Abstract
Selected arsenic-volatilizing indigenous soil bacteria were isolated and their ability to form volatile arsenicals from toxic inorganic arsenic was assessed. Approximately 37 % of AsIII (under aerobic conditions) and 30 % AsV (under anaerobic conditions) were volatilized by new bacterial isolates in 3 days. In contrast to genetically modified organism, indigenous soil bacteria was capable of removing 16 % of arsenic from contaminated soil during 60 days incubation period while applied with a low-cost organic nutrient supplement (farm yard manure).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is an environmental threat of geogenic and anthropogenic origin. It is prevalent in many regions of the world (Tripathi et al. 2007; Mandal and Suzuki 2002), especially in the lower Ganges river Plain of Bengal, India (Rahman et al. 2005) and Bangladesh (Smith et al. 2000). Use of As-contaminated groundwater for irrigation made the soil a major sink, affecting farmland ecosystems (Williams et al. 2009).
The solubility, mobility, and bioavailability (and hence toxicity) of arsenic in soil systems depends on the chemical form, primarily the oxidation state. Estimation of total As fails to determine the species-dependent toxicity problem of the metalloid As (Heikens et al. 2007). Trivalent arsenic inactivates enzymes and affects the respiratory system, while pentavalent arsenic is rapidly excreted from the body. Generally, inorganic arsenic species are believed to be more toxic than organic forms to living organisms, including humans and other animals (Goessler and Kuehnett 2002; Meharg and Hartley-Whitaker 2002).The toxicity of different arsenic species were often reported to vary in an order of arsenite > arsenate > mono-methylarsonate (MMA) > dimethylarsinate (DMA) (Penrose 1974; Stugeron et al. 1989). For many years it was believed that the acute toxicity of inorganic arsenic was greater than organic arsenic and hence, the methylation of inorganic arsenic was a detoxification reaction. This dogma was held because DMAV, the primary excreted metabolite of inorganic arsenic, is less acutely toxic than inorganic arsenic, until it was reported recently that derivatives of MMAIII (LD50 for mice is 2 mg As kg−1 oral feed) are much more toxic than arsenite (LD50 for mice is 26 mg As kg−1 of oral feed) (Hughes 2002).
Problems related to arsenic contamination have drawn attention worldwide and several physical and chemical remediation processes have been developed (Hering et al. 1996). However, these are expensive and have limited use as poverty and contamination coexist in most As-contaminated areas in the world (Zhu et al. 2009). Considering the limitations of conventional remediation techniques, biological methods using microbes, could be explored as alternative mitigation options (Singh et al. 2008; Chandraprabha et al. 2011).
Bioremediation of As by microorganisms has been widely hailed because of their potential advantage in providing a cost-effective, eco-friendly technology for heavy-metal removal (Valls and De Lorenzo 2002). Conversion of metal(loid)s to their volatile derivatives by organisms is a well-known phenomenon in nature (Challenger et al. 1945). During arsenic volatilization, some species of fungi and bacteria methylate inorganic As species to relatively less toxic volatile methylarsenicals (Rodriguez 1999; Cernansky et al. 2009). Arsenic biovolatilization starts with a reduction of AsV to AsIII and through a series of methylation reaction forms less toxic volatile organo-arsenicals (Turpeinen et al. 1999; Bentley and Chasteen 2002).
Formation of gaseous As has been reported (Abedin et al. 2002; Mahimairaja et al. 2005). As summarized by WHO (WHO 2001a, b), volatilization can substantially contribute to As removal from top soils, up to 12–35 % year−1. Several species of soil dwelling microorganisms have shown As-volatizing potential (Frankenberger 2002; Turpeinen et al. 2002, Islam et al. 2007; Mohapatra et al. 2008). This includes species of Penicillium and Aspergillus, which can volatilize both organic and inorganic As compounds, while different species of Pseudomonas were capable of volatilizing inorganic As. The rate of biovolatilization of AsV was observed to be 23 % while for AsIII it was 26 % by Staphylococcus sp. in 3 days from culture media (Srivastava et al. 2012). Most of the previous reports related to As volatilization involved either aerobic (Pseudomonas, Bacillus, and Alcaligenes) or anaerobic (Clostridium, Desulfovibrio, and Methanobacterium) bacteria (Bentley and Chasteen 2002).
Earlier attempts made to assess the capabilities of microorganism in decontaminating soil matrices have often dealt with genetically engineered organisms (Liu et al. 2011) or in simulated in situ situations of anthropogenic contamination. The As contamination in West Bengal is characteristically different, geogenic in origin, and widespread across waterlogged rice ecosystems which poses an unique soil micro-environment of staggered anaerobic and aerobic spells. No specific data for anaerobic soils such as flooded paddy fields have been reported, making it yet not possible to assess the importance of As behavior in paddy fields (Abedin et al. 2002; Mahimairaja et al. 2005). Thus, the present investigation has been aimed to characterize the arsenic decontaminating microorganisms, naturally isolated from the soils of the endemic area. The mechanism which coincidentally appears to regulate As may have evolved in an arsenic-rich environment and may help stake-holders of the contaminated area, principally marginal in socio-economic stratum, to provide for a low-cost, user-friendly technology for arsenic mitigation. To reach such an outcome the present study has been designed with the specific objectives to identify efficient As-volatilizing soil microbes isolated from the polluted soil and to evaluate their volatilizing potential when enriched with farm yard manure and in contaminated aerobic and anaerobic soil systems.
Materials and methods
Isolation and screening of arsenic-volatilizing microbes
Arsenic-contaminated surface soils (0–15 cm) were collected from different sites of Chakdah block of Nadia district, India. Total and extractable As concentrations were estimated (Sparks et al. 2006). For isolation and purification of As-resistant bacterial strains, diluted soil samples prepared in sterile saline solution, were plated on solidified Luria Bertani (LB) medium (an enriched medium for growth of the isolates under stressed conditions) with either 500 mg L−1 AsIII or 1,000 mg L−1 AsV and incubated at 30 °C for 48 h. Arsenic volatilization capacities of 65 purified bacterial isolates were analyzed by a modified trapping method (Gao and Burau 1997). Single colonies of As-resistant bacteria were grown in closed vials containing 50 mL LB medium with 25 mg L−1 AsV incubated at 30 °C for 3 days and capped with mercuric nitrate impregnated filter paper (Edvantoro et al. 2004). Trapping material (filter paper) was prepared by soaking Whatman 541 cellulose filter paper with Hg2 (NO3)2·2H2O in 10 % acetic acid water for 3 h and then air-dried (Pearce et al. 1998). Volatilized As was trapped in filter paper and analyzed for total As in an atomic absorption spectrophotometer (PerkinElmer, USA) coupled with FIAS 400 (Sarkar et al. 2012).
Tolerance to inorganic arsenic
Tolerance to AsV and AsIII were determined for each isolate by growing them separately in 20 mL LB liquid medium spiked with increasing concentrations of AsV (0 to 500 mM) and AsIII (0 to 100 mM) and incubated for 48 h at 30 °C. Controls for each concentration were inoculated with cell suspension grown in LB medium without As to obtain a cell density of approximately 106 CFU (colony forming unit) mL−1. Bacterial growth was monitored by measuring the optical density at 600 nm with a UV–vis spectrophotometer (Varian Cary-50 UV–vis). Bacterial isolates that could tolerate the highest As concentrations were selected and identified by their morphological features and biochemical properties (Holtz 1993).
Identification of the bacterial isolates based on 16S rDNA sequence analysis
Bacterial isolates showing considerable AsIII-volatilizing ability and higher As tolerance capacity were selected for molecular identification by 16S rDNA sequence analysis. Total genomic DNA was extracted (Sambrook 2001), and PCR amplification of 16S rRNA gene with forward primer 27F 5′-AGA GTT TGA TCM TGG CTC AG-3′ and the reverse primer 1492R 5′-GGY TAC CTT GTT ACG ACT-3′ (Chroumous Biotech Private Limited, India) were carried out. The reaction mixture composed of 1X PCR buffer, 0.2 mM dNTPs, 10 pmole of each primer, 60 ng of DNA template, 2 units of Taq DNA polymerase, and sterile deionized water to a final volume of 25 μL. Before amplification cycle, DNA was initially denatured at 94 °C for 5 min followed by 1 min denaturation at 94 °C. After amplification, a final extension step (10 min at 72 °C) was performed. The cycling parameters consisted of 35 cycles initiated through denaturation at 94 °C for 5 min, denaturation at 94 °C for 1 min, primer annealing at 52 °C for 1 min, and extension at 72 °C for 5 min. The PCR products were purified and held at 4 °C until verification through agarose gel electrophoresis (1 %).
The amplified and gel-eluted PCR fragments of the rDNA were sequenced in ABI 3100 Genetic Analyzer with primers 27F. Sequencing reaction was performed by using the Big Dye terminator cycle sequencing Kit V3.1 (Applied Biosystems, Foster City, USA) following the manufacturer’s protocol. The partial 16S rDNA sequences of the isolated strains were compared with those available in the GenBank database by BLASTN algorithm to identify sequences with a high degree (≥98 %) of similarity.
Biovolatilization of arsenic by efficient bacteria in culture medium
Volatilization of inorganic As species mediated through selected bacterial strains were estimated in aerobic and anaerobic situations. Volatilized As species were trapped in mercurous-nitrate-impregnated filter paper (Gao and Burau 1997). To investigate As volatilization, LB medium was spiked with AsV and AsIII (5, 10, 15, 20, 25, and 30 mg L−1) and incubated with different bacterial strains in closed vials with mercurous-nitrate-soaked filter papers as As-trapping device. Sodium arsenate (Na2HAsO4, 7H2O) and sodium arsenite (NaAsO2) were used. Freshly prepared 100 μL cells of each culture media (106 CFU mL−1) were added in 25 mL LB medium in filter paper capped closed vessel, incubated at room temperature 25 ± 3 °C for 3 days (Liu et al. 2011). Anaerobic condition was maintained through N2 sparging for 2 min to displace oxygen and the bottles were prepared under N2 atmosphere. Each set of experiments was established in triplicate and total As content of the trapped material was measured (Sarkar et al. 2012).
Arsenic species identification during volatilization
A microwave digestion system (Multiwave 3000, Anton Par) with a rotor of 48 Teflon digestion vessels was used for sample digestion and extraction. For total As analysis 5 mL of culture media and trapped filter paper disk were taken in a clean Teflon digestion vessel and 5 mL aqua regia was added to it. The vessel was then digested in a microwave followed by a 5-min hold and analyzed by inductively coupled plasma–mass spectrometry (ICP-MS; PerkinElmer ELAN DRCe 6000) for total As. For speciation analysis 5 mL of culture media (culture broth of the previous experiment spiked with 10 mg L−1 AsV was taken) and the trapped filter paper added with 10 mL of orthophosphoric acid (1 M) with ascorbic acid and placed in a microwave teflon vessel and the mixture was maintained at 60 W for 10 min and subjected for separation through an anion exchange column (Hamilton PRP-X100 F with a precolumn used for chromatographic separation). For the isocratic method, a PerkinElmer Series 200 Micro Pump was used instead of the quaternary pump and the detections and quantifications have been done through a PerkinElmer ELAN DRCe ICP-MS.
Volatilization of arsenic from arsenic-contaminated soil
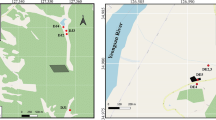
Arsenic-contaminated soil used for this experimental study was collected form Ghentugachi (N 23°02′04.4″ and E 088°34′55.5″) village of Nadia district, India. Total As concentration of the soil is 27 mg kg−1 and extractable As concentration is 7 mg kg−1 pH 7.51. To determine As volatilization by efficient bacterial strains, microcosm experiments were performed with 500 g of air-dried test soil in teflon-coated closed bottles through incubation with 5 mL culture of different bacterial strains. Formaldehyde (0.04 %) was used to kill the microbes in control soil. It inhibits the microbial activity but does not change the pH and dissolved nutrient concentration of the soil. Farm yard manure was used as organic source and added at 0, 2.5, and 5 g kg−1 and thoroughly mixed to 500 g of soil. Volatilized As evolved from the soils was trapped in mercurous-nitrate-impregnated filter papers placed in the caps of closed bottles, incubated at room temperature (25 ± 3 °C) for 30 and 60 days. Each experiment was performed in triplicate and appropriate moisture conditions of the soils were maintained according to the method of Edvantero et al. (2004). Arsenic trapped filter papers were removed at 10-day intervals and total soil As concentration was determined through the standard method (Sparks et al. 2006) at the end of the incubation period.
Results
Arsenic tolerance and molecular identification of bacterial strains
Selected bacterial strains, isolated from contaminated soil showed considerable resistance to As toxicity with varying levels of tolerance capacity (Table 1). Twelve bacterial isolates, having As-volatilizing activity, were subjected to As resistance tests and most of them were found tolerant to As concentration higher than 100 mM. The minimal inhibitory concentration (MIC) of inorganic As for selected strains were observed within a range of 106.6–400 mM for AsV and 10–53.3 mM for AsIII while highest tolerance to AsV (400 mM) and AsIII (53.3 mM) was exhibited by the isolate AMT-08 and AMT-04, respectively. Molecular characterization of the strains has been carried out based on sequencing of 16S rDNA and compared with existing GenBank databases (Table 1).
Biovolatilization of arsenic by efficient bacteria in culture medium
Incubation studies with 12 selected As-volatilizing bacterial strains were conducted in cultures spiked with 25 mg L−1 AsV for 72 h under aerobic and anaerobic condition to assess their As-volatilizing efficiency (Fig. 1). The bacterial isolates AMT-08 and AGH-09 showed higher As-volatilizing capacity under aerobic conditions, ADP-18 volatilized maximum As under anaerobic conditions while AMT-04 performed well in both conditions.
The As volatilization capacity of the selected bacterial strains has been initiated across a range of inorganic AsIII and AsV spiked in the system (Table 2). AMT-08 showed highest efficiency of removing both AsIII and AsV from culture media spiked with 5 mg L−1 of AsIII/AsV in aerobic situation while such efficiencies tend to decrease with increasing concentration of As in media (Table 2). Similar efficiencies have been shown by ADP-18 under anaerobic situation which volatilized about 35 % AsIII and 30 % AsV. Interestingly, AMT-04 simultaneously supported considerable arsenic volatilization from aerobic as well as anaerobic systems. It is a characteristic feature of facultative aerobe, showing better performance under anaerobic environment but lesser activity under aerobic condition.
Arsenic species identification during volatilization under aerobic system
Arsenic speciation has been measured by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry (Hymer and Caruso 2004; Sanz et al. 2007).
Arsenic speciation studies in culture broth (cells + LB medium; Table 3) and the filter trap (Table 4) showed recoveries up to 87 % of total As. Highest percent recovery in trapping filter paper was observed with strain ADP-18. All the As species recovered were AsIII and AsV with DMA and MMA at non-detectable levels. Significant proportions of AsIII have been recovered from the culture broth when the media has been solely spiked by AsV.
It is interesting to note that the As trapped in the filter paper did not show any recoveries of AsIII or DMA, MMA, arsenobetaine (As-B) which envisaged that whatever As has been volatilized during incubation was in the form of AsV, although, at the same time, AsIII and AsV coexist in the culture broth at the end of incubation inoculated with almost all bacterial strains. The unaccounted part of total As which could not be retrieved by summing up the individual species, may be due to other As species (DMA, MMA, As-B etc.) in nondetectable proportions both in filter-trapped accumulation and in the cell + LB medium.
Arsenic removal from contaminated soil by microbial volatilization
To take an account of in situ As volatilization efficiencies of selected bacterial strains, contaminated soils were incubated for 30 and 60 days with efficient As-volatilizing bacterial inoculants with or without organic supplementation through farm yard manure. Significant variations in As volatilization were observed with varying inoculants and organic supplementation as compared to respective controls (Table 5). Arsenic removal from the soil system through volatilization by bacterial strains were observed to increase with advancement of incubation period irrespective of the bacterial strains involved which has been further stimulated with increasing application of organic amendment.
AMT-08 has been found most successful in managing removal of the metalloid from soil throughout the entire incubation period (Fig. 2) and recorded a maximum 4.22 mg kg−1 at 60th day of incubation when the soil has been amended with 5 g kg−1 FYM of soil. It is interesting to note that significant arsenic recoveries in filter traps were also obtained from un-inoculated controls when amended with FYM at varying levels.
Discussion
Arsenic tolerance
Microbes developed various intrinsic As tolerance mechanism to sustain in the adverse environmental conditions. Metal resistant of bacteria often have genes located on plasmids. Genetic system named ars operon is the main functional unit for As resistance (Mukhopadhyay and Rosen 2002). arsR is the repressor of the operon, arsB can pump out AsIII present within the cell, arsC is the AsV reductase (Joshi et al. 2009). The bacterial strains isolated and studied through the present investigation have shown much higher resistance to AsV than those isolated from soil, gold mines, and geothermal effluents in the related researches throughout world (Saltikov and Olson 2002; Simeonova et al. 2004). The bacterial strains under the present investigation were isolated from anaerobic soil environment (submerged paddy soil) which is predominated by AsIII over AsV and hence showed much higher tolerance to AsIII as compared to findings from related research established as a model microorganism for bioremediation of arsenic and one of the most arsenic-resistant microorganisms (400 mM for AsV and 60 mM for AsIII) described to date (Mateos et al. 2006). The selected tolerant genera, Rhodobacter sphaeroides and Alcaligenes faecalis have not been previously isolated from arsenic-contaminated soil environments and no report exists about their role in arsenic bioremediation. Bacteria capable of arsenic volatilization isolated herein are genetically diverse which is in conformity with previous findings (Bentley and Chasteen 2002).
Bacteria mediated arsenic volatilization from culture media
Biovolatilization of As from culture media inoculated with 12 selected bacterial strains showed (Fig. 1) varying capabilities of As volatilization exhibited by different strains under aerobic and anaerobic conditions. Some strains (AMT-04, ADP-03) remained versatile in both the systems (aerobic and anaerobic). Similar observations have also been recorded by Mohapatra et al. (2008) who found that As volatilization decreased with increase in concentration of AsV and reached equilibrium after certain period of time.
The bacterial volatilization of As also depends on substrate and AsIII volatilization rates have been found to be relatively higher than AsV both from aerobic and anaerobic systems (Table 2, Fig. 3). Some microbes can take up AsV via phosphate transporter and then reduce AsV internally to AsIII which is then extruded from the cell (Joshi et al. 2009). This may be the probable reason of getting AsIII though we started the experiment with sole AsV. In case of methylation AsIII is a reaction intermediate of AsV reduction to MMA, DMA, TMA, and supposed to be more readily reduced than AsV. The rate of biovolatilization was 23 % (AsV) and 26 % (AsIII) in case of Staphylococcus sp (Srivastava et al. 2012) also supported the present findings. Fungal strains, Aspergillus niger, Aspergillus clavatus, and Neosartorya fischeri on the contrary, did not show any mentionable difference in volatilization output from AsIII or AsV system (Cernansky et al. 2009). This investigation identified the bacterial strain having As volatilization potential in both aerobic and anaerobic conditions from different inorganic As species.
Bacteria mediated arsenic volatilization from soil
With a view to develop an efficient biological tool for decontaminating As in soil matrices, volatilization was checked in naturally contaminated soil. Selected bacterial strains used in the present investigation showed varying capabilities of removing As from soil systems through volatilization which has been further stimulated when supplemented by external application of organic. These observations were supported by previous research reports that supplemented C source, which provided additional energy for microbes, was able to stimulate As volatilization (Edvantoro et al. 2004; Lee et al. 2005). Unlike other incubation study experiments, where the authors had sterilized the soils prior to microcosm inoculation (Turpeinen et al. 2002), soils were not sterilized in this study. We had tried to assess volatilization of As from soils by microcosms consisting of As-volatilizing soil bacteria under natural condition in presence of native microbial residents of soil. This would imitate the true conditions when the microcosms would be applied for As mitigation in contaminated area, since it would depict the competitive existence and volatizing activity of the applied bacterial strains in presence of other native soil bacteria.
Wide range of microorganisms, belonging to Methanobacterium, Bacillus, Pseudomonas, Streptococcus, Staphylococcus, Aspergillus, Penicillium, and Scopulariopsis strains, have been identified as potential producers of volatile As (Cullen and Reimer 1989). In the present investigation an indigenous soil bacterial strain AMT-08 have the ability to remove 10 % (in 30 days) and 16 % (in 60 days) As when supplemented with farm yard manure. Similarly, Woolson (1977) reported 18 % loss of As in 160 days under aerobic conditions, from artificially As spiked soils, whereas the reported strain AMT-08 is capable of removing twice As per day from soil. The strain AMT-08 has an increased As-volatilizing ability with exogenous nutrients (farm yard manure) and successful exploitation of these hyper-tolerant isolates may deliver an eco-friendly tool for As mitigation within manageable expenditure as compared to genetically engineered alternatives.
References
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Bentley R, Chasteen TG (2002) Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol Mol Biol Rev 66:250–271
Cernansky S, Kolencik M, Sevc J, Urik M, Hiller E (2009) Fungal volatilization of trivalent and pentavalent arsenic under laboratory conditions. Bioresource Technol 100:1037–1040
Challenger F (1945) Biological methylation. Chem Rev 36:315–361
Chandraprabha MN, Natarajan KA (2011) Mechanism of arsenic tolerance and bioremoval of arsenic by Acidithiobacilus ferrooxidans. J Biochem Tech 3(2):257–265
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Edvantoro BB, Naidu R, Megharaj M, Singleton I (2004) Microbial formation of volatile arsenic in cattle dip site soils contaminated with arsenic and DDT. Appl Soil Ecol 25:207–217
Frankenberger W, Arshad M (2002) Volatilization of arsenic in environmental chemistry of arsenic. In: W Frankenberger (eds). Marcel Dekker, New York. pp 363–380
Gao S, Burau RG (1997) Environmental factors affecting rates of arsine evolution from and mineralisation of arsenicals in soil. J Environ Qual 26:753–763
Goessler W, Kuehnett D (2002) Analytical methods for the determination of arsenic and arsenic compounds in the environment. In: Frankenberger Jr WTT (eds). Marcel Dekker, New York. pp 27–50
Heikens A, Panaullah GM, Meharg AA (2007) Arsenic behaviour from groundwater and soil to crops: impacts on agriculture and food safety. Rev Environ Contam Toxicol 189:43–87
Hering JG, Chen PY, Wilkie JA, Elimelech M (1996) Arsenic removal by ferric chloride. J Am Water Works Assoc 88:155–167
Holtz JD (1993) Bergey’s manual of determinative bacteriology, 9th edn. Williams and Wilkins, Baltimore
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Hymer CB, Caruso JA (2004) Arsenic and its speciation analysis using high-performance liquid chromatography and inductively coupled plasma mass spectrometry. J Chromatogr 1045:1–13
Islam SMA, Fukushi K, Yamamoto K, Saha GC (2007) Estimation of biologic gasification potential of arsenic from contaminated natural soil by enumeration of arsenic methylating bacteria. Arch Environ Contam Toxicol 52:332–338
Joshi N, Wang X, Montgomery L, Elfick A, French CE (2009) Novel approaches to biosensors for detection of arsenic in drinking water. Desalination 248(1):517–523
Lee JU, Lee SW, Kim KW, Yoon CH (2005) The effects of different carbon sources on microbial mediation of arsenic in arsenic-contaminated sediment. Environ Geochem Hlth 27:159–168
Liu S, Zhang F, Chen J, Sun G (2011) Arsenic removal from contajminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. J Environ Sci 23(9):1544–1550
Mahimairaja S, Bolan NS, Adriano DC, Robinson B (2005) Arsenic contamination and its risk management in complex environmental settings. Adv Agron 86:1–82
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mateos LM, Ordonez E, Letek M, Gil JA (2006) Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. Int Microbiol 9:207–215
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and non-resistant plant species. New Phytol 154:29–43
Mohapatra D, Mishrab D, Roy Chaudhury G, Das RP (2008) Removal of arsenic from arsenic rich sludge by volatilization using anaerobic microorganisms treated with cow dung. Soil Sediment Contam 17:301–311
Mukhopadhyay R, Rosen BP (2002) Arsenate reductases in prokaryotes and eukaryotes. Environ Health Perspect 110(Suppl 5):745–748
Pearce RB, Callow ME, Macaskie LE (1998) Fungal volatilisation of arsenic and antimony and the sudden infant death syndrome. FEMS Microbiol Lett 158:261–265
Penrose WR (1974) Arsenic in the marine and aquatic environments. Analysis, occurrence and significance. CRC Crit Rev Environ Control 4:465–482
Rahman MM, Sengupta MK, Kumar M, Ahamed S, Chowdhury UK, Hossain MK, Das B, Lodh D, Saha KC, Pati S, Kaies I, Barua AK, Chakroborty D (2005) The magnitude of arsenic contamination in groundwater and its health effects to the inhabitants of the Jalangi-one of the 85 arsenic affected blocks in West Bengal, India. Sci Total Environ 338(3):189–200
Rodriguez RR (1999) An in-vitro gastro-intestinal method to assess bioavailable arsenic in contaminated soils and solid media. Environ Sci Technol 33:642–649
Saltikov C, Olson B (2002) Homology of Escherichia coli R773 arsA, arsB, and arsC genes in arsenic-resistant bacteria isolated from raw sewage and arsenic-enriched creek waters. Appl Environ Microbiol 68:280–288
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Third edition. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sanz E, Muñoz-Olivas R, Cámara C, Sengupta MK, Ahamed S (2007) Arsenic speciation in rice, straw, soil, hair and nails samples from the arsenic-affected areas of middle and lower Ganga plain. J Environ Sci Health, Part A: Environ Sci Eng 42(12):1695–1705
Sarkar S, Basu B, Kundu CK, Patra PK (2012) Deficit irrigation; an option to mitigate arsenic load of rice grain in West Bengal India. Agric Ecosyst Environ 46:147–152
Simeonova D, Lievremont D, Lagarde F, Muller D, Groudeva V, Lett MC (2004) Microplate screening assay for detection of AsIII-oxidizing and AsV-reducing bacteria. FEMS Microbiol Lett 237:249–253
Singh S, Mulchandani A, Chen W (2008) Highly selective and rapid arsenic removal by metabolically engineered Escherichia coli cells expressing Fucus vesiculosus metallothionein. Appl Environ Microbiol 74(9):2924–2927
Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull WHO 78:1093–103
Sparks DL, Page AL, Helmke PA, Leoppert RH, Solthanpour PN, Tabatabai MA, Johnston CT, Sumner ME (2006) Methods of soil analysis, part 3, chemical methods. Soil Science Society of America, Inc, Madison, pp 811–831
Srivastava S, Verma PC, Singh A, Mishra M, Singh N, Sharma N, Singh N (2012) Isolation and characterization of Staphylococcus sp strain NBRIEAG-8 from arsenic contaminated site of West Bengal. Appl Microbial Biotechnol 95(5):1275–1291
Stugeron RE, Siu KWM, Willie SN, Berman SH (1989) Quantification of arsenic species in a river water reference material for trace metals by graphite furnace atomic absorption spectrometry technique. Analyst 114:1393–1396
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–164
Turpeinen R, Kallio MP, Kairesalo T (2002) Role of microbes in controlling the speciation of arsenic and production of arsines in contaminated soils. Sci Total Environ 285(1–3):133–145
Turpeinen R, Pantsar-Kallio M, Haggblom M, Kairesalo T (1999) Influence of microbes on the mobilization, toxicity and biomethylation of arsenic in soil. Sci Total Environ 236:173–180
Valls M, De Lorenzo V (2002) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26(4):327–338
WHO (2001a) Arsenic and Arsenic Compounds Environmental Health Criteria 224 Second ed World Health Organization, Geneva
WHO (2001b) Environmental Health Criteria 224: arsenic and arsenic compounds WHO, Geneva
Williams PN, Lei M, Sun GX, Huang Q, Lu Y, Deacon C, Meharg AA, Zhu YG (2009) Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Environ Sci Technol 43(3):637–642
Woolson EA (1977) Fate of arsenicals in different environmental substrates. Environ Health Perspect 19:73–81
Zhu YG, Rosen BP (2009) Perspectives for genetic engineering for the photoremediation of arsenic contaminated environments: from imagination to reality? Curr Opin Biotechnol 20:220–224
Acknowledgments
The authors acknowledge financial support from World Bank through ICAR (PIU-NAIP) and facilitation obtained from ICAR Niche Area of Excellence.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Majumder, A., Bhattacharyya, K., Kole, S.C. et al. Efficacy of indigenous soil microbes in arsenic mitigation from contaminated alluvial soil of India. Environ Sci Pollut Res 20, 5645–5653 (2013). https://doi.org/10.1007/s11356-013-1560-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1560-x