Abstract
Purpose
The present study aimed to evaluate the effects of an intradialytic multicomponent exercise program (IMEP) on respiratory muscle strength, functional capacities, and inflammatory markers in people with chronic kidney disease (CKD). Methods: This was a randomized clinical trial in which 38 people with CKD were randomly allocated to training (TG; n = 19) or a control group (CG; n = 19). The TG performed 12 weeks of IMEP which consisted of aerobic training (AT), inspiratory muscle training (IMT), and resistance training (RT), three times a week on nonconsecutive days. Before and after 12 weeks of follow-up respiratory muscle strength, functional capacities, and inflammatory markers were measured. Post–pre-values were calculated and covariance analysis was used with the Bonferroni post hoc test using preintervention values as a covariate and significance was set at p < 0.05. Results: Adherence to the protocol was 92%. TG showed improvements in the 30-s sit-to-stand test (p < 0.001), Timed Up and Go (TUG) (p = 0.02), 6-min walk test (p < 0.001), right and left-hand grip strength (p < 0.001), and respiratory muscle strength maximal inspiratory (p = 0.02) and expiratory (p = 0.02) pressures compared to CG. There were no significant group differences for inflammatory markers. Conclusion: Twelve weeks of IMEP resulted in improved functional capacity and respiratory muscle strength in people with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a disorder that affects the structure and functioning of the kidney. In 2017, approximately 1.2 million people died from the disease, and, in addition, there was a 41.5% increase in the mortality rate since 1990 [1]. It has been reported that people with CKD have a high morbidity and mortality rate from cardiovascular diseases, which are linked to CKD-related factors, treatment, and lifestyle. [2,3,4]. In addition, important pulmonary changes have been observed, such as airflow limitation, obstructive disorders, reduced pulmonary diffusion capacity, and respiratory muscle strength, which, in turn, results in atrophy, cramps, asthenia, and muscle weakness [5, 6]. CKD is also associated with a low level of a patient’s health-related quality of life (HRQoL), especially in dialysis patients. Among the factors that impact HRQoL in CKD, inactivity, and fragility in the physical domain stand out [7].
Previous studies have shown that people with CKD undergoing hemodialysis treatment do not reach the recommended levels of physical activity and are less active compared to those without the disease, mainly because they are physically inactive during hemodialysis sessions [8, 9]. The worst physical activity level was observed in hemodialysis (HD) compared to peritoneal dialysis (PD) patients [10], and a previous study [11] showed for the first time the progressive decrease in physical activity level since CKD stages 3–4. In a study with more than 5000 participants, Wilkinson et al. (2021) [12] show that low physical activity level is already present at the first stages of CKD and it worsens with disease progression. Furthermore, the level of physical activity in people with CKD is associated with disease progression, cardiovascular events, chronic inflammation, physical and functional capacity, reduced quality of life, and mortality[13,14,15].
In this sense, a growing number of studies have found a positive effect of both aerobic and strength training programs [16,17,18] performed during hemodialysis on cardiorespiratory capacity, muscle volume, and strength, reduction of cardiovascular risk, and reduction of CKD progression. [19,20,21,22]. On the other hand, approaches using an intradialytic multicomponent exercise program (IMEP), that performs in different days aerobic, respiratory, and strength training to improve functional capacity in this population remain scarce. Thus, the present study aimed to evaluate the effects of an IMEP on functional capacities, respiratory muscle strength, and inflammatory biomarkers in people with CKD.
Methods
This is a randomized clinical trial carried out between June and December 2019 at the Institute of Hemodialysis and Renal Transplantation of the Clinics Hospital from the Federal University of Triângulo Mineiro in Uberaba/Minas Gerais, Brazil.
Participants
Adult participants (≥ 18 years), men, and women who were on hemodialysis treatment for at least 3 months were included. Participants with fasting glucose > 300 mg/dL, unstable angina, cardiac arrhythmia, decompensated heart failure, uncontrolled hypertension, uremic pericarditis, respiratory diseases, acute systemic infection, visual impairment, or musculoskeletal limitations that compromised the performance of the proposed exercises were excluded from the study. Initially, patients with visual impairment would not be excluded. However, the ethics committee suggested the exclusion of these patients due to the risk of injury in the motor tests.
All information regarding the evaluation and training protocols was explained to the volunteers, who agreed and signed the free and informed consent form, approved by the Ethics and Research Committee (protocol no. 3426374). The study is included in the Brazilian Registry of Clinical Trials (protocol no. RBR-4xqpmm).
After medical release, volunteers were evaluated at baseline and after 12 weeks of intervention, immediately before hemodialysis sessions on dialysis days.
Procedures
Before starting the physical training program, all patients underwent anamnesis through a sociodemographic questionnaire, anthropometric assessments, physical capacities, respiratory muscle strength, and blood collection.
Participants’ characteristics
Demographic data were collected through a questionnaire. For this, the participants were taken to a reserved room, separated from the others, so that there was no interruption or embarrassment during the answers. Data regarding disease status were extracted from the participant's medical records.
Anthropometric assessment
Body mass and height were measured using a mechanical scale and coupled stadiometer, with a maximum capacity of 150 kg, a sensitivity of 100 g, and a precision of 0.1 cm, respectively (Filizola, Campo Grande/MS, Brazil). Then, the body mass index [(BMI) = body mass (kg)/height2 (m)] was calculated.
Physical capacity and respiratory muscle strength
Participants were submitted to maximum handgrip strength tests with a Jamar® dynamometer (Yangdeok – Dong, Sh5001, Masan, Korea) [23]; 30-s sit-to-stand test (STS-30) [24]; 6-min walk test (6MWT); [25] functional mobility test "Timed Up and Go Test (TUG)": in this test, the patient is asked to get up from a chair (seat height 45 cm), walk 3 m, return and sit down again, while the time spent in carrying out this task is timed [26].
Respiratory muscle strength was assessed using maximal inspiratory (PImax) and maximal expiratory (PEmax) pressure with a manovacuometer (Instrumentation Industries, São Paulo, Brazil), connected to a mouthpiece, which measures pressures from 0 to + 120 cmH2O for expiratory pressures and 0 to− 120 cmH2O for inspiratory pressures. The MIP is the strength index of the inspiratory muscles (diaphragm and external intercostals), while the MEP measures the strength of the expiratory muscles (abdominal and internal intercostals). The volunteers were seated, using a nose clip and keeping the mouthpiece between their lips. Three acceptable maneuvers were performed, maintained for at least 1 s and with a 1-min rest interval. The highest value was considered [27].
Blood collection and analysis
Blood samples were collected from the intermediate vein of the arm, in vacuum tubes (20 ml) (BD, London, England). The inflammatory biomarkers levels: CRP, IL-1b, IL-6, IL-10, and TNF-α were determined in the patients’ plasma by ELISA. The blood was centrifuged at 3000 rpm for 10 min and the serum was immediately separated in duplicate in Eppendorf and frozen at − 20 °C. A specific kit (BD Biosciences, San Jose, CA, USA) and automated equipment (Facscalibir, Becton Dickinson, USA) were used for the analyses.
Experimental protocol
Before starting the training session, blood pressure, resting heart rate, oxygen saturation, respiratory rate, and, in diabetic patients, capillary blood glucose was measured. For patient safety, the exercise session was only performed if systolic blood pressure (SBP) was between 110 and 180 mmHg and/or diastolic blood pressure (DBP) between 50 and 100 mmHg and, also, resting heart rate between 50 and 100 bpm. For diabetic patients, capillary blood glucose should be between 100 and 250 mg/dL.
The IMEP was performed three times a week for 12 weeks, totaling 36 sessions. The program consisted of aerobic training (AT), inspiratory muscle training (IMT) and resistance training (RT) supervised by an exercise-qualified professional with 2 years of experience in the field. In the execution of intradialytic exercises, care was taken to perform during the first 2 h of dialysis, and, in addition, blood pressure, heart rate, and oxygen saturation were monitored during training sessions.
The AT was performed in the first weekly session with a cycle ergometer (Mini Bike E5, ACTE Sports, São Paulo, Brazil) positioned in front of the volunteer's chair. Each session was divided into a warm-up, main activity, and cool-down. The intensity of each training was controlled by Borg's modified subjective perception of exertion (RPE) 0 to 10 scale [28, 29]. During the exercises, the participants were asked every five minutes about the fatigue score, to adjust the load on the cycle ergometer in Borg = 4 to 6 points. This simple method evaluates the individual on their global assessment of training load, considering central factors (lung ventilation, for example) and peripheral factors (muscles and joints). Blood pressure was monitored every 5 min, at rest and after cooling down. Heart rate and oxygen saturation were constantly monitored using a heart rate monitor (Polar H10, Finland) and portable oximeter (finger oximeter PM100C, New Tech, U.S.A), respectively. The proposed program followed a sequence of load and volume adjustments each week, with a progression from 10 min in the first week to 36 min in the last week.
The IMT was performed in the second weekly session using the Threshold IMT® (Respironics, Murrysville PA, USA), which consists of linear pressure load equipment for inspiratory muscle training. The load selected in the Threshold IMT for inspiratory muscle strengthening followed an increasing staggering through the results of the respiratory muscle strength test collections, with an initial load estimated at 10% of the manuvacuometry test and a final load of 40%. Load adjustment took place every three sessions.
The proposed RT program was performed in the third weekly session and consisted of exercises for the muscle groups: quadriceps [knee extension (shin guard)]; biceps brachii [unilateral curl (halter)]; shoulder [flexion, with front elevation (halter)] and iliopsoas, sartorius, rectus femoris [hip flexion (shin guards)]. The RT followed the principle of progressive load increase, with the initial use of a set of 10–15 repetitions, until reaching three sets of 10–15 repetitions. Load adjustments occurred monthly (every twelfth session) to maintain RPE between 6–7 points on the modified Borg Scale [30]. In all phases of the protocol, the participant was given a rest interval of 90 to 120 s between sets and between exercises. Finally, the exercises were performed following the alternating training method by segment to avoid early muscle fatigue.
Statistical analysis
Data normality was verified by the Shapiro–Wilk test. Levene's test was applied to analyze the homogeneity of variances between the groups. Group baseline differences were assessed by one-way analysis of variance (ANOVA). Δ (post—pre) values were calculated, and covariance analysis (ANCOVA) was used with the Bonferroni post hoc test using preintervention values as a covariate. Cohen’s d coefficient was used to estimate the magnitude of the effect (η2) of the intervention, which could be interpreted as small (η2 = 0.2), medium (η2 = 0.5), or large (η2 = 0.8) (COHEN, 1992). The established significance level of p < 0.05. A non-parametric partial correlation (adjusted for HD time) was performed between the Δ of the variables. The number of participants was based on the power and sample size calculation, using the GPower 3.1 software, with an effect size of 0.51, α = 0.05, and a power of 80%, totaling a sample of 34 volunteers. Taking into account the one-way ANOVA analysis, we used the effect size f = 0.50 to achieve a sample size close to other studies with a similar design [31,32,33,34,35].
Significance was set at p < 0.05. All analyses were performed using SPSS (version 20.0, SPSS Inc., Chicago, IL).
Results
Initially, 52 eligible patients were recruited and invited to participate in the research, with prior authorization from the team's physicians. Of these, 14 were excluded, 38 were randomized, employing a simple and random drawing, removing papers with the numbers in a basket, and allocated to the training group (TG = 19) and control group (CG = 19). During the 12 weeks of intervention, three patients from the TG died and two underwent kidney transplantation. Four patients in the CG withdrew from participating in the research for personal reasons, totaling 27 individuals at the end of the intervention, Fig. 1.
The demographic and clinical characteristics of the population studied at baseline are shown in Table 1. There is a predominance of males in the CG and TG in both groups. The mean age was higher for the TG compared to the CG (p = 0.03). There was no difference for the other variables. The main etiology related to CKD was glomerulonephritis for the CG (35%); hypertensive nephrosclerosis and others for TG (38%), respectively. The total intervention time was 12 weeks, totaling 36 individualized IMEP sessions. Adherence to the protocol was 92% for the TG. No significant complications were observed with the performance of the exercises during the training sessions, with the occurrence of only two cases: one of hematoma associated with an arteriovenous fistula, registered after the end of the exercise due to the patient's carelessness; and another due to the need to change the dialyzer device, when the patient felt a slight indisposition.
The characteristics of the functional profile, respiratory muscle strength, and inflammatory markers between groups at baseline can be seen in Table 1. There was no significant difference in the variables presented.
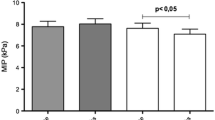
Table 2 shows the groups' comparison in physical capacities, respiratory muscle strength, and inflammatory markers after a 12-week IMEP intervention in people with CKD. For physical capacities, a statistical difference was observed between groups in all tests, STS-30 (p < 0.001); TUG (p = 0.02); 6MWT (p < 0.001); right (p < 0.001) and left (p < 0.001) handgrip strength. There was a significant difference for the respiratory muscle strength variables PI max and PE max, with (p = 0.02) for both results.
Regarding the inflammatory markers, the concentrations of the cytokines IL-10 and TNF-α were not detectable. No significant differences were observed for the cytokines, IL-6, IL1-β, IL-10, TNF-α, and CRP.
Table 3 presents partial correlations between physical function and respiratory muscle strength, controlled by hemodialysis time. There was a negative correlation between ∆STS-30 and ∆TUG (r = − 0.649; p < 0.001) and a positive correlation with ∆6MWT (r = 0.593; p < 0.001), ∆RHS (r = 0.602; p < 0.001), ∆LHS (r = 0.631; p < 0.001) and ∆PEmax. (r = 0.493; p < 0.05). ∆TUG showed only negative correlations with ∆6MWT (r = − 0.556; p < 0.001), ∆RHS (r = − 0.445; p < 0.05) and ∆PE max. (r = − 0.393; p < 0.05). The 6MWT showed positive correlations with ∆RHS (r = 0.484; p < 0.05), the ∆LHS (r = 0.446; p < 0.05), at ∆PI max. (r = 0.517; p < 0.05) and the ∆PE max. (r = 0.699; p < 0.001). In the parameters of hand grip strength, in ∆ RHS the correlations were positive with ∆LHS (r = 0.623; p < 0.001), the PI max. (r = 0.456; p < 0.05) and ∆PE max. (r = 0.484; p < 0.05). On the other hand, ∆LHS presented positive correlations with the ∆PI max. (r = 0.523; p < 0.05) and with ∆PE max. (r = 0.420; p < 0.05). The other correlations can be seen in Table 3.
Discussion
The present study investigated the effects of 12 weeks of IMEP on respiratory muscle strength parameters, functional capacities, and inflammatory markers in people with CKD. Our results showed improvement in physical capacity and respiratory muscle strength but no difference in inflammatory markers. Although advances related to dialysis treatment are of great value, the practice of exercises is still uncommon in hemodialysis clinics [8]. In this sense, the present study adds relevant information regarding the clinical safety of the intervention performed during hemodialysis sessions, taking individualized and supervised care into account. The adherence observed in our study was 92%, with minimal intercurrences throughout the development of the study, corroborating previous evidence [36, 37].
We found a significant improvement in the physical capacity of people with CKD after 12 weeks of exercise intervention. For example, muscular endurance, functional mobility, and handgrip strength improved significantly, albeit at a small effect size (0.38, 0.21, and ~ 0.44, respectively). On the other hand, aerobic resistance increased at a moderate effect size (0.51). We believe that the combination of varied exercise stimuli during dialysis contributes to the improvement of people with CKD motor function. The results reveal that the gains in muscular strength of the lower and upper limbs associated with an increase in respiratory muscular strength results in mobility improvements in this population. It is possible to affirm, therefore, that improving or maintaining physical aptitudes in people with CKD minimizes the loss of their ability to perform activities in daily life and occupational tasks. In line with our findings, a previous meta-analysis reported the benefits of exercise in improving physical function and other aspects [38]. In addition, Fernandes et al. (2019) [33], in a randomized clinical trial, treated 39 patients using aerobic training with a cycle ergometer for 8 weeks and found a significant difference between CG and TG groups in the parameters MIP, MEP, and 6MWT. Still in this line, Figueiredo et al. (2018), in an 8-week protocol of inspiratory muscle training (IMT) at 50% of maximal inspiratory pressure (PImax), low-intensity aerobic training (AT) or combined training (CT), observed that IMR, TA, and CT improved functional parameters and modulated inflammatory biomarkers; in addition, IMT elicited a similar response to low-intensity AT in hemodialysis patients.
People with CKD present chronic inflammation characterized by high serum levels of CRP and IL-6, which is due to a multifactorial cause and can be attributed to the transfer of endotoxins from the dialysis capillary membrane to the blood during sections, activation of pro-inflammatory cytokines, and by endothelial alterations, which lead to protein-energy malnutrition and decreased survival [17, 31]. Our results did not find significant differences capable of improving the parameters of inflammatory markers, IL-6, IL1-β, IL-10, TNF-α, and CRP. There is conflicting evidence in the literature on the ability of exercise to reduce markers of inflammation in people with CKD. Dungey et al. (2017) [39] performed 6 months of intradialytic aerobic exercises and were not able to improve CRP, IL-6, and TNF-α levels. On the other hand, Watson et al. (2017) [34] performed 8 weeks of resistance training where an increase in the modulation of IL-6, IL-15, MCP-1, and TNF-α was observed. Recently, Meléndez-Oliva et al. (2022) reported a significant reduction in plasma IL-6 and CRP levels after 4 months of intervention. Corroborating with our findings, a randomized clinical trial, where 17 patients were divided into a control and training group, performed intradialytic aerobic exercise for 4 months and found no significant differences in serum concentrations of CRP and IL-6 [35]. Similar findings can be found in our previous study [17]. A recent systematic review points out that there is no clear evidence that AT reduces inflammatory markers associated with CKD. Furthermore, there is clear evidence for reduced CPR in patients who performed high-intensity RT, but not for IL-6 [40].
An important data to verify the effectiveness of dialysis is the Kt/V index. Exercise has been presented as an important tool that contributes to this process, as it increases blood flow to peripheral muscles and improves muscle cell perfusion [21]. Our findings showed no significant change after 12 weeks of intervention regarding this variable, but previous studies showed divergences in this result. Fernandes et al. (2019) [33], found no significant difference in Kt/V after 24 sessions of aerobic training in 39 patients. Cruz et al. (2018), also in a cycle ergometer protocol, after 36 training sessions found a significant difference in this variable. These findings should be interpreted with caution due to the different exercise protocols used.
Regardless of the statistical significance, the present study results suggest that the intervention with exercises promotes a substantial improvement in the clinical condition of the patients, which reverberates in their physical disposition to face the treatment. Also, our findings are in line with other studies, for example, while we present an increase of 33.38 m for the 6MWT, Ferrari et al. (2019) [41] presented 36.37 m when considering the combined exercise. Concerning inflammation, the results presented here are also anchored in the literature, with insufficient evidence to prove the effectiveness of exercise in improving the inflammatory markers levels, except for the reduction of CRP after intervention with aerobic exercise Ferrari et al. (2019).
Some limitations of the present study include: (1) limited sample size, due to the low adherence to the proposal of physical exercises during HD, probably due to the individuals being used to the monotony of the treatment. It is worth noting that the number of participants in the present study was similar to other studies [32, 34, 39, 42, 43]. Even though it was not our object of study, it is worth remembering that in a recent meta-analysis [44] sought to investigate the benefits, obstacles, and results, of exercise in people with CKD and it was observed that among the 423 participants in the case of the best place to exercise, the majority (73%) preferred at home, followed by the neighborhood, at the gym and finally the renal therapy unit (RTU). Among the preferred modalities, combined training was the most cited. These results are in line with the previous study [45]. (2) The present study opted for simple randomization, because it had the advantage of increasing comparability between groups by keeping the ratio of the number of participants between groups almost the same. However, by not stratifying the randomization by gender and age, we failed to control for covariates that could interfere with the studied variables. (3) Although these patients are rigorous in terms of nutrition, treatment frequency, and monitoring of renal disease parameters, our study did not analyze the biochemical markers that can greatly influence the physical performance of these individuals, such as calcium, phosphate, albumin, PTG, hemoglobin, hematocrit, urea and others. In this sense, it would be relevant for future studies to analyze the adaptations of these parameters in a multicomponent exercise program.
It is valid to highlight that: (1) the present work was a pioneer in our hospital. Although the literature supports intervention with a lower degree of monitoring, it was essential not to have gaps for possible intercurrences. We believe that the present work's internal validity makes the future more clinically viable interventions possible; (2) in the present study, the proposal was to perform each exercise approach on separate days for logistical reasons (e.g., available equipment, the flow of people on site, and the quantity of qualified and available exercise specialists).
Twelve weeks of IMEP resulted in improved functional capacity, and respiratory muscle strength in people with CKD.
References
Collaboration GCKD (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733
Bansal N (2015) Stricter systolic blood pressure control is associated with higher all-cause mortality in patients with chronic kidney disease. Evid Based Med 20(2):68
Workeneh BT, Mitch WE (2010) Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 91(4):1128S-1132S
Major RW, Cheng MRI, Grant RA, Shantikumar S, Xu G, Oozeerally I, Brunskill NJ, Gray LJ (2018) Cardiovascular disease risk factors in chronic kidney disease: a systematic review and meta-analysis. PLoS ONE 13(3):e0192895
Bollenbecker S, Czaya B, Gutierrez OM, Krick S (2022) Lung-kidney interactions and their role in chronic kidney disease-associated pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 322(5):L625–L640
Mukai H, Ming P, Lindholm B, Heimburger O, Barany P, Stenvinkel P, Qureshi AR (2018) Lung dysfunction and mortality in patients with chronic kidney disease. Kidney Blood Press Res 43(2):522–535
Hussien H, Apetrii M, Covic A (2021) Health-related quality of life in patients with chronic kidney disease. Expert Rev Pharmacoecon Outcomes Res 21(1):43–54
Shimoda T, Matsuzawa R, Yoneki K, Harada M, Watanabe T, Matsumoto M, Yoshida A, Takeuchi Y, Matsunaga A (2017) Changes in physical activity and risk of all-cause mortality in patients on maintence hemodialysis: a retrospective cohort study. BMC Nephrol 18(1):154
Avesani CM, Trolonge S, Deleaval P, Baria F, Mafra D, Faxen-Irving G, Chauveau P, Teta D, Kamimura MA, Cuppari L et al (2012) Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant 27(6):2430–2434
Junqué Jiménez A, Esteve Simó V, Andreu Periz L, Segura Ortí E: The Relationship between Physical Activity Levels and Functional Capacity in Patients with Advanced Chronic Kidney Disease. 2021, 30(3):360-368.
Segura-Ortí E, Gordon PL, Doyle JW, Johansen KL: Correlates of physical functioning and performance across the spectrum of kidney function. 2018, 27(5):579-596.
Wilkinson TJ, Clarke AL, Nixon DGD, Hull KL, Song Y, Burton JO, Yates T, Smith AC (2021) Prevalence and correlates of physical activity across kidney disease stages: an observational multicentre study. Nephrol Dial Transplant 36(4):641–649
Zhang F, Wang H, Wang W, Zhang H: the role of physical activity and mortality in hemodialysis patients: A Review. 2022, 10.
Morishita S, Tsubaki A, Shirai N (2017) Physical function was related to mortality in patients with chronic kidney disease and dialysis. Hemodial Int 21(4):483–489
Mallamaci F, Pisano A, Tripepi G: Physical activity in chronic kidney disease and the EXerCise Introduction To Enhance trial. Nephrol Dial Transplant 2020, 35(Suppl 2):ii18-ii22.
Cheema BS, Abas H, Smith BC, O’Sullivan AJ, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B et al (2011) Effect of resistance training during hemodialysis on circulating cytokines: a randomized controlled trial. Eur J Appl Physiol 111(7):1437–1445
Cruz LGd, Zanetti HR, Andaki ACR, Mota GRd, Barbosa Neto O, Mendes ELJMRdEF: Intradialytic aerobic training improves inflammatory markers in patients with chronic kidney disease: a randomized clinical trial. 2018, 24.
Figueiredo PHS, Lima MMO, Costa HS, Martins JB, Flecha OD, Goncalves PF, Alves FL, Rodrigues VGB, Maciel EHB, Mendonca VA et al (2018) Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: A randomized clinical trial. PLoS ONE 13(7):e0200727
Vanden Wyngaert K, Van Craenenbroeck AH, Van Biesen W, Dhondt A, Tanghe A, Van Ginckel A, Celie B, Calders P (2018) The effects of aerobic exercise on eGFR, blood pressure and VO2peak in patients with chronic kidney disease stages 3–4: a systematic review and meta-analysis. PLoS ONE 13(9):e0203662
Heiwe S, Jacobson SH (2014) Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 64(3):383–393
Huang M, Lv A, Wang J, Xu N, Ma G, Zhai Z, Zhang B, Gao J, Ni C (2019) Exercise training and outcomes in hemodialysis patients: systematic review and meta-analysis. Am J Nephrol 50(4):240–254
Torres E, Aragoncillo I, Moreno J, Vega A, Abad S, Garcia-Prieto A, Macias N, Hernandez A, Godino MT, Luno J (2020) Exercise training during hemodialysis sessions: physical and biochemical benefits. Ther Apher Dial 24(6):648–654
Patrizio E, Calvani R, Marzetti E, Cesari M (2021) Physical functional assessment in older adults. J Frailty Aging 10(2):141–149
Jones CJ, Rikli RE, Beam WC (1999) A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 70(2):113–119
Segura-Orti E, Martinez-Olmos FJ (2011) Test-retest reliability and minimal detectable change scores for sit-to-stand-to-sit tests, the six-minute walk test, the one-leg heel-rise test, and handgrip strength in people undergoing hemodialysis. Phys Ther 91(8):1244–1252
Ortega-Perez de Villar L, Martinez-Olmos FJ, Perez-Dominguez FB, Benavent-Caballer V, Montanez-Aguilera FJ, Mercer T, Segura-Orti E: Comparison of intradialytic versus home-based exercise programs on physical functioning, physical activity level, adherence, and health-related quality of life: pilot study. Sci Rep 2020, 10 (1):8302.
American Thoracic Society/European Respiratory S: ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002, 166(4):518–624.
Foster C, Florhaug JA, Franklin J, Gottschall L, Hrovatin LA, Parker S, Doleshal P, Dodge C (2001) A new approach to monitoring exercise training. J Strength Cond Res 15(1):109–115
Guio BM, Gomes CP, Costa FBD, Oliveira ADS, Duarte MT, Leite MJ (2017) Beneficial effects of intradialytic cardiopulmonary rehabilitation. J Bras Nefrol 39(3):275–282
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Akchurin OM, Kaskel F (2015) Update on inflammation in chronic kidney disease. Blood Purif 39(1–3):84–92
Bae YH, Lee SM, Jo JI (2015) Aerobic training during hemodialysis improves body composition, muscle function, physical performance, and quality of life in chronic kidney disease patients. J Phys Ther Sci 27(5):1445–1449
Fernandes AO, Sens Y, Xavier VB, Miorin LA, Alves V (2019) Functional and respiratory capacity of patients with chronic kidney disease undergoing cycle ergometer training during hemodialysis sessions: a randomized clinical trial. Int J Nephrol 2019:7857824
Watson EL, Viana JL, Wimbury D, Martin N, Greening NJ, Barratt J, Smith AC (2017) The effect of resistance exercise on inflammatory and myogenic markers in patients with chronic kidney disease. Front Physiol 8:541
Wilund KR, Tomayko EJ, Wu PT, Ryong Chung H, Vallurupalli S, Lakshminarayanan B, Fernhall B (2010) Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant 25(8):2695–2701
Johansen KL (2007) Exercise in the end-stage renal disease population. J Am Soc Nephrol 18(6):1845–1854
Pu J, Jiang Z, Wu W, Li L, Zhang L, Li Y, Liu Q, Ou S (2019) Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open 9(1):e020633
Qiu Z, Zheng K, Zhang H, Feng J, Wang L, Zhou H (2017) Physical exercise and patients with chronic renal failure: a meta-analysis. Biomed Res Int 2017:7191826
Dungey M, Young HML, Churchward DR, Burton JO, Smith AC, Bishop NC (2017) Regular exercise during haemodialysis promotes an anti-inflammatory leucocyte profile. Clin Kidney J 10(6):813–821
Melendez Oliva E, Villafane JH, Alonso Perez JL, Alonso Sal A, Molinero Carlier G, Quevedo Garcia A, Turroni S, Martinez-Pozas O, Valcarcel Izquierdo N, Sanchez Romero EA: Effect of Exercise on Inflammation in Hemodialysis Patients: A Systematic Review. J Pers Med 2022, 12(7).
Ferrari F, Helal L, Dipp T, Soares D, Soldatelli A, Mills AL, Paz C, Tenorio MCC, Motta MT, Barcellos FC et al (2020) Intradialytic training in patients with end-stage renal disease: a systematic review and meta-analysis of randomized clinical trials assessing the effects of five different training interventions. J Nephrol 33(2):251–266
Dipp T, Macagnan FE, Schardong J, Fernandes RO, Lemos LC, Plentz RDM (2020) Short period of high-intensity inspiratory muscle training improves inspiratory muscle strength in patients with chronic kidney disease on hemodialysis: a randomized controlled trial. Braz J Phys Ther 24(3):280–286
Wong J, Davis P, Patidar A, Zhang Y, Vilar E, Finkelman M, Farrington K (2017) The effect of intra-dialytic exercise on inflammation and blood endotoxin levels. Blood Purif 44(1):51–59
Moorman D, Suri R, Hiremath S, Jegatheswaran J, Kumar T, Bugeja A, Zimmerman D (2019) Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol 14(2):268–276
Delgado C, Johansen KL (2012) Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant 27(3):1152–1157
Funding
There is no funding for the research.
Author information
Authors and Affiliations
Contributions
PLB and ELM had the idea for the article. PLB, EAB, and JCS performed the research. HRZ, LR, and ELM critically revised and contribute to the work. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest for all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barbosa, P.L., Blumer, E.A., Oliveira, J.C.S. et al. A multicomponent exercise program improves functional capacity and respiratory muscle strength in hemodialysis patients: a randomized clinical trial. Sport Sci Health 19, 1217–1225 (2023). https://doi.org/10.1007/s11332-023-01053-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-023-01053-z