Abstract
Heavy metals and arsenic are well-known carcinogens. However, few studies have examined whether soil heavy metals and arsenic concentrations associate with cancer in the general population. In this ecological study, we aimed to evaluate the association of heavy metals and arsenic in soil with cancer mortality rates during 2005–2010 in Suzhou, China, after controlling for education and smoking prevalence. In 2005, a total of 1683 soil samples with a sampling density of one sample every 4 km2 were analyzed. Generalized linear model with a quasi-Poisson regression was applied to evaluate the association between town-scale cancer mortality rates and soil heavy metal concentrations. Results showed that soil arsenic exposure had a significant relationship with colon, gastric, kidney, lung, and nasopharyngeal cancer mortality rates and soil nickel exposure was significantly associated with liver and lung cancer. The associations of soil arsenic and nickel exposure with colon, gastric, kidney, and liver cancer in male were higher than those in female. The observed associations of soil arsenic and nickel with cancer mortality rates were less sensitive to alternative exposure metrics. Our findings would contribute to the understanding of the carcinogenic effect of soil arsenic and nickel exposure in general population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposures to arsenic and heavy metals have been found to be associated with multiple cancer types, including bladder, colon, kidney, liver, lung, skin, and prostate cancer, in numerous epidemiological studies (Celik et al. 2008; Chen et al. 1992; Grimsrud et al. 2002; Silvera and Rohan 2007; Smith et al. 1992; Straif et al. 2009). However, most epidemiological evidence on arsenic and cancer risk in general population comes from drinking water arsenic (Straif et al. 2009). As the persistence of heavy metals in soils is a long process (Li et al. 2001), soil heavy metal and arsenic concentration serve as an indicator of long-term exposure to heavy metals and arsenic, which may pose an increased risk of cancer.
Arsenic in soil was almost entirely in the inorganic form (Hughes et al. 2011), which is classified by the International Agency for Research on Cancer as a human carcinogen (IARC 1980). As there is sufficient evidence showing that a biological threshold below which arsenic would not cause cancer does not exist (Smith et al. 2002), it seems that even low arsenic concentration in soil could possibly influence human cancers (Putila and Guo 2011). Recent studies have demonstrated that exposures of soil heavy metals and arsenic, even at low levels, were associated with incidence of oral and lung cancer in Taiwan (China) (Huang et al. 2013; Su et al. 2010), lung cancer in USA (Luo et al. 2011; Putila and Guo 2011), all cancers in Australia (Pearce et al. 2012), and gastric cancer in Northern Ireland (McKinley et al. 2013).
In addition, few studies have addressed the possible associations of heavy metals and arsenic with nasopharyngeal cancer. Nasopharyngeal cancer is a rare cancer in most parts of the world, whereas it has a high incidence rate in certain parts of Asia, particularly in China (Chang and Adami 2006; Yu and Yuan 2002). Previous studies found that nickel in drinking water had a significant positive correlation with nasopharyngeal cancer mortality rate in China (Xia et al. 1988), and arsenic and cadmium levels in the blood were associated with the risk of nasopharyngeal cancer in Tunisian population (Khlifi et al. 2014). Thus, the association of heavy metal and arsenic exposures with nasopharyngeal cancer mortality rates remains unclear.
With the rapid industrialization and urbanization during the past 20 years, soil heavy metal and arsenic pollution has become a serious issue in China (Wei and Yang 2010). A recent published national soil survey, which covered nearly 6.3 million km2, shows that 2.7, 7.0, 1.1, and 4.8 % of all soil samples exceeded the Chinese soil quality standard for arsenic, cadmium, chromium, and nickel, respectively (MEP and MLR 2014). Meanwhile, China is facing a high burden of cancer, with an age-adjusted mortality rate by world population of 115.65 per 100,000 person in 72 cancer registries (including 31 cities and 41 counties from 24 provinces and covering 6.4 % of the whole national population) in 2009 (Chen et al. 2013). To reduce the burden of cancer, it is crucial to identify related risk factors. However, the potential relationships of soil heavy metals and cancer have not been adequately studied in China.
In this study, we attempted to evaluate the association of soil heavy metals and arsenic exposures with site-specific cancer mortality rates during 2005–2010 in Suzhou, China.
Materials and methods
Suzhou, an important economic centre of Jiangsu Province, is located in the Yangtze River delta in eastern China and covers an area of 8488 km2. Our study area included 83 towns of Suzhou, covering both urban and rural areas (Fig. 1).
Soil sampling and heavy metal analysis
In 2005, a total of 1683 top-soil (0–20 cm) samples in Suzhou were collected with a sampling density of one sample per 4 km2 (Fig. 1). In each sample, four subsamples with a sampling density of one subsample per km2 were combined together for analysis. The <20 mesh (<0.84 mm) fraction of all samples were analyzed for four carcinogenic metals, including arsenic (As), cadmium (Cd), chromium (Cr), and nickel (Ni). The analytical technique used to measure the levels of metals was atomic fluorescence spectroscopy for As, X-ray fluorescence spectroscopy for Cr and Ni, and flameless atomic absorption spectroscopy for Cd. The mean concentrations of heavy metals and arsenic for each town were calculated by taking the average of all samples within each town. All measurements were expressed in terms of parts per million (ppm).
Cancer mortality rate data
We obtained records of all residents’ deaths in Suzhou from the Suzhou Municipal Centre for Disease Control and Prevention for a 6-year period from January 1, 2005 to December 31, 2010. Mortality counts were classified into the following categories using the International Classification of Diseases, Revision 10 (ICD-10): bladder cancer (code C67), colon cancer (C18-C20), gastric cancer (C16), kidney cancer (C64-C65), liver cancer (C22), lung cancer (C33-C34), nasopharyngeal cancer (C11), and prostate cancer (C61). These cancers were selected based on previous studies (Chen et al. 1992; Smith et al. 1992; Sridhara Chary et al. 2008). Cancer mortality rates for each town were calculated as a 6-year average from 2005 to 2010, since sparse deaths in some towns might result in unstable single-year estimates. Cancer mortality rates were then directly age-adjusted to the 2000 Chinese population using the following formula:
Where AARC i is the age-adjusted cancer mortality rate in town i; C i,age is the cancer mortality count in town i for each age bracket; Pop i,age is the population of town i for each age bracket in 2000; and PerPopage is the percentage of Chinese population for each age bracket in 2000.
Smoking prevalence and education level data
Smoking data was collected from a chronic disease survey in 2010 in Suzhou, conducted by the Suzhou Municipal Centre for Disease Control and Prevention. A multistage cluster random sampling design was implemented and 30,578 local persons over 20 years old were sampled in this survey. Since smoking prevalence in China is strongly affected by age and gender (Li et al. 2011), total smoking prevalence (including current smoking and ever smoking) by age and gender in Suzhou was then applied to calculate the town smoking prevalence using the following formula:
Where SP i is the total smoking prevalence in town i; SPmale,agej is the total smoking prevalence of male age bracket j; SPfemale,agej is the total smoking prevalence of female age bracket j; Male i,agej is the percentage of age bracket j in men for town i; Female i,agej is the percentage of age bracket j in women for town i; PerMale i is the percentage of men in town i in 2000; and PerFemale i is the percentage of women in town i in 2000.
As educational level has been demonstrated that to be significantly associated with cancer mortality (Albano et al. 2007), education level in each town was used as a confounder in this analysis. The average years of education was obtained from the 2000 population census of China.
Statistical analysis
Correlation test was first conducted to explore the possible relationships of heavy metals and arsenic with all eight types of cancer mortality rates. Heavy metals and cancer types that have significant correlations were then included in the regression analysis.
Because town-scale cancer mortality rates were low, overdispersed Poisson distribution was used to reflect the annual cancer mortality rate as a counting measure (Putila and Guo 2011). Thus, we used a generalized linear model (glm) with a quasi-Poisson regression to evaluate the association between cancer mortality rates and heavy metals. In the glm model, the natural logarithm of the expected age-adjusted cancer mortality rate was modelled as a linear function of soil metal exposures and the coefficient was estimated by the maximum likelihood method. The model coefficient was also the log relative risk associated with the exposure factor. Thus, the relative risk associated with a unit increase in the exposure variable was calculated as the natural exponential function of the model coefficient. Smoking prevalence and education level were controlled in the glm model as confounders. The glm model used the following formula:
where λ i is the expected age-adjusted annual cancer mortality rate in town i (i.e., E(AAR i )) with Var(AAR i ) = φλ i , φ is the overdispersion parameter; β j are the coefficients for possible risk factors; Metal j,i is the soil concentration for heavy metal j in town i; Smoking i is the smoking prevalence in town i; Education i is the average years of education in town i; and e error is the error term.
If spatial autocorrelation in the regression residuals was statistically significant, some important spatially structured explanatory variables might be missing in the multiple regression and spatial regression should be applied (Griffith 1987; Kissling and Carl 2008). Moran’s I statistic was used to test possible spatial autocorrelations of the regression residuals in the glm model.
In addition to defining the soil concentration of heavy metals as the average concentration of all sampling sites within in each town (the average method), the mean value of Kriging interpolated concentrations within each town (the Kriging method) was used to evaluate sensitivity to soil heavy metals concentrations.
All analyses were conducted in ArcGIS (version 10.0; ESRI, Redlands, CA) and R software (version 2.15.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Over the 6-year study period, a total of 46,675 deaths for eight study cancers (644 bladder; 4367 colon; 14,022 gastric; 351 kidney; 10,076 liver; 15,691 lung; 795 nasopharyngeal; and 729 prostate) were identified, which accounted for 65.3 % of total cancer deaths in Suzhou. The average annual age-adjusted cancer mortality rate (per 100,000 person) (AAR) from 2006 to 2010 was 1.19 for bladder cancer, 8.76 for colon cancer, 32.33 for gastric cancer, 0.61 for kidney cancer, 23.24 for liver cancer, 33.41 for lung cancer, 1.72 for nasopharyngeal cancer, and 1.44 for prostate cancer (Table 1). The AARs of gastric, liver, and nasopharyngeal cancer were more than twice the corresponding worldwide AARs in 2008 (10.3, 10.0, and 0.8, respectively) (Ferlay et al. 2010). The AARs of lung cancer in Suzhou was 1.7 times larger than the world average one (19.4). Bladder and prostate cancer had smaller AARs compared with the worldwide AARs (2.0 and 7.5) and had no significant correlation with soil carcinogenic heavy metals (Table 2).
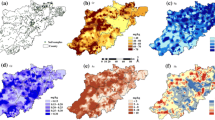
In 2005, the mean soil concentrations were 9.08 ppm for arsenic, 0.23 ppm for cadmium, 84.52 ppm for chromium, and 36.02 ppm for nickel (Table 1), which were below the grade II values (20, 0.30, 150, and 40 ppm, respectively) of Chinese Environmental Quality Standard for Soils (SEPA 1995). Spearman rank correlation test shows that only arsenic and nickel were significantly correlated with the cancer mortality rates in Suzhou (Table 2). Thus, these two metals were included in the glm models. Figure 2 shows the distribution of arsenic and nickel concentrations in top-soils in 2005 in Suzhou. Both arsenic and nickel concentrations were higher in central areas of Suzhou, where elevated mortality rates of colon, gastric, kidney, lung, and nasopharyngeal cancer were observed (Fig. 3).
After adjusting for smoking prevalence and education, arsenic concentrations in top-soils were significantly associated with the age-adjusted mortality rates of colon, gastric, kidney, lung, and nasopharyngeal cancer, while nickel concentrations were significantly associated with liver and nasopharyngeal cancer (Table 3). In the joint analysis of arsenic and nickel exposures, an increase of 1 ppm of arsenic concentration in top-soils was associated with 8.3 % increase in colon cancer mortality rate [relative risk (RR) 1.083, 95 % confidence interval (95 % CI) 1.027–1.142], 11.1 % increase in gastric cancer mortality rate (RR 1.111, 95 % CI 1.061–1.165), 12.9 % increase in kidney cancer mortality rate (RR 1.129, 95 % CI 1.039–1.228), 5.0 % increase in lung cancer mortality rate (RR 1.050, 95 % CI 1.001–1.102), and 8.6 % increase in nasopharyngeal cancer rate (RR 1.086, 95 % CI: 1.028–1.148). Given an increase of 1 ppm in nickel concentration in top-soils, the liver cancer mortality rate increased by 2.2 % (RR 1.022, 95 % CI 1.004–1.040) and the nasopharyngeal cancer mortality rate increased by 4.0 % (RR 1.040, 95 % CI 1.014–1.067). Generally, the effects of arsenic and nickel on cancer mortality rates were higher in men than in women. No significant association of female kidney and liver cancer mortality rate with arsenic or nickel was observed in the glm models.
Spatial autocorrelation analysis of deviance residuals revealed random patterns with p values of global Moran’s I statistics greater than 0.05 for all above glm regressions (see the Supplementary Material, Table S2). This implies that no key factors that influenced the spatial distribution of cancer mortality rates were missed in the glm regression and there was no need for spatial regression in this study.
The sensitivity analysis showed that the associations of soil arsenic and nickel exposures with cancer mortality rates remained similar when using the Kriging method to define the soil concentrations (Fig. 4).
Relative risks (95 % CI) for age-adjusted mortality rates of colon, gastric, kidney, liver, lung, and nasopharyngeal cancer associated with 1 ppm increase in soil arsenic (a) and nickel (b) concentrations using different methods for soil heavy metal exposure. Average stands for using the average concentration of sampling sites within each town; Kriging stands for using the mean value of Kriging interpolated concentrations within each town; NP stands for nasopharyngeal cancer
Discussion
This ecological study found that low-level soil arsenic exposure had a significant relationship with colon, gastric, kidney, lung, and nasopharyngeal cancer mortality rates in Suzhou, China, after controlling for age structure, education, and smoking prevalence. Significant associations of soil nickel exposure with liver and lung cancer were also observed. The associations of soil arsenic and nickel exposure with colon, gastric, kidney, and liver cancer in male were higher than those in female, whereas no associations between soil heavy metals and female kidney or liver cancer mortality rates were found.
The current epidemiological evidence on arsenic and cancer risk in general population mainly comes from the association of human cancer with arsenic in drinking water (Straif et al. 2009). However, recent studies revealed that arsenic exposure in other media could also pose cancer risks (Meharg et al. 2009; Zhang et al. 2009). We found that soil arsenic concentrations, even at low levels, were significantly associated with colon, gastric, kidney, lung, and nasopharyngeal cancer mortality rates. To our knowledge, this is one of the few ecological studies to report the effect of soil arsenic and nickel exposure on human cancer. The observed association between soil arsenic exposure and lung cancer is consistent with a similar study in the USA, which found that an increase of 1 ppm of soil arsenic concentration was associated with a 0.4 % increase in the lung cancer incidence rate (Putila and Guo 2011). Consistently, Pearce et al. also found significant excess risks for male colon (RR 1.180, 95 % CI 1.010–1.380) and female colon (RR 1.210, 95 % CI 1.020–1.440) cancer in disadvantaged areas in the highest quantile of soil arsenic exposure relative to the lowest in Victoria, Australia (Pearce et al. 2012). For gastric cancer, our finding of its association with soil arsenic confirms that observed by a spatial analysis study in the in Northern Ireland (McKinley et al. 2013).
A few epidemiological studies had reported the relationship of heavy metals and arsenic with nasopharyngeal cancer (Chang and Adami 2006). The positive association of nickel and arsenic concentration with nasopharyngeal cancer we reported was generally in accordance with previous studies. Nickel levels in drinking water, rice, and hairs of residents in counties with high nasopharyngeal cancer incidence were found two to three times higher than those in counties with low nasopharyngeal cancer incidence in southern China (Li et al. 1983). In a case-control study, nasopharyngeal cancer incidence was significantly associated with blood levels of arsenic (odds ratio 1.16, 95 % CI 1.06–1.28) in the Tunisian population (Khlifi et al. 2014). Another analysis showed that arsenic levels in tumour tissues from 34 nasopharyngeal cancer patients (48.5 μg/g) were significantly higher than those of healthy tissues (9.1 μg/g) (Khlifi et al. 2013). A study in Hong Kong also found significantly higher arsenic concentrations in the hair samples of nasopharyngeal cancer patients (0.195 ppm) than those of healthy people (0.133 ppm) (Man et al. 1996).
For kidney and liver cancer, arsenic and nickel were significantly associated with male cancer mortality rates, but not associated with female cancer mortality rates (Table 3). For colon and gastric cancer, the associations with soil arsenic were larger in men than those in women. These gender differences might be due in part to biological differences, such as the biotransformation of arsenic by methylation (Vahter et al. 2007), or gender-specific lifestyle factors (Pearce et al. 2012).
Results from the current study do not support an increased risk of bladder or prostate cancer mortality rate with increased heavy metal concentrations. This may because cancer mortality is an insensitive outcome to study these tumours since they usually have relatively good prognosis (García-Esquinas et al. 2013). Furthermore, bladder and prostate cancer have relatively low mortality rates compared with other cancers (Table 1); thus, the relatively rare cancers of bladder and prostate may be overshadowed by other cancers (Benbrahim-Tallaa and Waalkes 2008).
Education was negatively associated with age-adjusted colon, gastric, liver, lung, and nasopharyngeal cancer mortality rates (see the Supplementary Material, Table S3). The associations between education and gastric and lung cancer mortality rates were statistically significant, implying that people with a low socioeconomic level had higher gastric and nasopharyngeal risks than those with a high socioeconomic level. This was consistent with previous studies. Low socioeconomic status was found to be positively associated with nasopharyngeal cancer in China (Zheng et al. 1994), Thailand (Sriamporn et al. 1992), and Tunisia (Jeannel et al. 1990). This may be because people with low socioeconomic levels had a large consumption of carcinogenic preserved foods, since preserved foods were very cheap (Yu and Yuan 2002). Poor education was also found to be closely linked to the development of gastric cancer through a high Helicobacter pylori infection prevalence amongst people with low education level (Crew and Neugut 2006).
As an ecologic design, the main limitations of this study are the inherent limitations of ecological studies, such as possible misclassification of exposure, the inability to address exposure duration, and the inability to adjust for individual-level confounding factors. However, ecological studies are very important to generate hypotheses and provide necessary evidences for associations between environmental exposures and disease (Webb and Bain 2010). Although we were unable to control individual-level risk factors, the significant associations we found revealed previously unseen large carcinogenic effects of low-level soil arsenic and nickel exposure on a general population in China. Given the serious soil heavy metal contamination and the high burden of cancer mortality in China, this ecological study presents a direct evidence that cancer mortality rates in general population may be partially attributable to soil arsenic and nickel exposures. Further individual-level studies with accurate exposure measurement and detailed confounder information are needed to confirm our findings.
Conclusions
In conclusion, this ecological study found that town-scale arsenic concentrations in soils were associated with colon, gastric, kidney, lung, and nasopharyngeal cancer mortality rates in Suzhou, China. Significant associations of soil nickel exposure with liver and nasopharyngeal cancer mortality rates were also found in this study. This indicates that low-level exposure to arsenic and nickel in soil is responsible for excess cancer mortality risks in Suzhou. Our findings will contribute to the understanding of the relationship between arsenic/nickel and cancer risk in general population. More strict measures of soil heavy metal pollution control should be taken to reduce the high burden of cancer mortality rates in China.
Abbreviations
- As:
-
Arsenic
- Ni:
-
Nickel
- glm:
-
Generalized linear model
- RR:
-
Relative risks
- CI:
-
Confidence interval
- ppm:
-
Parts per million
References
Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, Henley J, Liff J, Thun MJ (2007) Cancer mortality in the United States by education level and race. J Natl Cancer Inst 99:1384–1394
Benbrahim-Tallaa L, Waalkes MP (2008) Inorganic arsenic and human prostate cancer. Environ Health Perspect 116:158
Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, Shiels M, Hammond E, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ (2008) Arsenic in drinking water and lung cancer: a systematic review. Environ Res 108:48–55
Chang ET, Adami H-O (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15:1765–1777
Chen C, Chen C, Wu M, Kuo T (1992) Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 66:888
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J (2013) Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res 25:10
Crew KD, Neugut AI (2006) Epidemiology of gastric cancer. World J Gastroenterol 12:354
Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, Howard B, Farley J, Best LG, Navas-Acien A (2013) Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiol Biomarkers Prev 22:1944–1953
Griffith DA (1987) Spatial autocorrelation. Association of American Geographers, Washington
Grimsrud TK, Berge SR, Haldorsen T, Andersen A (2002) Exposure to different forms of nickel and risk of lung cancer. Am J Epidemiol 156:1123–1132
Huang HH, Huang JY, Lung CC, Wu CL, Ho CC, Sun YH, Ko PC, Su SY, Chen SC, Liaw YP (2013) Cell-type specificity of lung cancer associated with low-dose soil heavy metal contamination in Taiwan: an ecological study. BMC Public Health 13:330
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332
IARC (1980) Some metals and metallic compounds. IARC Monogr Eval Carcinog Risk Chem Hum 23:1–415
Jeannel D, Hubert A, De Vathaire F, Ellouz R, Camoun M, Ben Salem M, Sancho-Garnier H, De-Thé G (1990) Diet, living conditions and nasopharyngeal carcinoma in Tunisia—a case-control study. Int J Cancer 46:421–425
Khlifi R, Olmedo P, Gil F, Hammami B, Chakroun A, Rebai A, Hamza-Chaffai A (2013) Arsenic, cadmium, chromium and nickel in cancerous and healthy tissues from patients with head and neck cancer. Sci Total Environ 452:58–67
Khlifi R, Olmedo P, Gil F, Feki-Tounsi M, Hammami B, Rebai A, Hamza-Chaffai A (2014) Risk of laryngeal and nasopharyngeal cancer associated with arsenic and cadmium in the Tunisian population. Environ Sci Pollut Res 21:2032–2042
Kissling WD, Carl G (2008) Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob Ecol Biogeogr 17:59–71. doi:10.1111/j.1466-8238.2007.00334.x
Li Z, Pan Q, Chen J (1983) Epidemiology of nasopharyngeal carcinoma. In: Li Z, Pan Q, Chen J (eds) Nasopharyngeal carcinoma: clinical and laboratory research. Guangdong Sciences and Technology Press, Guangzhou, pp 1–68
Li X, C-s P, Liu PS (2001) Heavy metal contamination of urban soils and street dusts in Hong Kong. Appl Geochem 16:1361–1368
Li Q, Hsia J, Yang G (2011) Prevalence of smoking in China in 2010. N Engl J Med 364:2469–2470
Luo J, Hendryx M, Ducatman A (2011) Association between six environmental chemicals and lung cancer incidence in the United States. J Environ Public Health 2011:9
Man CK, Zheng YH, Mak PK (1996) Trace element profiles in the hair of nasopharyngeal carcinoma (NPC) patients. J Radioanal Nucl Chem 212:151–160
McKinley JM, Ofterdinger U, Young M, Barsby A, Gavin A (2013) Investigating local relationships between trace elements in soils and cancer data. Spat Stat 5:25–41
Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu Y-G, Feldmann J, Raab A, Zhao F-J, Islam R, Hossain S, Yanai J (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43:1612–1617
MEP MLR (2014) National soil pollution survey bulletin. Ministry of Environmental Protection and Ministry of Land and Resources, Beijing
Pearce DC, Dowling K, Sim MR (2012) Cancer incidence and soil arsenic exposure in a historical gold mining area in Victoria, Australia: a geospatial analysis. J Expo Sci Environ Epidemiol 22:248–257
Putila JJ, Guo NL (2011) Association of arsenic exposure with lung cancer incidence rates in the United States. PLoS ONE 6:e25886
SEPA (1995) Environmental quality standards for soils (GB15618-1995). State Environmental Protection Administration of China
Silvera SAN, Rohan TE (2007) Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Cause Control 18:7–27
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259
Smith AH, Lopipero PA, Bates MN, Steinmaus CM (2002) Arsenic epidemiology and drinking water standards. Science 296:2145–2146
Sriamporn S, Vatanasapt V, Pisani P, Yongchaiyudha S, Rungpitarangsri V (1992) Environmental risk factors for nasopharyngeal carcinoma: a case-control study in northeastern Thailand. Cancer Epidemiol Biomarkers Prev 1:345–348
Sridhara Chary N, Kamala CT, Samuel Suman Raj D (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 69:513–524
Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Ba S, El Ghissassi F, Vr B, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10:453–454
Su C-C, Lin Y-Y, Chang T-K, Chiang C-T, Chung J-A, Hsu Y-Y, Lian I-B (2010) Incidence of oral cancer in relation to nickel and arsenic concentrations in farm soils of patients’ residential areas in Taiwan. BMC Public Health 10:67
Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M (2007) Gender differences in the disposition and toxicity of metals. Environ Res 104:85–95
Webb P, Bain C (2010) Essential epidemiology: an introduction for students and health professionals. Cambridge University Press, New York
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Xia L, Liang S, Jiang J-W, Zhou X-J, Li J (1988) Trace element content in drinking water of nasopharyngeal carcinoma patients. Cancer Lett 41:91–97
Yu MC, Yuan J-M (2002) Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 12:421–429
Zhang H, G-h H, G-m Z (2009) Health risks from arsenic-contaminated soil in Flin Flon-Creighton, Canada: integrating geostatistical simulation and dose-response model. Environ Pollut 157:2413–2420
Zheng Y, Tuppin P, Hubert A, Jeannel D, Pan Y, Zeng Y (1994) Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu County, Guangxi. China Br J Cancer 69:508
Acknowledgments
This research was funded by the Jiangsu Science and Technology Supporting Project (BE2013720) and National Natural Science Foundation of China (41271014 and 41171411).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Kai Chen and Qi Lin Liao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, K., Liao, Q.L., Ma, Z.W. et al. Association of soil arsenic and nickel exposure with cancer mortality rates, a town-scale ecological study in Suzhou, China. Environ Sci Pollut Res 22, 5395–5404 (2015). https://doi.org/10.1007/s11356-014-3790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3790-y