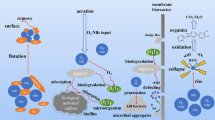
Abstract
Currently, there is a growing emphasis on wastewater reclamation and reuse all over the world due to restricted water resources. Among a variety of wastewater reuse technologies, the use of microfiltration membranes (MF) is one of the popular processes because it has the ability to successfully eliminate particulates and colloidal matters. However, successful fouling control is not easy because effluents from the activated sludge process still contain small particulates and colloidal matters such as extracellular polymeric substance (EPS) and soluble microbial products (SMP). On the other hand, microbubbles have advantageous properties compared to common bubbles, but there hasn’t been reporting of the use of microbubbles in physical cleaning instead of aeration. Encouraging results were obtained herein through the application of microbubbles for physical cleaning. In evaluation of the cleaning efficiency, the efficiency of microbubbles was observed to be twice as high as that of aeration, except during the course of the initial 30 min. Total organic carbon (TOC) concentration of the membrane tank after treatment with microbubbles was more than twice as high as that after aeration for physical cleaning. The membrane cleaned with microbubbles also had the smoothest surface, with a roughness of 42.5 nm. In addition, microbubbles were found to effectively remove EPS and make the structure of the gel layer loose. In particular, the microbubbles had the ability to remove proteins through the effect of pyrolytic decomposition. Therefore, in FT-IR spectra of the membrane surfaces taken before and after physical cleaning, while each treatment showed similar peak positions, the peak values of the membrane treated with microbubbles were the lowest. Through various analyses, it was confirmed that microbubbles can remove foulants on the gel layer in spite of their very low shear force. This means that microbubble cleaning has full potential for use as a physical cleaning method in the wastewater reclamation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water resources are becoming more and more deficient in many regions of the world owing to the increased demand from population growth and industrial development (Anonymous 2011; Pearce 2008; Petala et al. 2006). Wastewater reclamation and reuse can play an important role in the development of strategies for the utilization of water resources, as it is almost impossible to attain new sources of water (Abdel-Jawad et al. 1999). Although various wastewater treatment technologies like adsorption, filtration, and disinfection have been proposed to meet the regulations and guidelines associated with reclaimed water, microfiltration (MF) membranes are gaining popularity in the world (Jimenez et al. 2000). In addition, MF and ultrafiltration (UF) membranes are widely used in pre-treatment for reverse osmosis (RO) processes for the production of reclaimed water (Lazarova et al. 2008). However, effluents from the activated sludge process still contain dissolved matters and particulate substances that accelerate membrane fouling (Park et al. 2006). Membrane fouling causes a decrease in membrane performance and obstructs the application of membrane filtration techniques for wastewater reclamation.
Fouling is usually defined as reversible or irreversible based on the attachment strength of the foulants to the membranes. Reversible fouling is caused by loose attachment which can be removed by physical cleaning methods such as relaxation, aeration, and backwashing. On the other hand, irreversible fouling can only be removed through the application of chemicals due to their strength, forming a matrix-like gel layer (Huang et al. 2010; Meng et al. 2009). Smaller particles have been reported to adhere more persistently to the surface of membranes because they are influenced by lower shear forces than larger particles (Bourgeous et al. 2001). Therefore, while particle foulants are generally expected to be removable by physical cleaning, smaller particulates are apparently likely to be the matters contributing significantly to irreversible fouling (Psoch and Schiewer 2006). For this reason, it is not easy to control fouling through physical cleaning of the MF membranes, in which raw water is a secondary effluent.
Based on fouling removal mechanisms, membrane cleaning can be roughly categorized into two types: physical and chemical cleaning. In general, aeration and relaxation, which are the methods used widely for physical membrane cleaning, are adopted for the removal of reversible fouling. Relaxation allows diffusive back transport of membrane foulants, while aeration generates shear force on the membrane surface. These methods can effectively control fouling in most membrane water treatment processes. However, it is difficult to successfully reduce the fouling in MF membrane treatment for wastewater reuse because a considerable portion of the secondary effluents contain small particle or colloidal foulants, including EPS and SMP.
Because of the problems associated with cleaning, we attempted to utilize microbubbles, which have different properties compared with bubbles used in normal aeration, for physical cleaning. Microbubbles with a diameter of 10–50 um have been explored for applications in wastewater treatment for their improved gas–liquid mass transfer and contaminant removal (Agarwal et al. 2011). This is possible because microbubbles have large gas-liquid interfacial area, long residence time in the liquid phase, and a fast dissolution rate, including the generation of free radicals. Several scholars have published results of research on phenol removal by free radicals through the collapse of microbubbles, generation of hydroxyl radicals, improvement of the ozone mass transfer efficiency, and coagulation floatation process of dyeing wastewater (Chu et al. 2007, 2008; Li et al. 2009; Liu et al. 2010; Takahashi et al. 2007). Although microbubble technologies have been applied in various areas, no study has yet tried to use them for the cleaning of fouled membranes in MF membrane treatment system. It has been reported that microbubbles improved cleaning efficiency in RO processes (Fazel and Chesters 2014), but that was installed on a bypass loop of the cleaning in place (CIP) system and permeability growth was shown simply after microbubbles cleaning.
Therefore, the objective of this study was to evaluate the cleaning ability of microbubbles compared to relaxation and general aeration. To reasonably assess the cleaning efficiency, analysis of foulants in the membrane tank was carried out by examining turbidity, TOC, dissolved organic carbon (DOC) and FT-IR after physical cleaning. Several analysis instruments including scanning electron microscope (SEM), FT-IR, atomic force microscopy (AFM), and fluorescence excitation emission matrix (FEEM) were also used to investigate the fouled membranes.
Materials and methods
Membrane and lab-scale filtration system
The membranes used in this study were made of polytetrafluoroethylene (PTFE), which has high flux and durability compared with the widely used polyvinylidene fluoride (PVDF). The specifications of membranes used in this study are listed in Table 1. Each membrane was submerged in the tank of a filtration system which consisted of a 30 L membrane tank, a permeate pump, pressure gauges, and a microbubble generator as shown in Fig. 1. The whole lab-scale equipment was composed of three same membrane filtration systems except for a microbubble generator, and all experiments were carried out at the same time. The membrane systems were operated under a 9-min filtration and 1-min stop cycle without aeration because influent was more clean than wastewater and there weren’t activated sludge in the membrane tank. We chose operation conditions with 40 L/m2/h considering the performance of a flat sheet PTFE membrane and keep filtration in constant flux until TMP increased to about 40 kPa. Secondary effluents in the wastewater treatment system were used as raw water, for which the quality was characterized in Table 2. Evaluation of the cleaning efficiency was performed in each membrane tank after the membranes were fouled equally. The physical cleaning conditions were idle, and aeration with normal bubbles or microbubbles. All fouled membranes were formed through three membrane filtration systems, whenever foulants removal efficiency of physical cleaning was evaluated, and then each physical cleaning was applied simultaneously for the same amount of time. Aeration flow rates of the normal and microbubbles were 30 and 3 L/min, respectively. The cleaning efficiency was estimated by membrane resistance, derived from permeate pressure and flux.
Microbubble generator methods and conditions
The microbubble generator was composed of a pump, a mixing chamber, an air inhaler valve, and a nozzle. The microbubble formation process was as follows. First of all, air and water was pumped into the mixing chamber, and the air was dissolved in water at high pressure. Next, the water including air came out through the nozzle at normal pressure, at which time microbubbles were made (refer to Fig. S1 in the supplementary material). The pressure of the mixing chamber was 4 kgf/cm2, and the inhale air flow was 4 L/min. The size distribution of microbubble dispersions were characterized using a Mastersizer 2000 because a laser beam diffracts due to the presence of microbubbles in its path (Couto et al. 2009). The microbubbles size ranged from 10 to 200 um, and the average value was 44.8 um (refer to Fig. S2 in the supplementary material).
Physical cleaning efficiency assessment method
Cleaning efficiency was assessed by calculating the resistances that were gained from the pressure values and flux. The formula for calculation was as shown in Eq. (1).
where
- R t :
-
Total resistance (1/m)
- R T :
-
Total resistance after physical cleaning (1/m)
- R m :
-
Membrane inherent resistance (1/m)
Fouled membrane analytical methods
Foulants that were detached from membrane surface in the membrane tank were characterized in terms of turbidity, TOC, and FEEM. Turbidity of solution containing foulants, collected after each physical cleaning, was determined in nephelometric turbidity units (NTU) in a turbidity meter (2100N IS, Hach) calibrated in the range from 0.1 to 4000 NTU. TOC measurements were performed using TOC-V CSH (Shimadzu, Japan), and an average value of five measurements was used. The fluorescence intensity of membrane foulant extracts after physical cleaning was measured at excitation wavelengths between 230 and 500 nm, and emission wavelengths between 280 and 600 nm, with a LS-50B luminescence spectrophotometer (Perkin Elmer, USA). The excitation and emission slits were maintained at 10 nm. Fluorescence signals detected in specific areas are related to specific fractions of DOC, based on previous studies (Chen et al. 2003). AFM was employed to study the morphology of the membrane surface with foulants. The images were obtained over a scan areas of 10 μm × 10 μm, using AFM device (SPA 300HV, Japan). The external surface of each membrane sample was characterized in terms of mean roughness (Ra). The cross sectional images of the fouled membranes were observed by SEM (JSM7000F), after being fractured in liquid nitrogen and then coated with a thin layer of gold by a sputtering technique. Finally, IR spectra were obtained by the method ATR–FTIR spectroscopy using Nicolet 5700 (Nicolet, USA) to investigate foulants on the membrane. A spectrum, collected as the average of 32 scans with a resolution of 4 cm−1, was recorded from 4000 to 400 cm−1 in order to obtain the organic foulant information.
Results and discussion
Fouling removal efficiency according to physical cleaning methods and application time
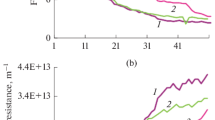
In the assessment of different physical cleaning methods for fouling control, as shown in Fig. 2, the cleaning efficiencies of aeration and microbubbles were higher than that of idle cleaning at every time point, as expected. However, although the shear force of the microbubbles was lower than that of aeration due to the slow liftoff speed of the microbubbles, the cleaning efficiency of microbubbles became higher than that of aeration after 60 min of cleaning time. Examination of the cleaning efficiency per unit time revealed that while physical cleaning was the most efficient for every condition during the course of 30 min, the cleaning efficiency of aeration was initially 2.1 percentage points higher than that of the microbubbles. On the other hand, the cleaning efficiency of aeration dropped to less than half that of the microbubbles in the other time sections. Aeration is usually used to reduce fouling by constantly scouring the membrane surface, and the tangential shear force can prevent large particles from attaching to the membrane surface. However, shear force has a limited effect on fouling control for the deposition of small particles (Wu et al. 2012). In addition, soluble and colloidal particles are likely to enter the membrane pores or be adsorbed onto the surface of the membrane. As shown in turbidity and SS of Table 2, there were few particulates present, and the soluble and colloid material was the main factor causing membrane fouling. In conclusion, the microbubbles could remove foulants on the membrane surface because of adhesion to the foulants.
Properties of removed foulants according to physical cleaning methods
As shown in Fig. 3, in the case of the concentration of organic foulants which were detached from the membrane surface, very low amounts were detected for idle cleaning, which hardly increased at all without reference to cleaning time. Meanwhile, the outflow foulants of the membrane in the microbubble cleaning system had a higher concentration than in aeration cleaning, except during the first 30 min. The gaps between aeration and microbubbles were featured especially prominently in the measurement of turbidity and TOC. Microbubbles were observed to be more efficient than aeration in the detachment of foulants, and the foulants were very relevant with particular or colloidal matters. Figure 4 shows the FEEM analysis. FEEM fluorescence spectra were obtained by collecting excitation (Ex) and emission (Em) spectra over a range from 200 to 600 nm. The FEEM of the idle treatment shows a peak related to the biological region (Ex/Em, 280/350 nm, 290/340 nm) and an aromatic protein region (230/310 nm) (Chen et al. 2003). The FEEM of the aeration treatment had a peak in every region. However, while the FEEM of the microbubble treatment had peak points in the soluble microbial by-product-like and humic acid-like regions, there was no peak observed in the aromatic protein region. This will be mentioned later (refer to “The influence of microbubbles on the protein”).
Physical cleaning evaluation by membrane surface analysis
Although SEM images of the surface of fouled membranes were not included herein, it is enough to identify foulants on the membrane surface. The foulants are likely not to form a cake layer but a gel layer, because the layer is formed by very small particulates and colloidal matters. A previous study showed that it is difficult to remove foulants from the membrane surface once a gel layer was formed (Wang et al. 2008). Therefore, it is impossible to confirm differences in the foulants on the membrane surface through membrane surface visualization. On the other hand, the thickness of each layer was 2.04, 1.68, and 2.00 um, as shown in Fig. 5. Particularly, it should be noted that while the cleaning efficiency of microbubbles was higher than that of aeration, the foulant layer of the membrane treatment with microbubbles was thicker than that of the membrane treated with aeration. This means that the microbubbles not only caused floating of the foulants on the membrane, but also decreased the density of the foulant layer.
The surface morphologies of the fouled membranes were characterized using AFM for evaluation of the fouling on the membrane surfaces. AFM 3D images of the membrane surfaces after each physical cleaning are shown in Fig. 6. The roughness parameters of the surfaces obtained from AFM measurements were 55.4, 45.7, 44.9, and 42.5 nm with no cleaning, idle treatment, aeration, and microbubbles, respectively. Based on the roughness values, microbubble cleaning resulted in the smoothest surface, which was also reflected visually in the 3D images. It was demonstrated that the microbubbles can grab foulants and cause them to float on the membrane surface, allowing better performance than that of aeration.
The FT-IR spectra of membrane foulants in the gel layer are illustrated in Fig. 7. On the whole, each spectrum had different values for similar peak positions. The spectra showed a broad region of adsorption around a peak at 3286 cm−1, which was attributed to stretching of the O-H bond in hydroxyl functional groups, and a sharper peak at 2924 cm−1, which was due to stretching of C-H bonds (Kumar et al. 2006; Wang et al. 2008). There were two peaks (1636 and 1541 cm−1) in the spectrum unique to the protein secondary structure, called amides I and II (Maruyama et al. 2001). In addition, a high peak at 1052 cm−1 exhibited the character of polysaccharides or polysaccharide-like substances. As a result, it was determined that proteins and polysaccharides, which are key substances of EPS, were the main foulants on the membranes. That is a probable result, considering that the raw water used was the supernatant from secondary sedimentation. Physical cleaning with microbubbles most effectively removed the foulants associated with EPS, according to peak intensity.
The influence of microbubbles on the protein
When the collapsing speed of microbubbles is higher than the speed of sound in water, the temperature inside the collapsing bubbles can increase drastically due to adiabatic compression (Adewuyi 2001). To confirm pyrolytic decomposition of protein, which takes place within the collapsing bubbles, membranes were contaminated with a representative protein, bovine serum albumin (BAS). The peaks at 3304, 1646, and 1542 cm−1, which were attributed to the O-H bond in the hydroxyl functional groups, amides I and II, were shown in the graph. However, all of them disappeared after the cleaning with microbubbles. In addition, protein concentration decreased from 72 to 14 mg/L when microbubbles were injected into a tank with protein for 180 min. This leads to the absence of foulants related to protein in the membrane tank after cleaning with microbubbles, which is one of the reasons why microbubbles could control membrane fouling.
Conclusions
In this study, the use of microbubbles, which are applied by floating, oxygen supply, and decomposition of contaminants, was attempted in order to remove foulants on the surface of membranes in the processing of membranes for wastewater reclamation. Microbubble cleaning could control membrane fouling better than cleaning by aeration after 30 min from the start time in spite of their low shear forces compared to the prevalent aeration. There are three possible reasons to explain this result. First, microbubbles can adhere to particulates or colloidal matters and cause them to float. Ultimately, the foulants are detached from the membrane surface, which could be verified herein through measurement of the turbidity and TOC of the solution in the membrane tank, and AFM images of the membrane surface after physical cleaning. Second, microbubbles can loosen the density of the gel layer. Although this could not be checked directly, the density decline of the gel layer can be recognized from the results, in which the gel layer of microbubbles was thicker than that of aeration. Lastly, because the temperature inside bubbles can become extremely high when the collapsing speed of the microbubbles is sufficient, the effect of pyrolytic decomposition of protein can be expected. Microbubbles cannot be applied to all membrane processes due to their generation process. However, they have sufficient potential for controlling membrane fouling in the wastewater reuse process.
References
Abdel-Jawad M, Ebrahim S, Al-Tabtabaei M, Al-Shammari S (1999) Advanced technologies for municipal wastewater purification: technical and economic assessment. Desalination 124:251–261
Adewuyi YG (2001) Sonochemistry: environmental science and engineering applications. Ind Eng Chem Res 40:4681–4715
Agarwal A, Ng WJ, Liu Y (2011) Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 84:1175–1180
Anonymous (2011) Pretreatment in reverse osmosis seawater desalination: a short review FAU - Valavala, Ramesh FAU - Sohn, Jinsik FAU - Han, Jihee FAU - Her, Namguk FAU - Yoon, Yeomin. Environ Eng Res 16:205–212
Bourgeous KN, Darby JL, Tchobanoglous G (2001) Ultrafiltration of wastewater: effects of particles, mode of operation, and backwash effectiveness. Water Res 35:77–90
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation—emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37:5701–5710
Chu LB, Xing XH, Yu AF, Zhou YN, Sun XL, Jurcik B (2007) Enhanced ozonation of simulated dyestuff wastewater by microbubbles. Chemosphere 68:1854–1860
Chu L-B, Xing X-H, Yu A-F, Sun X-L, Jurcik B (2008) Enhanced treatment of practical textile wastewater by microbubble ozonation. Process Saf Environ Prot 86:389–393
Couto HJB, Nunes DG, Neumann R, França SCA (2009) Micro-bubble size distribution measurements by laser diffraction technique. Miner Eng 22:330–335
Fazel M, Chesters S (2014) RO membrane cleaning using microbubbles at 6,800 m3/d wastewater RO plant in UAE. Desalination and Water Treatment, 1--9. doi:10.1080/19443994.2014.931527
Huang X, Xiao K, Shen Y (2010) Recent advances in membrane bioreactor technology for wastewater treatment in China. Front Environ Sci Eng China 4:245–271
Jimenez B, Chavez A, Leyva A, Tchobanoglous G (2000) Sand and synthetic medium filtration of advanced primary treatment effluent from Mexico City. Water Res 34:473–480
Kumar M, Adham SS, Pearce WR (2006) Investigation of seawater reverse osmosis fouling and its relationship to pretreatment type. Environ Sci Technol 40:2037–2044
Lazarova V, Gallego S, Garcia Molina V, Rouge P (2008) Problems of operation and main reasons for failure of membranes in tertiary treatment systems. Water Sci Technol 57:1777–1784
Li P, Takahashi M, Chiba K (2009) Enhanced free-radical generation by shrinking microbubbles using a copper catalyst. Chemosphere 77:1157–1160
Liu S, Wang Q, Ma H, Huang P, Li J, Kikuchi T (2010) Effect of micro-bubbles on coagulation flotation process of dyeing wastewater. Sep Purif Technol 71:337–346
Maruyama T, Katoh S, Nakajima M, Nabetani H, Abbott TP, Shono A, Satoh K (2001) FT-IR analysis of BSA fouled on ultrafiltration and microfiltration membranes. J Membr Sci 192:201–207
Meng F, Chae S-R, Drews A, Kraume M, Shin H-S, Yang F (2009) Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res 43:1489–1512
Park C, Kim H, Hong S, Choi S-I (2006) Variation and prediction of membrane fouling index under various feed water characteristics. J Membr Sci 284:248–254
Pearce GK (2008) UF/MF pre-treatment to RO in seawater and wastewater reuse applications: a comparison of energy costs. Desalination 222:66–73
Petala M, Tsiridis V, Samaras P, Zouboulis A, Sakellaropoulos GP (2006) Wastewater reclamation by advanced treatment of secondary effluents. Desalination 195:109–118
Psoch C, Schiewer S (2006) Resistance analysis for enhanced wastewater membrane filtration. J Membr Sci 280:284–297
Takahashi M, Chiba K, Li P (2007) Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J Phys Chem B 111:1343–1347
Wang Z, Wu Z, Yin X, Tian L (2008) Membrane fouling in a submerged membrane bioreactor (MBR) under sub-critical flux operation: membrane foulant and gel layer characterization. J Membr Sci 325:238–244
Wu J, He C, Zhang Y (2012) Modeling membrane fouling in a submerged membrane bioreactor by considering the role of solid, colloidal and soluble components. J Membr Sci 397–398:102–111
Acknowledgments
This research was supported by the R&D Program for Society of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (No.2014M3C8A4032345).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Lee, EJ., Kim, YH., Kim, HS. et al. Influence of microbubble in physical cleaning of MF membrane process for wastewater reuse. Environ Sci Pollut Res 22, 8451–8459 (2015). https://doi.org/10.1007/s11356-014-3928-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3928-y