Abstract
In the shallow environment, the nutrient and carbon exchanges at the sediment–water interface contribute significantly to determine the trophic status of the whole water column. The intensity of the allochthonous input in a coastal environment subjected to strong anthropogenic pressures determines an increase in the benthic oxygen demand leading to depressed oxygen levels in the bottom waters. Anoxic conditions resulting from organic enrichment can enhance the exchange of nutrients between sediments and the overlying water. In the present study, carbon and nutrient fluxes at the sediment–water interface were measured at two experimental sites, one highly and one moderately contaminated, as reference point. In situ benthic flux measurements of dissolved species (O2, DIC, DOC, N-NO3 −, N-NO2 −, N-NH4 +, P-PO4 3−, Si-Si(OH)4, H2S) were conducted using benthic chambers. Furthermore, undisturbed sediment cores were collected for analyses of total and organic C, total N, and biopolymeric carbon (carbohydrates, proteins, and lipids) as well as of dissolved species in porewaters and supernatant in order to calculate the diffusive fluxes. The sediments were characterized by suboxic to anoxic conditions with redox values more negative in the highly contaminated site, which was also characterized by higher biopolymeric carbon content (most of all lipids), lower C/N ratios and generally higher diffusive fluxes, which could result in a higher release of contaminants. A great difference was observed between diffusive and in situ benthic fluxes suggesting the enhancing of fluxes by bioturbation and the occurrence of biogeochemically important processes at the sediment–water interface. The multi-contamination of both inorganic and organic pollutants, in the sediments of the Mar Piccolo of Taranto (declared SIN in 1998), potentially transferable to the water column and to the aquatic trophic chain, is of serious concern for its ecological relevance, also considering the widespread fishing and mussel farming activities in the area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In shallow coastal ecosystems, nutrient regeneration at the sediment–water interface (SWI) plays a significant role in maintaining high primary production in the water column via benthic–pelagic coupling (Hyun et al. 2013). In fact, in systems receiving sufficient light to maintain a phytobenthic community, benthic microalgae have an important role as filter in the flux of dissolved nutrients by transforming, storing, and removing nutrients from the overlaying water (Sundbäck et al. 2000; Grenz et al. 2000; McGlathery et al. 2007). Thus, the autotrophic metabolism influences fluxes at the SWI by the uptake and temporary retention of nutrients, burial of recalcitrant organic matter and indirectly by oxygenating surface sediment (McGlathery et al. 2001). Sediments can therefore constitute either a source or a sink of nutrients, becoming the main factor in controlling the trophic level in aquatic systems (Spagnoli and Bergamini 1997). Benthic–pelagic coupling works in both directions, as the release of nutrients from sediments enhances primary production in the water column, and the organic matter produced in pelagic system sinks to the surface sediment providing labile substrate to be regenerated (Grenz et al. 2000). It has been reported (Kelly and Nixon 1984) that up to 80 % of annual carbon and nitrogen deposition may be remineralized and returned to the overlying water, thus supporting primary pelagic production.
Coastal areas are often subjected to a severe exposition to urban, industrial, and agricultural wastewaters that influence the volume and quality of water inputs. Rivers are the greatest contributors to coastal areas of organic materials, nutrients, heavy metals, and sediments, usually untreated and unfiltered (Dunn et al. 2013 and references therein; Ortega et al. 2008). This organic material is extremely reactive and induces higher rates of microbial processes, sometimes resulting in eutrophication of the receiving water body. The enhanced microbial activity can lead to a change in sediment status from oxic to hypoxic or even anoxic, and reduces oxygen levels in bottom waters (De Vittor et al. 2012). Hypoxia is a global key stressor affecting a huge portion of coastal zones and the persistence of these episodes could result in changes in ecosystem functioning (Emili et al. 2011), especially in the increase of nutrient and dissolved organic carbon exchange between sediments and water column (De Vittor et al. 2012; Dunn et al. 2013).
Establishing the magnitude of benthic exchanges, especially in conditions of hypoxia/anoxia, is important because dissolved oxygen is a key regulator for many biogeochemical cycles, such as sulfur, nitrogen, and heavy metals; therefore, changes in sediment redox status could enhance the release of contaminants (e.g., mercury, iron, sulfur) (Santos-Escheandia et al. 2009; Covelli et al. 2011; Emili et al. 2011; De Vittor et al. 2012). Hence, the study of nutrient and carbon fluxes at the SWI is fundamental to define the functionality of coastal ecosystems and to provide indications for managing conservation and development land uses.
The present study has been conducted with the aim of evaluating benthic metabolism, and carbon and nutrient exchange at the SWI of the Mar Piccolo of Taranto (Southern Italy), an example of a marine ecosystem contaminated by waste waters. As these processes are strongly related to contaminant cycling, the study was associated with simultaneous measurements of major and trace element fluxes using the same benthic chambers (Emili et al. 2015). In situ benthic chamber experiments were performed in two experimental sites, one highly and one moderately contaminated, as reference point. In parallel, sediments as well as supernatant and porewaters were characterized.
Methods
Study area
As part of the Taranto Gulf, Mar Piccolo is a semi-enclosed basin located in the north area of the town. Its surface area accounts for approximately 21 km2. It is divided into two inlets (called first and second inlet), and it is connected to Mar Grande and the Taranto Gulf (Ionian Sea) by two channels linked to the first inlet where the maximum depth of 13 m is achieved. Summer water stratification is usually detected due to its morphology and low water exchange. Hypoxic conditions at the bottom are often noticed as a consequence of oxygen consumption by microbial degradation of significant inputs of organic matter from wastewater discharges, mussel farming, and agricultural soil drainage which also supplies an excess of nutrients to the system (Spada et al. 2012). The sediments are made up of fine sands and muds with traces of shell fragments (Pastore 1993). In general, the benthic system of the Mar Piccolo is net heterotrophic. Microphytobenthic abundances are quite low in respect to other shallow environments, and diatoms are the most abundant group although their percentage decreases in relation to pollution (Rubino et al. 2015). The southeastern area of the second inlet is the most densely colonized by macroalgae, while in the first inlet a much lower areal coverage is present (Cibic et al. 2015). Macrobenthic community is generally dominated by polychaetes and molluscs, followed by crustaceans and other taxa (mainly anthozoans, sipunculids, and nemertines) (Franzo et al. 2015).
High sulfide concentration is present in bottom sediments throughout the year as a result of organic matter anaerobic degradation (Cardellicchio et al. 2006). Besides, this coastal environment has suffered from severe anthropogenic pressures (remarkably an iron and steel factory, a petroleum refinery, and the Italian Navy shipyard and arsenal), and it was declared a “Contaminated Site of National Interest (SIN)” in 1998.
A more exhaustive description of the study area is reported by Cardellicchio et al. (2015).
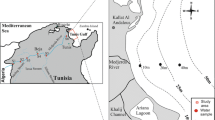
The study of biogeochemical fluxes at the SWI of the Mar Piccolo was carried out in June 2013 at two sites both at about 11-m depth (Fig. 1). Station 1E (sampled on June 12) is approximately located at the center of the first inlet. The second station (1I, sampled on June 14) is placed just in front of the Navy Arsenal, and it can be considered as the most contaminated by anthropogenic activities (Spada et al. 2012; Emili et al. 2015). The physical and chemical features of the two stations are described by Kralj et al. (2015) and environmental characteristics of bottom waters at the beginning of each sampling are reported in Table 1.
Sediment sampling and porewater collection
Undisturbed sediment cores, two for each sampling site (1E and 1I), were collected by divers pushing a Plexiglas tube (16-cm i.d; 30-cm length) into the sediment. Cores were stored at dark into refrigerate containers and carefully transported to the laboratory minimizing disturbance at the SWI. Upon return to the laboratory, within 2 h after core collection, the sediment cores were immediately extruded and sectioned into slices (0–1, 1–2, 2–3.5, 3.5–5, and 5–7 cm) in a N2-filled chamber and redox potential was measured at each slice. Measurement of Eh was performed at room temperature directly into the N2-filled chamber. After calibration, electrode was rinsed and an initial reading was made in the overlying seawater ~1 cm above the sediment surface after the reaching of a stable value (several minutes). Then, the overlying water was sampled with a syringe and the electrode was pushed in 0.5-cm depth into the surface sediment where a further reading was taken after several minutes. The sediment core was sliced and the operation repeated after each slicing. The redox electrode was rinsed with distilled water and dried between measurements. Porewaters were extracted from each sediment slice by centrifugation at in situ temperature. Samples were subsequently recovered in an inert atmosphere, filtered through Millipore Millex HA 0.45 μm pore size cellulose acetate filters and finally collected in acid-precleaned vials, which were stored frozen until analysis. Porewater samples and supernatant were analyzed for inorganic nutrients (nitrite, nitrate, ammonium, phosphate, and silicate), dissolved organic carbon (DOC), dissolved inorganic carbon (DIC), and hydrogen sulfide (H2S).
Sediment samples (five for each core) were freeze-dried, homogenized, and ground to fine powder for analyses of total and organic C (Ctot, Corg), total N (Ntot), total P (Ptot), and biopolymeric carbon (BPC, as sum of carbohydrate (CHO), protein (PRT), and lipid (LIP) carbon). Part of the intact samples was used to determine granulometry and sediment water content (Emili et al. 2015).
Diffusive fluxes
Diffusive fluxes (F) represent an instantaneous measure of the flux of solutes diffusing from sediment porewaters to the overlying water column, along a concentration gradient. Positive values are indicative of an efflux, while negative values represent an influx or scavenging from the water column. In the present study, the diffusive benthic fluxes of DIC, DOC, and N, P, Si nutrients and H2S were calculated by application of Fick’s first law as reported by De Vittor et al. (2012). In the absence of bioirrigation, Fick’s first law can be expressed as follows:
where F is the flux of a solute with concentration C at depth x and \( \mathit{\partial} \) C/\( \mathit{\partial} \) x is the concentration gradient of chemical species between porewater at the depth of 1 cm and the overlying water, Φ the sediment porosity (0.75), θ the tortuosity (dimensionless), and D w is the diffusion coefficient of the solute in water (Ullman and Aller 1982; Alperin et al. 1994) in the absence of the sediment matrix.
Benthic flux chamber experiments
In situ benthic flux measurements were conducted using benthic chambers already tested in the Gulf of Trieste (Bertuzzi et al. 1997; Covelli et al. 1999) and in the Marano and Grado Lagoon (Covelli et al. 2008; De Vittor et al. 2012). The chamber, consisting of a transparent Plexiglas box, covers a sediment area of 0.25 m2 containing ~53 L of seawater. The chamber waters were homogenized using a magnetic stirring mechanism, coupled to a rotating propeller on the chamber topside. Samples were periodically (t = 0 and every ≅2 h for a total of five samplings) collected at sites 1E and 1I using 50-mL polypropylene syringes during day deployments of about 8 h. The volume removed from the chamber was replaced by in situ water flowing into the chamber from a valve. Flux rates calculated from concentration data were not corrected for dilution because of the low ratio of the sample to total chamber volume (0.2 %). The hourly metabolism rates were scaled to the number of light hours for each experiment to calculate diurnal net metabolism (McGlathery et al. 2001). In situ flux rates of solutes across the SWI were calculated on square meter basis adapting Rizzo (1990) and Nixon et al. (1976) protocols.
Unfortunately, as a consequence of the sampling schedule and the lack of overnight samples, daily respiration could not be estimated; furthermore, also nutrients and DOC fluxes reflect only daylight processes.
Analyses
Analyses of Ctot and Ntot in freeze-dried and homogenized sediment samples were performed using a Perkin Elmer 2400 CHNS/O Elemental Analyser. Corg was determined after acidification of samples with 1 M HCl (Hedges and Stern 1984) at a combustion temperature of 975 °C. Ptot was analyzed by extraction from freeze-dried and homogenized sediment with 1 M HCl after ignition at 550 °C (Aspila et al. 1976). Dissolved phosphate in extracts was determined using the same method described for inorganic nutrients in overlying and porewaters (see at the end of the paragraph). The precision of Ctot, Corg, Ntot, and Ptot was about 3 %.
Two different CHO fractions (water soluble, CHOH2O and EDTA-extractable, CHOEDTA) were determined following Blasutto et al. (2005). The carbohydrate fractions were measured spectrophotometrically using the phenol-sulfuric acid assay (Dubois et al. 1956), modified by Gerchacov and Hatcher (1972) for sediment samples. CHO concentrations were calculated from calibration curves of d-glucose. CHO concentrations, obtained as equivalent-glucose, were transformed into carbon using a conversion factor of 0.49 g C g−1 (Fabiano et al. 1995).
PRT analyses were carried out on lyophilized sediment samples after extractions with NaOH (0.5 M, 4 h). PRT were determined according to Hartree (1972) modified by Rice (1982) to compensate for phenol interference. PRT concentrations were calculated from calibration curves of serum albumin. Even if determination could be affected by the interference of humics (Rice 1982), this method was chosen due to its ease and high sensitivity. Moreover, the wide application of this protocol makes our results comparable with other studies (Fabiano et al. 1995; Fabiano et al. 2001; Pusceddu et al. 2009). Concentrations obtained as albumin equivalents were transformed into carbon using a conversion factor of 0.50 g C g−1 (Fichez 1991). Total LIP were extracted by direct elution with chloroform and methanol following the procedure of Bligh and Dyer (1959) and analyzed according to Marsh and Wenstein (1966). LIP concentrations were calculated from calibration curves of tripalmitine. Concentrations obtained as tripalmitine equivalents were transformed into carbon using a conversion factor of 0.75 g C g−1 (Fichez 1991). All biochemical analyses were carried out in three to five replicates, with standard deviation lower than 5 %.
The sum of the carbon equivalents of CHO, PRT and LIP was referred as BPC (sensu Fichez 1991).
Dissolved O2 (DO) was analyzed by the Winkler method (Grasshoff et al. 1983) using an automated titration system (Mettler Toledo, DL 21). The reproducibility of the methods was 5 %. DIC and DOC were determined using the Shimadzu TOC-V CSH analyzer. For DIC, samples were injected into the instrument port and directly acidified with H3PO4 (25 %). For DOC analysis, water samples were previously acidified (automatically into instrument syringe, 2 %—6 M HCl) and after CO2 elimination, the concentration was determined using a high temperature catalytic method (Sugimura and Suzuki 1988). Phosphoric acidification for DIC and combustion conducted at 680 °C on a catalyst bed for DOC, generated CO2 that was carried to a nondispersive infrared detector (NDIR). Analysis showed a variation coefficient <2 %. The reproducibility of the method was between 1.5 and 3 %. Nutrient analyses, including nitrate (N-NO3 −) and nitrite (N-NO2 −), ammonium (N-NH4 +), phosphate (P-PO4 3−), and silicate (Si-Si(OH)4), were performed with a segmented flow Bran + Luebbe AutoAnalyzer 3 following standard colorimetric methods (Hansen and Koroleff 1999). The precision was 3 %. Aliquots of 2.5 ml of overlying or porewater and stored in a dark place until analysis. Samples for hydrogen sulfide determination were preserved by adding zinc acetate and measured spectrophotometrically using a VARIAN CARY 100 Scan spectrophotometer at 670 nm according to Fonselius (1983).
To test if the concentrations of the biochemical compounds differed significantly between the two stations (1E and 1I), an analysis of variance (ANOVA) test was performed using the Oneway Analysis Platform analysis. To highlight relationships between analyzed biochemical variables, the nonparametric Spearman’s rank correlation analysis was applied.
In order to verify the differences between impacted and control sites, principal component analysis (PCA) was applied on solid phase and porewater data.
All analyses were performed using the JMP 11 Pro statistics software package (SAS Institute Inc.).
Results and discussion
Sediment biogeochemistry
Surface sediment grain size at both stations was rather uniform over the 0–7-cm depth layers sampled, and it largely consisted of fine particles (<63 μm). Grain size distribution at station 1E (average 13.9 % sand, 72.6 % silt, and 13.5 % clay) lightly differed from station 1I characterized, on average, by 13.5 % sand, 76.7 % silt, and 9.8 % clay proportion (Emili et al. 2015).
The porewater oxygen penetration depth was between 0.16 and 0.29 cm (Rubino et al. 2015), indicating that the upper sample for porewater extraction and solid analyses included the whole oxic zone and the top of the anoxic sediment (Anschutz et al. 2007).
The redox potential (Eh) along sediment cores varied from −150 to −418 mV at station 1E and from −272 to −400 mV at 1I and are similar to those reported by Spada et al. (2012) who found values ranging from −348 to −457 mV in the surface sediments of the Mar Piccolo basin. Matijević et al. (2007) reported that the transition from suboxic to anoxic conditions (presence of HS− or S2−) takes place at potentials from 0 to to 150 mV. Thus, Eh values measured along the sediment cores indicate the presence of anoxic conditions, sulfate reduction and anaerobic organic matter degradation (Matijević et al. 2013). Vertical profiles displayed a clear decrease in Eh downcore at 1E while 1I, more subjected to anthropogenic inputs and characterized by more negative potential, showed an irregular trend (Fig. 2).
Similar Ctot contents (average 8.0 ± 0.2 vs 7.7 ± 0.3 %) and uniform trends with depth were encountered at sites 1E and 1I, while higher Corg contents (average 3.8 ± 0.1 vs 4.2 ± 0.3 %), with the exception of the surface layer, and Ntot contents (average 0.27 ± 0.01 vs 0.39 ± 0.03 %) were found at 1I (Fig. 2).
The ratio of total organic carbon to total nitrogen (Corg/Ntot) is widely applied for inferring the origin of organic matter (OM) in marine sediments. According to Goñi et al. (2003), Corg/Ntot (molar) ratios >14 are characteristic of vascular plant-derived OM dominated by carbon-rich (and nitrogen-poor) biochemical classes (i.e., lignin and cellulose), while Corg/Ntot (molar) values <10 may be defined as of autochthonous marine origin (from phytoplankton and bacterioplankton characterized by higher N content) (Ogrinc et al. 2003). The Corg/Ntot ratios (molar) were rather constant along sediment depth at site 1E, varying from 15.2 to 16.9 (average 16.3 ± 0.7) while at 1I a wide range of variation was observed, from 8.9 to 14.4 (average 12.6 ± 2.2), with a net increase with depth, mainly as a consequence of the decreasing Ntot trend downcore. The ratio in surface sediments (0–1 cm) found at 1E was twice higher (16.9) than at 1I (8.9), suggesting that OM is predominantly of marine origin at this station while 1E could be influenced by some terrestrial input. Concentrations of Ptot decreased slightly with depth from 809 ± 4 μg g−1 (average 0–2 cm) to 761 μg g−1 at 1E and from 724 to 679 μg g−1 at 1I (Fig. 2), suggesting a recent enrichment in the surface sediment most likely due to urban and/or aquaculture inputs (Anschutz et al. 2007). Corg/Ptot ratio (Fig. 2) was quite constant along sediments of 1E with values, on average, of 123 ± 1.4, typical of suboxic and intermittently anoxic environments (Algeo and Ingall 2007). At 1I, similar conditions (Corg/Ptot = 115) were found only in surface sediments (0–1 cm) while in the deeper ones (1–7 cm), the ratio reached a mean value of 161 ± 2.7, characteristic of permanently anoxic environments (Algeo and Ingall 2007).
The sediment concentrations of biochemical compounds, CHOH2O and CHOEDTA, PRT, LIP, and BPC (as sum of the carbon of the four components) differ significantly between stations 1E and 1I (CHOH2O: p < 0.01; CHOEDTA: p < 0.01; PRT: p < 0.01; LIP: p < 0.005; BPC: p < 0.005) (Fig. 2). On average, 1I was characterized by 1.5–2-fold higher concentrations of all components, even if the relative percentage composition is similar between the two stations. At both stations, the BPC was dominated by LIP (67–77 % of total BPC), followed by PRT (20–28 %) and CHOEDTA (3–6 %). CHOH2O contributed only for 1–2 % to total BPC. At 1E, LIP ranged from 2758 to 4244 μg C g−1 showing an increasing gradient with depth, PRT ranged from 1045 to 1193 μg C g−1 with a uniform trend along depth, CHOEDTA varied between 123 and 190 μg C g−1 increasing slightly with depth, while CHOH2O profile was quite uniform with concentrations ranging from 49 to 66 μg C g−1. At 1I, LIP ranged from 4099 to 6523 μg C g−1 (maximum was reached at the deepest sediment layer of 5–7 cm), PRT varied between 1607 and 1927 μg C g−1 displaying an irregular vertical profile, CHOEDTA decreased with depth from 439 to 226 μg C g−1, while CHOH2O showed a highly variable profile with a concentrations range of 73–144 μg C g−1.
LIP concentrations found in our study, especially those of site 1I, are definitely higher than those reported worldwide for marine sediments (for a review, see Pusceddu et al. 2009). However, they are comparable to those measured by Cibic et al. (2012) at 2–6-cm sediment depth of an Adriatic lagoon, contaminated by hydrocarbon and heavy metal and by Pusceddu et al. (1999) in the top sediments (0–1 cm) at a station of the Marsala lagoon (Mediterranean Sea) characterized by Posidonia oceanica (and detritus of Posidonia) coverage. Similar concentrations with a maximum of more than 20,000 μg g−1 (lipid carbon = 15,400 μg C g−1) were also reported by Neira et al. (2001) in an upwelling region off Central Chile. The high lipid contribution to BPC (lipids are always the dominant component, up to 77 %) found in our study is the highest ever reported in the literature (for a review, see Pusceddu et al. 2009, 2010). Generally, the composition of the sedimentary organic detritus shows a dominance of carbohydrates or proteins (Pusceddu et al. 2009, 2010; Cibic et al. 2012) depending on its origin (primary organic carbon from microalgae vs aged and/or nonliving organic matter). The cause of the high concentration of lipids found especially at site 1I remains unknown. Although one possibility could be the natural production from diatoms like Coscinodiscus, one of the most important phytoplanktonic taxa in Mar Piccolo of Taranto (Caroppo and Cardellicchio 1995; Caroppo et al. 2006), deposited shortly before sampling (Neira et al. 2001), we believe that they derive from anthropogenic sources (e.g., untreated sewage discharges and petroleum-related compounds).
BPC vertical profiles reflected patterns observed for lipids concentrations. The BPC represented, on average, the 13.0 ± 2.4 % of Corg at station 1E and its contribution to Corg increased regularly with depth. At station 1I, the biopolymeric available fraction of total organic carbon constituted, on average, the 18.2 ± 3.6 % of Corg showing the highest value of 23.2 % at the uppermost layer of sediment (0–1 cm), a clear decrease at 1–2 cm and then a regular increase up to the bottom layers (Fig. 2). Increasing accumulation of BPC in marine sediments is associated even in highly productive systems (such as estuaries, ponds, and fish farm sediments) with low algal carbon contributions to BPC. This suggests that, in eutrophic sediments characterized by high BPC concentrations and benthic algal biomass, algal carbon is progressively diluted in a complex and heterogeneous organic matrix (Pusceddu et al. 2009).
A PCA was performed on the correlation matrix of variables describing the solid phase of the two sediment cores to highlight differences between the two stations (Fig. 3). The first two eigenvalues explain 81.3 % of the original data variance. By plotting the original datapoints (each point representing a single sediment layer) onto a biplot with the first two principal components as the axes, two groups can clearly be distinguished, corresponding to the two stations. Principal component 1 is responsible for this separation, and it is well correlated with all original variables, except for Corg (factor loading < 0.5). The highest loadings (>|0.94|) were associated with variables Ptot, BPC, and PRT. Differences among the different sediment layers in the same core are explained by the second principal component, which is mostly represented by the percentage of sand in the sediment.
Porewater chemistry
The concentration and lability (freshness) of organic matter determine the efficiency of its mineralization and the concentrations of its by-products. Metabolite accumulation in porewaters results from the balance between reaction rates and transport processes (Metzger et al. 2007). Thus, porewater concentrations provide an image of ongoing diagenetic reactions and physical processes (Metzger et al. 2007).
The DIC profiles exhibited two different patterns: a regular but minor increase of concentration with depth along the sediment core (from 2879 μM, in the supernatant, to 3347 μM, at 5–7-cm depth) at 1E, and a marked increasing concentration downcore with a clear positive gradient at the SWI going to a maximum of 5714 μM at the deepest layer (5–7 cm), at 1I (Fig. 4). In the porewater concentration versus depth profiles, the increasing DIC concentrations are attributed to processes accompanying the decomposition of sedimentary organic matter (Lojen et al. 2004) as confirmed by the positive correlation found between DIC and P-PO4 3− (p < 0.05 and p < 0.01 at 1E and 1I, respectively) and between DIC and N-NH4 + (p < 0.01 at both stations). As DIC represents the ultimate product of organic carbon oxidation, the increasing DIC concentrations downcore evidenced at station 1I indicate that significant decomposition occurs within the sediment column (Alperin et al. 1999).
Porewater DOC concentrations reflect a balance between production and consumption reactions; dissolved compounds produced by hydrolysis of solid-phase organic matter are consumed by biotic and abiotic processes (Alperin et al. 1999). DOC concentrations in porewaters varied from 119 to 809 μM and from 345 to 714 μM, at 1E and 1I, respectively. Values increased irregularly downcore especially at 1E indicating DOC diagenetic formation within sediments (Alperin et al. 1999) with prevalently macromolecular (humic) nature and, hence, lower mobility (Burdige et al. 1992) (Fig. 4).
At both stations, the porewater N-NH4 + profiles showed a large increase in concentration with depth with a strong positive gradient at the SWI, but concentrations at each sediment depth of station 1I were always approximately 3-fold higher compared to 1E, reaching a maximum of 291 μM at the deepest sediment layer (Fig. 4). This behavior suggests the presence of more intense denitrification processes and organic matter mineralization rate at site 1I, characterized by higher biopolymeric carbon concentrations (i.e., proteins).
At station 1E, a considerable N-NO2 − concentration gradient existed between the surficial interstitial water and the overlying seawater; immediately below, the concentrations decreased substantially to reach a quasi-constant value below 3-cm depth. N-NO3 − concentrations increased sharply in the first centimeter of the sediment and then decreased to reach a constant concentration of about 2.5 μM at layers between 1 and 3 cm to increase again below 4-cm depth. At 1I, N-NO2 − concentrations increased in the 1–2-cm layer of the sediment and then gradually declined to reach a steady state below 2-cm depth. The concentration of N-NO3 − increased from 2.9 μM in the supernatant to 6.3 μM at 5–7-cm depth, except for a decrease at 2–4-cm depth (Fig. 4). This increasing N-NO3 − concentration with depth suggests that bioturbation is actively reworking sediment as confirmed by the high presence of mobile species, which normally move in the first 10 cm of the sediment like the polychaetes Lumbrineris latreilli, Glycera rouxii, and Eunice vittata and the amphipod Microdeutopus anomalus. These bioturbators were not abundant at station 1E characterized by higher macrozoobenthic biomass but dominated by surface deposit feeders (Auriemma, personal communication; Franzo et al. 2015). The presence of N-NO2 − and N-NO3 − along the sediment depth, albeit in low concentrations, suggests an accumulation of the products of OM oxidation in the interstitial water. However, the presence of very high levels of N-NH4 + also in the upper sediment layers indicates that the ammonia produced during heterotrophic breakdown of OM was not immediately oxidized and accumulated in porewaters prior to its transport predominantly to the sediment below (Ram and Zingde 2000). As found by Lojen et al. (2004), the concentration vs. depth profiles of nitrate and ammonia (Fig. 4) can be explained in relation to the observed influx of nitrate and ammonia into the sediment in benthic chamber experiments (see “In situ benthic fluxes”). The increase of ammonia and nitrate with depth is typical of environments where rapid decomposition of sedimentary organic matter takes place (Lojen et al. 2004). Ram and Zingde (2000) suggested also that N-NO3 − increase in the sediment depth could be due to a nonsteady-state condition derived from either the two following factors: the sediment could have been buried at a time when the bottom water N-NO3 − concentrations were high or, else, the nitrate was consumed less rapidly than in surface sediments, due to the differences in OM and/or microbial populations.
The concentration profiles of P-PO4 3− reflect the behavior of N-NH4 + with positive correlation coefficients of 0.94 (p < 0.01), and 0.96 (p < 0.01) at stations 1E and 1I, respectively, showing a large increase in concentration with sediment depth and higher concentrations (up to 6-fold) at each sediment depth at 1I (Fig. 4). The linear relationship indicates that porewater P-PO4 3− is released in relation to ammonia by organic matter decomposition (Ogrinc and Faganeli 2006). The release of phosphate to porewaters is mostly due to the loss of capacity by the sediment to adsorb phosphate as it undergoes burial and to the aerobic and anaerobic mineralization of organic matter during early diagenesis, which produces dissolved phosphate (Anschutz et al. 2007). The observed differences between the two stations can be attributed to the different content of biopolymeric carbon and as a consequence of the different grade of OM mineralization in the cores.
The ratio between dissolved ammonium and phosphate can provide information about the processes of phosphate release and uptake in the sedimentary cores. A constant ratio indicates a stoichiometric nutrient regeneration due to organic matter mineralization. Variable ratios suggest that reactions of P removal or addition take place (Anschutz et al. 2007 and references therein). The ratio varied between 17 and 52 at 1E and from 17 to 64 at 1I (Fig. 4). This variability indicates that the mineralization of marine organic matter alone cannot explain the profiles and that, in the surface sediments (0–2 cm), reactions of P removal, such as adsorption on iron oxides (De Vittor et al. 2012), precipitation as authigenic phosphate mineral, and biological uptake within the sedimentary column occurred (Anschutz et al. 2007). Remarkably, Mar Piccolo sediments are rich in iron (Petronio et al. 2012). The decrease of the NH4 +/PO4 3− ratio with depth instead confirms the release of phosphate from iron oxides as these are reduced, and the anaerobic mineralization of organic P (Sundby et al. 1992).
Silicate concentration, at station 1E, was 15.5 μM in the overlaying water, resulted homogenous in the first 3 cm of sediments with values of 48–51 μM, and then increased to 165 and 157 μM in the deeper layers. At 1I, a considerable concentration gradient of Si-Si(OH)4 existed between the surficial interstitial water (236 μM) and the overlying seawater (22 μM); silicate increased up to 400 μM in the middle layer of the sediment core and then declined in the deeper layers (Fig. 4). The observed increasing Si-Si(OH)4 concentrations with sediment depth at station 1I may be the result of biogenic silica dissolution in the sediment (Zhang et al. 2013 and references therein) which depends on temperature, pH, the nature and concentration of aqueous salt solutions, and Al content of the surrounding solution (Pastuszak et al. 2008). On the other hand, biogenic silica settled on the seafloor can be transformed into aluminosilicate materials (Ren et al. 2013) and precipitation of authigenic aluminosilicate minerals gives a low apparent solubility and contributes to low silicic acid concentrations, which could be the case of station 1E.
H2S concentrations were very low at both stations, ranging from 0.2 to 0.8 μM and from 0.5 to 1.5 μM at 1E and 1I, respectively (Fig. 4). This could be due to bioturbation, as suggested by the presence of N-NO3 − throughout the core lengths, which rules out the possibility of sulfate reduction (Ram and Zindge 2000), but also to very quickly reactions of reactive iron with sulfide, which leads to FeS precipitation (Middelburg and Levin 2009) reducing sulfide diffusion from the deeper anoxic sediment layers.
Similar to solid phase data, a PCA performed on porewaters (Fig. 5) showed that sediment layers can also be distinguished in two groups, corresponding to the two experimental stations. However, the distinction is not so sharp due to the deepest layer of 1E (5–7-cm depth) falling in the 1I group. This could be due to its high concentration of silicates and ammonia and the large negative value of Eh (−418 mV). Principal component 1 mostly summarizes the variables N-NH4 + (0.94), P-PO4 3−, Si-Si(OH)4, DIC, and Eh (factor loading >|0.87|) while N-NO3 − has the strongest loading to the second component. Together, PC1 and PC2 explain 73.3 % of the original dataset variance.
Diffusive fluxes
The diffusive fluxes of all analyzed parameters at the two study sites are reported in Table 2.
Following the concentration gradient, the flux of all analytes occurred in one direction from the sediment porewater to the overlying bottom waters with the only exceptions of H2S and N-NO2 − at stations 1E and 1I, respectively. Excluding DOC and nitrite, the flux of all other inorganic nutrients, DIC and H2S were considerably higher at station 1I than at 1E. The reason of this is related to the higher concentrations of this analytes in the porewater of 1I enhanced by the higher biopolymeric carbon content and biological activity. The lower DOC flux associated with the higher DIC flux registered at station 1I suggests that DOC was utilized as fast as it was produced (De Vittor et al. 2012). Looking at the diffusion of the inorganic nitrogen species, nitrite fluxes are negligible and nitrate ones appear marginal (up to one order of magnitude lower) compared to the fluxes of ammonium. Low N-NO3 − fluxes toward the water column or N-NO3 − influxes have already been reported by several authors (Al-Rousan et al. 2004; Engelsen et al. 2008; De Vittor et al. 2012) and are related to the fact that N-NH4 + is the first inorganic product in the regeneration of nitrogenous organic material by microorganisms. N-NO2 − and N-NO3 − are formed in sediment only under well-oxygenated conditions by nitrifying bacteria throughout the nitrification processes (Al-Rousan et al. 2004 and references therein). The higher fluxes of N-NH4 + and P-PO4 3− found at station 1I could be related both to the more anoxic conditions, which increased the release rate of these species from sediments to the overlaying water (Al-Rousan et al. 2004 and references therein), and to the dependence of phosphate and ammonium regeneration on the quality of organic matter in the sediment (Clavero et al. 2000). Also, the higher Si-Si(OH)4 flux is related to the increased porewater concentration of silicate, probably due to enhanced dissolution of biogenic Si under more reducing conditions, as suggested by the H2S efflux at 1I compared to the influx at 1E. Hydrogen sulfide release from anoxic sediments, in fact, has been widely reported (Middelburg and Levin 2009).
Concentration variations in benthic chambers
A very good linear correlation (p < 0.01) was observed between analyte concentration and incubation time only for DIC at station 1I, suggesting the absence of a constant flux during the duration of the deployment (Berelson et al. 2013). The concentration of oxygen did not fall below 249 μM during the time the benthic chambers were on the seafloor, showing slightly higher concentrations at station 1I (ranging from 295 to 335 μM) compared to 1E (249–279 μM). During the 8-h sediment incubation at both sites (Fig. 6), O2 concentration showed temporal little decrease/increase or nearly constant levels, suggesting that the production was balanced by respiration. The trends in DIC versus time (Fig. 6) showed some scatter but in both stations were fit with a linear decreasing trend-line showing a decoupling with O2, probably due to nonphotosynthetic utilization of CO2, chemosynthetic removal of O2 (Johnson et al. 1981), and carbonate precipitation, as previously postulated for the sediments of the Gulf of Trieste and the Marano and Grado lagoons (De Vittor et al. 2012 and references therein).
DOC values, whose release should be compatible with DIC and O2 based rates for net system production (Johnson et al. 1981), showed substantial differences between the two stations (Fig. 6) and resulted not related to O2 and DIC changes. The lack of DOC/O2 and DOC/DIC correlations during the incubation time suggests that DOC may originate more from OM aerobic degradation and from dissolved OM (perhaps humic substances) benthic release rather than from extracellular photosynthetates (Ogawa et al. 2003), even if the inverse correlation (p < 0.05) with silicates found at 1I supported the hypothesis of a diatoms role in DOC release. Looking at the inorganic nitrogen pool (Fig. 6), the N-NO3 − levels were low, often close to 0.5 μM, which is considered limiting to phytoplankton growth (Fasham et al. 1990) but always higher (1–2 orders of magnitude) than N-NO2 − (whose concentration was negligible) at both the sites, ranging from 0.3 to 0.9 μM and from 0.7 to 2.0 μM, at 1E and 1I, respectively. The changes of N-NO3 − concentrations, which could be also attributed to benthic infauna causing either increases or decreases in nitrate release from the sediment, depending on the depth of their burrowing and their amount of irrigation activity (Fields et al. 2014), showed that at both sites, nitrate, although low in concentration, did not become completely consumed during incubations.
Ammonium concentrations ranged from 1.1 to 2.8 μM and from 1.0 to 1.7 μM at 1E and 1I, respectively (Fig. 6). Even if a significant correlation was not found at both sites, the variation of N-NH4 + inside the chambers followed that of nitrate, suggesting a simultaneous influence of denitrification (sediment)/nitrification (oxygenated water) processes and algal uptake.
P-PO4 3− concentrations (Fig. 6) were low at both stations (0.01–0.03 μM), suggesting that phosphate is the limiting nutrient for microalgal production. This hypothesis is supported by the high DIN/P-PO4 3− ratios, always higher than the Redfield ratio (De Vittor et al. 2012).
Silicate levels (Fig. 6) varied from 2.3 to 3.8 μM at 1E and from 3.6 to 3.8 μM at 1I. At both stations, silicate trends were highly correlated with nitrate (p < 0.05 and p < 0.01, at 1E and 1I, respectively), suggesting that benthic diatoms preferentially assimilate ammonium rather than nitrate. On the other hand, the fact that ammonium trends follow nitrate (even if not significantly related) suggests that nutrient fluxes are also controlled by external factors, such as the bioirrigation rate (Berelson et al. 2013).
In situ benthic fluxes
Fluxes calculated from chamber incubations, which represent the total fluxes (Rasheed et al. 2006) differed substantially from diffusive ones (Table 2). Unexpectedly, the flux of almost all analytes were negative, i.e., directed to the sediment porewater, with the only exceptions of P-PO4 3− at both stations and N-NO2 − at station 1I. This great difference suggests the enhancement of fluxes by bioturbation and that biogeochemically important processes occur at the SWI (De Vittor et al. 2012). Furthermore, total fluxes, in addition to diffusive fluxes, may include fluxes from advective porewater exchange (out of the chamber) although in mud sediments, like those of our study, this contribution can be minor (Rasheed et al. 2006). In our study, the discrepancy between benthic and diffusive fluxes is also due to the lack of data from overnight samples; thus, our calculations only reflect daylight processes without taking into account night respiration and fluxes, which generally differ substantially from the daylight ones. Several authors, in fact, have reported differences in night and day fluxes or during light and dark incubations (Rizzo 1990; Billerbeck et al. 2007; De Vittor et al. 2012; Dunn et al. 2013; Sospedra et al. 2015).
During the 8 h of diurnal incubation experiments, the oxygen uptake rate of −1.6 and −5.6 mmol m−2day−1 at sites 1E and 1I, respectively, showing daily net negative production, suggested that sediments were more heterotrophic at 1I, due to the increased respiratory demands for decomposition of the higher quantity of organic matter. On the other hand, the apparent community production measured from DIC reached highly negative values of −27.8 and −30.4 mmol m−2day−1, at 1E and 1I, respectively, thus suggesting that respiration processes exceeded the primary production but also that carbonate precipitation could have occurred.
The diurnal DOC influx was one order of magnitude higher at 1E (−51.8 mmol m−2day−1) than at 1I (−5.8 mmol m−2day−1), suggesting that larger amounts of DOC are removed at 1E, during daylight by biotic and abiotic pathways. For example, the photolysis of complex organic compounds may produce labile DOC from biologically less reactive riverine and marine DOC (Moran and Zepp 1997). On the other hand, the lack of new production of reactive DOC and different sources of organic matter, not equivalent in terms of biodegradability for heterotrophic bacteria, could have differently affected the functional performance of bacterial communities between the two stations. In addition, irrigation may be an important mass transfer process. Nevertheless, it seems that at both stations DOC utilization, within incubation time, exceeds DOC production. Differences between benthic and diffusive fluxes, which were directed out of the sediments, also suggest that the majority of porewater DOC was resistant towards microbial oxidative degradation and/or that porewater DOC differs in terms of molecular composition (i.e., different molecular weight with different diffusion coefficient) from bottom-water DOC; thus, the diffusive fluxes could have been overestimated.
Diurnal influxes of nitrate (nitrite fluxes were negligible) reached similar values at the two stations while the ammonium flux was double at station 1I. This behavior could be the consequence of intense autotrophic and heterotrophic assimilation but also of nitrification processes. Our data also support the idea that benthic microalgal community can function as a “filter” that controls the flux of dissolved nutrients at the sediment/water interface through assimilation and photosynthetic oxygenation of the sediment surface Sundbäck et al. (2000). Phosphate release, whose regeneration from sediments is a slow process (Liu et al. 2004) and whose exchanges are largely controlled by the oxygen status of the sediment (Rizzo 1990), was observed only at 1I probably due to degradation of higher content of sedimentary organic P. The measured influx of silicate was 6-fold greater at 1E suggesting a higher uptake by microphytobenthos as confirmed by the definitely higher abundance of benthic diatoms evidenced at this station (43,186 ± 8861 cells cm−3 at 1E vs 9576 ± 1732 cells cm−3 at 1I; Rubino et al. 2015). The differences in diffusive and benthic flux of silicate likely depend also on the remineralization of moribund, i.e., not actively photosynthesizing diatoms at the SWI (Warnken et al. 2007).
Summary and conclusion
-
1.
In the Mar Piccolo of Taranto, the station 1I, placed just in front of the Navy Arsenal and more contaminated by anthropogenic activities, is characterized by more negative redox potential, higher biopolymeric carbon content (most of all lipids), and lower C/N ratios.
-
2.
Steep near-surface gradients of porewater concentrations were observed for almost all analytes with stronger gradient again localized at 1I where diffusive fluxes were, with the exception of DOC and N-NO2 −, always higher. These results highlight the role of the higher contaminated site in enhancing benthic fluxes.
PCA clearly emphasized the differences between impacted and control sites in relation of their different solid phase and porewater contents characteristics.
The higher content of DIC, nutrients, and H2S in the porewater of station 1I suggest the presence of higher diagenetic activities enhancing the release of this analytes which could result in a higher release of contaminants as already reported by several authors (Emili et al. 2012; Dang et al. 2014).
-
3.
At both stations, diurnal benthic fluxes of O2, DIC, DOC, N-NH4 +, N-NO3 −, and Si-Si(OH)4 were negative while P-PO4 3− efflux was negligible at 1E and low at 1I. Based on oxygen diurnal influx, station 1I seems more heterotrophic with respect to 1E.
-
4.
The low release of P-PO4 3−, evidenced both by diffusive and benthic fluxes, suggests P to be a limiting nutrient for primary production in the Mar Piccolo of Taranto.
The great difference observed between diffusive and in situ benthic fluxes could be due to the lack of data from overnight samples during benthic incubation, but also suggests an important role of biological activity at or near the SWI.
-
5.
The severe anthropogenic pressures suffered by the Mar Piccolo of Taranto (declared SIN in 1998), which caused a multi-contamination of both inorganic and organic pollutants in the sediments potentially transferable to the water column and to the aquatic trophic chain is of serious concern for its ecological relevance, also considering the widespread fishing and mussel farming activities in the area.
More research effort toward detailed analysis of the mobility and biogeochemical fate of dissolved porewater compounds in relation to contaminants (focusing in a larger study area and on seasonal variability) are needed to obtain a better understanding on the role of the sediment of the Mar Piccolo of Taranto as source of pollutants/nutrients toward the seawater column.
References
Algeo TJ, Ingall E (2007) Sedimentary Corg:P ratios, paleocean ventilation and phanerozic atmospheric pO2. Palaeogeogr Palaeocl 256:130–155
Alperin MJ, Albert DB, Martens CS (1994) Seasonal variations in production and consumption rates of dissolved organic carbon in an organic-rich coastal sediment. Geochim Cosmochim Ac 58:4909–4930
Alperin MJ, Martens CS, Albert DB, Suayah IB, Benninger LK, Blair NE, Jahnke RA (1999) Benthic fluxes and porewater concentration profiles of dissolved organic carbon in sediments from the North Carolina continental slope. Geochim Cosmochim Ac 63:427–448
Al-Rousan S, Rasheed M, Badran M (2004) Nutrient diffusive fluxes from sediments in the northern Gulf of Aqaba, Red Sea. Sci Mar 68(4):483–490
Anschutz P, Chaillou G, Lecroart P (2007) Phosphorus diagenesis in sediment of the Thau Lagoon. Estuar Coast Shelf Sci 72:447–456
Aspila KI, Agemian H, Chau AS (1976) A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Berelson WM, McManus J, Severmann S, Reimers CE (2013) Benthic flux of oxygen and nutrients across Oregon/California shelf sediments. Cont Shelf Res 55:66–75
Bertuzzi A, Faganeli J, Welker C, Brambati A (1997) Benthic fluxes of dissolved inorganic carbon, nutrients and oxygen in the Gulf of Trieste (Northern Adriatic). Water Air Soil Poll 99:305–314
Billerbeck M, Røy H, Bosselmann K, Huettel M (2007) Benthic photosynthesis in submerged Wadden Sea intertidal flats. Estuar Coast Shelf Sci 71:704–716
Blasutto O, Cibic T, De Vittor C, Fonda Umani S (2005) Microphytobenthic primary production and sedimentary carbohydrates along salinity gradients in the lagoons of Grado and Marano (Northern Adriatic sea). Hydrobiologia 550:47–55
Bligh EG, Dyer W (1959) A rapid method for total lipid extraction and purification. Can J Biochem Phys 37:911–917
Burdige DJ, Alperin MJ, Homstead J, Martens CS (1992) The role of benthic fluxes of dissolved organic carbon in oceanic and sedimentary carbon cycling. Geophys Res Lett 19:1851–1854
Cardellicchio N, Buccolieri A, Di Leo A, Spada L (2006) Heavy metals in marine sediments from the Mar Piccolo of Taranto (Ionian Sea, Southern Italy). Ann Chim-Rome 96:727–741
Cardellicchio N, Annicchiarico C, Di Leo A, Giandomenico S, Spada L (2015) The Mar Piccolo of Taranto: an interesting marine ecosystem for the environmental problems studies. Environ Sci Pollu R, in this issue
Caroppo C, Cardellicchio N (1995) Preliminary study on phytoplankton communities of Mar Piccolo in Taranto (Jonian sea). Oebalia 21:61–76
Caroppo C, Turicchia S, Margheri MC (2006) Phytoplankton assemblages in coastal waters of the northern Ionian Sea (eastern Mediterranean), with special reference to cyanobacteria. J Mar Biol Assoc UK 86:927–937
Cibic T, Franzo A, Celussi M, Fabbro C, Del Negro P (2012) Benthic ecosystem functioning in hydrocarbon and heavy-metal contaminated sediments of an Adriatic lagoon. Mar Ecol Prog Ser 458:69–87. doi:10.3354/meps09741
Cibic T, Bongiorni L, Borfecchia F, Di Leo A, Franzo A, Giandomenico S, Karuza A, Micheli C, Rogelja M, Del Negro P (2015) Ecosystem functioning nearby the largest steelworks in Europe and the main Italian naval base: the study case of the Mar Piccolo of Taranto. Environ Sci Pollu R, in this issue
Clavero V, Izquierdo JJ, Fernández NFX (2000) Seasonal fluxes of phosphate and ammonium across the sediment-water interface in a shallow small estuary (Palmones River, southern Spain). Mar Ecol Prog Ser 198:51–60
Covelli S, Faganeli J, Horvat M, Brambati A (1999) Porewater distribution and benthic flux of mercury and methylmercury in the Gulf of Trieste (northern Adriatic Sea). Estuar Coast Shelf Sci 48:415–428
Covelli S, Faganeli J, De Vittor C, Predonzani S, Acquavita A, Horvat M (2008) Benthic fluxes of mercury species in a lagoon environment (Grado Lagoon, northern Adriatic Sea, Italy). Appl Geochem 23:529–546
Covelli S, Emili A, Acquavita A, Koron N, Faganeli J (2011) Benthic biogeochemical cycling of mercury in two contaminated northern Adriatic coastal lagoons. Cont Shelf Res 31:1777–1789
Dang DH, Lenoble V, Durrieu G, Mullot JU, Mounier S, Garnier C (2014) Sedimentary dynamics of coastal organic matter: an assessment of the porewater size/reactivity model by spectroscopic techniques. Estuar Coast Shelf Sci 151:100–111
De Vittor C, Faganeli J, Emili A, Covelli S, Predonzani S, Acquavita A (2012) Benthic fluxes of oxygen, carbon and nutrients in the Marano and Grado lagoon (Northern Adriatic sea, Italy). Estuar Coast Shelf Sci 113:57–70
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Dunn RJK, Robertson D, Teasdale PR, Walthman NJ (2013) Benthic metabolism and nitrogen dynamics in an urbanized tidal creek: domination of DNRA over denitrification as a nitrate reduction pathway. Estuar Coast Shelf Sci 131:271–281
Emili A, Koron N, Covelli S, Faganeli J, Acquavita A, Predonzani S, De Vittor C (2011) Does anoxia affect mercury cycling at the sediment–water interface in the Gulf of Trieste (northern Adriatic Sea)? Incubation experiments using benthic flux chambers. Appl Geochem 26:194–204
Emili A, Acquavita A, Koron N, Covelli S, Faganeli J, Horvat M, Žižek S, Fajon V (2012) Benthic flux measurements of Hg species in a northern Adriatic lagoon environment (Marano & Grado Lagoon, Italy). Estuar Coast Shelf Sci 113:71–84
Emili A, Acquavita A, Covelli S, Spada L, Di Leo A, Giandomenico S, Cardellicchio N (2015) Water-sediment interactions controlling heavy metals mobility in a contaminated coastal environment (Taranto, Italy). Environ Sci Pollut R in this issue
Engelsen A, Hulth S, Pihl L, Sundbäck K (2008) Benthic trophic status and nutrient fluxes in shallow-water sediments. Estuar Coast Shelf Sci 78:783–795
Fabiano M, Danovaro R, Fraschetti S (1995) A three-year time series of elemental and biochemical composition of organic matter in subtidal sandy sediments of the Ligurian Sea (North-western Mediterranean). Cont Shelf Res 15:1453–1469
Fasham MJR, Ducklow HW, McKelvie SM (1990) A nitrogen based model of plankton dynamics in the oceanic mixed layer. J Mar Res 48:591–639
Fichez R (1991) Composition and fate of organic matter in submarine cave sediments; implications for the biogeochemical cycle of organic carbon. Oceanol Acta 14:369–377
Fields L, Nixon SW, Oviatt C, Fulweiler RW (2014) Benthic metabolism and nutrient regeneration in hydrographically different regions on the inner continental shelf of Southern New England. Estuar Coast Shelf Sci 148:14–26
Fonselius SH (1983) Determination of hydrogen sulphide. In: Grasshof K, Ehrhardt M, Kremling K (eds) Methods of seawater analysis, 2nd edn. Weinheim, Verlag Chemie, pp 73–80
Franzo A, Auriemma R, Nasi F, Vojvoda J, Pallavicini A, Cibic T, Del Negro P (2015) Benthic ecosystem functioning in the severely contaminated Mar Piccolo of Taranto (Ionian Sea, Italy): focus on heterotrophic pathways. Environ Sci Pollu R, in this issue
Gerchacov SM, Hatcher PG (1972) Improved technique for analysis of carbohydrates in sediments. Limnol Oceanogr 17:938–943
Goñi MA, Teixeira MJ, Perkey DW (2003) Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA). Estuar Coast Shelf Sci 57:1023–1048
Grasshoff K, Ehrhardt M, Kremling K (1983) Methods of seawater analysis, 2nd edn. Weinheim, Verlag Chemie
Grenz C, Cloern JE, Hager SW, Cole BE (2000) Dynamics of nutrient cycling and related benthic nutrient and oxygen fluxes during a spring phytoplankton bloom in South San Francisco Bay (USA). Mar Ecol Prog Ser 197:67–80
Hansen HP, Koroleff F (1999) Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhardt M (eds) Methods of seawater analysis, 3rd edn. Wiley-VCH, Weinheim, pp 159–228
Hartree EF (1972) Determination of proteins: a modification of the Lowry method that give a linear photometric response. Anal Biochem 48:422–427
Hedges JI, Stern JH (1984) Carbon and nitrogen determinations of carbonate-containing solids. Limnol Oceanogr 29:657–663
Hyun JH, Kim SH, Mok JS, Lee JS, An SU, Lee WC, Jung RH (2013) Impacts of long-line aquaculture of Pacific oysters (Crassostrea gigas) on sulfate reduction and diffusive nutrient flux in the coastal sediments of Jinhae–Tongyeong, Korea. Mar Pollut Bull 74:187–198
Johnson KM, Burney CMMN, Sieburth J (1981) Enigmatic marine ecosystem metabolism by direct diel CO2 and O2 flux in conjunction with DOC release and uptake. Mar Biol 65:49–60
Kelly JR, Nixon SW (1984) Experimental studies of the effect of organic deposition on the metabolism of a coastal marine bottom community. Mar Ecol Prog Ser 17:157–169
Kralj M, De Vittor C, Comici C, Relitti F, Alabiso G, Del Negro P (2015) Recent evolution of the physical-chemical characteristics of a Site of National Interest - the Mar Piccolo of Taranto (Ionian Sea) - and changes over the last 20 years. Environ Sci Pollu R, in this issue
Liu SM, Zhang J, Li DJ (2004) Phosphorus cycling in sediments of the Bohai and Yellow Seas. Estuar Coast Shelf Sci 59:209–218
Lojen S, Ogrinc N, Dolenec T, Vokal B, Szaran J, Mihelčić G, Branica M (2004) Nutrient fluxes and sulfur cycling in the organic-rich sediment of Makirina Bay (Central Dalmatia, Croatia). Sci Total Environ 327:265–284
Marsh JB, Weinstein WJ (1966) A simple charring method for determination of lipids. J Lipid Res 7:574–576
Matijević S, Kušpilić G, Kljaković-Gašpić Z (2007) The redox potential of sediment from the Middle Adriatic region. Acta Adriat 48(2):191–204
Matijević S, Bogner D, Bojanić N, Žuljević A, Despalatović M, Antolić B, Nikolić V, Bilić J (2013) Biogeochemical characteristics of sediments under the canopy of invasive alga Caulerpa racemosa var. cylindracea (Pelješac peninsula, Adriatic Sea). Fresen Environ Bull 22(10a):3030–3040
McGlathery KJ, Anderson IC, Tyler AC (2001) Magnitude and variability of benthic and pelagic metabolism in a temperate coastal lagoon. Mar Ecol Prog Ser 216:1–15
McGlathery KJ, Sundbäck K, Anderson IC (2007) Eutrophication in shallow coastal bays and lagoons: the role of plants in the coastal filter. Mar Ecol Prog Ser 348:1–18
Metzger E, Simonucci C, Viollier E, Sarazin G, Prévot F, Jézéquel D (2007) Benthic response to shellfish farming in Thau lagoon: pore water signature. Estuar Coast Mar S 72:406–419
Middelburg JJ, Levin LA (2009) Coastal hypoxia and sediment biogeochemistry. Biogeosciences 6:1273–1293
Moran MA, Zepp RG (1997) Role of photoreactions in the formations of biologically labile compounds from dissolved organic matter. Limnol Oceanogr 42:1307–1316
Neira C, Sellanes J, Soto A, Gutiérrez D, Gallardo VA (2001) Meiofauna and sedimentary organic matter off Central Chile: response to changes caused by the 1997–1998 El Niño. Oceanol Acta 24:313–328
Nixon SW, Oviatt CA, Garber J, Lee V (1976) Diel metabolism and nutrient dynamics in a salt marsh embayment. Ecology 57:740–750
Ogawa H, Usui T, Koike I (2003) Distribution of dissolved organic carbon in the East China Sea. Deep-Sea Res Pt II 5:353–366
Ogrinc N, Faganeli J (2006) Phosphorus regeneration and burial in near-shore marine sediments (the Gulf of Trieste, northern Adriatic Sea). Estuar Coast Shelf Sci 67:579–588
Ogrinc N, Faganeli J, Pezdic J (2003) Determination of organic carbon remineralization in near-shore marine sediments (Gulf of Trieste, Northern Adriatic) using stable carbon isotopes. Org Geochem 34:681–692
Ortega T, Ponce R, Forja J, Gómez-Parra A (2008) Benthic fluxes of dissolved inorganic carbon in the Tinto-Odiel system (SW of Spain). Cont Shelf Res 28:458–469
Pastore M (1993) Mar Piccolo. Nuova Eolitrice Apulia, Martina Franca (In Italian)
Pastuszak M, Conley DJ, Humborg C, Witek Z, Sitek S (2008) Silicon dynamics in the Oder estuary, Baltic Sea. J Mar Syst 73:250–262
Petronio BM, Cardellicchio N, Calace N, Pietroletti M, Pietrantonio M, Caliandro L (2012) Spatial and temporal heavy metal concentration (Cu, Pb, Zn, Hg, Fe, Mn, Hg) in sediments of the Mar Piccolo in Taranto (Ionian Sea, Italy). Water Air Soil Poll 223:863–875
Pusceddu A, Sarà G, Armeni M, Fabiano M, Mazzola A (1999) Seasonal and spatial changes in the sediment organic matter of a semi-enclosed marine system (W-Mediterranean Sea). Hydrobiologia 397:59–70
Pusceddu A, Dell’Anno A, Fabiano M, Danovaro R (2009) Quantity and bioavailability of sediment organic matter as signatures of benthic trophic status. Mar Ecol Prog Ser 375:41–52
Pusceddu A, Bianchelli S, Canals M, Sanchez-Vidal A, De-Madron XD, Eussner S, Lykousis V, de-Stigter H, Trincardi F, Danovaro R (2010) Organic matter in sediments of canyons and open slopes of the Portuguese, Catalan, Southern Adriatic and Cretan Sea margins. Deep-Sea Res I 57:441–457
Ram A, Zingde MD (2000) Interstitial water chemistry and nutrients fluxes from tropical intertidal sediment. Indian J Mar Sci 29:310–318
Rasheed M, Al-Rousan S, Manasrah R, Al-Horani F (2006) Nutrient fluxes from deep sediment support nutrient budget in the oligotrophic waters of the Gulf of Aqaba. J Oceanogr 62:83–89
Ren H, Brunelle BG, Sigman DM, Robinson RS (2013) Diagenetic aluminum uptake into diatom frustules and the preservation of diatom-bound organic nitrogen. Mar Chem 155:92–101
Rice DL (1982) The detritus nitrogen problem: new observations and perspectives from organic geochemistry. Mar Ecol Prog Ser 9:153–162
Rizzo WM (1990) Nutrient exchange between the water column and the subtidal benthic microalgal community. Estuaries 13:219–226
Rubino F, Cibic T, Belmonte M, Rogelja M, Del Negro P (2015) The microbenthic community structure and the trophic status of sediments in Mar Piccolo of Taranto (Ionian Sea). Environ Sci Pollu R, in this issue
Santos-Escheandia J, Prego R, Cobelo-García A, Millward GE (2009) Porewater geochemistry in a Galician Ria (NW Iberian Peninsula): implications for benthic fluxes of dissolved trace elements (Co, Cu, Ni, Pb, V, Zn). Mar Chem 117:77–87
Sospedra J, Falco S, Morata T, Gadea I, Rodilla M (2015) Benthic fluxes of oxygen and nutrients in sublittoral fine sands in a north-western Mediterranean coastal area. Cont Shelf Res 97:32–42
Spada L, Annicchiarico C, Cardellicchio N, Giandomenico S, Di Leo A (2012) Mercury and methylmercury concentrations in Mediterranean seafood and surface sediments, intake evaluation and risk for consumers. Int J Hyg Envir Heal 215:418–426
Spagnoli F, Bergamini MC (1997) Water-sediment exchange of nutrients during early diagenesis and resuspension of anoxic sediments from the Northern Adriatic Sea shelf. Water Air Soil Poll 99:541–556
Sugimura Y, Suzuki Y (1988) A high temperature catalytic oxidation method for the determination of non-volatile dissolved organic carbon in seawater by direct injection of liquid sample. Mar Chem 24:105–131
Sundbäck K, Miles A, Göransson E (2000) Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: an annual study. Mar Ecol Prog Ser 200:59–76
Sundby B, Gobeil C, Silverberg N, Mucci A (1992) The phosphorus cycle in coastal marine sediments. Limnol Oceanogr 37(6):1129–1145
Ullman WJ, Aller RC (1982) Diffusion coefficients in nearshore marine sediments. Limnol Oceanogr 27:552–556
Warnken KW, Santschi PH, Roberts LA, Gill GA (2007) The cycling and oxidation pathways of organic carbon in a shallow estuary along the Texas Gulf Coast. Estuar Coast Shelf Sci 76(1):69–84
Zhang L, Wang L, Yin K, Lü Y, Zhang D, Yang Y, Huang X (2013) Pore water nutrient characteristics and the fluxes across the sediment in the Pearl River estuary and adjacent waters, China. Estuar Coast Shelf Sci 133:182–192
Acknowledgments
The activities described in this publication were funded by the Project Bandiera RITMARE - La Ricerca Italiana per il Mare coordinated by the National Research Council and funded by the Ministry for Education, University and Research within the National Research Programme 2011–2013. The authors are warmly grateful to Nicola Cardellicchio and the team of the IAMC institute, section of Taranto, for their logistical support and hospitality. Thanks are due to M. Bussi and C. Landucci from DMG Trieste for their help in grain-size and CHN analyses and to T. Cibic for environmental data reported in Table 1. We are also grateful to three anonymous reviewers for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
De Vittor, C., Relitti, F., Kralj, M. et al. Oxygen, carbon, and nutrient exchanges at the sediment–water interface in the Mar Piccolo of Taranto (Ionian Sea, southern Italy). Environ Sci Pollut Res 23, 12566–12581 (2016). https://doi.org/10.1007/s11356-015-4999-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4999-0