Abstract
Nutrient load into the ocean can be retained during the process of plant uptake and sedimentation in marshes along the bay zone. Seasonal variations of biomass and nutrient concentration in three dominated plant assemblages and associated sediments were monitored in this study area to determine effects of salt marsh on nutrient retention. Results showed that plant aboveground biomass displayed a unimodal curve with nutrient concentration generally decreased from spring to winter. Belowground biomass was relatively low during the rapid growth period with nutrient concentration tending to decrease and then increase during this period. Plant total nitrogen (TN) pools are higher than total phosphorus (TP) pools, and both pools showed significant seasonal variations. Water purification coefficients (WPC) of nutrients by plant assimilation were 34.4/17.3, 19.3/24.0, and 5.14/6.04 t/(m2 year) (TN/TP) for Phragmites australis, Spartina alterniflora, and Scirpus mariqueter, respectively. Overall, these results suggest that higher annual plant biomass and nutrient assimilation contribute to greater nutrient retention capacity and accumulation in sediments, thereby enabling reduced eutrophication in transitional waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In coastal areas, population growth and rapid economic development contribute to many point and nonpoint source nutrient inputs, both directly or indirectly, which largely lead increases in nutrient concentration in ocean waters. Nitrogen (N) and phosphorus (P) are essential nutrient elements in ecosystems, and P is a limiting nutrient for phytoplankton growth (Redfield 1958; Boers et al. 1998). Eutrophication in aquatic ecosystems caused by excessive input of N and P is recognized as a major water quality problem globally (Smith 2003; Schindler 2006).
Coastal wetland is one type of wetlands, which is located between ocean and terrestrial land, and is composed of a complex and assemblage of swamps, marshes, mudflats, etc. (Bai et al. 2012a). Salt marsh is typically dominated by halophytic (salt-tolerant) plants such as herbs, grasses, or low shrubs, with daily tidal flow that occurs and continuously floods the area (Adam 1990). As a transitional zone between terrestrial and marine ecosystems, salt marshes are one of the most biologically productive habitats that drive nutrient cycles and food webs supporting estuarine and coastal ecosystems (Marinucci 1982).
Plants and sediment nutrient pools may greatly affect nutrient cycles in salt marshes. Salt marsh plants have high assimilative capacity for N, P, and heavy metals (Quan et al. 2007; González-Alcaraz et al. 2012; Negrin et al. 2012). Sediment N mineralization, nitrification, and denitrification are critical processes of the N cycle (Bai et al. 2010, 2012a; Gao et al. 2012). Nitrification is an important source of NO3 − for denitrification, while denitrification was the main mechanism associated with removal of nitrate in wetlands (DeLaune et al. 2005; Bai et al. 2010; González-Alcaraz et al. 2011). There was a higher contribution of Spartina maritima marshes and cypress swamps to N removal through denitrification, highlighting the role of the wetlands in this ecosystem service (Lindau et al. 2008; Sousa et al. 2012). Sediments play an important role in P retention by sorption and precipitation reactions (Pant and Reddy 2001; Lai and Lam 2008). Under oxic and anoxic conditions, Ca compounds were the main inorganic soil components contributing to P retention in the salt marsh, except in sites with low CaCO3 concentration, where Fe and Al oxides contribute more to P retention (Álvarez-Rogel et al. 2007). Retention of suspended particles in sediment colonized by marsh vegetation also acts as a trap for nutrients and contaminants (Cartaxana and Catarino 1997; Palomo et al. 2004; Quan et al. 2007; Sousa et al. 2010). Differences in nutrient accumulation levels occur among plant species as well as between sediments (Cartaxana and Catarino 1997; Palomo et al. 2004). Considering the role of plants in this process, salt marshes are crucial to N and P remediation. Nutrient cycling and accumulation processes partitioned by salt marsh halophytes have been found to reduce eutrophication and lower atmospheric CO2 (Sousa et al. 2010).
There are several threats to existence and functioning of salt marshes, particularly direct and indirect anthropogenic impacts such as leveeing of rivers, beach reclamation, aquaculture and marine construction, and other activities (DeLaune et al. 2005; Bromberg-Gedan et al. 2009). Notably, these effects have resulted in the sharp decline of natural intertidal beach and increased pollutant loads to intertidal and coastal waters in the vicinity of Hangzhou Bay, adjacent to the East China Sea.
In recent years, increased delivery of N and P resulting in severe eutrophication of Hangzhou Bay has been documented in various government reports (MEPC 2010; OFBZ 2011), which indicate that the bay's environmental quality is below Grade IV Sea Water Quality Standard of China (0.50 and 0.045 mg/kg for inorganic N and orthophosphate, respectively). In addition, land reclamation is decreasing the size of Hangzhou Bay area. For example, 70 % of the total land area (1,154 km2) in Cixi County, located in south Hangzhou Bay, was reclaimed from salt marshes (Feng and Bao 2006). The decline in salt marshes minimizes the buffer zone between the continent and the ocean that acts as a biological filter, thereby accelerating the eutrophication process as the marsh nutrient retention capacity is reduced.
To date, limited studies have been conducted to evaluate the role of N and P cycling in the impacted salt marshes of Hangzhou Bay. Hence, the aims of this study were to evaluate the nutrient (N and P) pools and the seasonal changes in plants (Phragmites australis (Cav.) Trin. ex Steud., Spartina alterniflora Loisel., and Scirpus mariqueter Wang et Tang.) and sediments of the Hangzhou Bay salt marsh.
Material and methods
Study site
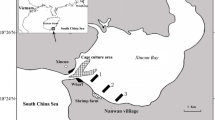
Hangzhou Bay is in the northern Zhejiang Province of China (Fig. 1). The bay is situated in the outer part of the Qiantang River Estuary, adjacent to the East China Sea, which is the world's largest tidal bore. Hangzhou Bay is characterized by a north subtropical maritime monsoon climate, with four distinct seasons, a mean annual temperature of 16 °C, annual rainfall of 1,273 mm, and water depth of 8–10 m. The study area is located in the south Hangzhou Bay. The area's strong tidal currents have a high carrying capacity for suspended sediments that were carried downstream from the Yangtze River and Qiantang River (Zhou and Gao 2004) and allow rapid accumulation of sediments that extend the shoreline at a velocity of 292 m/year (Yang et al. 2004). Semidiurnal tides regularly flood the low marsh and mudflat zones, while the high marsh is irregularly inundated, and spring high tides may also flood the entire salt marsh area.
The main characteristics of the sediment indicated by a previous investigation in the research area were shown in Table 1. The water in Hangzhou bay estuary is a mixture of seawater and fresh water and its salinity can range from 5 to 15 g/kg. There are only a few plant species in natural marsh. Native dominant plant species includes P. australis and S. mariqueter, each of which can form a dense monoculture in the marsh zone (Shao et al. 2011). Monocultures of P. australis are often found around the high marsh zone, while S. mariqueter, a pioneer plant that is endemic to China, dominates the low marsh zone. S. alterniflora often invades the midmarsh zone and then quickly expands toward the high and low marsh zones (Quan et al. 2007).
Sampling strategy
Three randomly selected 0.5 × 0.5 plots representative of P. australis, S. alterniflora, and S. mariqueter were located at the high, mid, and low marsh separated by at least 100 m. Plant biomass was collected bimonthly in March, May, July, September, and November 2009, and these months generally corresponded to the four seasons in our research area. During each sampling event, live aboveground plant was clipped at the ground level. Belowground samples (roots and rhizomes) were collected from the same plots using a 0.5 × 0.5 × 0.6-m box corer. Sediment cores were washed over a 2-mm mesh screen and retained roots and rhizomes were collected for analysis. Additionally, three sediment cores with two layers (0–5 cm as surface sediment and 5–20 cm as rooted sediment) were sampled using an Eijkelkamp gouge auger (50 cm length by 6 cm diameter) from each plant species. Sediment in bare mudflat that was located near the plots of S. mariqueter was taken as control.
Analytical procedures
Plant samples were rinsed thoroughly with tap water and then cleaned with deionized water. Samples were first dried at 105 °C for 30 min and then dried at 60 °C to constant weight for approximately 48 h. The above- and belowground biomass of the three salt marsh plants were calculated on a dry weight basis (grams per square meter), respectively. Sediment was dried at room temperature. Each of the dry sediment and plant samples was homogenized by grinding to powder for analysis of total N (TN) and total P (TP) concentration. TN concentration of the sediment and plant was determined by the Kjeldahl method (Nelson and Sommers 1980). TP was measured using a molybdophosphoric blue colorimetric method after acid digestion (Murphy and Riley 1962).
Calculation of nutrient pools and production
Elemental concentration and above- and belowground biomass were multiplied to yield total amounts of above- and belowground nutrient pools for each plant species, respectively. Annual net primary production for aboveground was estimated by peak live aboveground biomass, and belowground was calculated based on the differences between the maximum and minimum belowground biomass recorded during the study period (De la Cruzz and Hackney 1977). Annual net nutrient primary production (ANNPP) was estimated similarly.
Data analysis
Repeated ANOVA (analysis of variance) measurements were carried out in order to compare the evolution over time and among different sampling plots for the parameters analyzed in sediment and plant samples, separately, using SPSS 16.0. Dependent variables were above- and belowground biomass, plant and sediment nutrient concentrations, and plant nutrient pools. Data were log-transformed, when necessary, to achieve homogeneity of variance (Levene's test). When data did not fulfill the sphericity requirement for Mauchly's sphericity test, univariate F statistics using a corrector index of epsilon were applied, based on Greenhouse–Geisser, Huynh–Feldt, or lower-bound corrections. Pearson's correlation analyses were performed among plant biomass, nutrient concentrations, and nutrient pools. Differences were considered significant if probabilities for nonsignificance were <0.05.
Results
Above- and belowground biomass
The ANOVA of repeated measures indicated that plant aboveground and belowground biomass differed significantly across seasons (Fig. 2, p < 0.05). The aboveground biomass of all species showed similar seasonal patterns with a unimodal curve. P. australis and S. mariqueter reached the highest average aboveground biomass (3,731.7 and 487.4 g/m2, respectively) in July, whereas S. alterniflora achieved its highest level (3,105.9 g/m2) in September. Overall, the belowground biomass was relatively high in the early stage of plant growth or in the fall during wilting and decreased during the rapid growth phase.
The aboveground biomass of the three species was relatively low compared to belowground biomass during the first sampling. Subsequently, the aboveground biomass was higher than belowground biomass (p < 0.05), accounting for more than 60 % of total plant biomass between May and September. In November, the trend was the same for P. australis and S. alterniflora, while for S. mariqueter, the aboveground biomass was lower than belowground as it withered earlier than the other two species. The mean belowground/aboveground biomass ratios during the growing season for P. australis, S. alterniflora, and S. mariqueter were 2.44, 1.38, and 0.62, respectively. However, the mean ratios were 0.38, 0.31, and 0.62, respectively, if we don't include the date of the early stage in March.
Plant biomass showed a significant difference between plants (p < 0.05). The aboveground biomass of P. australis and S. alterniflora was significantly greater than those of S. mariqueter (Fig. 2, p < 0.05), while there was no significant difference between the native plant (P. australis) and invasive plant (S. alterniflora). Belowground biomass displayed a trend of S. alterniflora > P. australis > S. mariqueter during the entire study period.
Nitrogen and phosphorus concentrations in plants
Seasonal patterns of TN and TP concentrations in plant of three species are shown in Fig. 3. TN and TP concentrations in aboveground part of P. australis and S. alterniflora were highest at the early period of growth and gradually decreased along the study period. The variation of S. mariqueter is similar for TP concentration, while the changes were not significant for TN concentration. The concentrations of TN and TP in the belowground part showed a decreasing and then an increasing tendency over the year. The concentrations in the belowground part of P. australis and S. mariqueter were lowest (3.64 and 6.20 g/kg for TN, 0.655 and 1.65 g/kg for TP, respectively) in July when the aboveground biomass was highest. The turning point for S. alterniflora was in September (2.26 and 0.25 g/kg for TN and TP, respectively) when its aboveground biomass reached the highest value (Fig. 2).
The TN concentration of the three plant species was much greater in the aboveground than the belowground part (Fig. 3), and the TP concentration had varying trends between above- and belowground parts across seasons (Fig. 3). The ANOVA of repeated measures indicates significant differences of plant TN and TP concentrations among the species (p < 0.05). Both TN and TP concentrations were significantly greater in S. mariqueter than in P. australis and S. alterniflora (Fig. 3, p < 0.05), except in March when S. mariqueter had no burgeoned aboveground growth.
Nitrogen and phosphorus concentrations in sediment
The TN and TP concentrations and seasonal dynamics in vegetated and unvegetated sediments are shown in Fig. 4. Study results indicate that sediment TN and TP concentrations varied significantly across seasons (p < 0.05) but did not differ significantly between depths. The sediment TN concentration had significant variation among plant species and bare mudflat (p < 0.05). TN concentration was significantly greater in the plots of P. australis, S. alterniflora, and S. mariquete than in the plots of bare mudflats (Fig. 4, p < 0.05). The sediment TN variation among plots of plant species was in conformity to the variation of plant aboveground biomass (r = 0.59, p < 0.01) than aboveground concentration (r = −0.00, p > 0.05). Sediment TP concentration also showed variation among plant species. While these differences were not constant over time, there were several repetitive trends with TP concentration in sediment of P. australis > S. mariqueter > S. alterniflora > bare mudflats.
The coefficients of variation of TN and TP in 0–5 cm sediment between different plant species and bare mudflat were 18.2–29.1 and 1.9–3.9 %, respectively. The coefficients of variation of TN and TP in 5–20 cm sediment were 12.9–25.7 and 2.9–6.8 %, respectively. Overall, the change of TP in wetland sediment was not as apparent as that for TN.
Allocation of nitrogen and phosphorus in salt marsh
Pool allocation of TN and TP in plant above- and belowground parts is shown in Fig. 5. Generally, the TN pools are higher than TP pools in salt marsh. Both TN and TP pools showed significant (p < 0.05) and similar seasonal variation patterns throughout the growing season in plants. Both nutrient pools of the aboveground part were lowest in March and highest in July at the peak of the growing season. For the pools belowground, the highest values were often in March or November. The seasonal variation patterns of total TN and TP pools (above- and belowground) of these plants were similar to that of the aboveground part.
For different parts of the two native species, the aboveground part contained significantly more TN and TP than belowground during the growing season, except in March when the plants began to sprout or November when wilting. This trend was similar for the invasive plant S. alterniflora, except in November which pools in the aboveground part were still higher than belowground.
Discussion
Capacity of nutrient retention in different plants
Biomass is an important parameter in evaluating ecosystem structure and function. Capacity of nutrient retention in salt marshes is directly related to plant biomass production and nutrient levels in the ambient environment. Compared with other results (Quan et al. 2007; Li et al. 2009), this study found that the peak aboveground biomass of P. australis and S. alterniflora was significantly greater in the Hangzhou Bay wetland than in the Yangtze River estuary (3,731 vs. 1,246 g/m2 for P. australis and 3,105 vs. 2,759 g/m2 for S. alterniflora), while the trend was opposite for S. mariqueter (487 vs. 548g/m2), even though both sites have similar climates. There was also a marked difference in the aboveground biomass between invasive plant S. alterniflora and native P. australis (S. alterniflora > P. australis in the Yangtze River estuary but no significant difference in this study site). The aboveground biomass of S. alterniflora was also higher than that in North American coastal wetlands, which was from 176 g/(m2 year) in New Jersey to 938 g/(m2 year) in Texas (Kirwan, et al. 2009). Therefore, aboveground primary production varies widely in salt marsh plants from various sites and may also result in a different nutrient retention capacity. Such findings may be related to different latitudes, varying growth years of plants as well as various salinity and nutrient levels in the ambient water in different estuaries (Brix, et al. 2001; Kirwan, et al. 2009).
S. alterniflora reached the highest aboveground biomass slightly later than P. australis and S. mariqueter. This is likely due to different phenology among these species, as P. australis and S. mariqueter burgeon only in spring, whereas S. alterniflora has a slow continuous growth in autumn and winter (Li et al. 2009). Plant aboveground biomass increased, while nutrient concentrations decreased during the growing season. Similar results also have been reported for other marsh species (Boyer et al. 2001; Neves et al. 2007). Nutrient pools were greatest when biomass was high but nutrient concentration was low in each plant. This study also found that for the aboveground part, although the nutrient concentration in S. mariqueter was higher than both P. australis and S. alterniflora, nutrient pools were lower for S. mariqueter than both P. australis and S. alterniflora. As such, the nutrient pools of the aboveground part were mainly influenced by the biomass rather than the nutrient concentration itself for the salt marsh plants in this study area (r = 0.94 and 0.85 for N and P, respectively, p < 0.01).
Seasonal changes of TN and TP concentrations in the aboveground part adequately explain the pattern of nutrient transfer in salt marsh plants. Aboveground growth began in March with a high nutrient concentration in the newly burgeoned photosynthetic leaves and reached the highest aboveground biomass at the expense of rhizome resources. Subsequently, the plants started to senesce and a large portion of the aboveground nutrients were slowly translocated to the belowground part in winter (White and Howes 1994; Quan et al. 2007) and reserved for the next year's growth. Further evaluation of the S. alterniflora marsh indicated that recycling of N through translocation from aboveground to belowground parts and remineralization of dead belowground part are the major pathways in the sediment N cycle, equivalent to 67–79 % of the annual plant N demand (White and Howes 1994). In addition, belowground biomass and nutrient reserves of the marsh plants maintain a certain level throughout the year, while most of the aboveground biomass is broken down or brought off the coast by tide with a small amount of residue in the sediment (Howarth 1993). Consequently, the belowground part plays an important role in ecosystem nutrient cycling and energy conversion (Schalles and Shure 1989). This is particularly true for plants such as P. australis and S. alterniflora, which have significantly higher belowground biomass than that of S. mariqueter. However, the belowground/aboveground biomass ratios were lower in P. australis and S. alterniflora than S. mariqueter. This might be related to different flooding conditions and that the more unfavorable conditions in lower marsh of S. mariqueter caused greater investment in belowground biomass. Also, the belowground/aboveground biomass ratios were lower than that in European (Brix et al. 2001) and South American (Negrin et al. 2012). This might lie with the different physical characteristics among different research areas. The lower growth years of plants in our research area caused by intensive and rapid reclamation would be another reason that leads to relatively undeveloped roots system.
From this study, the salt marsh plant assimilated the greatest amount of nutrients during the growing season and lower amounts in the winter. All three species reached the highest nutrient pools in summer (July). Given that nutrients could be re-released through the decomposition of senescent plant materials at the end of the growing season with little residues remaining in the sediment (Howarth 1993), this would be an opportune time to remove excess nutrients from the salt marsh in summer through best management practices such as harvesting or grazing (Kang et al. 2002). Harvesting could also be a potentially useful and effective approach for the control and management of the exotic and invasive plant S. alterniflora.
The influence of plants on sediment nutrient retention
Distribution and accumulation of N and P in sediment are related to allochthonous and autochthonous sources, sediment characteristics (composition, grain size, hydrology, Eh, and pH), and nutrient properties (Pant and Reddy 2001; Álvarez-Rogel et al. 2007; Song et al. 2007; Zhou et al. 2007; Bai et al. 2012b). Changes in species composition also may alter ecosystem structure (e.g., vertical root profile) and processes (e.g., nutrient cycling and carbon allocation) (Liao et al. 2008; Ehrenfeld 2010), suggesting that sediment nutrient concentration may consequently be changed with different plant species.
In this study, the variation of sediment TN concentration among plant species was more consistent for plant aboveground biomass, as plants remove N from water and enhance TN storage in sediment by increasing the organic fraction. Phosphorous concentration is affected less by plant species because it is dominated by sediment adsorption rather than biological processes (Picard et al. 2005). The variation of sediment TP concentration is more related to P fractionation and is affected by sediment characteristics such as grain size composition. Preliminary studies (Liang et al. 2012) in this area have shown that calcium carbonate-bound P was the main form of P, which was relatively evenly distributed in the sediment of different grain sizes. Therefore, the spatial variation of TP in sediment is not as apparent as TN.
Sediment reflects historic accumulation of nutrient input and storage. The variation among plant species may be due to heterogeneity of sediments or external sources. Sedimentation rate in vegetated sediment should also be considered. Vegetated salt marshes often show rapid sediment accretion and growth of marshlands because of slow flow rate caused by especially short, stiff species and by directly trapping sediment on leaves and stems and within joints (Stumpf 1983; Leonard et al. 1995; Li and Yang 2009). For example, a 27-cm net accretion in 100 years for an unstable Barataria Bay S. alterniflora marsh of Louisiana and a 45-cm net accretion in 100 years for a stable S. alterniflora marsh in Four League Bay have been estimated (Mitsch and Gosselink 2000). In Dongtai (China), S. alterniflora has accelerated natural land formation with vertical accretion rates of 48.5–52.1 cm compared to 10.5–16.9 cm in bare mudflat in 3.4 years (Chung et al. 2004). The contribution of vegetation-trapped sediment to the local total deposition rate was likely responsible for >10 % of the total depositional rate in the S. alterniflora marsh and <10 % of the total depositional rate in the S. mariqueter marsh (Li and Yang 2009). The retained P by increasing sedimentation of plant materials contributed up to 25 % of the total monthly P retention (Schulz et al. 2003). Although the sedimentation rate in this study was not investigated, it is speculated from the above reports that vegetated marshes have higher nutrient retention than bare mudflats.
Implication for reducing eutrophication in Hangzhou Bay
The N and P used by the plants ultimately come from the surface water. Therefore, N and P uptake from sediment interstitial waters and their incorporation in plant biomass imply nutrient sequestration and retention, thereby decreasing nutrient availability in the water column and potentially reducing eutrophication (Sousa et al. 2010). This study has a preliminary assessment of the contribution of yearly nutrient retention created by salt marsh growth in the system to reduce eutrophication by dividing the annual net TN and TP primary productions (ANNPP) by reduced nutrient concentration. The results (Table 2) indicate that the water purification coefficients (WPC) of dissolved inorganic N (DIN) and orthophosphate (DP) from water by plant were 34.4/17.3, 19.3/24.0, and 5.14/6.04 t/(m2 year) for P. australis, S. alterniflora, and S. mariqueter, respectively. Although the TN pools are higher than TP pools in the salt marsh, the WPC of DIN is lower than DP in most cases. As P is a limiting factor for phytoplankton growth, these results further illustrate the importance of protecting salt marshes.
Overall, salt marsh plants display high sequestration for N and P, thereby helping reduce eutrophication in the estuarine ecosystem. It should be pointed out that for TN, except plant uptake and immobilization in plant biomass, another major mechanism for reducing mineral N loading is denitrification (DeLaune et al. 2005; González-Alcaraz et al. 2011). González-Alcaraz et al. (2011) have compared denitrification considering the presence or absence of plant rhizhosphere. Results indicated that in fine-textured basic wastes, the process is intense regardless of the existence of plant rhizosphere, while the role of vegetation is more relevant in sandier acidic wastes. However, according to the research done by Sousa et al. (2012), it seems that it cannot be concluded whether or not annual denitrification was significantly different between the vegetated sediment and the bare mudflats. N and P in water can also be absorbed by sediment. The maximal phosphate sorption capacity (PSC) is a useful measure of the P removal capacity of sediment–water system (Lai and Lam 2008). The PSC determined in our study area (shown in Table 1) reveals that the sediment can theoretically bind more P and could be about 1.5-fold higher than is currently reflected in sediment. Yet, considering the denitrification of nitrate and sorption of orthophosphate in sediment, the actual purification of water N and P by the whole system is expected to be even greater.
Conclusions
Our research showed significant distribution patterns and seasonal variations of N and P concentrations and pools in different parts of marsh plants. This can provide detailed information about changes in salt marsh retention capacity and contribute to explain seasonal variations in nutrient retention. It can also help to determine the optimal biomass harvesting time of salt marsh plants for maximum nutrient removal.
There was a significant relationship between plant net primary productivity and wetland nutrient retention, so the protection of localized wetlands would create more favorable environmental conditions for increasing plant biomass and help improve deteriorated water quality. However, in order to assess the nutrient retention capacity in the whole system, we should take into account of the nutrient quantities coming in and out of the salt marsh in water, the denitrification process of N cycling, and the maximal PSC in sediment.
References
Adam P (1990) Salt marsh ecology. Cambridge University Press, Cambridge
Álvarez-Rogel J, Jiménez-Cárceles FJ, Egea-Nicolás C (2007) Phosphorus retention in a coastal salt marsh in SE Spain. Sci Total Environ 378:71–74
Bai JH, Gao HF, Deng W, Yang ZF, Cui BS, Xiao R (2010) Nitrification potential of marsh soils from two natural saline–alkaline wetlands. Biol Fertil Soils 46:525–529
Bai JH, Gao HF, Xiao R, Wang JJ, Huang C (2012a) A review of soil nitrogen mineralization as affected by water and salt in coastal wetlands: issues and methods. Clean Soil Air Water 40:1099–1105
Bai JH, Wang QG, Deng W, Gao HF, Tao WD, Xiao R (2012b) Spatial and seasonal distribution of nitrogen in marsh soils of a typical floodplain wetland in Northeast China. Environ Monit Assess 184:1253–1263
Boers PCM, Van-Raaphorst W, Van-der-Molen TD (1998) Phosphorus retention in sediments. Water Sci Technol 37:31–39
Boyer KE, Fong P, Vance RR, Ambrose RF (2001) Salicornia virginica in a southern California salt marsh: seasonal patterns and nutrient-enrichment experiment. Wetlands 21:315–326
Brix H, Sorrell BK, Lorenzen B (2001) Are Phragmites-dominated wetlands a net source or net sink of greenhouse gases? Aquat Bot 69:313–324
Bromberg-Gedan K, Silliman BR, Bertness MD (2009) Centuries of human driven change in salt marsh ecosystems. Annu Rev Mar Sci 1:117–141
Cartaxana P, Catarino F (1997) Allocation of nitrogen and carbon in an estuarine salt marsh in Portugal. J. J Coast Conserv 3:27–34
Chung CH, Zhuo RZ, Xu GW (2004) Creation of Spartina plantations for reclaiming Dongtai, China, tidal flats and offshore sands. Ecol Eng 23:135–150
De-la-Cruz AA, Hackney CT (1977) Energy value, elemental composition, and productivity of belowground biomass of a Juncus tidal marsh. Ecology 58:1165–1170
DeLaune RD, Jugsujinda A, West JL, Johnson CB, Kongchum M (2005) A screening of the capacity of Louisiana freshwater wetlands to process nitrate in diverted Mississippi River water. Ecol Eng 25:315–321
Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Ann Rev Ecol Evol Syst 41:59–80
Feng LH, Bao YX (2006) Relationship between population, resource, environment and development of reclamation area of tidal flats—a case of Cixi City. Mar Sci 30:88–91 (in Chinese)
Gao HF, Bai JH, Xiao R, Yan DH, Huang LB, Huang C (2012) Soil net nitrogen mineralization in salt marshes with different flooding periods in the Yellow River Delta, China. Clean-Soil Air Water 40:1111–1117
González-Alcaraz MN, Egea C, Jiménez-Cárceles FJ, Párraga I, María-Cervantesa A, Delgado MJ, Álvarez-Rogel J (2012) Storage of organic carbon, nitrogen and phosphorus in the soil–plant system of Phragmites australis stands from a eutrophicated Mediterranean salt marsh. Geoderma 185–186:61–72
González-Alcaraz MN, Egea C, María-Cervantesa A, Jiménez-Cárceles FJ, Álvarez-Rogel J (2011) Effects of eutrophic water flooding on nitrate concentrations in mine wastes. Ecol Eng 37:693–702
Howarth RW (1993) Microbial processes in salt-marsh sediments. In: Ford ET (ed) Aquatic microbiology. Blackwell, Oxford, pp 239–261
Kang S, Kang H, Ko D, Lee D (2002) Nitrogen removal from a riverine wetland: a field survey and simulation study of Phragmites japonica. Ecol Eng 18:467–475
Kirwan M, Guntenspergen GR, Morris JT (2009) Latitudinal trends in Spartina alterniflora productivity and the response of coastal marshes to global change. Glob Chang Biol 15:1982–1989
Lai DYF, Lam KC (2008) Phosphorus retention and release by sediments in the eutrophic Mai Po Marshes, Hong Kong. Mar Pollut Bull 57:349–356
Leonard LA, Hine AC, Luther ME (1995) Surficial sediment transport and deposition processes in a Juncus roemarianus marsh, West-Central Florida. J Coast Res 11:322–336
Li B, Liao CZ, Zhang XD, Chen HL, Wang Q, Chen ZY, Gan XJ, Wu JH, Zhao B, Ma ZJ, Chen XL, Jiang LF, Chen JK (2009) Spartina alterniflora invasions in the Yangtze River estuary, China: an overview of current status and ecosystem effects. Ecol Eng 35:511–520
Li H, Yang SL (2009) Trapping effect of tidal marsh vegetation on suspended sediment, Yangtze Delta. J Coast Res 25:915–924
Liang W, Shao XX, Wu M, Li WH, Ye XQ, Jiang KY (2012) Phosphorus fraction in the sediments from different vegetation type in Hangzhou Bay coastal wetlands. Acta Ecol Sin 32:5025–5033 (in Chinese)
Liao CZ, Peng RH, Luo YQ, Zhou XH, Wu XW, Fang CM, Chen JK, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714
Lindau CW, Delaune RD, Scaroni AE, Nyman JA (2008) Denitrification in cypress swamp within the Atchafalaya River Basin, Louisiana. Chemosphere 70:886–894
Marinucci AC (1982) Trophic importance of Spartina alterniflora production and decomposition to the marsh–estuarine ecosystem. Biol Conserv 22:35–58
Ministry of Environmental Protection of the People's Republic of China (MEPC) (2010) Bulletin of China's coastal sea environmental status. http://www.mep.gov.cn/gzfw/xzzx/wdxz/201206/P020120613558299715101.pdf
Mitsch WJ, Gosselink JG (2000) Wetlands, 3rd edn. Wiley, New York, p 919
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Negrin VL, de Villalobos AE, González-Trilla G, Botté SE, Marcovecchio JE (2012) Above- and belowground biomass and nutrient pools of Spartina alterniflora (smooth cordgrass) in a South American salt marsh. Chem Ecol 28:391–404
Nelson DW, Sommers LE (1980) Total nitrogen analysis of soil and plant tissues. J Assoc Offic. Anal Chem 63:770–778
Neves JP, Ferreira LF, Simões MP, Gazarini LC (2007) Primary production and nutrient content in two salt marsh species, Atriplex portulacoides L. and Limoniastrum monopetalum L., in Southern Portugal. Estuar Coasts 30:459–468
Ocean and Fisheries Bureau of Zhejiang Province (OFBZ) (2011) Bulletin of marine environmental status in Zhejiang Province, China. http://www.zjoaf.gov.cn/attaches/2012/07/11/2012071100007.pdf
Palomo L, Clavero V, Izquierdo JJ, Avilés A, Becerra J, Niell FX (2004) Influence of macrophytes on sediment phosphorus accumulation in a eutrophic estuary (Palmones River, Southern Spain). Aquat Bot 80:103–113
Pant HK, Reddy KR (2001) Phosphorus sorption characteristics of estuarine sediment under different redox conditions. J Environ Qual 30:1474–1480
Picard CR, Fraser LH, Steer D (2005) The interacting effects of temperature and plant community type on nutrient removal in wetland microcosms. Bioresour Technol 96:1039–1047
Quan WM, Han JD, Shen AL, Ping XY, Qian PL, Li CJ, Shi LY, Chen YQ (2007) Uptake and distribution of N, P and heavy metals in three dominant salt marsh macrophytes from Yangtze River estuary, China. Mar Environ Res 64:21–37
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–211
Schalles JF, Shure DJ (1989) Hydrology, community structure, and productivity patterns of a dystrophic Carolina Bay wetland. Ecol Monogr 59:365–385
Schindler DW (2006) Recent advances in the understanding and management of eutrophication. Limnol Oceanogr 151:356–363
Schulz M, Kozerski HP, Pluntke T, Rinke K (2003) The influence of macrophytes on sedimentation and nutrient retention in the lower River Spree (Germany). Water Res 37:569–578
Shao XX, Yang WY, Wu M (2011) Soil organic carbon content and its distribution pattern in Hangzhou Bay coastal wetlands. Chin J Appl Ecol 22:658–664 (in Chinese)
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems: a global problem. Environ Sci Pollut Res 10:126–139
Song KY, Zoh KD, Kang H (2007) Release of phosphate in a wetland by changes in hydrological regime. Sci Total Environ 380:13–18
Sousa AI, Lillebø AI, Pardal MA, Caçador I (2010) Productivity and nutrient cycling in salt marshes: contribution to ecosystem health. Estuar Coast Shelf Sci 87:640–646
Sousa AI, Lillebø AI, Risgaard-Petersen N, Pardal MA, Caçador I (2012) Denitrification: an ecosystem service provided by salt marshes. Mar Ecol Prog Ser 448:79–92
Stumpf RP (1983) The process of sedimentation on the surface of a salt marsh. Estuar Coast Shelf Sci 17:495–508
White DS, Howes BL (1994) Long-term 15N-nitrogen retention in the vegetated sediments of a New England salt marsh. Limnol Oceanogr 39:1878–1892
Yang JZ, Zhao YL, Wang Y (2004) Remote sensing dynamic monitoring of tidal banks in the Hangzhou Bay. Chin J Geol 39:168–177 (in Chinese)
Zhou JL, Wu Y, Kang QS, Zhang J (2007) Spatial variations of carbon, nitrogen, phosphorous and sulfur in the salt marsh sediments of the Yangtze Estuary in China. Estuar Coast Shelf Sci 71:47–59
Zhou XJ, Gao S (2004) Spatial variability and representation of seabed sediment grain sizes: an example from the Zhoushan-Jinshanwei transect, Hangzhou Bay, China. Chin Sci Bull 49:2503–2507
Acknowledgments
This research was supported by the National Natural Science Foundation of China (21077088; 31000296) and the National Major Science and Technology Programs for Water Pollution Control and Treatment (2012ZX07506-006). We thank Stacey Ollis for language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Shao, X., Wu, M., Gu, B. et al. Nutrient retention in plant biomass and sediments from the salt marsh in Hangzhou Bay estuary, China. Environ Sci Pollut Res 20, 6382–6391 (2013). https://doi.org/10.1007/s11356-013-1698-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1698-6