Silva-Filho E, Villas-Boas R (eds) Environmental geochemistry in the tropics, vol 72. Lecture Notes in Earth Sciences. Springer Berlin Heidelberg, pp 247–272. doi:10.1007/BFb0010918
Artaxo P, Martins JV, Yamasoe, MA, Procópio AS, Pauliquevis TM, Andreae MO, Guyon P, Gatti LV, Leal AM (2002) Physical and chemical properties of aerosols in the wet and dry seasons in Rondônia, Amazonia. J Geophys Res Atmos 107:LBA 49-1-LBA 49–14. doi:10.1029/2001JD000666
ASTDR (2002) Toxicological profile for DDT, DDE, DDD. Department of Health and Human Services, Public Health Service, Atlanta
Azeredo A (2007) Organochlorine pesticides and PAHs: A study of two groups of organic pollutants. Dissertation, Universidade Federal do Rio de Janeiro (in Portuguese)
Azeredo A, Torres JPM, de Freitas Fonseca M, Britto JL, Bastos WR, Azevedo e Silva CE, Cavalcanti G, Meire RO et al. (2008) DDT and its metabolites in breast milk from the Madeira River basin in the Amazon, Brazil. Chemosphere 73:S246-S251. doi:10.1016/j.chemosphere.2007.04.090
Azevedo e Silva CE (2011) Study of the biomagnification of mercury in fish from Puruiznho Lake (AM) through the use of carbon and nitrogen stable isotopes. Dissertation, Universidade Federal do Rio de Janeiro (in Portuguese)
Bahm K, Khalil MAK (2004) A new model of tropospheric hydroxyl radical concentrations. Chemosphere 54:143–166. doi:10.1016/j.chemosphere.2003.08.006
Barra R, Colombo JC, Eguren G, et al (2006) Persistent Organic Pollutants (POPs) in Eastern and Western South American Countries. In: Ware DGW, Nigg DHN, Doerge DDR (eds) Reviews of Environmental Contamination and Toxicology. Springer New York, pp 1–33. doi:10.1007/0-387-30638-2_1
Becker L, Scheringer M, Schenker U, Hungerbühler K (2011) Assessment of the environmental persistence and long-range transport of endosulfan. Environ Pollut 159:1737–1743. doi:10.1016/j.envpol.2011.02.012
Boethling RS, Howard PH, Beauman JA, Larosch ME (1995) Factors for intermedia extrapolation in biodegradability assessment. Chemosphere 30:741–752. doi:10.1016/0045-6535(94)00439-2
Bogdal C (2012) Report on passive air sampling under the global monitoring plan for persistent organic pollutants—GMP projects 2010–2011. United Nations Environment Programme, Division of Technology, Industry, and Economics
Bogdal C, Schmid P, Kohler M, Müller CE, Iozza S, Bucheli TD, Scheringer M, Hungerbühler K (2008) Sediment record and atmospheric deposition of brominated flame retardants and organochlorine compounds in lake Thun, Switzerland: lessons from the past and evaluation of the present. Environ Sci Technol 42:6817–6822. doi:10.1021/es800964z
Bogdal C, Scheringer M, Abad E, Abalos M, van Bavel B, Hagberg J, Fiedler H (2013) Worldwide distribution of persistent organic pollutants in air, including results of air monitoring by passive air sampling in five continents. TrAC Trends Anal Chem 46:150–161. doi:10.1016/j.trac.2012.05.011
Bornman MS, Barnhoorn IEJ, Genthe B (2010) DDT for malaria control: effects in indicators and health risk. Water Research Comission, Report No. 1674/1/09
Bouwman H, Kylin H (2009) Malaria control insecticide residues in breast milk: the need to consider infant health risks. Environ Health Perspect 117:1477–1480. doi:10.1289/ehp.0900605
Bouwman H, van den Berg H, Kylin H (2011) DDT and malaria prevention: addressing the paradox. Environ Health Perspect 119:744–747. doi:10.1289/ehp.1002127
Buser AM, MacLeod M, Scheringer M, Mackay D, Bonnell M, Russell MH, DePinto JV, Hungerbühler K (2012) Good modeling practice guidelines for applying multimedia models in chemical assessments. Integr Environ Assess Manag 8:703–708. doi:10.1002/ieam.1299
Camenzuli L, Scheringer M, Gaus C, Ng CA, Hungerbühler K (2012) Describing the environmental fate of diuron in a tropical river catchment. Sci Total Environ 440:178–185. doi:10.1016/j.scitotenv.2012.07.037
Eggen T, Majcherczyk A (2006) Effects of zero-valent iron (Fe0) and temperature on the transformation of DDT and its metabolites in lake sediment. Chemosphere 62:1116–1125. doi:10.1016/j.chemosphere.2005.05.044
Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, et al. (2009) The pine river statement: human health consequences of DDT use. Environ Health Persp 117:1359–1367. doi:10.1289/ehp.11748
FAO, WHO (2000) Pesticide residues in food. Report of the Joint Meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO Core Assessment Group. Food and Agriculture Organization Plant Production and Protection Paper 163. http://www.who.int/foodsafety/publications/jmpr-reports/en/. Accessed January 20 2015
Fenner K, Scheringer M, Hungerbühler K (2000) Persistence of parent compounds and transformation products in a level IV multimedia model. Environ Sci Technol 34:3809–3817. doi:10.1021/es0000347
Ferreira CP, De-Oliveira ACAX, Paumgartten FJR (2011) Serum concentrations of DDT and DDE among malaria control workers in the amazon region. J Occup Health 53:115–122. doi:10.1539/joh.O10026
Foght J, April T, Biggar K, Aislabie J (2001) Bioremediation of DDT-contaminated soils: a review. Bioremediation J 5:225–246. doi:10.1080/20018891079302
Ge J, Woodward LA, Li QX, Wang J (2013) Composition, distribution and risk assessment of organochlorine pesticides in soils from the Midway Atoll, North Pacific Ocean. Sci Total Environ 452–453:421–426. doi:10.1016/j.scitotenv.2013.03.015
GEF (2006) Development of a national implementation plan in Brazil as a first step to implement the Stockholm Convention on persistent organic pollutants (POPs). Global Environmental Facility United Nations Environmental Program. http://www.thegef.org/gef/project_detail?projID=2096. Accessed 10 September 2012
Harner T, Wideman JL, Jantunen LMM, Bidleman TF, Parkhurst WJ (1999) Residues of organochlorine pesticides in Alabama soils. Environ Pollut 106:323–332. doi:10.1016/S0269-7491(99)00110-4
Herrera-Portugal C, Ochoa H, Franco-Sánchez G, Yáñez L, Díaz-Barriga F (2005) Environmental pathways of exposure to DDT for children living in a malarious area of Chiapas, Mexico. Environ Res 99:158–163. doi:10.1016/j.envres.2005.03.010
Hitch R, Day H (1992) Unusual persistence of DDT in some Western USA soils. Bull Environ Contam Toxicol 48: 259–264. doi:10.1007/BF00194381
Hollander A, Huijbregts MAJ, Ragas AMJ, Meent D (2006) BasinBox: a generic multimedia fate model for predicting the fate of chemicals in river catchments. Hydrobiologia 565:21–38. doi:10.1007/s10750-005-1903-9
Humphries MS (2013) DDT residue contamination in sediments from lake Sibaya in northern KwaZulu-Natal, South Africa: implications for conservation in a world heritage site. Chemosphere 93:1494–1499. doi:10.1016/j.chemosphere.2013.07.047
Hussain A, Maqbool U, Asi M (1994a) Studies on dissipation and degradation of 14C-DDT and 14C-DDE in Pakistani soils under field conditions. J Environ Sci Health Part B 29:1-15. doi:10.1080/03601239409372853
Hussain A, Maqbool U, Asi M (1994b) Studies on the dissipation of 14C-DDT from water and solid surfaces. J Environ Sci Health B 29:177–184. doi:10.1080/03601239409372870
Hussain A, Tirmazi SH, Maqbool U, Asi M, Chaughtai FA (1994c) Studies of the effects of temperatures and solar radiation on volatilization, mineralization and binding of 14C-DDT in soil under laboratory conditions. J Environ Sci Health B 29:141–151. doi:10.1080/03601239409372866
INMET (2012) Climatological Normals of Brazil 1961-1990. http://www.inmet.gov.br/portal/index.php?r=clima/normaisClimatologicas. Accessed 10 October 2013 (in Portuguese)
INMET (2015) Automatic stations. http://www.inmet.gov.br/portal/index.php?r=home/page&page=rede_estacoes_auto_graf. Accessed 1 February 2014 (in Portuguese)
Kadir HA (1988) Dissipation and degradation of 14C-DDT in Malaysian soils. International Atomic Energy Agency (IAEA). Technical Document 476:41–45
Kenneth DR (2003) What do we know about the fate of pesticides in tropical ecosystems? In: Environmental fate and effects of pesticides, vol 853. ACS Symposium Series, vol 853. American Chemical Society, pp 96–123. doi:10.1021/bk-2003-0853.ch006
Kuo-Ching Ma DM, Sum CL, Wan YS (2006) Insecticides. In: Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, second edition. CRC Press, pp 3711–4022. doi:10.1201/9781420044393.ch18
Lalah JO, Acholla FV, Wandiga SO (1994) Fate of 14C-p,p’-DDT in Kenyan tropical soils. J Environ Sci Health B 29:57–64. doi:10.1080/03601239409372858
Lawrence Boul H (1996) Effect of soil moisture on the fate of radiolabelled DDT and DDE in vitro. Chemosphere 32:855–866. doi:10.1016/0045-6535(96)00018-5
Linders J, Mensink H, Stephenson G, Wauchope D, Racke K (2000) Foliar interception and retention values after pesticide application. A proposal for standardized values for environmental risk assessment. Pure Appl Chem 72:2199–2218
Loiola C, da Silva C, Tauil P (2002) Malaria control in Brazil: 1965 to 2001. Rev Panam Salud Publica 11:235–244
Longnecker MP (2005) Invited commentary: why DDT matters now. Am J Epidemiol 162:726–728. doi:10.1093/aje/kwi277
Mackay D (2001) Multimedia environmental models: the fugacity approach, second edition. CRC Press
MacLeod M, Fraser AJ, Mackay D (2002) Evaluating and expressing the propagation of uncertainty in chemical fate and bioaccumulation models. Environ Toxicol Chem 21:700–709. doi:10.1002/etc.5620210403
Mariën K, Laflamme DM (1995) Determination of a tolerable daily intake of DDT for consumers of DDT contaminated fish from the lower Yakima River, Washington. Risk Anal 15:709–717. doi:10.1111/j.1539-6924.1995.tb01343.x
Martínez F-B, Trejo-Acevedo A, Betanzos A, Espinosa-Reyes G, Alegría-Torres J, Maldonado I (2012) Assessment of DDT and DDE levels in soil, dust, and blood samples from Chihuahua, Mexico. Arch Environ Contam Toxicol 62:351–358. doi:10.1007/s00244-011-9700-0
Martínez-Salinas R, Díaz-Barriga F, Batres-Esquivel L, Pérez-Maldonado I (2011) Assessment of the levels of DDT and its metabolites in soil and dust samples from Chiapas, Mexico. Bull Environ Contam Toxicol 86:33–37. doi:10.1007/s00128-010-0174-y
Meire RO, Lee SC, Yao Y, Targino AC, Torres JPM, Harner T (2012) Seasonal and altitudinal variations of legacy and current-use pesticides in the Brazilian tropical and subtropical mountains. Atmos Environ 59:108–116. doi:10.1016/j.atmosenv.2012.05.018
MMA (2015) Brazilian national implementation plan: Stockholm convention. Brazilian Ministry of Environment, Brasília (in Portuguese)
Moreira-Turcq P, Jouanneau JM, Turcq B, Seyler P, Weber O, Guyot JL (2004) Carbon sedimentation at Lago Grande de Curuai, a floodplain lake in the low Amazon region: insights into sedimentation rates. Palaeogeogr Palaeoclimatol Palaeoecol 214:27–40. doi:10.1016/j.palaeo.2004.06.013
Nowell LH (1999) Analysis of key topics? Sources, behavior, and transport. In: Pesticides in Stream Sediment and Aquatic Biota. CRC Press. doi:10.1201/9781439822708.ch5
Oliveira RC, Dorea JG, Bernardi JV, Bastos WR, Almeida R, Manzatto AG (2010) Fish consumption by traditional subsistence villagers of the Rio Madeira (Amazon): impact on hair mercury. Ann Hum Biol 37:629–642. doi:10.3109/03014460903525177
Oliveira-Ferreira J, Lacerda M, Brasil P, Ladislau J, Tauil P, Daniel-Ribeiro C (2010) Malaria in Brazil: an overview. Malar J 9:115
Pereira WE, Domagalski JL, Hostettler FD, Brown LR, Rapp JB (1996) Occurrence and accumulation of pesticides and organic contaminants in river sediment, water and clam tissues from the San Joaquin River and tributaries, California. Environ Toxicol Chem 15:172–180. doi:10.1002/etc.5620150216
Pérez-Maldonado IN et al (2010) Assessment of DDT levels in selected environmental media and biological samples from Mexico and Central America. Chemosphere 78:1244–1249. doi:10.1016/j.chemosphere.2009.12.040
Peters AJ, Jones KC, Flower RJ, Appleby PG, Ramdani M, Kraïem MM, Fathi AA (2001) Recent environmental change in North African wetland lakes: a baseline study of organochlorine contaminant residues in sediments from nine sites in the CASSARINA project. Aquat Ecol 35:449–459. doi:10.1023/A:1011980226851
Pozo K, Harner T, Lee SC, Wania F, Muir DCG, Jones KC (2008) Seasonally resolved concentrations of persistent organic pollutants in the global atmosphere from the first year of the GAPS study. Environ Sci Technol 43:796–803. doi:10.1021/es802106a
Qiu X, Zhu T, Li J, Pan H, Li Q, Miao G, Gong J (2004) Organochlorine pesticides in the air around the Taihu Lake, China. Environ Sci Technol 38:1368–1374. doi:10.1021/es035052d
Ricking M, Schwarzbauer J (2012) DDT isomers and metabolites in the environment: an overview. Environ Chem Lett 10:317–323. doi:10.1007/s10311-012-0358-2
Ritter R, Scheringer M, MacLeod M, Hungerbuhler K (2011) Assessment of nonoccupational exposure to DDT in the tropics and the north: relevance of uptake via inhalation from indoor residual spraying. Environ Health Perspect 119:707–712. doi:10.1289/Ehp.1002542
Saldanha GC, Bastos WR, Torres JPM, Malm O (2010) DDT in fishes and soils of lakes from Brazilian Amazon: case study of Puruzinho Lake (Amazon, Brazil). J Braz Chem Soc 21:306–311
Samuel T, Pillai MKK (1988) Persistence and fate of 14C-p,p’-DDT in an Indian sandy loam soil under field and laboratory conditions. International Atomic Energy Agency (IAEA). Technical Document 476:27–39
Santschi PH, Presley BJ, Wade TL, Garcia-Romero B, Baskaran M (2001) Historical contamination of PAHs, PCBs, DDTs, and heavy metals in Mississippi River Delta, Galveston Bay and Tampa Bay sediment cores. Mar Environ Res 52:51–79. doi:10.1016/S0141-1136(00)00260-9
Schenker U, Scheringer M, Hungerbühler K (2007) Including degradation products of persistent organic pollutants in a global multi-media box model. Environ Sci Poll Res Int 14:145–152. doi:10.1065/espr2007.03.398
Schenker U, Scheringer M, Hungerbühler K (2008) Investigating the global fate of DDT: model evaluation and estimation of future trends. Environ Sci Technol 42:1178–1184. doi:10.1021/es070870h
Schenker U, Scheringer M, Sohn MD, Maddalena RL, McKone TE, Hungerbühler K (2009) Using Information on uncertainty to improve environmental fate modeling: a case study on DDT. Environ Sci Technol 43:128–134. doi:10.1021/es801161x
Sjoeib F, Anwar E, Tungguldihardjo MS (1994) Behaviour of DDT and DDE in Indonesian tropical environments. J Environ Sci Health B 29:17–24. doi:10.1080/03601239409372854
Sommerfreund JK et al (2010) Contaminant fate and transport in the Venice Lagoon: results from a multi-segment multimedia model. Ecotoxicol Environ Saf 73:222–230. doi:10.1016/j.ecoenv.2009.11.005
Stephens J, Maeda DN, Ngowi AV, Moshi AO, Mushy P, Mausa E (1994) Dissipation and degradation of 14C-p, p’-DDT and 14C-p,p’-DDE in Tanzanian soils under field conditions. J Environ Sci Health B 29:65–71. doi:10.1080/03601239409372859
Su N-Y, Ban PM, Scheffrahn RH (1999) Longevity and efficacy of pyrethroid and organophosphate termiticides in field degradation studies using miniature slabs vol 92(4):890–898. doi:10.1093/jee/92.4.890
SVS (2013) Epidemiological situation of malaria in Brazil, 2000 to 2011. Epidemiological Bulletin 44 (1). Health Surveillance Secretariat, Brazilian Ministry of Health. http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/leia-mais-o-ministerio/197-secretaria-svs/11955-boletins-epidemiologicos-arquivos. Accessed 21 March 2015 (in Portuguese)
Tayaputch N (1988) Fate of 14C-DDT in Thailand under field and laboratory conditions. International Atomic Energy Agency (IAEA). Technical Document 476:63–68
Torres JPM, Pfeiffer WC, Markowitz S, Pause R, Malm O, Japenga J (2002) Dichlorodiphenyltrichloroethane in soil, river sediment, and fish in the Amazon in Brazil. Environ Res 88:134–139. doi:10.1006/enrs.2001.4312
Torres JPM et al (2009) Persistent toxic substances in the Brazilian Amazon: contamination of man and the environment. J Braz Chem Soc 20:1175–1179
USEPA (2014) Estimation Programs Interface SuiteTM for Microsoft (R) Windows, v 4.11. United States Environmental Protection Agency, Washington, DC, USA.
van den Berg H (2009) Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Perspect 117:1656–1663. doi:10.1289/ehp.0900785
Van Dyk JC, Bouwman H, Barnhoorn IEJ, Bornman MS (2010) DDT contamination from indoor residual spraying for malaria control. Sci Total Environ 408:2745–2752. doi:10.1016/j.scitotenv.2010.03.002
Vieira EDR, Torres JPM, Malm O (2001) DDT environmental persistence from its use in a vector control program: a case study. Environ Res 86:174–182. doi:10.1006/enrs.2001.4258
Wang F et al (2007) Organochlorine pesticides in soils under different land usage in the Taihu Lake region. Chin J Environ Sci 19:584–590. doi:10.1016/S1001
Wania F, Breivik K, Persson NJ, McLachlan MS (2006) CoZMo-POP 2—a fugacity-based dynamic multi-compartmental mass balance model of the fate of persistent organic pollutants. Environ Model Softw 21:868–884. doi:10.1016/j.envsoft.2005.04.003
Whelan MJ (2013) Evaluating the fate and behaviour of cyclic volatile methyl siloxanes in two contrasting North American lakes using a multi-media model. Chemosphere 91:1566–1576. doi:10.1016/j.chemosphere.2012.12.048
WHO (2011) DDT in indoor residual spraying: human health aspects (Environmental Health Criteria 241). World Health Organization, Switzerland
WHO (2012) World malaria report. World Health Organization, Switzerland
WHO (2013) WHO recommended insecticides for indoor residual spraying for malaria vectors. World Health Organization. http://www.who.int/whopes/Insecticides_IRS_Malaria_25_Oct_2013.pdf. Accessed 15 September 2013
Xia X, Hopke PK, Holsen TM, Crimmins BS (2011) Modeling toxaphene behavior in the Great Lakes. Sci Total Environ 409:792–799. doi:10.1016/j.scitotenv.2010.10.051
Xu B, Jianying G, Yongxi Z, Haibo L (1994) Behaviour of DDT in Chinese tropical soils. J Environ Sci Health B 29:37–46. doi:10.1080/03601239409372856
Yáñez L, Ortiz-Pérez D, Batres LE, Borja-Aburto VH, Dı́az-Barriga F (2002) Levels of dichlorodiphenyltrichloroethane and deltamethrin in humans and environmental samples in malarious areas of Mexico. Environ Res 88:174–181. doi:10.1006/enrs.2002.4333
Zayed SMAD, El‐Axab AE, Soliman SM (1994a) Dissipation of DDT in natural water under field conditions. J Environ Sci Health B 29:185–188. doi:10.1080/03601239409372871
Zayed SMAD, Mostafa IY, El‐Arab AE (1994b) Degradation and fate of 14C-DDT and 14C-DDE in Egyptian soil. J Environ Sci Health B 29:47–56. doi:10.1080/03601239409372857
Zhang G, Parker A, House A, Mai B, Li X, Kang Y, Wang Z (2002) Sedimentary records of DDT and HCH in the pearl River Delta, South China. Environ Sci Technol 36:3671–3677. doi:10.1021/es0102888
Acknowledgments
The authors would like to acknowledge the financial support of the Brazilian Swiss Joint Research Programme in Switzerland, and from CNPq/MCTi and FAPERJ/SECT/RJ in Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Marcus Schulz
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2.32 mb)
Rights and permissions
About this article
Cite this article
Mendez, A., Ng, C.A., Torres, J.P.M. et al. Modeling the dynamics of DDT in a remote tropical floodplain: indications of post-ban use?. Environ Sci Pollut Res 23, 10317–10334 (2016). https://doi.org/10.1007/s11356-015-5641-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5641-x