Abstract
We investigated the potential role of silicon in improving tolerance and decreasing cadmium (Cd) toxicity in durum wheat (Triticum turgidum L. durum) either through a reduced Cd uptake or exclusion/sequestration in non-metabolic tissues. For this, plants were grown in hydroponic conditions for 10 days either in presence or absence of 1 mM Si and for 11 additional days in various Cd concentrations (0, 0.5, 5.0 and 50 μM). After harvesting, morphological and physiological parameters as well as elemental concentrations were recorded. Cadmium caused reduction in growth parameters, photosynthetic pigments and mineral nutrient concentrations both in shoots and roots. Shoot and root contents of malate, citrate and aconitate increased, while contents of phosphate, nitrate and sulphate decreased with increasing Cd concentrations in plants. Addition of Si to the nutrient solution mitigated these adverse effects: Cd concentration in shoots decreased while concentration of Cd adsorbed at the root cell apoplasmic level increased together with Zn uptake by roots. Overall, total Cd uptake decreased in presence of Si. There was no co-localisation of Cd and Si either at the shoot or at the root levels. No Cd was detected in leaf phytoliths. In roots, Cd was mainly detected in the cortical parenchyma and Si at the endodermis level, while analysis of the outer thin root surface of the plants grown in the 50 μM Cd + 1 mM Si treatment highlighted non-homogeneous Cd and Si enrichments. These data strongly suggest the existence of a root localised protection mechanism consisting in armoring the root surface by Si- and Cd-bearing compounds and in limiting root–shoot translocation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a non-essential element, highly toxic at very low concentrations for plants and animals. It enters the environment mainly through industrial processes, sewage sludge and phosphate fertilisers (Sanita di Toppi and Gabrielli 1999). Cadmium causes several biochemical and physiological disorders in plants (Das et al. 1997), and even when plants are cultivated on moderately Cd-polluted soils, Cd can be considerably accumulated in edible shoot parts as leaves or grains (Zorrig et al. 2010; Uraguchi et al. 2011). The main pathway for heavy metals to enter the plants is through root uptake from the soil solution, and it is mediated by numerous proteins localised in the cell plasma membrane (Lasat 2002; Lux et al. 2011). Roots are therefore the first target of Cd, which inhibits root growth and affects root morphology (Ci et al. 2009). Organic compounds may participate in the uptake and translocation of Cd in many plant species: metal-chelate complexes involving organic acids have been shown to mediate Cd uptake (Cieslinski et al. 1998) or solubilise soil-bound Cd into the soil solution. Similarly, Zorrig et al. (2010) suggested that in lettuce, citrate might play a role in the translocation of Cd through the xylem vessels from roots to shoots. When Cd is translocated to shoots via the xylem sap (Uraguchi et al. 2009), it can inhibit the photosynthesis via reduction of chlorophyll biosynthesis and function of photosystems (Ouzounidou et al. 1997; Ci et al. 2009, 2010; Vaculík et al. 2015). Cadmium toxicity also causes various nutrient imbalances by decreasing the uptake of essential mineral nutrients by plants (Jalil et al. 1994; Clemens et al. 2002).
It has been reported that durum wheat accumulates Cd in grains to a greater extent than bread wheat (Meyer et al. 1982; Hart et al. 1998, 2002; Greger and Löfstedt 2004) so that it may exceed the concentration limits of international regulatory agencies (Chaney et al. 1996; Norvell et al. 2000), such as the European Food Safety Authority (EFSA) which defines a tolerable weekly intake of 2.5 μg Cd kg−1 body weight. This high Cd uptake and translocation rate from root to shoot is the main source of Cd contamination in food (Hart et al. 2002; Greger and Löfstedt 2004; Liu et al. 2007). Consequently, there is a need to develop agronomic management practices to reduce Cd transfer from soil to plant that may impact yield (Hasan et al. 2009; Gallego et al. 2012) and translocation of Cd to grain (Naeem et al. 2015).
While its concentration in plants can reach 10 % on a dry weight basis, silicon (Si) is not considered to be the part of the 17 so-called essential elements for higher plants, although it has been proven primarily to be structurally beneficial for the growth and development of many plants (Epstein 1994; Ma et al. 2006). Recently, it has been suggested that Si may diminish Fe deficiency symptoms (Hernandez-Apaolaza 2014). Silicon is also able to reduce the negative effect of toxic metals (Adrees et al. 2015). However, the mechanisms involved in Si mitigation of toxicants are still not clearly understood. For example, Si decreases Mn toxicity by increasing the binding of Mn to the cell wall or by homogenising the distribution of Mn in the leaves of cucumber (Rogalla and Römheld 2002) and maize (Doncheva et al. 2009). It has an effect on reducing Zn toxicity in rice seedlings by enhancing antioxidant defense capacity and by reducing Zn translocation to shoots (Song et al. 2011). Silicon application decreased the toxic effects of Cd in maize (da Cunha et al. 2008; Vaculík et al. 2009), cucumber (Feng et al. 2010), strawberry (Treder and Cieslinski 2005) and rice (Shi et al. 2005; Nwugo and Huerta 2008; Liu et al. 2009). In contrast to these studies, experiments on Si and Cd toxicity in wheat plant are scarce. Rizwan et al. (2012) reported that Si application in soil in the form of amorphous silica can reduce Cd toxicity in durum wheat by decreasing soil Cd mobility, immobilising Cd in roots and/or by increasing plant biomass. Naeem et al. (2015) found that Cd concentration in wheat grain could be decreased by applying Si to soil, but the extent of the beneficial effect depended on Cd accumulating abilities of cultivars. However, we lack information on the localisation of Cd and Si in plant organs and in cell compartments, which respective distribution may help to better understand the Si-induced Cd tolerance mechanism. In addition, we have little information on the effect of Cd on the photosynthesis apparatus of wheat plant (amount and type of chlorophylls) as well as on the balance of organic and inorganic anions which are important nutritional/osmotic factors and the possible recovery of the associated physiological functions induced by Si addition.
This study was designed to test whether Si addition is capable to improve Cd tolerance and thus decrease toxicity either through a reduced Cd uptake or exclusion/sequestration in non-metabolic tissues. We thus investigated the effects of Si addition on Cd uptake and Si and Cd (co)-localisation in durum wheat grown in hydroponics with various combinations of Cd and Si concentrations through (i) inspection by micro X-ray fluorescence (micro-XRF), scanning electron microscopy coupled with energy-dispersive X-ray spectrometry (SEM-EDX) and X-ray photoelectron spectroscopy (XPS) of wheat (Triticum turgidum L. cv. Claudio) tissues and (ii) measurements of various morphological and physiological parameters, namely biomass, shoot and root lengths, photosynthetic pigments, micronutrients (Zn, Mn) concentration and major inorganic and organic anions and essential cations homeostasis including Si itself.
Materials and methods
Plant culture and treatments
Durum wheat (T. turgidum L. ssp. durum cv. Claudio W.) was grown in hydroponic condition. Caryopses were surface sterilised with sodium hypochlorite containing 2.6 % active chloride for 3 min and then thoroughly washed with distilled water five to six times to remove excess chloride. Thereafter, they were imbibed in aerated water for 4 h at room temperature then transferred for germination in petri dishes for 3 days at 23 °C (day)/20 °C (night). Seedlings were then transferred to 12-L plastic containers filled with a modified Hoagland nutritive solution continuously aerated and containing the following: 0.5 mM KH2PO4, 0.5 mM K2HPO4, 0.5 mM MgSO4·7H2O, 1 mM KNO3, 1 mM Ca(NO3)2·4H2O, 0.125 mM KCl, 50 μM H3BO3, 12 μM MnSO4·H2O, 0.7 μM CuSO4·5H2O, 1 μM ZnSO4·7H2O, and 0.25 μM MoO4Na2·2H2O. For Fe, 100 μM FeIII-EDTA-Na was used for 10 days and then replaced by 10 μM Fe-N,N′-bis (2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid (HBED) when Cd treatments were started to avoid the complexation of Cd with EDTA (Chaney et al. 1998). Plants were grown under a short-day cycle (8/16 h 23 °C/20 °C day/night), 70 % humidity and 187 μmol photon m−2 S−1 light intensity. Eight containers each containing 100 wheat seedlings were installed, and four of these containers were supplied with 1 mM Si in the form of monosilicic acid from potassium-metasilicate Si(KOH)2, which has a K/Si mole ratio of 2 (Metso 400-YARA) (Voogt et al. 2001). Additional K produced by Si(KOH)2 was subtracted from KNO3, and the resultant nitrate loss was supplemented with dilute nitric acid. The pH of the solution was checked every day and adjusted to 6.5 ± 0.2 with 1 mM 2-morpholinoethanesulphonic acid (MES) buffer when required. After 10 days of culture in presence of silicon, the plants were exposed to 0, 0.5, 5.0 and 50 μM Cd(NO3)2·4H2O added to the nutrient solution. These concentrations were chosen according to previous reports (Ci et al. 2009, 2010) indicating Cd stress on wheat seedlings within this concentration range. Cadmium concentrations were tested without and with 1 mM Si application for 11 additional days. Nutrient solutions were changed every 3 days during the first 10 days (nutrient solution with Si alone when required) and every second day during the last 11 days (nutrient solution with added Cd and Si when required).
During each renewal, the nutritive solution was sampled at the beginning (before contact with wheat plants) and just before the renewal, filtered on 0.2 μm membrane and analysed for dissolved organic carbon (DOC), total Cd and Si to check for any possible contamination or element depletion.
Plant harvesting and biomass
After 21 days of culture, the plants of each treatment were harvested and separated into shoots and roots. Analyses were performed on individual plants with shoots and roots analysed separately. Shoots were washed with distilled water and then divided into two parts: one subsample was frozen in liquid N2 prior to measuring its organic and inorganic contents and localising Cd and Si by microscopy/spectroscopy; another subsample was used to measure fresh weight then oven-dried at 80 °C for approx. 48 h until constant dry weight was reached, weighted (for water content) and finely ground and homogenised with a Retsch mixer mill for further analysis. Harvested roots were also divided into two groups of whole mixed roots. One subsample was washed with dilute HCl to measure metals bound to root cell walls (root apoplasmic metals) as described by Chaignon et al. (2002): briefly, 0.4 g of roots (on a fresh weight basis) was shaken end-over-end with 20 mL of 1 mM HCl for 3 min, then 180 μl of 1 M HCl was added to yield a final concentration of 10 mM HCl. After shaking for another 3 min, the roots were recovered and gently dried between two layers of filter paper then oven-dried at 80 °C. Another subsample was washed only with distilled water, and some fresh roots were stored in liquid nitrogen, while others were oven-dried at 80 °C and used to measure fresh weight as described for the shoots.
Anion assays
Anionic profiling was performed for both shoots and roots: fresh plant samples were weighted, incubated in Milli-Q ultrapure water in a 1:8 FW/water ratio, heated at 70 °C for about 40 min, homogenised, cooled at room temperature and the supernatant solution was recovered after the extracted solution was centrifuged three times at 14 × 103 g at 4 °C for 10 min. The concentration of major inorganic and organic anions in the supernatant was determined by high-performance ionic chromatography (LC20, Dionex) using an IonPaq AS11 column and a NaOH linear gradient. NaOH concentration was raised linearly from 3.5 to 5 mM over 3 min then from 5 to 33.5 mM over 8 min following the procedure described in El Kassis et al. (2007). Measurement of each anion was performed by comparing the retention times and peak areas, using the Chromeleon software (Dionex), with the standards.
Determination of photosynthetic pigments
Chlorophylls (a and b) and carotenoid contents in the fresh leaves (about 1.0 g) were extracted in the dark with 10 mL of 80 % acetone by continuous shaking until colour had completely disappeared from the leaves. Light absorbance at 663, 645 and 470 nm was determined by a spectrophotometer (Hitachi U-2800). The concentrations of chlorophylls and carotenoids were calculated by using the adjusted extinction coefficients (Lichtenthaler 1987).
Determination of Cd, micro and macro elements and silicon in plant samples
For the determination of Cd, micro (Mn and Zn) and macro (K, P, Ca and Mg) elements, finely ground plant part samples (0.25 g) were digested in 4 mL of concentrated HNO3 70 % in a block of mineralisation with a rise in temperature up to 95 °C for 6 h then filtered at 0.2 μm (Whatman cellulose nitrate membrane) (modified from Keller et al. 2003).
Silicon concentration in plant shoots and roots was measured using the Tiron extraction method (Guntzer et al. 2010). In brief, 50 mg of each plant sample (part above or underground) was extracted by adding 30 mL of 0.1 M Tiron, [4,5-dihydroxy-1,3-benzene-disulfonic acid disodium salt, (OH)2C6H2(SO3Na)2], solution buffered at pH 10.5 in polypropylene tubes, which were placed in a water bath at 85 °C and horizontally shaken for 2 h with the caps loosely closed to prevent evaporation. The tubes were then cooled, and 10 mL was sampled into pre-labeled 40-mL sample vials containing 10 mL of 30 % H2O2 in order to destroy Tiron. This new solution was shaken again at 85 °C in the water bath for about half an hour till the colour had disappeared and the solution was filtered at 0.2 μm.
The micro (Mn and Zn), macro (K, P, Ca and Mg) elements and Cd and Si concentrations were measured in the plant digests and extracts by inductively coupled plasma optical emission spectroscopy (ICP-OES, Jobin-Yvon, Ultima-C).
Extraction of phytoliths
Phytoliths were extracted from shoots of treatments with and without Si addition according to Parr et al. (2001a, b). This technique included the following steps: (1) rinsing 10–20 g of plant material in Milli-Q ultrapure water, drying it in an oven at 50 °C and grinding during 3 min in an agate mill; (2) heating the plant material in the crucible at 500 °C for 8 h; (3) removing from crucibles and transferring the content to polypropylene tubes (0.2 g per tube); (4) adding 10 mL of 10 % HCl to each tube, heating in water bath at 70 °C for 20 min; (5) centrifuging at 3,500 rpm for 5 min followed by decantation; (6) rinsing with Milli-Q ultrapure water and centrifuging at 3,500 rpm for 5 min followed by decantation; (7) adding 10 mL of 15 % H2O2, heating in water bath at 70 °C for 20 min; (8) centrifuging at 3,500 rpm for 5 min and decanting; (9) rinsing with Milli-Q ultrapure water via repeated centrifugation at 3,500 rpm during 5 min; and (10) drying at 50 °C during 48 h in an oven. The residual solid phases were then thoroughly rinsed in de-ionised water and freeze-dried at −55 °C. The resulting mineral phases were characterised by scanning electron microscopy (SEM) using a JEOL JSM840a and by X-ray diffraction using an INEL CPS 120 Coκα, with a scan speed of 0.02 s−1. This method of phytolith extraction did not produce any preparation artefacts: the electron microscopic observations of freshly extracted phytoliths did not reveal any heat-generated smoothing and melting features on the surface, and the X-ray diffraction analysis on the phytolith material did not identify the presence of any crystalline phases (data not shown).
Localisation of Si and Cd at the tissue level by micro X-ray fluorescence (μXRF)
One shoot sample containing 1 mM Si in the nutrient solution and two root, 50 μM Cd and 50 μM Cd + 1 mM Si, samples were selected for chemical micro-analyses by μXRF. Frozen samples were freeze-dried at −55 °C for about 72 h and then embedded in an epoxy resin. The embedded samples were then cut transversely with a diamond wire saw at about 10 cm from root apex to get the comparable root cross sections. The thickness of the obtained cross sections was about 200 μm. The measurements were carried out on a microscope (HORIBA XGT7000) equipped with an X-ray guide tube producing a finely focused and high-intensity beam with a 10-μm spot size Rh X-ray tube, accelerating voltage of 30 kV and a current of 1 mA. X-rays emitted from the irradiated sample were detected with EDX spectrometer equipped with a liquid nitrogen cooled high-purity Si detector. Only elements with an atomic number Z > 11 (Z Na) can be detected. Analyses were performed in sample chamber vacuum mode. Elemental maps (256 px2, pixel size of 8 μm), showing in particular Cd, Si, S and K distribution in cross sections, were recorded with a total counting time of 30 × 1,000 s under total vacuum mode (to enhance Si detection). Images were obtained from Kα emission line intensities of Cd, Si, K, Ca, Zn and Mn.
Analysis at the cellular level: scanning electron microscopy (SEM-EDX)
SEM observations were performed on the 50 μM Cd + 1 mM Si-treated roots. The root sample was divided into two parts. The first part was freeze-fractured in a cryo-specimen chamber and sputter-coated with a mixture of gold and palladium in argon atmosphere. The other part was freeze-dried at −55 °C for about 72 h and then examined by cutting horizontally ca. 5 mm away from root apex and vertically the root at different positions. Both samples were examined using a Philips XL30 SFEG SEM at nitrogen liquid temperature (North Billerica, MA) coupled to an Oxford Instruments (Oxfordshire, UK) EDX. The SEM was operated at 20 kV with a counting time of 300 s per point. With SEM-EDX, chemical microanalysis results were obtained with a spatial resolution of about 2 to 5 μm and a penetration depth of about the same range and are expressed in percent weight.
Analysis at the root surface: XPS
Apart from the desorption experiment, root surface was microscopically examined to identify possible Cd and Si localisation depending on Cd and Si concentrations in solution. We used the XPS technique, which is known to probe approximately the first 10 nm of a sample (65 % of the signal originates from the first 30 Å and about 95 % of the signal comes from 100 Å). It is used to measure elementary composition of freeze-dried surfaces. Experiments were carried out in a Kratos Axis UltraDLD vacuum chamber, fitted with a monochromatic AlKα X-ray source (hν = 1486.6 eV and power = 150 W). Wide spectra and high-resolution scans were recorded with pass energy of 160 and 20 eV, respectively. Data were analysed using the commercial software casaXPS, with Shirley background correction and binding energy calibration using C 1s at 285 eV. Different chemical environment for one given atom will give rise to shift in binding energy of emitted electrons (for example, it is possible to distinguish a C atom bound to another C atom with one bound to an O atom). Samples were placed across a hole in a sample holder made of Mo, so that the collected signal was only coming from the sample. No X-ray beam damage was noticed during the analysis.
Statistical analysis
All the data were statistically analysed using one-way ANOVA at a significance level of P ≤ 0.05 with SPSS 16.0 software for Windows. Single-step multiple comparisons of means were performed via Tukey’s post hoc test, while Student’s t test was used for comparison against control (in ESM). Different letters on the histograms indicate that the means were statistically different at P ≤ 0.05 level. Data presented are from eight treatments with three to five replicates as described above.
Results
Wheat growth
Increasing Cd concentrations in the nutrient solution reduced shoot length and induced slight chlorosis symptoms on leaves of plants treated with 5 μM Cd and pronounced chlorosis on plants grown in 50 μM Cd (Fig. 1a, c), while there were no visible symptoms on the leaves in the presence of Si (Fig. 1a). Cadmium treatments reduced the length of primary seminal roots as compared to control (Fig. 1b, c), and the effect was significant for the highest Cd treatment. Differences were also observed in the fresh and dry weights of shoots and roots among the treatments (Fig. 11, ESM): higher (5.0 and 50 μM) Cd treatments significantly reduced the shoots and roots fresh and dry weights. Addition of Si significantly increased root length especially in 5.0 and 50 μM Cd compared to Cd-only treated plants (Fig. 1b, c) but had no effect on fresh and dry weights (Fig. 11, ESM).
Effect of exogenous Cd and silicon on morphology of shoots and roots of wheat seedlings grown in hydroponics with 0, 0.5, 5 and 50 μM Cd and 0 or 1 mM Si. a Second fully developed wheat leaf aspect: chlorosis induced by increasing concentrations of Cd- on Cd-only-treated plants as compared to plants treated with Cd + Si exhibiting no visible symptoms. b Primary root length according to Cd and silicon treatments (distance between two lines is 3 cm). a, b Cd0 = 0 μM Cd, Cd0.5 = 0.5 μM Cd, Cd5 = 5.0 μM Cd, Cd50 = 50 μM Cd. c Length of shoots and primary seminal roots of wheat plants. Bars represent SD of five replicates. Different letters indicate significant differences among the treatments at a P < 0.05 for shoots and roots separately
Chlorophylls and carotenoid concentrations
Total chlorophyll (Chl a + Chl b) and carotenoid concentrations measured in leaves significantly decreased in similar proportions with increasing Cd concentrations in the nutrient solution (decrease of 8 % for 0.5 μM Cd, 31 % for 5 μM Cd and 67 % for the 50 μM Cd treatment) (Fig. 2a). In the presence of 1 mM Si, all pigment concentrations were higher than the respective concentrations found in the Cd-only treated plant (from +10 % for Chl b in the 0.5 μM Cd + 1 mM Si treatment and up to +53 % for carotenoids in the 5 μM Cd + 1 mM treatment), while Chl a/Chl b ratio was not modified (Fig. 2b).
Effect of exogenous Cd and silicon on pigment concentrations in shoots of wheat seedlings grown in hydroponics with 0, 0.5, 5 and 50 μM Cd and 0 or 1 mM Si. a Chlorophyll a + b and carotenoids concentrations (in g kg−1 DW) and b chlorophyll a/b ratio in shoots of wheat seedlings grown in hydroponics with 0, 0.5, 5 and 50 μM Cd and 0 or 1 mM Si. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05
Si, Cd and micro nutrient (Mn, Zn) concentrations in shoots and roots
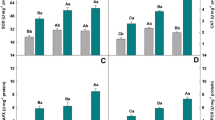
Silicon concentration in shoots significantly decreased with increasing Cd levels in the nutrient solution, while no significant effect was observed in roots (Fig. 3). Cadmium concentrations significantly increased in shoots with increasing Cd levels in the nutrient solution (Fig. 4). Silicon application decreased Cd concentrations in shoots as compared to the same Cd treatments without Si application, the decrease being significant in the highest Cd treatment. Mn concentrations significantly decreased in shoots with increasing Cd levels, while Zn concentrations significantly decreased only in highest Cd treatment as compared to control (Fig. 4). Silicon addition did not change significantly both Mn and Zn concentrations as compared to same treatments without Si.
Effect of exogenous Cd on silicon concentration in wheat shoots and roots grown in hydroponics with 0, 0.5, 5.0 and 50 μM Cd and addition of 1 mM Si. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05 for shoots and roots separately
Cadmium contents significantly increased in diluted acid-washed (symplasmic Cd) roots with increased Cd concentrations in solution containing or not Si (Fig. 5a). Manganese concentration decreased, while Zn concentration remained constant or increased with increasing Cd levels in solution. A decrease in Mn concentration was 14, 66 and 71 % for 0.5, 5.0 and 50 μM Cd treatments, respectively, as compared to control (Cd = 0 μM). Zinc concentration increased 3 and 22 % for 0.5 and 50 μM Cd treatments, respectively, as compared to control (Cd = 0 μM). Silicon application did modify neither Cd nor Mn uptake by the roots. Silicon addition only significantly increased Zn concentrations at higher Cd treatments, and this increase was 158 and 60 % for 5 and 50 μM Cd, respectively.
Cadmium, Zn and Mn concentrations (mg kg−1 DW) in a acid-washed roots and b apoplasmic adsorbed Cu, Zn and Mn concentrations (mg kg−1 DW) calculated as the difference between concentrations in water-washed and acid-washed roots of wheat seedlings grown in hydroponics with 0, 0.5, 5 and 50 μM Cd and 0 or 1 mM Si. Bars represent SD of three replicates. Different letters indicate significant differences among the treatments at a P < 0.05
Cadmium adsorption onto the root surface increased with increasing Cd concentrations in the nutrient solution (Fig. 5b), and Si application further increased Cd adsorption on the roots surface especially in the 50 μM Cd treatment. Manganese adsorption did not vary much across Cd treatments, while Si application significantly decreased adsorption of Mn onto the root surface only in the highest Cd treatment. Zinc adsorption increased in all Cd treatments, and this increase was 8, 193 and 245 % for the 0.5, 5.0 and 50 μM Cd treatments, respectively, as compared to control (Cd = 0 μM). Silicon application significantly decreased Zn adsorption of 52 and 76 % for the 5 and 50 μM Cd treatments, respectively, as compared to control.
The total plant Cd contents (Cd mass per plant) increased with Cd concentration in the nutrient solution, but no significant difference was observed between the –Si and +Si treatments for the same Cd concentration in solution except for three cases: shoots and roots for the 0.5 μM Cd and roots for the 5 μM Cd treatments (Table 2, ESM).
Major cations and organic and inorganic anions
Increasing Cd concentrations in the nutrient solution decreased K, P, Ca, Mg and inorganic anion concentrations in both shoots and roots, except for Ca in roots at the highest Cd concentration in solution and chlorine in roots, which significantly increased with increasing Cd levels (Fig. 12, ESM). Addition of Si did not significantly change both concentrations and trends (data not shown). Phosphate concentration remained constant across the Cd treatments, but PO4 concentrations in roots were larger in all treatments with Si.
Concentrations of malate, citrate and aconitate were larger in shoots than in roots and increased both in shoots and roots in response to increasing Cd levels in solution with a significant increase in both shoots and roots under the highest Cd stress as compared to control (Fig. 6). Consequently, they only decreased in Cd + Si-treated as compared to Cd-only-treated roots at the highest Cd concentration.
Concentrations of a malate, b citrate, c and d aconitate (mmol kg−1 DW) in shoots and roots of wheat seedlings grown in hydroponics with 0, 0.5, 5 and 50 μM Cd and 0 or 1 mM Si. Bars represent SD of four replicates. Different letters indicate significant differences among the treatments at a P < 0.05 for shoots and roots separately
Cadmium and Si localisation in shoots
Analysis by μXRF of cross sections of shoot treated with 1 mM Si showed that Si was mainly detected inside the cells of abaxial and adaxial epidermis of leaf in the form of dense spots (Fig. 7). No Cd was detected because concentrations were below detection limits and because of interferences between Cd, K and Rd used as detector (data not shown). Analysis of individual phytoliths extracted from leaves indicated that, as expected, Si was present as amorphous silica (Fig. 8). SEM and optical observations of these phytoliths showed bodies of various shapes, and EDX microanalysis revealed that they contained mostly Si and O. Traces of magnesium, Ca, K and Cl were also detected but no Cd regardless of the Cd loading.
Cd and Si localisation in roots
SEM observations were only performed on 50.0 μM Cd + 1 mM Si-treated roots. Elements analysed by EDX were Si, Cd, C, O, Na, Mg, P, S, Cl, K, Ca, Mn, Fe and Zn, and the semi-quantitative concentrations of these elements are presented in percent weight (Fig. 10). Relatively large amounts of Cd were present in the cortical parenchyma region near rhizodermis and in the rhizodermis (Figs. 9 and 10). Small amounts of Cd were detected in the xylem or in phloem, while in endodermis cells, only trace amounts were detected. On the contrary, Si was mainly detected in endodermis cells, while small amounts were present in the cortical parenchyma cells. Only traces of Si were detected in the rhizodermis cells. Thus, we did not observe any Cd-Si co-localisation in the roots (Fig. 10).
Scanning electron microscope (SEM) microphotographs of root cross or longitudinal sections of wheat plant grown in 50 μM Cd + 1 mM Si solution. a−d Scanning electron microscopy micrographs. Different letters indicate different tissues of root cross section, i.e. r rhizodermis, cp cortical parenchyma, en endodermis, pe pericycle, cc central cylinder, mx metaxylem. Different numbers indicate locations at which EDX spectra were taken. a ×500, b ×350, c ×6,500, d ×250
Correlation between Si and Cd concentrations (in percent weight) at different locations in roots of wheat plants grown in hydroponics with increasing Cd treatments with or without addition of 1 mM Si. Numbers labelling the points correspond to the numbers indicated on the SEM micrographs (Fig. 9)
Chemical composition of the outer thin root surface layer by XPS analysis
Results of microscopic analysis described above were further corroborated by XPS analyses that allowed examining the outer thin surface layer (<100 Å) elementary composition of freeze-dried roots: the surface concentration of Si for roots grown in Si-rich (1 mM) solutions was significantly higher than that of roots grown without Si addition in the nutrient solutions (Table 1). The most important result was the enrichment in Cd observed on roots grown in +Si solutions as compared to roots grown in Cd-only solutions (0.156 and 0.079 atomic percentage, respectively). Similarly, Cd/C ratio on the surfaces of root grown in 50 μM Cd solution decreased from 0.0025 in the +Si solution to 0.0014 in Cd-only solution. Interestingly, P concentration (and P/C ratio) increased in roots grown in 50 μM Cd + Si solution by a factor of 2 as compared to the Cd-only solution. In addition, Cd and Si distribution at the root surface was highly heterogeneous: for sample in 50 μM Cd + 1 mM Si treatment, Cd concentration measured at two different spots in two different roots ranged from 0.08 to 0.31. The concentration of other measured components did not exhibit any significant variation among samples subjected to different treatments. High-resolution spectra of C 1s revealed three types of carbon at the surface: C−C bond at 285 eV, C atom bond to 1 O atom (C−O) at 286.5 eV and C atom bond to 2 O atoms (O−C=O) at 289 eV. No significant changes in the ratio of these functional groups were noticed in all treatments as compared with the untreated roots (Table 1).
Discussion
Diagnosis of Cd toxicity in wheat and alleviation by Si addition
Increasing Cd concentrations in solution induced visible toxicity symptoms on both roots and shoots (Fig. 1) including decreased shoot and root fresh and dry weights (Fig. 11, ESM), shoot and root lengths and increased chlorosis (Fig. 1). These results are in agreement with previous studies in which toxicity symptoms were observed in wheat plants with 50 μM Cd concentration in the nutrient solution (Ci et al. 2009, 2010) but also in a broad set of plants such as maize (Wójcik and Tukiendorf 2005; Zhang et al. 2008; Vaculík et al. 2009, 2012, 2015), tomato (Haouari et al. 2012), rice (Shi et al. 2005; Liu et al. 2009) and cucumber (Feng et al. 2010). Chlorosis symptoms were observed in leaves of tomato (Lycopersicon esculentum) and pea plants with 10 and 50 μM Cd concentration in the nutrient solution, respectively (López-Millán et al. 2009; Bavi et al. 2011). The inhibitory effect of high Cd concentration on root length has been previously explained by alteration of cell division at root apex, inhibition of several enzyme activities (Zhang et al. 2008) and breakdown of root cortex cells (Sridhar et al. 2007) leading to an increasing Cd influx (Cosio et al. 2005).
Addition of 1 mM Si in solution only had an alleviating effect on growth of primary seminal roots especially when Cd was the highest in solution (Fig. 1). These results are in agreement with the studies reporting that Si addition under Cd stress increased root elongation (Vaculík et al. 2009, 2012). They speculated that Si mediated increase in cell wall extensibility of young apical parts of maize roots may be considered as one of the causal factors for the better root elongation in Cd + Si treatment, while there were less lateral roots. As a consequence, the root biomass did not change with silicon application. The effect of Si on Cd concentration in shoot was more evident when calculating the root-to-shoot ratios, which increased from ca. 12, 29 and 20 (without Si) to 22, 33 and 29 (with Si) in the 0.05, 5.0 and 50 μM Cd treatments, respectively. Similar results have been reported previously in rice (Shi et al. 2005; Zhang et al. 2008), cucumber (Feng et al. 2010) and maize (Liang et al. 2005) and more recently in wheat (Naeem et al. 2015). In the later study, they also found a reduction of the root-to-grain ratio.
Photosynthetic pigments can be used as early indicators of growth or recovery under stress conditions as they are the key components of photosynthesis. Cadmium treatments significantly decreased carotenoids as well as total chlorophyll concentrations in wheat leaves (Fig. 2) in accordance with the visual chlorosis symptoms. The effects of Cd on chlorophyll biosynthesis have been commonly observed in wheat (Ouzounidou et al. 1997), maize (Vaculík et al. 2015), tomato (López-Millán et al. 2009) and in pea plants (Sandalio et al. 2001). This reduction in chlorophyll biosynthesis may be due to the substitution of a central magnesium ion of chlorophyll structure with Cd due to their similar charges (Küpper et al. 1998) and/or may be due to damage or functional loss of the photosynthetic machinery (Feng et al. 2010). This hypothesis is in accordance with the lower Mg concentration measured in shoots of plants having the largest Cd concentration in shoots (data not shown). The carotenoids are accessory pigments that help in light harvesting and also protect chlorophylls and photosynthetic apparatus from oxidative damage and excess heat (Britton 1995). Due to decreased carotenoid concentration under Cd stress, chlorophyll damage may be increased. Unlike for morphological parameters and Cd concentrations, Si had an immediate effect on Chl a + b and carotenoid contents: the addition of Si significantly increased the concentration of these pigments as compared to Cd-only treatments (Fig. 2) probably contributing to a reduction of chlorosis. Similar effects of Si on photosynthetic pigments have also been observed in cucumber under 100 μM Cd stress supplied with 1 mM Si (Feng et al. 2010) and in maize grown in 5 and 50 μM Cd with 5 mM Si (Vaculík et al. 2015). These authors also demonstrated that Si plays an important role in the protection of chloroplast ultrastructure and nitrogen metabolism. Nwugo and Huerta (2008) reported that Si addition improved chlorophyll-fluorescence parameters and light use efficiency in rice under 5 μM Cd stress. The lower (non-significant) Si effect in the highest Cd treatment may be related to the lower Si concentration in shoots.
The effect of Si on cation and anion uptake
Micronutrient distribution was impacted by the increase in Cd concentration in solution (Figs. 4 and 5) and not corrected by the addition of Si. Decrease in Zn concentrations in shoots and roots with increasing Cd concentrations in plant is traditionally explained by a competitive inhibition of Zn uptake when there is an excess Cd and vice versa (Grant et al. 1998; Harris and Taylor 2001). Indeed, Zn and Cd share a common transport system at the root cell plasma membrane in both bread and durum wheat (Hart et al. 2002). Especially in the highest treatments, larger amounts of Zn were internalised and less were adsorbed onto the apoplasm (Fig. 5a versus b) in plants supplied with Si. Larger Zn absorption in the presence of Si may increase Zn/Cd competition at the plasma membrane helping to reduce Cd concentration in the symplasm.
A significant increase of malate, citrate and aconitate concentrations in shoots and/or roots of durum wheat exposed to 50 μM Cd (Fig. 6) agrees with the previous studies with Al and Cd (Saber et al. 1999) and with Al (Ma et al. 1998, 2001). Higher concentration of organic anions in the presence of Cd may indicate a plant physiological response against Cd toxicity. Organic anions may chelate Cd in the cytoplasm and detoxify Cd while allocating it to metabolically inactive parts of the cells. In the presence of Si, the concentration of organic anions decreased both in shoots and roots of the 50 μM treatment (Fig. 6). It is possible that the detoxification mechanisms of organic ligand production are altered in the presence of Si, because of a smaller total uptake of Cd in shoots. Additional explanation for lower organic anion production could be a larger adsorbed Cd pool onto roots as proven by XPS analyses that may induce a lower potentially available “sensed” Cd concentration (Figs. 4 and 5) as discussed below.
The concentration of organic anions is also important for Cd translocation from root to shoot. Larger production of organic anions in roots under Cd stress without Si application may complex Cd in roots and may facilitate its translocation from root to shoot. In rape seed plants, the level of amino acids and organic acids detected was found to be enough to bind Cd transported via the xylem (Nakamura et al. 2008). Similarly, a significant increase in citrate synthesis and Cd-citrate complexes has been reported to be present in the xylem sap of tomato plants treated with Cd (Senden et al. 1995; López-Millán et al. 2009). Zorrig et al. (2010) also reported that cadmium-citrate complexes control Cd translocation from root to shoot in lettuce. Our findings indicate that increased Cd concentration in shoots without Si addition might be due to enhanced Cd transport mediated by organic ligands and chloride complexes.
Cadmium-Si distribution in plant: coprecipitation vs. compartmentalisation
The results also highlighted the changes in the uptake and distribution of Si in wheat plants (Fig. 2) indicating that Si uptake is adversely affected by Cd concentration in the medium. With a shoot-to-root ratio shifted from 6.5 to 2.5 between the 0 and the 50 μM Cd in solution, reduced Si uptake with increasing Cd concentration in the nutrient solution indicated that Cd may disturb the plant metabolism which resulted in reduced Si uptake by plant roots. Since Si potentially alleviates a whole range of stresses, this suggests that a Cd contamination may weaken the Si effect and, as a consequence, may reduce the resistance of wheat to other stresses. But unlike for shoots, there was no effect of Cd on Si concentrations either in the bulk of the roots (Fig. 2) or at the thin outer surface layer (Table 1). This resulted in a shift in the Si shoot/root ratio towards lower values, which may indicate an increasing Si immobilisation in roots with increasing Cd concentration in roots. Whether this reduced Si transfer to the shoot is a consequence of Cd toxicity or is an active process aiming at reducing Cd toxicity at the root level remains to be clarified.
The increase of the root surface Cd concentration in the presence of Si as inferred from XPS analysis may also suggest some armoring protection mechanisms consisting in non-homogeneously distributed granular-like Cd, Si-bearing precipitates. The extremely small thickness of these newly formed compounds does not allow detecting them with usual microscopic/macroscopic techniques. Interestingly, the increase of surface phosphorus concentration in the presence of Si (Table 1) may also lead to additional immobilisation of Cd in the form of Cd-phosphates; however, the synergetic mechanism of Si and P immobilisation remains unclear.
An additional effect of Si investigated in this study was whether Si would induce change in Cd allocation within the plant shoot tissues. Specifically, without change in its total concentration, Cd could be located in less metabolically active compartments inducing less toxicity. One of those compartments could be the phytoliths. Indeed, when treated with Si, shoots were the main sites for Si accumulation in the form of phytoliths (Fig. 7), which were mainly deposited in the adaxial and abaxial epidermis, visible in the form of dense spots (Fig. 8). Silicon deposition in the epidermis may be associated with stomata and specific bulliform cells, where water evaporates from the plants (Motomura et al. 2000; Sommer et al. 2006; Currie and Perry 2007). Ma et al. (2001) proposed that Si deposition may decrease the transpiration in the surface layers of rice. Such a reduction in transpiration would reduce Cd translocation to shoots as observed in Fig. 4. Other mechanisms associated to the decrease in Cd toxicity when plants are supplied with Si could be (i) enhanced apoplasmic binding of Cd as proposed for maize (Vaculík et al. (2012)) and rice (Wang et al. 2000; Liu et al. 2009), (ii) altered Cd subcellular distribution and (iii) reduced Cd content in cell organelle fractions of leaves as identified for peanuts (Shi et al. 2010). Neumann and zur Nieden (2001) also reported that Zn toxicity in Cardaminopsis halleri leaves might be reduced by co-precipitation of Zn and Si in the form of Zn silicate in the vacuole and the cell wall. Although apoplasmic binding could not be ruled out, the EDX spectra obtained from phytoliths of the studied wheat plants contained no Cd, or in concentrations below EDX detection limits, which indicates that there was no significant co-precipitation of Cd and Si in wheat leaves. Consequently, compartmentalisation of Cd in shoots rather than co-precipitation may be a possible mechanism of Si-reduced Cd toxicity in wheat plants, together with reduced total Cd concentration and uptake both in shoots and roots.
In wheat roots, Si may also act as a physical barrier and/or reduce Cd transfer by compartmentalisation of Cd in metabolically inactive parts such as root apoplasm. Although the mechanism is unclear, the modified Cd distribution observed in roots grown in presence of Si disagrees with previous reports on other plants indicating that Si did not affect the distribution of apoplasmic and symplasmic Cd in roots of peanut (Shi et al. 2010), rice (Shi et al. 2005) and maize plants (Vaculík et al. 2012). The change however was also observed by Keller et al. (2015) in wheat roots exposed to Cu and Si.
Furthermore, SEM-EDX analysis indicated that Si was mostly in the endodermis cell walls, while Cd was mainly found in the cortical parenchyma region, in small amount in the central cylinder and in trace amounts in the vicinity of endodermis (Figs. 9 and 10) of water-washed roots. The consequences are twofold: (i) Si and Cd do not seem to co-precipitate in roots unlike suggested in previous reports for rice (Wang et al. 2000; Shi et al. 2005) and maize (da Cunha and do Nascimento CWA 2009) and (ii) massive Si deposition in the vicinity of the endodermis may reduce the cell wall porosity of the Casparian strips and reduce metal apoplasmic transport as already suggested for Cd tolerance in rice (Shi et al. 2005) and maize (da Cunha and do Nascimento CWA 2009). It may provide an increased selectivity against metal entering the stele of roots (MacFarlane and Burchett 2000) and consequently reduce translocation to shoots as indicated by reduced shoot Cd concentration in the presence of Si (Fig. 4).
In conclusion, in hydroponics, total Cd uptake was reduced when wheat plants were supplemented with Si. Our results suggest that the reduction of the toxicity symptoms can be explained by the existence of a root localised protection mechanism consisting in armoring the root surface by Si- and Cd-bearing compounds and in limiting root–shoot translocation.
References
Adrees M, Ali S, Rizwan M, Rehman MZ, Ibrahim M, Abbas F, Farid M, Qayyum MK, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Bavi K, Kholdebarin B, Moradshahi A (2011) Effect of cadmium on growth, protein content and peroxidase activity in pea plants. Pak J Bot 43:1467–1470
Britton (1995) Structure and properties of carotenoids in relation to function. FASEB J 9:1551–1558
Chaignon V, Di Malta D, Hinsinger P (2002) Fe-deficiency increases Cu acquisition by wheat cropped in a Cu-contaminated vineyard soil. New Phytol 154:121–130
Chaney RL, Ryan JA, Li YM, Welch RM, Reeves PG, Brown SL, Green CE (1996) Phyto-availability and bio-availability in risk assessment of cadmium in agricultural environments. In: Proceedings of sources of cadmium in the environment. Organization for Economic Co-Operation and Development, Paris, pp 49-78
Chaney R, Brown S, Angle J (1998) Soil-root interface: ecosystem health and human food-chain protection. Soil chemistry and ecosystem health. P. Huang. Madison, Wisconsin, Soil Sci Soc Am, pp 279-311
Ci D, Jiang D, Dai T, Jing Q, Cao W (2009) Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 77:1620–1625
Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W (2010) Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiol Plant 32:365–373
Cieslinski G, Van Rees KCJ, Szmigielska AM, Krishnamurti GSR, Huang PM (1998) Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant Soil 203:109–117
Clemens S, Palmgren MG, Kramer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7:309–315
Cosio C, DeSantis L, Frey B, Diallo S, Keller C (2005) Distribution of cadmium in leaves of Thlaspi caerulescens. J Exp Bot 56:765–775
Currie HA, Perry CC (2007) Silica in plants: biological, biochemical and chemical studies. Ann Bot 100:1383–1389
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197:323–330
da Cunha KPV, do Nascimento CWA, da Silva AJ (2008) Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on contaminated soil. J Plant Nutr Soil Sci 171:849–853
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Doncheva SN, Poschenrieder C, Stoyanova ZI, Georgieva K, Velichkova M, Barcelo J (2009) Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Environ Exp Bot 65:169–197
El Kassis E, Cathala N, Rouached H, Fourcroy P, Berthomieu P, Terry N, Davidian JC (2007) Characterization of a selenate-resistant Arabidopsis thaliana mutant. Root growth as a potential target for selenate toxicity. Plant Physiol 143:1231–1241
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci U S A 91:11–17
Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H (2010) Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Scient Horti 123:521–530
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Grant CA, Buckley WT, Bailey LD, Selles F (1998) Cadmium accumulation in crops. Can J Plant Sci 78:1–17
Greger M, Löfstedt M (2004) Comparison of uptake and distribution of cadmium in different cultivars of bread and durum wheat. Crop Sci 44:501–507
Guntzer F, Keller C, Meunier JD (2010) Determination of the silicon concentration in plant material using Tiron extraction. New Phytol 188:902–906
Haouari CC, Nasraoui AH, Bouthour D, Houda MD, Daieb CB, Mnai J, Gouia H (2012) Response of tomato (Solanum lycopersicon) to cadmium toxicity: growth, element uptake, chlorophyll content and photosynthesis rate. Afr J Plant Sci 6:1–7
Harris NS, Taylor GJ (2001) Remobilization of cadmium in maturing shoots of near isogenic lines of durum wheat that differ in grain cadmium accumulation. J Exp Bot 52:1473–1481
Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV (1998) Characterization of cadmium binding, uptake and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol 116:1413–1420
Hart JJ, Welch RM, Norvell WA, Kochian LV (2002) Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol Plant 116:73–78
Hasan SA, Fariduddin Q, Ali B, Hayat S, Ahmad A (2009) Cadmium: toxicity and tolerance in plants. J Environ Biol 30:165–174
Hernandez-Apaolaza L (2014) Can silicon partially alleviate micronutrient deficiency in plants? A review. Planta 240:447–458
Jalil A, Selles F, Clarke JM (1994) Effect of cadmium on growth and the uptake of cadmium and other elements by durum wheat. J Plant Nutr 17:1839–1858
Keller C, Hammer D, Kayser A, Richner W, Brodbeck M, Sennhauser M (2003) Root development and phytoextraction efficiency: comparison of different species. Plant Soil 249:67–81
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241:847–860
Küpper H, Küpper F, Spiller M (1998) In situ detection of heavy metal substituted chlorophylls in water plants. Photosyn Res 58:123–133
Lasat MM (2002) Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual 31:109–120
Liang YC, Wong JWC, Long W (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Lichtenthaler HK (1987) Chlorophylls and carotenoids—pigments of photosynthetic biomembranes In: Colowick SP, Kaplan NO (ed.) Methods in enzymology. Academic Press, San Diego-New York-Berkeley-Boston-London-Sydney-Tokyo-Toronto, 148:350-382
Liu JG, Qian M, Cai GL, Yang JC, Zhu QS (2007) Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J Hazard Mater 143:443–447
Liu C, Li F, Luo C, Liu X, Wang S, Liu T, Li X (2009) Foliar application of two silica sols reduced cadmium accumulation in rice grains. J Hazard Mater 161:1466–1472
López-Millán AF, Sagardoy R, Solanas M, Abadía A, Abadía J (2009) Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot 65:376–385
Lux A, Martinka M, Vaculik M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Ma JF, Hiradate S, Matsumoto H (1998) High aluminium resistance in buckwheat. II. Oxalic acid detoxifies aluminium internally. Plant Physiol 117:753–759
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
MacFarlane GR, Burchett MD (2000) Cellular distribution of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.) Vierh. Aquatic Bot 68:45–59
Meyer MW, Fricke FL, Holmgren GGS, Kubota J, Chaney RL (1982) Cadmium and lead in wheat grain and associated surface soils of major wheat production areas of the United States. pp 34. In: Agronomy abstracts, the Am Soc Agron, Madison
Motomura H, Fujii T, Suzuki M (2000) Distribution of silicified cells in the leaf blades of Pleioblastus chino (Franchet et Savatier) Makino (Bambusoideae). Ann Bot 85:751–757
Naeem A, Saifullah, Ghafoor A, Farooq M (2015) Suppression of cadmium concentration in wheat grains by silicon is related to its application rate and cadmium accumulating abilities of cultivars. J Sci Food Agric 95:2467–2472
Nakamura SI, Akiyama C, Sasaki T, Hattori H, Chino M (2008) Effect of cadmium on the chemical composition of xylem exudates from oilseed rape plants (Brassica napus L.). Soil Sci Plant Nutr 54:118–127
Neumann DE, Zur Nieden U (2001) Silicon and heavy metal tolerance of higher plant. Phytochem 56:685–692
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelate-extractable soil cadmium. Soil Sci Soc Am J 64:2162–2168
Nwugo CC, Huerta AJ (2008) Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 311:73–86
Ouzounidou G, Moustakas M, Eleftheriou EP (1997) Physiological and ultrastructural effects of cadmium on wheat (Triticum aestivum L.) leaves. Arch Environ Contam Toxicol 32:154–160
Parr JF, Lentfer CJ, Boyd WE (2001a) A comparative analysis of wet and dry ashing techniques for the extraction of phytoliths from plant material. J Archaeol Sci 28:875–886
Parr JF, Dolic V, Lancaster G, Boyd WE (2001b) A microwave digestion method for the extraction of phytoliths from herbarium specimens. Rev Palaeobot Palynol 116:203–212
Rizwan M, Meunier JD, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209–210:326–334
Rogalla L, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Saber NE, Abdel-Monein AM, Barakat SY (1999) Role of organic acids in sunflower tolerance to heavy metals. Biol Plant 42:65–73
Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, Del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sanita di Toppi L, Gabrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Senden MHMN, Van Der Meer AJGM, Verburg TG, Wolterbeek HT (1995) Citric acid in tomato plant roots and its effect on cadmium uptake and distribution. Plant Soil 171:333–339
Shi X, Zhang C, Wang H, Zhang F (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Shi G, Cai Q, Liu C (2010) Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul 61:45–52
Sommer M, Kaczorek D, Kuzyakov Y, Breuer J (2006) Silicon pools and fluxes in soils and landscapes: a review. J Plant Nutr Soil Sci 169:310–329
Song A, Li P, Li Z, Fan F, Nikolic M, Liang Y (2011) The alleviation of zinc toxicity by silicon is related to zinc transport and antioxidative reactions in rice. Plant Soil 344:319–333
Sridhar BBM, Han FX, Diehl SV, Monts DL, Su Y (2007) Effects of Zn and Cd accumulation on structural and physiological characteristics of barley plants. Braz J Plant Physiol 19:15–22
Treder W, Cieslinski G (2005) Effect of silicon application on cadmium uptake and distribution in strawberry plants grown on contaminated soils. J Plant Nutr 28:917–929
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T (2011) Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci U S A 108:20959–20964
Vaculík M, Lux A, Luxova M, Tanimoto E, Lichtscheid I (2009) Silicon mitigates cadmium inhibitory effects in young maize plants. Environ Exp Bot 67:52–58
Vaculík M, Landberg T, Greger M, Luxova M, Stolarikova M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize. Ann Bot 110:433–443
Vaculík M, Pavlovič A, Lux A (2015) Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol Environ Saf 120:66–73
Voogt W, Sonneveld C, Datnoff LE, Korndrfer GHS (2001) Silicon in horticultural crops grown in soilless culture, chapter 6. In: Studies in plant science, Elsevier, pp 115–131
Wang LJ, Wang YH, Chen Q, Cao WD, Li M, Zhang FS (2000) Silicon induced cadmium tolerance of rice seedlings. J Plant Nutr 23:1397–1406
Wójcik M, Tukiendorf A (2005) Cadmium uptake, localization and detoxification in Zea mays. Biol Plant 49:237–245
Zhang CH, Wang L, Nie Q, Zhang W, Zhang F (2008) Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ Exp Bot 62:300–307
Zorrig W, Rouached A, Shahzad Z, Abdelly C, Davidian JC, Berthomieu P (2010) Identification of three relationships linking cadmium accumulation to cadmium tolerance and zinc and citrate accumulation in lettuce. J Plant Physiol 167:1239–1247
Acknowledgments
This work was financed by the French Institut National des Sciences de l’Univers (INSU, CNRS) programme Ecosphère continentale et côtière (EC2CO). Muhammad Rizwan was financed by the Higher Education Commission (HEC) of Pakistan. We thank Stéphane Jézequel (Arvalis-Institut du Végétal, France) for his help in the selection of the durum wheat cultivar and for providing the seeds, Perrine Chaurand (Aix-Marseille University, France) for the XRF analysis and Wim Voogt (Wageningen University, NL) for providing Si(KOH)2 and expertise concerning Si in nutrient solution. Partial support from BIO-GEO-CLIM project (14.B25.31.0001) of the MinObrNauki is also acknowledged (OP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Rizwan, M., Meunier, JD., Davidian, JC. et al. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23, 1414–1427 (2016). https://doi.org/10.1007/s11356-015-5351-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5351-4