Abstract
The study was conducted to assess the effects of in utero di-butyl phthalate (DBP) and butyl benzyl phthalate (BBP) exposure during late gestation on offspring’s development and reproductive system of male rats. Pregnant rats were treated orally with DBP (2, 10, 50 mg/kg), BBP (4, 20, 100 mg/kg), and diethylstilbestrol (DES) 6 μg/kg (positive control) from GD14 to parturition. A significant reduction in dams’ body weight on GD21 in DBP-, BBP-, and DES-treated groups was observed. The gestation length was considerably elevated in the treated groups. Decline in male pups’ body weight was significant at PND75 in DBP- (50 mg/kg), BBP- (20,100 mg/kg), and DES-treated groups. The weight of most of the reproductive organs and sperm quality parameters was impaired significantly in DBP- (50 mg/kg) and BBP- (100 mg/kg) treated groups. Further, a non-significant decline in testicular spermatid count and daily sperm production was also monitored in treated groups. A significant reduction in serum testosterone level in BBP (100 mg/kg), whereas the testicular activity of 17β-HSD was declined non-significantly in the treated groups with respect to control. The data suggests that DBP and BBP exposure during late gestation period might have adverse effects on offspring’s development, spermatogenesis, and steroidogenesis in adult rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters are a class of widely distributed industrial chemicals, used as plasticizers to soften the polyvinyl chloride-based products. Phthalates have become ubiquitous environmental contaminants because large-scale production, widespread use, and their high concentration in cosmetics and personal care products, toys, pharmaceuticals, medical devices, food packaging, vinyl flooring, and building materials (Hernandez-Diaz et al. 2009). Phthalates are generally lipophilic, which influences their leaching and environmental partitioning characteristics. They cannot covalently bind to the polymers matrix. Therefore, migration from the products into the environment is likely to occur (Schettler, 2006). The potential route of human exposure to phthalates is via ingestion, inhalation, and dermal contact. Phthalates and their metabolites have been detected in different human body fluids such as maternal urine (Koch et al. 2006), milk (Lottrup et al. 2006), and amniotic fluid (Silva et al. 2004). Phthalates can be transferred from maternal blood into the developing fetus via placenta (Mose et al. 2007) and into neonates via breast milk (Main et al. 2006). Thus, the possibility of these compounds entering into the biological system has generated concern about their reproductive and developmental toxic potential in humans. Reports are available on in vitro estrogenic potential of di-butyl phthalate (DBP) and butyl benzyl phthalate (BBP) in estrogen-responsive human breast cancer cells (Jobling et al. 1995; Harris et al. 1997) and in recombinant yeast screen assay (Coldham et al. 1997). Thus, exposure to these chemicals during this critical period of development might affect offspring development and function. The declining trends in semen quality reported from different parts of the world, especially from industrialized countries, suggest a possible role of some of the industrial chemicals. The declining trend in sperm quality in men during the last few decades could be the result of several environmental factors (Miguel et al. 2006).
There are reports of the adverse effects of DBP such as pathological changes in testes and male reproductive accessory glands, hypospadias, cryptorchidism, retention of nipples, reduced anogenital distance, and reduced sperm production in animals (Duty et al. 2004; Lyche et al. 2009). Zhang et al. (2004) reported that in utero DBP exposure might cause a significant reduction in the litter size, pups body weight at birth, anogenital distance (AGD) in male pups, organ weight, and sperm quality. Further, BBP was also shown to be developmentally toxic in mice and rats (Nagao et al. 2000). BBP in utero exposure showed a decrease in size of testis and a reduction in daily sperm production in adult rats (Sharpe et al. 1995). Owing to these, this study was conducted to investigate the effects of DBP and BBP exposure during late gestation period on offspring’s development and male reproductive system of rats.
Material and methods
Animals
Albino rats were obtained from the institute’s animal house facility. They were maintained on standard rat diet (Amrit Feed, Pune, India) and tap water ad libitum. Virgin female rats, about 10 weeks old, were mated overnight with male of the same age. The day when sperm were detected in the vaginal smear was considered gestation day zero (GD0). Successfully mated females were divided into nine groups randomly with a minimum of six rats in each group.
Chemicals and treatment
The pregnant rats were treated orally with 20, 100, and 500 times of reference dose (RfD)* of DBP (i.e., 2, 10, and 50 mg/kg) and BBP (i.e., 4, 20, and 100 mg/kg), procured from M/s. Greyhound chromatography and allied chemicals, UK in corn oil by gavage from GD14 up to parturition. Positive control (diethylstilbestrol (DES) 6 μg/kg obtained from M/s. Sigma chemicals, USA); vehicle control (corn oil M/s. MP Biomedical, France), and control group were also maintained in similar fashion. The volume of each dose was adjusted to 1 ml/kg based on animal body weight.
Observations in dams and F1 offsprings
Pregnant rats were examined daily for obvious signs of toxicity, weight gain, and gestation length. The litter size, live and dead pups were recorded from each dam at postnatal day (PND1) (live birth index), PND4 (viability index), and PND21 (weaning index) and sex ratio (PND1) from each dam were recorded. Pups were examined for gross external abnormalities. The physical developmental landmarks, i.e., eye opening, fur formation, pinna detachment, and testis descend were also recorded. Anogenital distance (the distance between the anus and the genital tubercle) was measured for each offspring in all the groups at PND5 and PND25.
Pups body weight was recorded on PND1, PND21, and thereafter, biweekly until necropsy (PND75). Representative number of male animals from each dam was sacrificed on PND75. Reproductive organs (viz. testes, epididymis, prostate, vas deference, and seminal vesicle) and vital organs (viz. liver, kidney, and adrenal gland) were dissected out, cleaned, and weighed using digital balance.
Sperm quality parameters
The sperm quality parameters, i.e., sperm motility (Linder et al. 1986), sperm count (Wyrobek et al. 1983; Kumar et al. 1999), testicular spermatid count (Thayer et al. 2001), daily sperm production (Robb et al. 1978), and sperm head shape abnormality (Feuston et al. 1989) were evaluated.
(RfD*, Reference dose - An estimate, with uncertainty spanning perhaps an order of magnitude, of a daily oral exposure to the human population, including sensitive subgroups that are likely to be without an appreciable risk of deleterious effects during a lifetime) (www.epa.gov/tln/atw/hltef/hapglossaryrev.html).
17 β-Hydroxy steroidehydrogenase (HSD)
For the estimation of 17β-HSD, the testis was homogenized (1:10) in chilled Tris–KCl buffer, (pH 7.4), containing 0.05 % Triton X- 100 and the homogenate was centrifuged at 8,000 RPM for 30 min at 4 °C by ultracentrifuge (Sigma, Germany). The supernatant was used for HSD estimation (Jana et al. 2006).
Serum testosterone
The serum testosterone concentration was measured using commercial available ELISA kits according to manufacturer’s instructions (Testosterone Cat. N0. 582701, M/S Cayman Chemical Company, 1180 E. Ellsworth Road, USA).
Statistical analysis
Study variables of experimental animals were compared for significant differences from control. Data are expressed as mean ± SE. Statistical analysis was performed using Graph pad prism software (V. 5.01). In order to evaluate the significance level between control and exposed groups, unpaired t test was used.
Results
Effects of DBP and BBP exposure on dams’ body weight gain and gestation length
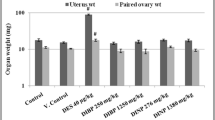
There was no mortality recorded in any of the groups. Dams’ body weight gain increased steadily from their initial body weight in all the treated and non-treated groups. However, the weight gain (percentage) was significantly lower in DBP-, BBP-, and DES-treated groups on GD21 as compared to control. The gestation length was considerably elevated in all the treated groups with respect to control (Fig. 1).
Effects of DBP and BBP exposure on rat offsprings development
No significant difference was observed in the litter size, live pups, fetal mortality rate, and sex ratio (M/F) between treated and non-treated groups. The body weight of male pups at birth and PND21 (weanling) decreased significantly in all the DBP-, BBP-, and DES-treated groups, but was non-significant in 2 mg/kg DBP-treated group at birth and their respective control (Table 1). Anogenital distance in male pups was slightly lower in both DBP- and BBP-treated groups and it was significant at PND25 in DES-treated group. The data on developmental indices, i.e., live birth, viability, and weanling indices showed slight alteration between treated and non-treated groups (Table 1). The data on the age of physical developmental landmarks (i.e., pinna detachment, fur formation, eye opening, testis descend) indicated that these landmarks were slightly delayed in higher doses of DBP, BBP, and also in DES-treated groups as compared to control (Table 2).
Body and organ weight changes in F1 adult male rats
The male rats were sacrificed on PND75; data on body and organ weight are shown in Table 3. A significant decline in body weight was observed in DBP- (50 mg/kg) BBP- (20 and 100 mg/kg), and in DES-treated groups; whereas in DBP- (2 and 10 mg/kg) and BBP- (4 mg/kg) treated groups, the alterations were statistically non-significant with respect to the control (Table 3).
The data on organ weight showed a declining trend in all the treated groups. The weight of epididymis and prostate significantly decreased in DBP- (50 mg/kg), BBP- (100 mg/kg), and in DES-treated group. The testis, adrenal gland, and seminal vesicle weight was decreased in DBP- (50 mg/kg) and in DES-treated groups. However, the kidney weight was significantly decreased in DBP- (2 and 50 mg/kg) and BBP- (100 mg/kg) treated groups. The liver weight also significantly declined in DBP- (50 mg/kg) treated group with respect to the control (Table 3).
Epididymal sperm count, motility, testicular spermatid count, and daily sperm production in F1 adult rats
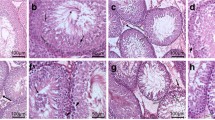
The cauda epididymal sperm count and sperm motility (percentage) were significantly reduced in DBP-(50 mg/kg), BBP- (100 mg/kg), and in DES-treated groups. Further, the testicular spermatid count and daily sperm production (DSP) also showed a declining trend (statistically non-significant) in the treated groups as compared to the control (Table 4). Different types of sperm head shape abnormalities (i.e., amorphous, hook absent, bent neck, straight head, small hook, banana shape, pin head/two tailed) were observed in treated and non-treated animals using phase contrast microscope (Zeiss CAXIO-Scope A1, 10X). These abnormalities were higher in treated groups with respect to control. The percentage of sperm abnormalities elevated significantly in DBP- (50 mg/kg), BBP- (100 mg/kg) and DES-treated groups as compared to control (Table 4).
Effects of DBP and BBP exposure on testicular 17β-Hydroxysteroid dehydrogenase activity
DBP and BBP exposure showed a declining trend in the testicular activity of steroidogenic enzyme (i.e., 17β-HSD) in all the DBP-, BBP-, and DES-treated groups. But, the alteration was more pronounced (statistically non-significant) in BBP-treated groups as compared to DBP-treated groups (Fig. 2).
Effects of DBP and BBP exposure on serum testosterone levels in F1 adult rats
The data on serum testosterone revealed that the testosterone levels were decreased in all the DBP-, BBP-, and DES-treated groups, but it was significantly declined in BBP higher dose (100 mg/kg) treated group with respect to the control (Fig. 3). However, at a dose of 2 mg/kg did not reveal any significant changes.
Discussion
The study indicated that DBP and BBP exposure during late gestation interferes in the development of offsprings as evidenced by a reduction in the body weight of F1 male rats, and might have also affected the male reproductive system of adult rats as observed by reduction in reproductive organs weight, deterioration in sperm quality parameters and also in testosterone levels. However, no marked difference in developmental landmarks and indices were found between DBP-, BBP-treated, and non-treated groups. Ema and Miyawaki (2002) reported that BBP at 500 and 1,000 mg/kg significantly decreased the maternal (body weight gain) and food consumption. Dams treated with BBP 300, 600, and 900 mg/kg from GD 8–18, showed a significant reduction in body weight. DBP at 50 mg/kg also caused a reduction in dams body weight gain (Howdeshell et al. 2008). The present study also indicated that dams exposed to DBP and BBP at late gestation may have adverse effect on body weight gain and developing fetus.
The gestation length was significantly elevated in all DBP, BBP, and DES-treated groups. A recent study on di-n-hexyl phthalate exposure during GD6–20, showed an increase in the gestation length (Saillenfait et al. 2009). Exposure to DBP (GD14–18 at 750 mg/kg) also delayed the gestation period in rats. Wistar rats treated with 1 % of DBP in the diet from GD6 to PND28 prolonged the gestation length and decreased the body weight of male and female rats (Zhu et al. 2009). The results on litter size and live pups/litter are in accordance with the findings of Nagao et al. (2000). They reported that chronic BBP exposure at 20, 100, and 500 mg/kg showed no marked alteration in the litter size, live pups, and sex ratio. DBP exposure (GD1–PND21) at 50 and 250 mg/kg showed minor variations in litter size but at 500 mg/kg, it was significantly reduced (Zhang et al. 2004). In the present study, DBP and BBP exposure slightly increased the rate of fetal mortality. Howdeshell et al. (2008) also showed an increase in fetal mortality in a dose-dependent manner in DBP- and BBP-treated groups. The data on developmental landmarks (i.e., pinna detachment, fur formation, eye opening, and testes descend) and indices (i.e., live birth, viability, and weanling index) did not show any significant alteration. No marked difference in the age of pinna detachment, eye opening, or incisor eruption in the pups’ of DBP-treated groups was also reported (Li et al. 2009). However, Nagao et al. (2000) reported that the chronic BBP exposure at 20 and 100 mg/kg significantly delayed the pinna opening or eyelid opening in male and female pups. Further, Ema et al. (2000) found that the duration of testis descent significantly increased in DBP 1,500 mg/kg treated group. The difference in the time of testis descent in the present study might be due to different doses used in both the studies. The results on viability index and weanling index, is in agreement of the study of Giribabu et al. (2012). They observed that DBP exposure caused a slight reduction in pups’ survival. However, Zhang et al. (2004) reported that DBP exposure at 50 and 250 mg/kg showed no marked difference in pups’ survival on PND1 while it was significantly reduced in 500 mg/kg treated group.
AGD is a developmental landmark for the differentiation of the external genitalia and is commonly used as hormonally sensitive parameter of sex differentiation in rodent. Masculinization of external genitalia in males is controlled by dihydrotestosterone, a metabolite of testosterone (Imperato-Mcginley et al. 1985; Clark et al. 1990). In the present study, DBP and BBP exposure slightly reduced the AGD at PND5 and PND25 in male pups. Earlier studies reported that BBP at 20 and 100 mg/kg showed no change in AGD at PND0 in male pups but it was significantly reduced at 500 mg/kg (Nagao et al. 2000). Exposure to DBP resulted in decreased AGD in male pups at PND4 which was statistically significant in 250 and 500 mg/kg treated groups (Zhang et al. 2004). Ema and Miyawaki (2002) also showed that BBP exposure at 250 mg/kg (GD15–17) slightly reduced the AGD while at BBP 500 and 1,000 mg/kg caused a significant reduction in the AGD of male fetuses. The data on pups’ body weight revealed that it was lower at PND1 and PND21 in all the treated groups, and it was significant in some of the DBP- and BBP-treated groups at PND75. Recently, Liu et al. (2012) reported that DBP exposure (GD14–18 at 750 mg/kg) caused a significant reduction in body weight of male fetuses at PND. Similarly, Giribabu et al. (2012) showed that DBP in utero exposure caused a reduction in the weight of fetuses at birth. Gray et al. (2000) also suggested that di(2-ethylhexyl)phthalate (DEHP) and BBP exposure (GD14 - PND3 at 750 mg/kg) significantly decreased the pups’ body weight at birth. Later, Zhang et al. (2004) also reported that exposure of DBP at 250 and 500 mg/kg to pregnant rat caused a dose related reduction in pup’s body weight at birth. Ema et al. (1998) showed a significant reduction in live fetus weight in BBP 500, 750, and 1,000 mg/kg (GD0-8) treated groups. These, coupled with the data obtained, suggest that prenatal DBP and BBP exposure affects the fetus development.
In utero DBP exposure produced several abnormal responses in male reproductive organs and these effects may be due to disruption of the stage-specific expression of genes related to androgen-dependent organs development (Kim et al. 2010). The weight of reproductive organs (i.e., epididymis, testis, prostate, and seminal vesicle) and vital organs (i.e., liver, kidney, and adrenal gland) was found to be lower in DBP (50 mg/kg) and BBP (100 mg/kg) higher dose-treated groups after prenatal exposure. Gray et al. (2000) showed that DEHP and BBP in utero exposure (GD14–PND3) caused a significant reduction in testis, seminal vesicle, prostate, and epididymis weight but the weight of liver, kidney, and adrenal gland reduced non-significantly. Nagao et al. (2000) reported that BBP exposure at 500 mg/kg significantly decreased the weight of epididymis, testis, and ventral prostate. Further, DBP at 500 mg/kg (GD1–PND21) caused a significant reduction in epididymis, liver, and kidney weight while prostate weight decreased non-significantly in DBP 250 mg/kg treated group.
Experimental studies showed a decrease in sperm production and increase in morphological abnormalities following prenatal and/or neonatal exposure to DBP in rodents (Wang et al. 2005; Mahood et al. 2007; Auharek et al. 2010). Earlier, Jeng and Yu (2008) also reported that phthalates exposure might cause a significant reduction in sperm motility and daily sperm production in rats. In our study, sperm count and motility showed a decrease while sperm head shape abnormalities increased significantly in DBP (50 mg/kg) and BBP (100 mg/kg) higher dose-treated groups (Fig. 4). A declining trend was also observed in testicular spermatid count and DSP in the treated groups. These data lends support to the notion that DBP and BBP exposure affects the programming of spermatogenesis, resulting in lower sperm density and inferior sperm quality at adulthood. Recently, Giribabu et al. (2012) reported that DBP exposure from GD1, 7, and 14 caused a significant reduction in sperm count, motility, viability, hypoosmotic swelling coil tailed, and increase in abnormal sperm number. It is known that testicular 17β-HSD is a gonadotropin-dependent enzyme (Murono and Payne, 1979), thus 17β-HSD activities might be dependent upon the gonadotropin secretion. Earlier, Steinberger (1971) reported that the increased and decreased in the activity of 17β-HSD is due to the increase and decrease in pituitary gonadotropin secretion, as LH plays an important role in testicular androgenesis. In the present study, the testicular activity of 17β-HSD enzymes declined in all the DBP-, BBP-, and DES-treated groups; this might be due to the reduction in gonadotropin secretion. Recently, Giribabu et al. (2012) also reported that prenatal DBP exposure caused a significant reduction in the testicular activity of 3 and 17β-HSD. In the present study, DBP, BBP, and DES exposure also caused a reduction in serum testosterone levels. These results coincide with previous studies, which showed decreased serum testosterone levels and impairment of steroid hormone metabolism after DBP exposure (Hirosawa et al. 2006; Xiao-feng et al. 2009). The data on serum testosterone is also supported by the Lehmann et al. (2004) study, which showed decrease in testosterone level after prenatal DBP exposure. Xiao-feng et al. (2009) suggested that DBP inhibits the testosterone production through a glucocorticoid-mediated pathway in pre-pubertal male rats. The present result also corroborates the observation of Kim et al. (2010). They reported that DBP exposure from GD 10–19, caused a reduction in dihydrotestosterone and testosterone level in F1 male rats. The observed reduction in serum testosterone level in DBP- and BBP-exposed rats might be responsible for alterations in the reproductive functions. It is known that inside the Sertoli cells, testosterone is selectively bound to the androgen receptor and activation of receptor will result in initiation and maintenance of spermatogenic process and inhibition of germ cell apoptosis (Dohle et al. 2003).
Conclusion
Prenatal exposure to DBP and BBP at late gestation might have adverse effects on offspring development and reproductive health, as evidenced by decline in offspring’s body and tissues weight and impairment in spermatogenesis and steroidogenesis.
References
Auharek SA, Franca LR, McKinnell C, Jobling MS, Scott HM, Sharpe RM (2010) Prenatal plus postnatal exposure to di (n-butyl) phthalate and/or flutamide markedly reduces final Sertoli cell number in the rat. Endocrinology 151:2868–2875
Clark RL, Antonello JM, Grossman SJ, Wise LD, Anderson C, Bagdon WJ, Prahalada S, MacDonald JS, Robertson RT (1990) External genitalia abnormalities in male rats exposed in utero to finasteride, a 5α-reductase inhibitor. Teratology 42:91–100
Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer MJ (1997) Evaluation of a recombinant yeast cell estrogen-screening assay. Environ Health Perspect 105:734–742
Dohle GR, Smit M, Weber RF (2003) Androgen and male fertility. World J Urol 21:341–345
Duty SM, Calafat AM, Silva MJ, Brock JW, Ryan L, Chen Z, Overstreet J, Hauser R (2004) The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl 25:293–302
Ema M, Miyawaki E (2002) Effects on development of the reproductive system in male offspring of rats given butyl benzyl phthalate during late pregnancy. Reprod Toxicol 16:71–76
Ema M, Miyawaki E, Kawashima K (1998) Reproductive effects of Butyl benzyl phthalate in pregnant and pseudo pregnant rat. Reprod Toxicol 12:127–132
Ema M, Miyawaki E, Kawashima K (2000) Critical period for development of reproductive system in male offspring of rats given di-n-butyl phthalate during late pregnancy. Toxicol Lett 111:271–278
Feuston MH, Bodnar KR, Kerstetter SL, Grink CP, Belcak MJ, Singer EJ (1989) Reproductive toxicity of 2- methoxy ethanol applied dermally to occluded and non-occluded sites in male rats. Toxicol Appl Pharmacol 100:145–161
Giribabu N, Sainath SB, Sreenivasula Reddy P (2012) Prenatal di-n-butyl phthalate exposure alters reproductive functions at adulthood in male rats. Environ Toxicol 1–11. In press.
Gray JR, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L (2000) Perinatal exposure to the phthalates DEHP, BBP and DINP but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58:350–365
Harris CA, Henttu P, Parker MG, Sumpter JP (1997) The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105:802–811
Hernandez-Diaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R (2009) Medications as a potential source of exposure to phthalates in the US population. Environ Health Perspect 117:185–189
Hirosawa N, Yano K, Suzuki Y, Sakamoto Y (2006) Endocrine disrupting effect of di-(2-ethylhexyl) phthalate on female rats and proteome analyses of their pituitaries. Proteomics 6:958–971
Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss K, Gray LE (2008) A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague–Dawley rat in a cumulative dose additive manner. Toxicol Sci 105:153–165
Imperato-Mcginley J, Binienda Z, Arthur A, Mininberg DT, Vaughan ED, Quimby FW (1985) The development of a male pseudohermaphroditic rat using an inhibitor of the enzyme 5α-reductase. Endocrinol 116:807–812
Jana K, Jana S, Samanta PK (2006) Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reprod Biol Endocrinol 4:1–13
Jeng HA, Yu L (2008) Alterations in sperm quality and hormone levels by polycyclic aromatic hydrocarbons on airborne particulate. J Environ Sci Health A Tox Hazard Subst Environ Eng 43:675–681
Jobling S, Reynolds T, White R, Parker MG, Sumpter JP (1995) A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect 103:582–587
Kim TS, Jung KK, Kim SS, Kang IH, Baek JH, Nam HS, Hong SK, Lee BM, Hong JT, Oh KW, Kim HS, Han SY, Kang TS (2010) Effects of in utero exposure to di (n-butyl) phthalate on development of male reproductive tracts in Sprague–Dawley rats. J Toxicol Environ Health A 73(21–22):1544–1559
Koch HM, Preuss R, Angerer J (2006) Di - (2-ethylhexyl) phthalate (DEHP): human metabolism and internal exposure-an update and latest results. Int J Androl 29:155–165
Kumar S, Patel KG, Gautam AK, Agarwal K, Shah BA, Saiyed HN (1999) Detection of germ cell genetic toxic potential of carbon disulphide using sperm head shape abnormality test. Hum Exp Toxicol 18:731–734
Lehmann KP, Phillips S, Sar M (2004) Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci 81:60–68
Li Y, Zhuang M, Li T, Shi N (2009) Neurobehavioral toxicity study of dibutyl phthalate on rats following in utero and lactational exposure. J Appl Toxicol 29:603–611
Linder RE, Strader LF, McElroy WK (1986) Measurement of epididymal sperm motility as a test variable in the rat. Bull Environ Contam Toxicol 36:317–324
Liu SB, Ma Z, Sun WL, Sun XW, Hong Y, Ma L, Qin C, Stratton HJ, Liu Q, Jiang JT (2012) The role of androgen - induced growth factor (FGF8) on genital tubercle development in a hypospadiac male rat model of prenatal exposure to di-n-butyl phthalate. Toxicology 293:53–58
Lottrup G, Andersson AM, Leffers H, Mortensen Toppari J, Skakkebaek NE, Main M (2006) Possible impact of phthalates on infant reproductive health. Int J Androl 29:172–180
Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU (2009) Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev 12:225–249
Mahood IK, Scott HM, Brown R, Hallmark N, Walker M, Sharpe RM (2007) In utero exposure to di (n-butyl) phthalate and testicular dysgenesis: Comparison of fetal and adult end points and their dose sensitivity. Environ Health Perspect 115:55–61
Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson M, Toppari J, Skakkebaek NE (2006) Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect 114:270–276
Miguel B, Alicia R, Eduardo C (2006) Effect of two insecticide and two herbicides on the porcine sperm motility patterns using computer-assisted semen analysis (CASA) in vitro. Reprod Toxicol 22:508–512
Mose T, Knudsen LE, Hedegaard M, Mortensen GK (2007) Transplacental transfer of monomethyl phthalate and mono (2-ethylhexyl) phthalate in a human placenta perfusion system. Int J Toxicol 26:221–229
Murono FF, Payne AH (1979) In vivo effect of gonadotropin on steroidogenic enzymes in hypophysectomized immature rats. Biol Reprod 20:911–916
Nagao T, Ohta R, Marumo H, Shindo T, Yoshimura S, Ono H (2000) Effect of butyl benzyl phthalate in Sprague–Dawley rats after gavage administration: a two-generation reproductive study. Reprod Toxicol 14:513–532
Robb GW, Amann RP, Killian GJ (1978) Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil 54:103–107
Saillenfait AM, Gallissot F, Sabate JP (2009) Differential developmental toxicities of di-n-hexyl phthalate and dicyclohexyl phthalate administered orally to rats. J Appl Toxicol 29:510–521
Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29:134–139
Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP (1995) Gestational and lactational exposure of rats to xenoesterogens results in reduced testicular size and sperm production. Environ Health Perspect 103:1136–1143
Silva MJ, Reidy JA, Herbert AR, Preau JL, Needham LL, Calafat AM (2004) Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol 72:1226–1231
Steinberger E (1971) Hormonal control of spermatogenesis. Physiol Rev 51:1–22
Thayer KA, Ruhlen RL, Howdeshell KL, Buchanan DL, Cooke PS, Preziosi D, Welshons WV, Haseman J, Vom Saal FS (2001) Altered prostate growth and daily sperm production in male mice exposed prenatally to sub clinical dose of 17-ethenyl oestradiol. Hum Reprod 16:988–996
Wang YB, Song L, Zhu ZP, Chen JF, Wang XR (2005) Effects of dibutyl phthalate on Sertoli cells of rat testis. Zhonghua Yu Fang Yi Xue Za Zhi 39:179–181
Wyrobek AJ, Gordon LA, Burkhart JG, Francis MW, Jr K, Letz G, Malling HV, Topham JC, Whorton MD (1983) An evaluation of the sperm morphology test and other sperm tests in non-human mammals. Mutat Res 115:1–72
Xiao-feng Z, Nai-qiang Q, Jing Z, Zi L, Yang Z (2009) Di (n-butyl) phthalate inhibits testosterone synthesis through a glucocorticoid-mediated pathway in rats. Int J Toxicol 28:448–456
Zhang Y, Jiang X, Chen B (2004) Reproductive and developmental toxicity in F1 Sprague–Dawley male rats exposed to di-n-butyl phthalate in utero and during lactation and determination of its NOAEL. Reprod Toxicol 18:669–676
Zhu YJ, Jianga JT, Ma L, Zhang J, Hong Y, Liao K, Liu Q, Liu GH (2009) Molecular and toxicologic research in newborn hypospadiac male rats following in utero exposure to di-n-butyl phthalate (DBP). Toxicology 260(1–3):120–125
Acknowledgments
The financial support in the form of a research grant to one of us (SK) received from the Indian Council of Medical Research (ICMR), New Delhi, is thankfully acknowledged. Authors are also thankful to the Ex. Director of the NIOH and the staff of the Animal House Facility and Division of Reproductive and Cytotoxicology for their support during the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ahmad, R., Gautam, A.K., Verma, Y. et al. Effects of in utero di-butyl phthalate and butyl benzyl phthalate exposure on offspring development and male reproduction of rat. Environ Sci Pollut Res 21, 3156–3165 (2014). https://doi.org/10.1007/s11356-013-2281-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2281-x