Abstract
Plastics from cathode ray tube (CRT) casings were sampled in Nigeria and analysed for their polybrominated dibenzo-p-dioxin and dibenzofuran (PBDD/F) content. PBDD/Fs, consisting mainly of PBDFs, were detected in BFR containing plastic with a median (mean) concentration of 18,000 ng/g (41,000 ng/g). The PBDD/Fs levels were highest in samples containing PBDEs, but the levels of PBDFs were two orders of magnitude higher than the levels reported in the technical PBDE mixtures and where frequently exceeding 1000 μg/g of PBDE content. These higher levels are likely to arise from additional transformation of PBDEs during production, use, recycling, or storage, but the processes responsible were not identified in this study. PBDD/Fs in CRT casings containing1,2-bistribromophenoxyethane (TBPE) were dominated by tetrabrominated dibenzo-p-dioxin (TBDDs) with concentrations around 10 μg/g of the TBPE content. The PBDD/Fs in CRT casings containing tetrabromobisphenol A (TBBPA) were found at concentrations around 0.1 μg/g of TBBPA levels. Casings treated with TBPE or TBBPA often contained PBDEs (and PBDF) as impurities—probably originating from recycled e-waste plastics. It was estimated that the 237,000 t of CRT casings stockpiled in Nigeria contain between 2 and 8 t of PBDD/Fs. The total PBDD/F contamination in polymers arising from total historic PBDE production/use is estimated in the order of 1000 t. TEQ values of CRT samples frequently exceeded the Basel Convention’s provisional low POPs content of 15 ng TEQ/g. Due to the significant risks to health associated with PBDD/Fs, more detailed studies on the exposure routes from PBDD/Fs in stockpiles are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brominated flame retardants (BFRs) including polybrominated diphenyl ethers (PBDEs), tetrabromobisphenol A (TBBPA), 1,2-bistribromophenoxyethane (TBPE) and hexabromocyclododecane (HBCD), and others (Arcadis and EBRC 2011) are added to synthetic materials such as plastic or foams to reduce their flammability (Shaw et al. 2010). Major application of BFRs include plastic casings of electronics, cables and circuit boards, polyurethane foams in upholstery in transport and furniture, and insulation foam in construction (Simonsen et al. 2000; BSEF 2000). Releases of BFRs from products and during production and end-of-life treatment and disposal have resulted in widespread environmental and human contamination (Labunska et al. 2014; Ma et al. 2009; Shaw et al. 2010; Takigami et al. 2008: Watanabe and Sakai 2003; Weber et al. 2011; Wong et al. 2007). Several BFRs including certain PBDEs, hexabromobiphenyl (HBB) and HBCD have already been listed as persistent organic pollutants in the Stockholm Convention (POP-BFRs) (Secretariat of the Stockholm Convention 2012a, b; Secretariat of the Basel Convention 2014).
It has been demonstrated that products and materials containing BFRs often also contain polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) (WHO 1998; Ebert and Bahadir 2003; Hale et al. 2002; Hagberg et al. 2006). This is a result of their unintentional formation and release throughout the life cycle of BFRs (Shaw et al. 2010). PBDD/Fs can be formed or released during the production of BFRs (WHO 1998; Hanari et al. 2006; Ren et al. 2011), during the manufacture of BFR containing products (Luijk et al. 1992; Ota et al. 2009; Ren et al. 2008), and in the recycling and disposal of BFR-containing polymers (Ebert and Bahadir 2003; Zennegg et al. 2014). For some flame retardants (notably PBDEs), the levels of PBDD/F contamination can increase during the use phase of the product by, for example, exposure to sunlight as has been observed in plastic matrices (Kajiwara et al. 2008) and in textiles (Kajiwara and Takigami 2010; Kajiwara et al. 2013). DecaBDE even formed PBDF when a spiked fish sample was heated to 200 °C (Vetter et al. 2014).
PBDD/Fs have physicochemical properties and toxicities similar to those of the highly toxic polychlorinated dibenzo-p-dioxin and dibenzofurans (PCDD/F) and are therefore of concern (Behnisch et al. 2003; Birnbaum et al. 2003; van den Berg et al. 2013). PBDD/Fs have been detected in various environmental and biological matrices (Litten et al. 2003; Terauchi et al. 2009; Jogsten et al. 2010; Sepulveda et al. 2010; Hayakawa et al. 2004) and food sources such as shellfish and fish (Fernandes et al. 2008; Ashizuka et al. 2008). The UK food authority has recently presented a comprehensive survey on PCDD/Fs, PBDD/Fs and PXDD/Fs in British food and highlighted that up to 30 % of TEQ could stem from PBDD/Fs, and additional 20–50 % could come from PXDD/Fs (Mortimer et al. 2013). PBDFs are also important contributors to dioxin-like exposure from house dust in Japan and the USA (Suzuki et al. 2007, 2010; Tue et al. 2013). In Sweden, the PBDD/F-TEQ contribution in human background contamination reached up to 15 % of TEQ (Ericson Jogsten et al. 2010), and fire fighters in the USA had 20 times higher TEQ levels from PBDD/Fs compared to PCDD/F in their blood (Shaw et al. 2013).
The largest amount of PBDD/Fs and their mixed brominated-chlorinated analogues (PXDD/Fs) are probably formed and released in the end-of-life stage of BFR containing plastics. In particular, the PBDD/F and PXDD/F contamination of sites where e-waste have been treated and burned in the open are described in a range of studies (Chi et al. 2011; Li et al. 2007; Ma et al. 2009; Shaw et al. 2010; Yu et al. 2008) or from related metal industries (Wang et al. 2010). Initial studies on PCDD/F and PBDD/F levels in humans at e-waste recycling sites were assessed in Vietnam and were higher compared to background sites (Tue et al. 2014).
Recently, the WHO toxic equivalency factor (TEF) expert panel concluded that PBDD, PBDF and some dioxin-like biphenyls (dl-PBBs) may contribute significantly to the daily human background exposure to total dioxin toxic equivalents (TEQs) (van den Berg et al. 2013).
These recent findings demonstrate that PBDD/Fs (and PXDD/Fs) seem relevant contaminants that humans are exposed to and highlight the need to assess and control their sources. A mini-review on management of PBDE-treated plastic has been published for China (Deng et al. 2014).
The knowledge on the total amount of PBDD/F present in material flows is important to understand and control the sources of PBDD/Fs and the related risk for human exposure. While for PCDD/Fs, the Stockholm Convention has developed a methodology to develop inventories in the frame of the Stockholm Convention (UNEP 2013), inventories of PBDD/Fs and a methodology to estimate the total amount of e.g. PBDD/Fs in material flows of BFR containing materials are missing. Since the largest share of BFRs is present in plastic from electronics, the assessment of these material and the related recycling streams seems of key importance, in particular, considering that plastic from e-waste is partly recycled into sensitive applications such as children toys, household goods or food container materials (Chen et al. 2009, 2010; Samsonek and Puype 2013; Diamond 2014).
The objective of this study was to investigate the presence and levels of PBDD/Fs in plastics from waste electrical and electronic equipment (WEEE), including cathode ray tube (CRT) casings of computer and televisions (TV), collected from various e-waste sites in Nigeria. The plastics investigated have been screened for the presence and levels of brominated flame retardants, including PBDEs during a previous study (Sindiku et al. 2014), and preliminary results were presented at conferences (Sindiku et al. 2013). One aim was also to derive for key brominated flame retardants and the related PBDD/F levels in e-waste plastic initial contamination factors which might allow a rough estimate of PBDD/F volume for key BFRs.
Material and methods
Sampling and selection of samples
A total of 382 plastic samples from 158 TV CRT casings and 224 computer CRT casings imported from different regions were collected from eight locations in Ogun State and Lagos State in southwest Nigeria. Sampling was carried out between January and March 2011 for the analysis of brominated flame retardants (Sindiku et al. 2014). The samples were specifically selected from waste storage sites, electronic workshops, roadsides, dumpsites and dismantling sites, thus covering the locations where a large portion of e-waste in Nigeria is stored or dumped (Sindiku et al. 2014). The labels on the TV and computer monitor casings were examined for information on the manufacturer, brand, model, serial number, year (1983–2006) and the country/region of production (for details, see Sindiku et al. 2014). From these set of 382 TV and computer CRT casings, 52 plastic samples were selected for the analysis of PBDD/Fs based on the following:
-
1.
Four BFRs previously analysed by gas chromatography electron capture detector (GC-ECD) (commercial OctaBDE, commercial DecaBDE, TBBPA and TBPE)
-
2.
Bromine content screened by using X-ray fluorescence (XRF) analysis (<5 -106,400 μg/g) (Sindiku et al. 2014)
Chemicals
All solvents used were of analytical grade quality. Toluene, n-hexane, dichloromethane and acetone were purchased from Merck (Darmstadt, Germany), tetrahydrofuran from VWR International (Fontenay-sous-Bois, France) and tetradecane from Fluka (Steinheim, Germany).
Silica gel 60 (0.063–0.200 mm; Merck, Darmstadt, Germany) of chromatography grade was used. It was washed with methanol and dichloromethane and activated and stored at 120 °C prior to use. Anhydrous sodium sulphate, sulphuric acid and potassium hydroxide were of pro analysis grade from Merck (Darmstadt, Germany). Similarly, sodium sulphate was dried at 550 °C for 48 h.
Sand, Fontainebleau (VWR International, Prolabo) used for accelerated solvent extraction (ASE), was kept in the oven for 24 h at 550 °C. Glass microfiber filters were from Whatman International Ltd. (Maidstone, England). The Florisil (0.150–0.250 mm), supplied by Merck (Darmstadt, Germany) was kept in the oven for 24 h at 550 °C.
The 13C-labelled PBDD/Fs and PCDD/Fs internal standard solution used for quantification originated from Wellington Laboratories (Guelph, Canada), while the native standard was from Cambridge Isotope Laboratories (Massachusetts, United States). The PBDD/F and PCDD/F homologues include the total of 2,3,7,8-substituted and the non-2,3,7,8-substituted congeners.
Sample extraction and analysis
Samples were extracted by dissolution and precipitation method using two subsequent solvents, tetrahydrofuran and n-hexane, to efficiently recover all target compounds. About 0.5 g of plastic samples of TV and computer were dissolved in 5 mL of tetrahydrofuran and vortexed for 20 min until the plastic fully dissolved. About 2 mL of the dissolved polymer solution was spiked with 13C-labeled Tetra-Octa PBDD/Fs and PCDD/Fs standard solutions. Then, by the stepwise addition of about 6–8 mL n-hexane, the entire polymer was precipitated. The supernatant was separated from the precipitated polymer, and the extract was concentrated and exchanged to hexane using a rotary evaporator.
The extracts were purified and fractionated on two different open chromatographic columns. First, a multilayered silica gel column consists of (from top to bottom) 1 g anhydrous sodium sulphate, 4 g H2SO4-impregnated silica gel (40 %), 1.4 g neutral silica gel, and 3 g KOH-impregnated silica gel and finally a plug of glass wool (everything pack in 16 mm i.d. glass column) to remove fats or any polar interfering substances, followed by a column (same type as above) consisting of 5 g Florisil (deactivated with 1 % water) mainly to separate the dioxins (both PCDD/Fs and PBDD/Fs) from the PBDEs (Li et al. 2007). Both columns were rinsed with two bed volumes of n-hexane before use. After sample application, the first column was eluted with 140 mL of n-hexane to recover the target contaminants, while the second column was eluted with 90 mL of n-hexane to recover the PBDEs followed by 150 mL of n-hexane/dichloromethane (40:60, v/v) to recover the dioxins. After each column, the collected eluates were evaporated to 1 mL using a rotary evaporator. Recovery standards (RS) consisting of 13C-labelled 1,2,3,4,6,8,9-HpCDF for the dioxins and 13C-labelled NonaCB (PCB#208) for the PBDEs were added to the final evaporated samples, after which they were further evaporated and the solvents were exchanged to tetradecane 100 μL for the PBDE fraction and 40 μL for the dioxin fraction. The samples were transferred to 2-mL GC vials with 150-μL inserts and subjected to GC/HRMS analysis.
Instrumental analysis
All targeted compounds (PBDD/Fs and PCDD/F) were analysed by gas chromatography high-resolution mass spectroscopy (GC-HRMS) using a Hewlett-Packard 6890N GC (Agilent Technologies, Palo Alto, CA) connected to a Waters Autospec Ultima MS (Waters Corp., Milford, MA, USA) operated in electron impact (EI) mode (34 eV) at resolution of ×10,000. The analyses were performed by using isotope-dilution technique according to SS-EN 1948–3. The two most intense ions of each molecular ion isotope distribution cluster were monitored, and the selected ion recording (SIR) descriptor was divided in time segments, during which only one homologue group was monitored, to enhance the sensitivity.
For PBDD/Fs, a 15 m × 0.25 mm × 0.25 μm J&W Scientific DB-5MS column (Agilent, Palo Alto, CA, USA) was used for the GC separation. Each analysis was initiated by injecting a 2-μL aliquot of the sample at an injector temperature of 280 °C, a constant flow of helium carrier at 1.0 mL/min, and a slightly different GC oven temperature program: 190 °C for 2 min, raise at 3 °C/min to 280 °C and hold for 10 min.
A 60 m × 0.25 mm × 0.25 μm J&W Scientific DB 5MS fused silica column (Agilent, Palo Alto, CA, USA) was used for the GC separation of PCDD/Fs. Each analysis was initiated by injecting a 2-μL aliquot of the sample in split-less mode at an injector temperature of 280 °C. The GC oven was temperature programmed as follows: 200 °C for 2 min, raised at 3 °C/min to 300 °C and held for 3 min. The helium gas was set to a constant flow of 1.2 mL/min using the electronic pressure control. The performance of the analyses fulfilled the requirements laid down in the European commission directive 2002/69/EC.
All compounds were quantified by comparing ratios of native compounds and added 13C-labeled isotopes of the same compounds in the samples and known reference standards, using the isotope dilution method. Eleven congeners of PBDD/Fs (2,3,7,8-TBDF, 2,3,7,8-TBDD, 1,2,3,7,8,-PeBDF, 2,3,4,7,8-PeBDF, 1,2,3,7,8-PeBDD, 1,2,3,4,7,8-HxBDF, 1,2,3,7,8,9-HxBDD, 1,2,3,4,6,7,8-HpBDF, 1,2,3,4,6,7,8-HpBDD, OBDF and OBDD) were quantified based on the response factors obtained from the corresponding standards. All 17 2,3,7,8-PCDD/Fs were quantified. For TEQ calculationFootnote 1, the TEF factors according to WHO (2005) were used (van den Berg et al. 2006). For PBDD/Fs, the TEFs of the similarly substituted PCDD/Fs were used following the recent recommendation of the WHO group (van den Berg et al. 2013). Only values higher than the limits of detection (LOD) for the respective compounds were included in the calculations.
Quality assurance/quality control
A procedural blank, a spiked blank consisting of all chemicals and a duplicated sample were run with each batch of 10 samples to assess potential sample contamination and the repeatability of analysis. Results showed that no target compounds were detected in the blanks. The limits of detection (LOD) were estimated as three times the noise level.
For TEQ calculations, values below the LOD were set to zero. The recovery of the individual PBDD/Fs congeners ranged from 52 to 143 %. The recovery of the 17 individual PCDD/Fs compounds ranged from 43 to 116 %. All data were expressed on a dry-weight basis (nanograms per gram dry wt).
Results and discussion
Concentrations of PBDD/Fs in plastics of TV and computer casings
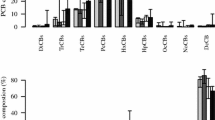
PBDD/Fs were detected in all 52 analysed television and computer CRT casings, with concentrations ranging from 21 to 350,000 ng/g and a mean (median) concentration of 41,000 ng/g (18,000 ng/g), which are in the parts per minute range for the average plastics investigated. Overall, there was no significant difference between the CRT casings produced in the different regions (Table 1), but computer casings from Europe had in average lower levels compared to all the other casings, including TV casings from Europe. The reason is the relatively low overall usage of PBDEs in these CRT casings (see supporting information of Sindiku et al. 2014). The highest PBDD/F levels (see Figs. 1 and 2) were found in the plastics containing high PBDE levels. However, plastics that contained TBPE as the main BFR had PBDD/F levels in the low parts per minute range only (Fig. 3). Overall, the congener pattern for PBDD/Fs in the TVs was dominated by highly brominated PBDFs, and PBDDs were most often only minor components (see Figs. 1 and 2 below).
PBDD/F homologue pattern and concentration in CRT casings of computer (CP) and TV CRT casings treated with c-OctaBDE and related PBDE concentrationsFootnote
The GC/MS calculation for TV/EU/292 resulted in an overestimation of the actual c-OctaBDE concentration by a factor of 2 compared to XRF and EDC assessment of 30 % BFR (see supporting information of Sindiku et al. 2014.
and patternsThe presence of PBDD/Fs in plastics has been reported previously, both for e-waste and other recycled materials (Ebert and Bahadir 2003; Luijk et al. 1992; Schlummer et al. 2007; Donnelly et al. 1989; Sakai et al. 2001a, b). Similarly high PBDD/F levels were found in waste television casings in Japan from the 1990s and in some cases even higher levels in printed circuit boards (Sakai et al. 2001a, b; Sakai 2000). Seven Japanese TV casings manufactured in early 1990s (l990~1994) were thus found to contain PBDD/Fs between 21,000 and 200,000 ng/g, while four casings from TVs manufactured in the late 1990s contained levels between 2000 and 14,000 ng/g Sakai et al. (2001a). They concluded that for Japan, the PBDE used in the late 1990s might have contained less PBDD/Fs compared to the PBDEs used in the early 1990s. We investigated if we could see a similar relationship between the years of manufacture and the levels of PBDD/Fs in the present study. The CRT casings of the seven televisions and one computer manufactured in the 1980s contained 970–126,000 ng/g of PBDD/Fs with a mean concentration of 45,000 ng/g, while the 10 television and 19 computer CRT casings manufactured in the 1990s contained 14–98,000 ng/g of PBDD/Fs with a mean concentration of 13,000 ng/g. Finally, the 3 TV and 11 computer CRT casings manufactured between 2000 and 2003 contained 270–127,000 ng/g, with a mean concentration of 49,000 ng/g. As seen, no obvious correlation with the year of manufacture can be discerned from this data, and it would also be difficult to draw any conclusions about the PBDD/F content in the original BFR mixtures used (as in the Japanese study). More recently, Ortuño et al. (2015) reported lower levels of PBDD/DFs in TV casings with average 2,3,7,8-substituted PBDD/F levels of 5450 ng/g.
Homologue profiles of PBDD/Fs in plastics of CRT casings containing PBDEs
The PBDD/Fs congener patterns in the TV and computer CRT casings are largely dependant on the flame retardant used. PBDEs are excellent PBDF precursors since they only need an elimination step to form the PBDF (Watanabe and Tatsukawa 1987; Weber and Kuch 2003). The plastics containing the high levels of PBDD/Fs contained also high levels of PBDE dominated by highly brominated PBDFs, such as octa-, hepta- and hexa-congeners (Figs. 1 and 2). Lower brominated PBDFs (tetra- and penta-congeners) accounted for less than 3 % of the total PBDFs in these samples, and PBDDs were more or less absent (Figs. 1 and 2).
For DecaBDE-treated samples, the PBDD/F homologue pattern was clearly dominated by OctaBDF and other highly brominated PBDF (Fig. 1). For some samples, OctaBDF constituted more than 90 % of total PBDFs which is similar to the PBDF homologue pattern of technical mixtures of DecaBDE (Hanari et al. 2006). In two commercial DecaBDE lots, they detected mainly OctaBDF (96 and 97 % of PBDF) with small quantities of HeptaBDF (3 to 4 %), while all lower brominated PBDF homologues were below the detection limit (Hanari et al. 2006). However, in the present study, most CRT casings with commercial DecaBDE contained more than 10 % of HeptaBDE and also contained HxBDFs to TeBDFs (see Fig. 1). This is most likely the result of secondary formation via debromination of formed OBDF and/or via ring closure of lower brominated PBDEs which have been formed via debromination of DecaBDE. The PBDE homologue pattern in apparent DecaBDE-treated CRT casings showed considerable contribution of NonaBDE and OctaBDE as well, and in some DecaBDE-treated CRTs, the contribution of HeptaBDE and HexaBDE listed as POPs in the Stockholm Convention was also significant (Supporting information Fig. S1; Sindiku et al. 2014). This also demonstrates that DecaBDE in e-waste plastics can be debrominated to POP-PBDEs listed under the Stockholm Convention, and the present study shows that these can also be further transformed into toxic PBDFs.
Such debromination of DecaBDE to lower brominated PBDE has been observed before under various conditions (UNEP 2010; Watanabe and Tatsukawa 1987) including plastic (Kajiwara et al. 2008, 2013; Sindiku et al. 2014). However, it is still unknown if the debromination and transformation mainly occurs here during production (extrusion), use, recycling of the plastics or exposure of the casings under sunlight. All these options are possible (Ebert and Bahadir 2003), and one study on the formation of PBDF from DecaBDE containing plastic from sunlight has been published (Kajiwara et al. 2008).
The CRTs that, according to their PBDE profile, had been treated with commercial OctaBDE (c-OctaBDE) had a PBDD/F homologue pattern with less brominated congeners (Fig. 2), compared to the c-DecaBDE-treated plastics (Fig. 1). Most of these profiles were dominated by HxBDFs and were comparable to the homologue profile Hanari et al. (2006) reported for the PBDFs in two c-OctaBDE formulations. However, some of these c-OctaBDE-treated casings had still PBDD/F profiles that were dominated by OctaBDF, e.g. CP/AM/341 (Fig. 2). One explanation to this can be that c-OctaBDE contains some DecaBDE (see Fig. 2), which more readily transforms into OBDF than the lower brominated PBDE transforms into PBDFs. That the higher brominated PBDEs form easier PBDFs compared to lower brominated PBDEs can also be seen from the relatively low levels of PBDF in commercial penta-BDE formulation (0.28 μg/g) compared to commercial DecaBDE containing approx 50 times more PBDF (Hanari et al. 2006) (see below). Another explanation is that in the reactors where c-OctaBDE was produced, also c-DecaBDE might have been produced and the reactors might have been contaminated with OBDF contaminating the following c-OctaBDE batch. Something similar is observed for chlorinated pesticides where e.g. 2,4,5-T/2,4-D mixture also contained relative high levels of OCDD (see supporting information of Holt et al. 2010) which cannot be explained from the structure of the dichlorinated and trichlorinated phenol-precursors in 2,4,5-T/2,4-D.
Homologue profiles of PBDD/Fs in plastics of CRT casings containing other BFRs or mixtures
A range of plastic samples contained a mixture of flame retardants indicating that they have been produced from recycled WEEE polymers. This includes samples with BFR concentration below 1 % which were obviously produced from e-waste plastic and depending on the region of production accounted to 5 to 20 % of the assessed 382 samples (see Sindiku et al. 2014). But, also some samples contained relatively high levels of BFRs (above 3 %) and contained BFR mixtures and were obviously produced on purpose from BFR containing e-waste plastic.Footnote 3 For example, a computer CRT casing from North America contained 3.6 % TBPE, 1.3 % TBBPA, 0.95 % OctaBDE and 0.5 % DecaBDE (Fig. 3). This gave rise to a PBDF pattern that was likely a mixture of those found in commercial OctaBDE and DecaBDE formulations, with a predominance of OBDF and HxBDF with lower concentration of HpBDF (and PeBDFs). Additionally, it also contained significant amounts of TBDDs (84.6 % of PBDD/F) (Fig. 3) most likely originating from TBPE and to a minor part also from TBBPA (see below). TBPE contains two tribromophenol moieties which can function as precursor for brominated PBDD in particular TBDD.
Another example of a CRT casing from a European TV containing residues of different BFRs is shown in Fig. 3. The PBDD/F profile of this casing was dominated by TBDD, which again indicates influence of the TBPE (present at 17.5 %), but contained also minor amounts of Hexa- and Hepta-BDFs. The latter stemming from the commercial OctaBDE which was present at 0.66 %.
These examples demonstrate that the fingerprint of PBDD/Fs can indicate the BFR used, and vice versa, the detected BFR gives an indication on the likely presence of PBDD/F (see below). More research is need for further insight of such correlations by assigning single PBDD/F congeners (including non-2,3,7,8-PBDD/F) which have not been elaborated as for the PCDD/F.
From the 52 investigate CRT casings, 12 samples (23 %) had higher PBDDs levels compared to PBDFs. These were mainly CRT casings flame retarded with TBPE and TBBPA and a few where the BFR could not be identified (see also Sindiku et al. 2014). PBDDF profiles of such samples, i.e. with TBPE or TBBPA as major flame retardants, are shown in Figs. 3 and 4 having TBDD as a major contaminant.
For the TBPE samples, the TBDD levels were around 1 μg/g in the plastic (Fig. 3) and approximately 5 to 10 μg/g in relation to the TBPE levels. For the TBBPA containing plastics, the TBDD levels were lower, i.e. around 0.01 μg/g or approx 0.1 μg/g relative to the TBBPA levels (Fig. 4). TBBPA does not contain a PBDD/F precursor moiety and therefore does not have a PBDD/F formation potential at temperatures/circumstances where chemical bonds are not degraded. However, when TBBA is thermally degraded, PBDD/Fs can be formed (Ortuño et al. 2011).
In all these samples, also PBDFs were detected at low levels (Fig. 4). According to the GC-ECD measurementsFootnote 4 performed PBDE were not detected in these samples (see supporting information of Sindiku et al. 2014). However, after re-running these samples with GC-MS, PBDEs were detected at parts per minute levels. This could explain the presence of highly brominated PBDFs (in the low and sub-ppm range) and the unusual PBDD homologue pattern with relevant TBDD (14 ng/g) and measurable OBDD (2 ng/g) and HpBDD (0.3 ng/g) but virtually no PeBDD and HxBDD (below 0.1 ng/g) (Fig. 3). Also, in a recent BFR screening study in plastic from electronics in Australia, PBDE was detected at parts per minute levels in many of the investigated polymers when using GC-MS (Gallen et al. 2014). The same was found in a preliminary study of the Mongolian Academy of Science, in which 16 of 17 investigated TBBPA-treated plastic samples from CRT casings were found to contain PBDEs at parts per minute levels (Bayarmaa et al. 2014). The most abundant PBDEs in the Mongolian study were BDE-183 and BDE-197, measured at around 100 µg/g. This highlights the importance of also determining traces of PBDE (and possibly other BFRs) when analysing PBDD/Fs in BFR-treated plastics with the aim of assigning the origin of the PBDD/Fs. If only the major flame retardant in a product or recycled product would be measured, then the PBDD/F contamination might be assigned to the wrong flame retardant; if not, PBDEs are quantified at low levels. The recycling of e-waste plastic can be expected to increase considering efforts of the plastic industry to increase recycling quota and considering the aim of society to move to more circular economies. For WEEE plastic recycling, PBDD/Fs need to be considered as a pollutant of concern.
Comparison of PBDD/F levels in Nigerian plastic samples and commercial PBDE formulations
While the total PBDD/F content in the plastics is relevant for the calculation of total PBDD/F amounts in certain plastic volumes, or for exposure assessment of PBDD/F, the relative PBDD/F content in relation to the flame retardant content is interesting to understand possible additional formation over the lifetime or recycling of the plastic.
Levels of PBDD/Fs have been reported in commercial mixtures of PBDEs (Hanari et al. 2006; Ren et al. 2011). Hanari et al. (2006) reported between 30 and 50 μg/g PBDD/Fs in commercial DecaBDE, with between 10 and 19 μg/g in commercial OctaBDE. These levels are somewhat higher than the later results from Ren et al. (2011) where PBDD/F levels in commercial DecaBDE ranged between 3.4 and 13.6 μg/g with a mean concentration of 7.8 μg/g. The lowest PBDD/F levels were detected in the commercial penta-BDE at 0.26 μg/g (Ren et al. 2011). The differences may reflect improvements in production processes between the two studies.
The levels of PBDD/Fs in the CRT casings treated with PBDEs frequently exceeded 1000 μg/g and were in some cases close to 2 % (mainly PBDF). This means that the PBDD/F levels in these polymers were considerably higher than would be expected from the levels reported in technical PBDE mixtures. In some cases, however, the PBDD/F levels were found at levels similar to those which would be expected from contamination in the technical mixtures. This was true even in some cases where the cases included recycled WEEE plastic (see e.g. Fig. 3 TV/EU/31 with 0.66 % c-OctaBDE).
The higher PBDD/F levels can be explained either by the use of PBDE mixtures which originally contained considerably higher levels of PBDD/Fs or by post-formation of PBDD/Fs (in particular PBDFs) during the production, use and storage of the plastics. There was no evidence of a significant difference in levels of the CRT casings from the 1980s and early 2000 (see above) which indicates that the elevated PBDF levels were mainly due to formation during the life-cycle of the plastics. The formation of PBDF from PBDE might have occurred here during processing of the plastics into CRTs (Ebert and Bahadir 2003; Weber and Kuch 2003) or in the recycling and remoulding of the polymers (Ebert and Bahadir 2003; Zennegg et al. 2014). It is also possible that PBDFs could have been formed during the normal usage of the TV/computer CRTs and afterwards during stockpiling the casings in particular by sunlight exposure. If this was a significant mechanism, then PBDD/F levels might increase further over time in e-waste plastic stockpiles kept in the open. Debromination from exposure to sunlight (Watanabe and Tatsukawa 1987) could also increase the toxicity of the mixtures. On the other hand, the PBDD/F can also be degraded over time by sunlight (Watanabe and Tatsukawa 1987; Kajiwara et al. 2008) and might reach an equilibrium. Further studies are needed to establish the significance of this mechanism and to model the long-term fate.
Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs)
Also, PCDD/Fs were measured in the CRT casings and were detected at low nanograms per gram concentrations, which is about four to six orders of magnitude lower compared to the PBDD/F levels (Table 1). PCDDs and PCDFs were present in similar proportions but with a slight predominance of PCDDs.
The origin of the PCDD/Fs present could not be determined. They could either come from the manufacturing processes or be a result of secondary contamination e.g. by adsorption of PCDD/Fs from open burning at sampling sites and related adsorption. Anyhow, these low PCDD/F levels are not relevant for exposure or management consideration.
TEQ considerations and limitations
Also, the 2,3,7,8-substituted PBDD and PBDF were determined in the CRT casings. TEQ concentrations for PBDD/Fs were estimated using WHO (1998) TEF factors for PCDD/Fs as recommended by the WHO TEF group (van den Berg et al. 2013). The mean (range) concentrations of 2,3,7,8-substituted PBDD/Fs detected in the TV and computer samples from the three regions (Asia, Europe and USA) are shown in Table 2.
When using the WHO TEFs as suggested by the WHO TEF expert panel, then a considerable share of the CRT casings (>20 %) could and possibly also the average mixed CRT casing waste could have TEQs above the provisional Basel Convention low POPs limit of 15 ng TEQ/g developed for PCDD/Fs containing wastes and defining a waste as POPs waste. The highest levels were from casings with PBDEs with frequent levels of several 100-ng TEQ/g polymer (Fig. 5). Therefore, these CRT casings could be considered POPs waste also from the perspective of dioxin-like TEQ content. Also, some of the CRT casings flame retarded with TBPE contained 2,3,7,8 substituted PBDDs with TEQs above the high provisional Basel low POPs limit for PCDD/F. The main TEQ contribution came here from 2,3,7,8-TBDD, although this congener only accounts for 1 % or less of the total TBDDs in these samples.
The samples were also orders of magnitude above the German regulation for hazardous chemicals in mixtures and products regulating also level of PBDD/Fs (German Government 2003).
There are however some uncertainties in TEQ calculation that needs to be stressed. While all 2,3,7,8-substituted congeners are available as standards for assignment of respective congener peaks in the GC analysis, it is currently not described to which extent non-2,3,7.8-substituted congeners overlap with these peaks. Considering the few separated peaks in the PBDD/F analysis with short GC columns usually used for PBDE and PBDD/F analysis (see supporting information Fig. S2), it is most likely that several of non-2,3,7,8-substituted PBDD/F congeners overlap with the 2,3,7,8-PBDD/Fs with the consequence that the calculated TEQ might be overestimated.
On the other hand, it should be mentioned that also non-2,3,7,8-substituted PBDD/Fs can have dioxin-like toxicity (e.g. even the 2,3,7-TriBDD has a aryl hydrocarbon receptor-mediated potency) (Behnisch et al. 2003), while the persistence of the non-2,3,7,8-congeners regarding metabolism might be lower.
Due to the possible human exposure risk of PBDD/Fs in e-waste plastic and recycled products from e-waste plastic, a more refined congener-specific analysis would be important for an appropriate risk assessment. The same is true for the assessment of PBDD/Fs in food (Mortimer et al. 2013) and in house dust (Suzuki et al. 2010; Tue et al. 2013)
Total PBDD/F amount in e-waste plastic in Nigeria and in stockpiles from historic PBDE production
From the measured median (mean) PBDD/F concentration of the 52 samples analysed (18,000 ng/g (41,000 ng/g)), and considering that these samples had higher BFR concentration than the average randomly collected 382 CRT casing samples (Sindiku et al. 2014) from which they were selected, it is estimated that the stockpiled 237,000 t of CRT casings in Nigeria contain approx. 2 to 8 t of PBDD/Fs. The total amount of plastics in EEE and e-waste imported to Nigeria between 2000 and 2010 has been estimated to 2.4 million tonnes (Ogungbuyi et al. 2012; Babayemi et al. 2014a, b). However, since the CRT casings contain in average higher levels of PBDEs compared to plastic from other e-waste (Waeger et al. 2010), the PBDD/Fs amount estimated here cannot be extrapolated to the total amount of e-waste plastic in Nigeria. For a total PBDD/F estimate, additional measurements of other (W)EEE-plastics fractions are therefore needed.
Ren et al. (2011) calculated on the basis of the global demand for commercial DecaBDE in 2001 and their measured concentration in technical PBDE the annual potential emissions of PBDD/Fs to approximately 0.43 t (range 0.21–0.78 t) for that year. Considering that the PBDD/F levels in used plastic might be rather in the order of 1000 µg/g relative to PBDEs, the total amount of PBDD/Fs from the historically produced approx. 1.25 million tonne DecaBDE and 100,000 tonnes of c-OctaBDEand 100,000 t of c-OctaBDE (Secretariat of the Stockholm Convention 2012a, b) could have generated PBDD/Fs in the order of 1000 t. However, also for a robust estimate, more research is needed in this respect since DecaBDE has been and is also applied in other uses than EEE plastic. However, also an increase of PBDD/Fs has been detected in deca-BDE-treated textiles (Kajiwara et al. 2013).
In any case, this large PBDD/F and PBDE stockpile require further assessments of exposure and health risk in particular considering the end-of-life treatment and recycling.
Considerations in respect to end-of-life treatment of e-waste plastic
The most relevant releases of contaminants (e.g. heavy metals, PBDD/F, PXDD/F, PBDEs) from EEE plastics are probably taking place during the end-of-life stage and in particular during the rudimental recycling activities occurring in developing and transition countries. These include, for example, manual disassembly of e-waste on the ground, open burning for recovery of metals, landfill fires, acid extraction of metals in open containers, shredding, melting and extrusion of plastics and dumping of residual materials (Li et al. 2007; Swedish EPA 2011; Wong et al. 2007; Yu et al. 2008; Zennegg et al. 2009). This practice has resulted in large contaminated sites in Asia over the last 2 decades (Labunska et al. 2014; Ma et al. 2009; Weber et al. 2008; Wong et al. 2007). A recent assessment of such a site showed, for highly exposed children, the exceedance of the US Environmental Protection Agency reference doses for major the PBDE congeners (BDE-47 and BDE-99) by factors of approx. 2.5 and 1.5, respectively (Labunska et al. 2014), but have not been assessed for dioxins.
For Nigeria, it has been estimated, by material and substance flow analysis, that 140 t of POP-PBDEs have already been subjected to open burning and that 640 t of POP-PBDE have ended up in dumpsites (Babayemi et al. 2014a; Supporting information Fig. S3). The total amount of DecaBDE in these open burning and dumpsites is approximately an order of magnitude higher compare to the POP-PBDE content. These largely unmanaged dumpsites frequently catch fire and are often continuously smouldering. Studies in the USA have shown that landfill fires release brominated and chlorinated dioxins at similar levels (Gullett et al. 2010) revealing that PBDE/BFR containing materials are an important dioxin sources during landfills/dump fires. In areas with long-term e-waste recycling utilising open burning, the dioxin contamination in soil far exceeded international dioxin limit values for soils (Yu et al. 2008) and can therefore be categorised as dioxin/POP-contaminated sites (Weber et al. 2008). Also in Nigeria, the practice of open burning of e-waste has been used for more than a decade with severe contamination as a consequence. The PBDD/F content of e-waste plastic and associated release additional formation in the end-of-life phase might result in such contamination in Nigeria (Babayemi et al. 2014b). The associated areas therefore need urgent assessment of contamination levels and human exposure to the wide range of pollutants present in e-waste (Sepulveda et al. 2010; Swedish EPA 2011) also considering dioxins.
The Stockholm Convention recommendations in respect to POP-PBDE risk reduction highlight that in particular, the industrial countries should take the lead in this work since they have the technologies and capacities to manage these material flows (Stockholm Convention 2011). In particular, the e-waste export and the export of e-waste plastic to developing countries need to be better controlled. Considering that the recycling flows of e-waste plastic are largely uncontrolled, the contaminants may end-up even in toys (Chen et al. 2010), household utensils (Chen et al. 2010) or food contact materials such as thermo-cup lids (Samsonek and Puype 2013) or salad servers (Diamond 2014). The documentation of the current study on high levels of PBDD/F in e-waste plastic fractions gives one more reason to urgently improve the waste management practice and better control the recycling flows. This is in particular also true for Nigeria considering their plan to establish approx. 20 recycling facilities for used plastics. A first assessment of recycling options of flame retarded plastic in Nigeria has been performed (Nnorom and Osibanjo 2008).
Conclusion
Plastics from CRT casings in Nigerian e-waste contain in average high concentrations of PBDD/Fs. It is estimated that the 237,000 t of CRT casings in Nigeria contain between 2 and 8 t of PBDD/DFs. Further, PBDD/F measurements are needed to estimate the total PBDD/F content in the 2.4 million tonnes of e-waste plastic in Nigeria from all EEE.
PBDE-treated plastic contained ca 1000 ppm of PBDD/F when related to the PBDE content. Taking into account the total global production of DecaBDE (ca 1.25 million tonnes) and c-OctaBDE (ca 110,000 t), it is possible that the total quantity of PBDD/Fs in these products might be around 1000 t. Further research in other PBDE-treated material flows is needed to refine this estimate.
The high levels of PBDD/Fs in e-waste plastics highlight the pollution potential from stockpiled PBDE/BFR-treated polymers in Nigeria and elsewhere. This clearly has to be taken into account in the management of these stockpiles. An urgent assessment of exposure risk and environmental pathways should therefore be undertaken—including an assessment of recycling and end-of-life treatment of these materials. The formation of more PBDF during storage and treatment along with the impact of debromination over time (thus forming lower brominated PBDF and POP-PBDEs with higher toxicity) increases exposure risk and must also be addressed.
Deca-BDE is still being produced but is currently being assessed for listing as a POP by the Stockholm Convention’s POPs Review Committee (UNEP 2013, 2014). The difficult challenges of safely handling and disposing of deca-BDE-treated polymers highlight the pressing need for a global phase out to avoid adding to the existing burden of PBDFs.
There is increasing evidence about the relevance of PBDD/Fs for human exposure (van den Berg et al. 2013), and further studies on the possible pathways to humans from these large PBDD/F stockpiles are needed. At the same time, the PBDD/F formation potential and their relevance to all BFRs should be critically reviewed.
The current process of developing inventories of POP-PBDE, HBB and HBCD containing materials as required by the Stockholm Convention will help to show the volumes of polymers treated with these POPs. This could be a good starting point for developing overall policies to manage (POP)-BFR containing polymers including e-waste plastic. The implementation of the Stockholm and Basel Conventions and the recently established Stockholm and Basel Convention guidance and guidelines on recycling and disposal of POP-BFR containing materials and articles (Stockholm Convention 2012a, b; Basel Convention 2014) could result in a better control of these material flows.
Considering the need for, and policy measures to move towards, a circular economy (European Commission 2015) material flows need to be diverted from landfilling to recycling where possible (and recovery only when recycling is not the environmentally preferable option). These stockpiles of POPs and their associated PBDD/Fs (together with the hundreds of other persistent toxic substances in use (Scheringer et al. 2012)) must be better controlled and, where possible, phased-out in order to enable safe recycling without exposure of hazardous chemicals to humans. The BAT/BEP guidance for managing PBDE containing materials (Stockholm Convention 2012a, b), the related Basel Convention guidelines (Secretariat of the Basel Convention 2014) and the compilation of information on POPs phase-out opportunities and alternatives as part of the implementation of the Stockholm Convention (Stockholm Convention Regional Center for Asia and the Pacific 2015) might help to achieve this.
The management of these POPs stockpiles and other halogenated chemicals is, however, a real challenge for all developing countries as most have little or no suitable destruction capacity for these compounds. Most also lack an appropriate waste management framework and related regulatory resources (Weber et al. 2013). Strategies should therefore be developed for developing countries to ensure that (former) producers and importers of articles containing PBDEs and other POPs can take responsibility for the management of the wastes according to the principle of extended producer responsibility (OECD 2001).
Notes
The TEQ for a sample is the sum of the products of the concentrations of the individual PCDD/F and the respective TEF (Van den Berg et al. 1998)
The GC/MS calculation for TV/EU/292 resulted in an overestimation of the actual c-OctaBDE concentration by a factor of 2 compared to XRF and EDC assessment of 30 % BFR (see supporting information of Sindiku et al. 2014.
In the recycling of e-waste plastic it was observed that in a developing country in Asia BFR containing plastic was partly separated from other plastic by simple sink/float technology due to the request of customers for flame retarded plastic for recycling (Schluep 2014).
For the study PBDE/BFR in e-waste plastic GC-ECD was preferably used (Sindiku et al. 2014) since the system can be heated to temperature above 300 °C to clean the system and for minimising BFR contamination/blanks in the equipment since the BFR levels vary by 5 orders of magnitude resulting in sometimes high BFR loads in the injection.
References
Arcadis and EBRC (2011) Identification and evaluation of data on flame retardants in consumer products – Final report 3|402 for European Commission Health and Consumers DG. Contract number 17.020200/09/549040
Ashizuka Y, Nakagawa R, Hori T, Yasutake D, Tobiishi K, Sasaki K (2008) Determination of brominated flame retardants and brominated dioxins in fish collected from three regions of Japan. Mol Nutr Food Res 52:273–283
Babayemi J, Sindiku O, Osibanjo O, Weber R (2014a) Substance flow analysis of polybrominated diphenyl ethers in plastic from EEE/WEEE in Nigeria in the frame of Stockholm Convention as a basis for policy advice. Env Sci Pollut Res. doi:10.1007/s11356-014-3228-6
Babayemi J, Sindiku O, Osibanjo O, Lundstedt S, Weber R (2014b) Material flow and substance flow analysis of POP-PBDEs in Nigeria and the risk of dioxin formation and release. Organohalogen Compd 76:1453–1456
Basel Convention (2014) Technical guidelines for the environmentally sound management of wastes consisting of, containing or contaminated with commercial octabromodiphenyl ether (hexabromodiphenyl ether and heptabromodiphenyl ether), commercial pentabromodiphenyl ether (tetrabromodiphenyl ether and pentabromodiphenyl ether) and Hexabromocyclododecane (Draft)
Bayarmaa B, Gan-Erdene T, Weber R, Jargalsaikhan L (2014) Analysis of Polybrominated diphenyl ether and Tetrabromobisphenol A in plastic samples in Mongolia. Rep Mongolian Acad Sci
Behnisch PA, Hosoe K, Sakai S (2003) Brominated dioxin-like compounds: in vitro assessment in comparison to classical dioxin-like compounds and other polyaromatic compounds. Environ Int 29(6):861–877
Birnbaum LS, Staskal DF, Diliberto JJ (2003) Health effects of polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs). Environ Int 29:855–860
BSEF (2000) An introduction to Brominated Flame Retardants, p. 4, BSEF, Bromine Science and Environmental Forum, www.bsef.com
Chen SJ, Ma YJ, Wang J, Chen D, Luo XJ, Mai BX (2009) Brominated flame retardants in children’s toys: concentration, composition, and children's exposure and risk assessment. Environ Sci Technol 43:4200–4206
Chen SJ, Ma YJ, Wang J, Tian M, Luo XJ, Chen D, Mai BX (2010) Measurement and human exposure assessment of brominated flame retardants in household products from South. China J Hazard Mater 176:979–984
Chi XW, Streicher-Porte M, Wang MYL, Reuter MA (2011) Informal electronic waste recycling: a sector review with special focus on China. Waste Manag 31:731–742
Deng C, Li Y, Li JH, Li HF (2014) A Mini-Review on Disposal of WEEE Plastics Containing PBDEs with a Special Focus on China. Adv Mater Res 878:600–608
Diamond M (2014) Personal communication 06. April 2014
Donnelly JR, Grange AH, Nunn NJ, Sovocool GW, Brumley WC, Mitchum RK (1989) Analysis of thermoplastic resins for brominated dibenzofurans. Biomed Environ Mass Spectrom 18:884–896
Ebert J, Bahadir M (2003) Formation of PBDD/F from flame-retarded plastic materials under thermal stress. Environ Int 29:711–716
Ericson Jogsten I, Hagberg J, Lindström G, Bavel B (2010) Analysis of POPs in human samples reveal a contribution of brominated dioxin of up to 15% of the total dioxin TEQ. Chemosphere 78:113–120
European Commission (2015) Roadmap Circular Economy. April 2015
Fernandes A, Dicks P, Mortimer D, Gem M, Smith F, Ield MD, White S, Rose M (2008) Brominated and chlorinated dioxins, PCBs and brominated flame retardants in Scottish shellfish: methodology, occurrence and human dietary exposure. Mol Nutr Food Res 52:38–249
Gallen C, Banks A, Brandsma S, Baduel C, Thai P, Eaglesham G, Heffernan A, Leonards P, Bainton P, Mueller JF (2014) Towards development of a rapid and effective non-destructive testing strategy to identify brominated flame retardants in the plastics of consumer products. Sci Total Environ 491–492:255–265
German Government (2003) Chemikalien-Verbotsverordnung in der Fassung der Bekanntmachung vom 13. Juni 2003 (BGBl. I S. 867), die zuletzt durch Artikel 5 Absatz 40 des Gesetzes vom 24. Februar 2012 (BGBl. I S. 212) geändert worden ist. (in German)
Gullett BK, Wyrzykowska B, Grandesso E, Touati A, Tabor DG, Ochoa GS (2010) PCDD/F, PBDD/F, and PBDE emissions from open burning of a residential waste dump. Environ Sci Technol 44:394–399
Hagberg J, Olsman H, van Bavel B, Engwall M (2006) Chemical and toxicological characterisation of PBDFs from photolytic decomposition of decaBDE in toluene. Environ Int 32(7):851–857
Hale RC, La Guardia MJ, Harvey EP, Mainor TM (2002) The potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere 46:729–735
Hanari NKK, Miyake Y, Okazawa T, Kodavanti PRS, Aldous KM, Yamashita N (2006) Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and polybrominated dibenzofurans as impurities in commercial polybrominated diphenyl ether mixtures. Environ Sci Technol 40:4400–4405
Hayakawa K, Takatsuki H, Watanabe I, Sakai S (2004) Polybrominated diphenyl ethers (PBDEs), polybrominated dibenzo-p-dioxins/dibenzofurans (PBDD/Fs) and monobromo-polychlorinated dibenzo-p-dioxins/dibenzofurans (MoBPXDD/Fs) in the atmosphere and bulk deposition in Kyoto, Japan. Chemosphere 57:343–356
Holt E, Weber R, Stevenson G, Gaus C (2010) Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) impurities in pesticides: a neglected source of contemporary relevance. Environ Sci Technol 44:5409–5415
Jogsten IE, Hagberg J, Lindström G, van Bavel B (2010) Analysis of POPs in human samples reveal a contribution of brominated dioxin of up to 15% of the total dioxin TEQ. Chemosphere 78:113–120
Kajiwara N, Takigami H (2010) Behavior of additive brominated flame retardants in textile products. Proceedings of the 5th International Symposium on Brominated Flame Retardants. 7–9 April 2010, Kyoto, Japan
Kajiwara N, Noma Y, Takigami H (2008) Photolysis studies of technical decabromodiphenyl ether (DecaBDE) and ethane (DeBDethane) in plastics under natural sunlight. Environ Sci Technol 42:4404–4409
Kajiwara N, Desborough J, Harrad S, Takigami H (2013) Photolysis of brominated flame retardants in textiles exposed to natural sunlight. Environ Sci Processes Impacts 15:653–660
Labunska I, Harrad S, Wang M, Santillo D, Johnston P (2014) Human dietary exposure to PBDEs around e-waste recycling sites in Eastern China. Environ Sci Technol 48:5555–5564
Li HR, Yu LP, Sheng GY, Fu JM, Peng PA (2007) The severe PCDD/Fs and PBDD/Fs pollution in air of electronic wastes dismantling area in China. Environ Sci Technol 41:5641–5646
Litten S, Mcchesney DJ, Hamilton MC, Fowler B (2003) Destruction of the World Trade Center and PCBs, PBDEs, PCDD/Fs, PBDD/Fs, and chlorinated biphenylenes in water sediment and sewage sludge. Environ Sci Technol 37:5502–5510
Luijk R, Govers HAJ, Nelissen L (1992) Formation of polybrominated dibenzofurans during extrusion of high-impact polystyrene/decabromodiphenylether/Antymony (III) oxide. Environ Sci Technol 26:2191–2198
Ma J, Addink R, Yun S, Cheng J, Wang W, Kannan K (2009) Polybrominated dibenzo-p-dioxins/ dibenzofurans and polybrominated diphenyl ethers in soil, vegetation, workshop-floor dust, and electronic shredder residue from an electronic waste recycling facility and in soils from a chemical industrial complex in eastern China. Environ Sci Technol 43:7350–7356
Mortimer D, Acheampong R, Fernandes A, Rose M (2013) Consumer exposure to chlorinated and brominated dioxins and biphenyls and polybromnated diphenyl ethers: new UK total diet study. Organohalogen Compd 75:1138–1141
Nnorom IC, Osibanjo O (2008) Sound management of brominated flame retarded (BFR) plastics from electronic wastes: state of the art and options in Nigeria. Resour Conserv Recycl 52:1362–1372
OECD (2001) Extended producer responsibility. A guidance manual for governments. Paris, France: OECD Publication Service
Ogungbuyi O, Nnorom IC, Osibanjo O, Schluep M (2012) Nigeria e-waste country assessment. Basel Convention Coordinating Centre for Africa (BCCC-Nigeria) and Swiss EMPA, Ibadan, Nigeria and St. Gallen, Switzerland May 2012. http://www.e-wasteguide.info/Ogungbuyi_2012_BCCC-Empa
Ortuño N, Font R, Moltó J, Conesa JA (2011) Thermal degradation of tetrabromobishenol A: emission of polybrominated dibenzod-p-dioxin and dibenzofurans and other organic compounds. Organohalogen Compd 3:511–514
Ortuño N, Lundstedt S, Lundin L (2015) Emissions of PBDD/Fs, PCDD/Fs and PBDEs from flame-retarded high-impact polystyrene under thermal stress. Chemosphere 123:64–70
Ota S, Aizawa H, Kondo Y, Takigami H, Hirai Y, Sakai S (2009) Current status of polybrominated dibenzo-p-dioxin and furans (PBDD/DFs) emissions in Japan. Organohalogen Compd 71:1323–1328
Ren M, Peng PA, Li XM, Li HR (2008) Review on environmental research of polybrominated dibenzo-p-dioxins and dibenzofurans. Environ Pollut Control 192:75–79 (in Chinese)
Ren M, Peng P, Cai Y, Chen D, Zhou L, Chen P, Hu J (2011) PBDD/F impurities in some commercial deca-BDE. Environ Pollut 159:1375–1380
Sakai S (2000) Thermal behavior of brominated flame retardants and PBDDs/DFs. Organohalogen Compd 47:210–213
Sakai S, Honda Y, Takatsuki H, Watanabe J, Aoki I, Nakamura K et al (2001a) Polybrominated substances in waste electrical and electronic plastics and their behavior in the incineration plants. Organohalogen Compd 52:35–38
Sakai S, Watanabe J, Honda Y, Takatsuki H, Aoki I, Futamatsu M, Shiozaki K (2001b) Combustion of brominated flame retardants and behavior of its byproducts. Chemosphere 42:519–531
Samsonek J, Puype F (2013) Occurrence of brominated flame retardants in black thermo cups and selected kitchen utensils purchased on the European market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. doi:10.1080/19440049.2013.829246
Scheringer M, Strempel S, Hukari S, Ng CA, Blepp M, Hungerbühler K (2012) How many persistent organic pollutants should we expect? Atmos Pollut Res 2012:383–391
Schluep (2014) Personal communication with Roland Weber 21.10.2014
Schlummer M, Gruber L, Mäurer A, Wolz G, Van Eldik R (2007) Characterisation of polymer fractions from waste electrical and electronic equipment (WEEE) and implications for waste management. Chemosphere 67:1866–1876
Secretariat of the Basel Convention (2014) Draft Technical guidelines for the environmentally sound management of wastes consisting of, containing or contaminated with commercial octabromodiphenyl ether (hexabromodiphenyl ether and heptabromodiphenyl ether), commercial pentabromodiphenyl ether (tetrabromodiphenyl ether and pentabromodiphenyl ether) and hexabromocyclododecane. November 2014
Sepulveda A, Schluep M, Renaud FG, Streicher M, Kuehr R, Hagelüken C, Gerecke AC (2010) A review of the environmental fate and effects of hazardous substances released from electrical and electronic equipments during recycling: examples from China and India. Environ Impact Assess Rev 30:28–41
Shaw SD, Blum A, Weber R, Kannan K, Rich D, Lucas D, Koshland CP, Dobraca D, Hanson S, Birnbaum LS (2010) Halogenated flame retardants: do the fire safety benefits justify the risks? Reviews on Environ Health 25:261–305
Shaw SD, Berger ML, Harris JH, Yun SH, Wu Q, Liao C, Blum A, Stefani A, Kannan K (2013) Persistent organic pollutants including polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans in firefighters from Northern California. Chemosphere 91:1386–1394
Simonsen FA, Moller LM, Madesn T, Stavnsbjerg M (2000) Brominated flame retardants: toxicity and ecotoxicity, Danish Environmental Protection Agency, Project No. 568
Sindiku O, Tysklind M, Osibanjo O, Babayemi JO, Schlummer M, Weber R, Lundstedt S (2013) Polybrominated dioxins and furans (PBDD/PBDF) in e-waste plastic in Nigeria. Abstract 6th International Symposium on Flame Retardants, April 7–10, 2013, San Francisco
Sindiku O, Babayemi JO, Osibanjo O, Schlummer M, Schluep M, Weber R (2014) Polybrominated diphenyl ethers listed as Stockholm Convention POPs and other brominated flame retardants in e-waste plastic in Nigeria. Environ Sci Pollut Res. doi:10.1007/s11356-014-3266-0
Stockholm Convention (2011) Work programmes on new persistent organic pollutants. 5th Conference of Parties meeting. UNEP/POPS/COP5/15
Secretariat of the Stockholm Convention (2012a) Guidance for the inventory of commercial pentabromodiphenyl ether (c-PentaBDE), commercial octabromodiphenyl ether (c-OctaBDE) and hexabromobiphenyls (HBB) under the Stockholm Convention on Persistent Organic Pollutants (Draft)
Secretariat of the Stockholm Convention (2012b) Guidance on best available techniques and best environmental practice for the recycling and disposal of articles containing polybrominated diphenyl ethers (PBDEs) under the Stockholm Convention on persistent organic pollutants (Draft)
Stockholm Convention Regional Center for Asia and the Pacific (2015) POPs in Articles and Phasing-Out Opportunities. http://map.bcrc.cn/
Suzuki G, Takigami H, Nose K, Takahashi S, Asari M, Sakai S-I (2007) Tentative identification of dioxin-like compounds in house dusts collected from Japan. Organohalogen Compd 69:2180–2183
Suzuki G, Someya M, Takahashi S, Tanabe S, Sakai S, Takigami H (2010) Dioxin-like activity in Japanese indoor dusts evaluated by means of in vitro bioassay and instrumental analysis: brominated dibenzofurans are an important contributor. Environ Sci Technol 44(21):8330–8336
Swedish EPA (2011) Recycling and disposal of electronic waste. Health hazards and environmental impacts. Report 6417 of the Swedish Environmental Protection Agency. March 2011
Takigami H, Suzuki G, Hirai Y, Sakai S (2008) Transfer of brominated flame retardants from components into dust inside television cabinets. Chemosphere 73:161–169
Terauchi H, Takahashi S, Lam PKS, Min BY, Tanabe S (2009) Polybrominated, polychlorinated and monobromo-polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls in marine surface sediments from Hong Kong and Korea. Environ Pollut 157:724–730
Tue NM, Suzuki G, Takahashi S, Kannan K, Takigami H, Tanabe S (2013) Dioxin-related compounds in house dust from New York State: occurrence, in vitro toxic evaluation and implications for indoor exposure. Environ Pollut 181:75–80
Tue NM, Katsura K, Suzuki G, le Tuyen H, Takasuga T, Takahashi S, Viet PH, Tanabe S (2014) Dioxin-related compounds in breast milk of women from Vietnamese e-waste recycling sites: levels, toxic equivalents and relevance of non-dietary exposure. Ecotoxicol Environ Saf 106:220–225
UNEP (2010) Debromination of brominated flame retardants. 6th POP Reviewing Committee meeting Geneva 11–15. October 2010 (UNEP/POPS/POPRC.6/INF/20)
UNEP (2013) Proposal to list decabromodiphenyl ether (commercial mixture, c-decaBDE) in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants. 6 June 2013
UNEP (2014) Report of the Persistent Organic Pollutants Review Committee on the work of its tenth meeting. Rome, 27–30 October 2014. UNEP/POPS/POPRC.10/10
van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE (2006) The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93:223–241
Van den Berg M, Birnbaum L, Bosveld BTC, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FXR, Liem AKD, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T (1998) Toxic Equivalency Factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environmental Health Perspectives, 106 (12):775–792
van den Berg M, Denison MS, Birnbaum LS, DeVito MJ, Fiedler H, Falandysz J, Rose M, Schrenk D, Safe S, Tohyama C, Tritscher A, Tysklind M, Peterson RE (2013) Review polybrominated dibenzo-p-dioxins, dibenzofurans, and biphenyls: inclusion in the toxicity equivalency factor concept for dioxin-like compounds. Toxicol Sci 133:197–208
Vetter W, Bendig P, Blumenstein M, Hägele F, Behnisch PA, Brouwer A (2014) Formation of polybrominated dibenzofurans (PBDFs) after heating of a salmon sample spiked with decabromodiphenyl ether (BDE-209). Environ Sci Pollut Res Int. doi:10.1007/s11356-014-3267-z
Waeger P, Schluep M, Müller E (2010) RoHS substances in mixed plastics from waste electrical and electronic equipment final report St. Gallen, September 17, 2010 http://www.ewasteguide.info/files/Waeger_2010_Empa-WEEEForum.pdf
Wang LC, Wang YF, Hsi HC, Chang-Chien GP (2010) Characterizing the emissions of polybrominated diphenyl ethers (PBDE) and polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) from metallurgical processes. Environ Sci Technol 44:1240–1246
Watanabe I, Sakai S (2003) Environmental release and behavior of brominated flame retardants. Environ Int 29:665–682
Watanabe I, Tatsukawa R (1987) Formation of brominated dibenzofurans from the photolysis of flame retardant decabromobiphenyl ether in hexane solution by UV and sun light. Bull Environ Contam Toxicol 39(6):953–959
Weber R, Kuch B (2003) Relevance of BFRs and thermal conditions on the formation pathways of brominated and brominated-chlorinated dibenzodioxins and dibenzofurans. Environ Int 29:699–710
Weber R, Gaus C, Tysklind M, Johnston P, Forter M, Hollert H, Heinisch H, Holoubek I, Lloyd-Smith M, Masunaga S, Moccarelli P, Santillo D, Seike N, Symons R, Torres JPM, Verta M, Varbelow G, Vijgen J, Watson A, Costner P, Wölz J, Wycisk P, Zennegg M (2008) Dioxin- and POP-contaminated sites—contemporary and future relevance and challenges. Environ Sci Pollut Res 15:363–393
Weber R, Watson A, Forter M, Oliaei F (2011) Persistent organic pollutants and landfills—a review of past experiences and future challenges. Waste Manag Res 29(1):107–121
Weber R, Aliyeva G, Vijgen J (2013) The need for an integrated approach to the global challenge of POPs management. Environ Sci Pollut Res Int 20:1901–1906, http://springerlink.bibliotecabuap.elogim.com/content/pdf/10.1007%2Fs11356-012-1247-8.pdf
Wong MH, Wu SC, Deng WJ, Yu XZ, Luo Q, Leung AOW, Wong CSC, Luksemburg WJ, Wong AS (2007) Export of toxic chemicals—a review of the case of uncontrolled electronic-waste recycling. Environ Pollut 149:131–140
World Health Organization (WHO) (1998) Polybrominated dibenzo-p-dioxins and dibenzofurans. Environ Health Criteria 205, Geneva
Yu X, Zennegg M, Engwall M, Rotander A, Larsson M, Wong MH, Weber R (2008) E-waste recycling heavily contaminates a Chinese city with chlorinated, brominated and mixed-halogenated dioxins. Organohalogen Compd 70:813–817, http://www.dioxin20xx.org/pdfs/2008/08-367.pdf
Zennegg M, Yu X, Hung WM, Weber R (2009) Fingerprints of chlorinated, brominated and mixed halogenated dioxins at two e-waste recycling sites in Guiyu/China. Organohalogen Compd 71:2263–2267
Zennegg M, Schluep M, Streicher-Porte M, Lienemann P, Haag R, Gerecke AC (2014) Formation of PBDD/F from PBDE in electronic waste in recycling processes and under simulated extruding conditions. Chemosphere 116:34–39
Acknowledgments
Funding from the Norwegian government to the Secretariat of the SC for partly financing the stay of Omotayo Sindiku at Umea University is appreciated. The views expressed in this paper do not necessarily reflect the views of the funding institutions. The authors declare that there were no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 258 kb)
Rights and permissions
About this article
Cite this article
Sindiku, O., Babayemi, J.O., Tysklind, M. et al. Polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) in e-waste plastic in Nigeria. Environ Sci Pollut Res 22, 14515–14529 (2015). https://doi.org/10.1007/s11356-015-5260-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5260-6