Abstract
Background and aims: Current state of evidence recommends beneficial effects of branched chain amino acids (BCAAs) on exercise performance; however, randomized controlled trials (RCTs) of BCAA supplementation yield discordant results. The objective of this study was to clarify the effects of BCAA supplementation in exercise through meta-analysis of all relevant RCTs.
Methods: A comprehensive search of PubMed, Embase, ISI web of science, and the Cochrane library has been conducted from inception to September 2016. This meta-analysis includes 31 primary trials of the effect of BCAA supplementation on central fatigue, fatigue substances (lactate and ammonia), energy metabolites (glucose and free fatty acids) and, muscle damage substances (LDH and CK). The estimates were either obtained from a fixed-effects model or a random-effects model. The studies’ heterogeneity was calculated by Cochrane’s test and I2 index.
Results: BCAA had no effect on central fatigue (SMD − 0.31, 95% CI − 0.72 to 0.09; p = 0.1; heterogeneity I2 = 0%, p = 0.9). However, a significant reduction was detected in the lactate levels (WMD − 0.16, 95% CI − 0.26 to − 0.53; p = 0.003; heterogeneity I2 = 47.9%, p = 0.023). Moreover, BCAA supplementation had beneficial effects on ammonia, glucose, FFA, and CK, but had no effects on LDH.
Conclusion: BCAA supplementation did not have any effect on the feeling of fatigue; however, it led to a favorable effect on fatigue substances, energy metabolites and muscle soreness substances. Therefore, it can be concluded that the ingestion of the BCAA can play a helpful role in the enhancement of the exercise performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past 2 decades, numerous studies have shown the advantageous effects of branched chain amino acids (BCAAs) on exercise performance [1]. BCAA as a nutritional supplement may improve serum concentration of fatigue substances (lactate, ammonia and 5-HT), energy metabolites (glucose and free fatty acids) and muscle soreness substances (LDH and CK) [2]. Fatigue during exercise can be attributable to both peripheral and central fatigue factors [3]. Fatigue of central origin translates into impulse conduction which promotes a reduction in the number of active motor units and a decrease in the firing frequency of the motoneurons.
Decrease in blood glucose levels due to liver glycogen depletion is thought to be one factor in the development of central fatigue. Also, serotonin or 5-hydroxytryptamine (5-HT) is another factor known to affect the central fatigue. Brain 5-HT increases during exercise which is assumed to be a cause of central fatigue [3, 4]. Increased 5-HT synthesis occurs in response to an increased transport of free tryptophan (f-Trp) across the blood–brain barrier. Furthermore, transport of free tryptophan is influenced by the serum levels of BCAAs. BCAA and f-TRP compete for the same carrier system [4]. Therefore, the f-Trp/BCAA ratio is a key factor that reflects the amount of free tryptophan that is transported to the brain. As a result, ingestion of BCAAs may be able to decrease the uptake of free tryptophan by the brain and also brain 5-HT synthesis and consequently delay central fatigue [3]. Moreover, increasing serum levels of free fatty acids (FFAs) during exercise causes a parallel increase in plasma-free tryptophan unbound to albumin [5, 6].
Earlier studies indicate that BCAA catabolism is increased during exercise [7]. BCAA enters the tricarboxiylic acid (TCA) cycle as a metabolite. When BCAA catabolism is increased, BCAA significantly suppresses the lactate production [8]. The challenge in some studies suggested that the ammonia produced from BCAAs leads to reaction that maintains the energy source of the cells. Therefore, BCAA supplementation during exercise is the key factor that changes the arterial \({NH}_{3}\) production [9,10,11]. Also, CK and LDH are useful blood measurements that reflect the degree of muscle soreness and damage [12]. CK and LDH control the ATP-PC system and maintain the balance of sugar catabolism and anabolism, respectively [2]. According to some studies, BCAA supplementation reduces serum concentration of LDH and CK [13,14,15]. There are many RCT studies carried out to investigate the effect of BCAA supplementation on exercise performance, and the results of these investigations are inconsistent. This lack of consistency may be caused by variations in the protocols of exercise and different protocols of BCAA supplementation among trials. In addition, no systematic analysis focused on BCAA supplementation on fatigue substances (lactate and ammonia), energy metabolites (glucose and free fatty acids) and muscle soreness substances (LDH and CK) have been done. Motivated by the controversy about the benefits of BCAA supplementation, the present meta-analysis aimed to evaluate the effects of BCAA supplementation on fatigue, energy metabolism, and muscle damage during exercise.
Methods
Search strategy, study selection, and data extraction
This meta-analysis was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. A comprehensive search of PubMed, Embase, ISI web of science, and the Cochrane library has been conducted from inception to September 2016. The search included English language publications. The phrase branched chain amino acid or BCAA was used in combination with the words fatigue, exhaustion, performance, training, endurance, sport, or exercise in the literature search. Two reviewers (AM and RH) independently reviewed the title and abstract of all relevant studies to identify eligible ones. After excluding the irrelevant studies, the full texts of the qualified studies were assessed to determine if they met the inclusion criteria of the proposed meta-analysis. The reference lists of original and review studies were also searched manually to ensure that further eligible publications were included. This meta-analysis study has been submitted in PROSPERO (ID: CRD42017063873).
Original studies were included with regard to the following inclusion criteria:
-
1.
Methodological design: randomized clinical trial
-
2.
Participants: healthy human females and males, professional athletes and untrained male and female
-
3.
Intervention: BCAA supplementation versus a control feed
-
4.
Outcome: they reported at least one of the results of interest including fatigue, lactate, ammonia, glucose, FFA, CK, and LDH.
The relevant data were extracted: first author’s name, publication year, country of trial, sample population in intervention and control groups, characteristics of the subjects (i.e., age, gender), details of the methodological design (i.e., crossover or parallel design), evaluation of quality score of trials, the dosage and protocol of intervention and placebo in trials, exercise intervention, extraction of mean differences, and SD changes of following outcome variables: fatigue, lactate, ammonia, glucose, FFA, CK, and LDH.
Quality assessment
Two reviewers (AM and RH) performed the quality assessment of all studies independently. The Jadad scale was employed to assess the methodological quality of all studies based on methods pertinent to randomization, double blinding, and descriptions of withdrawals [17]. The range of possible score is 0–5. The scores ranging from 0 to 2 were considered as lower scores and the scores ranging from 3 to 5 were considered as higher scores.
Data analysis
Mean and standard deviation (SD) of the pre- and postintervention were obtained from the original studies. Graph digitizer was used to obtain data from graphs when original data values were not available [18]. In any study that standard error of the mean (SEM) was reported, SD was calculated as SD = SEM × √n, where n equals group sample size. Mean differences and SD changes have been used to assess the treatment effects. The following formula has proved to be a reliable measure for the calculation of SD change:
Also, the correlation coefficients of the studies were obtained. If the correlation coefficient was not mentioned in a study, a correlation of 0.5 was assumed. The data analyses were conducted with a fixed-effects model, unless a study with heterogeneity random-effects model was used [19]. Forest plots were generated to show the mean effect size with 95% confidence interval (CI). The weighted mean difference (WMD) was calculated with 95% CI when all of the studies had measured the outcome variable on the same measurement scale. Since different measurement scales were used across studies, the standard mean difference (SMD) was calculated with 95% CI. The heterogeneity among studies was measured by Cochrane’s test (Q test) and I2 index. Percentages of around 25% (I2 = 25), 50% (I2 = 50), and 75% (I2 = 75) would mean low, medium, and high heterogeneity, respectively. Furthermore, subgroup analyses were performed to explore the source of heterogeneity and assess the possible effects of BCAA supplementation on study outcomes. Subgroup analyses were performed according to the duration of BCAA supplementation [< 1 days (short-term) or > 1 days (long-term)]. Additionally, sensitivity analysis was performed using one-study removed (leave-one-out) approach. Risk of publication bias in meta-analysis was assessed by both the visual funnel plot and Egger’s test [21]. All analyses were performed using STATA (version 12; college station, TX, USA). p < 0.05 was considered as significant.
Results
Summary of the included studies
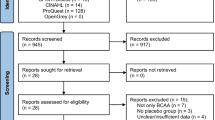
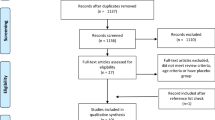
The flow diagram for selection of trials is summarized in Fig. 1. A total of 120 articles were identified from database searching. In addition, nine articles were identified from the reference lists of the relevant studies. After removing 33 duplicate articles, 96 articles were left for selection assessment. After assessing the titles and abstracts, 51 full text articles were initially selected. After further evaluation, 20 articles were excluded for the following reasons; reporting insufficient data (9 articles) [41,42,43,44,45,46,47,48,49], using duplicate data (2 articles) [50, 51], using BCAA infusion (1 article) [52], using leucine alone (4 articles) [53,54,55,56], and combining BCAAs with another amino acid supplement (4 articles) [57,58,59,60]. Finally, 31 articles were adopted for inclusion in this meta-analysis. A total of 576 subjects, 378 subjects received BCAA supplements and 379 subjects received a control feed. Mean age of the participants recruited in this study was 25.62 ± 5.5. Characteristics of eligible studies are listed in Table 1. Of the 31 eligible studies, 26 studies were performed on men only, 2 studies were performed on women only [14, 29] and 3 studies were performed on both males and females [13, 25, 38]. Methodological design of 12 studies was parallel and 19 studies were crossover. The variation in the dosage and mixture of BCAAs (leucine, isoleucine and valine) was observed between trials. Moreover, included trials in this meta-analysis reported different lengths of time that BCAA supplementation was applied. The protocols of BCAA supplementation were different across included studies. Therefore, subgroup classification was performed based on the duration of BCAA supplementation. According to the subgroup classification, 20 studies assumed to be short-term BCAA supplementation and 11 studies assumed to be long-term BCAA supplementation. Summary of the trials included in current meta-analysis is presented in Table 2.
The extracted data from trials were pooled in this meta-analysis. The main focus of this review was placed on the following outcome variables: fatigue, lactate, ammonia, glucose, FFA, CK, and LDH. The forest plots of all the outcome variables are presented in Figs. 2, 3, 4, 5, 6, 7 and 8.
Effect of BCAA supplementation on fatigue and fatigue substances (lactate and ammonia)
The central fatigue was evaluated via different scales across studies. Portier et al. [6] employed an analog visual scale with the crew members. Also, this traditional scale has been validated (Lagarde and Batejat) [61]. Chevrount et al. [23] and Mittelman et al. [25] used profile of mood states (POMS) scale consisting of depression, tension, anger, confusion vigor, and fatigue. Struder et al. [28] used EZ-scale comprising drive, confidence, mood, and fatigue. Blomstrand et al. [22] used a category-ratio scale CR-10 (Borg) [62]. To remove the effects of different measurement scales used in the trials, the standardized mean differences (SMD) were calculated. Five trials had recorded central fatigue, pooled results of which are presented in Fig. 2. The Fig. 2 shows results with low heterogeneity; the confidence interval around I2 contains near 0% value and can hold the homogeneity and the hypothesis of the meta-analysis. A good relationship favoring BCAA that had no effect on central fatigue (SMD − 0.31, 95% CI − 0.72 to 0.09; p = 0.1; heterogeneity I2 = 0%, p = 0.9). The levels of lactate were also analyzed. Fixed-effects model showed that the BCAA supplementation had a reduction effect on lactate Levels (WMD − 0.16, 95% CI − 0.26 to − 0.53; p = 0.003; heterogeneity I2 = 47.9%, p = 0.02). Pooled results from these studies are presented in Fig. 3. Regarding the ammonia, the heterogeneity was observed by fixed-effects model among trials. Therefore, random-effects model was calculated (Table 2) which showed a statistically significant positive effect (WMD 19.52, 95% CI 6.84–32.2; p = 0.003). Pooled results from these studies are presented in Fig. 4. Also, there was an insufficient number of trials in the subgroup classification which researchers performed calculation of the subgroup meta-analysis to explore the source of heterogeneity.
Effect of BCAA supplementation on energy metabolite substance (glucose and free fatty acids)
Concerning the glucose, random-effects model was calculated because of the heterogeneity that was observed (Table 2). Also, subgroup analysis was performed to explore the source of heterogeneity. Subgroup analysis of short-term BCAA supplementation showed a significant reduction effect on glucose levels (WMD − 0.41, 95% CI − 0.53 to − 0.29; p < 0.001). However, in the subgroup of long-term BCAA supplementation, no effect on glucose levels was found (WMD − 0.01, 95% CI − 0.23 to 0.2). Therefore, the reduction effects of BCAA supplementation are related to trials with a short-term of BCAA supplementation. Additionally, duration of trials is the source of heterogeneity. Pooled results from these studies are presented in two subgroups in Fig. 5. Regarding the free fatty acids, a beneficial effect was observed (WMD − 0.059, 95% CI − 0.112 to − 0.005; p = 0.03; heterogeneity I2 = 24.3%, p = 0.19). Pooled result from these studies is presented in Fig. 6.
Effect of BCAA supplementation on muscle soreness substances (LDH and CK)
Five trials had recorded levels of LDH. Pooled results from these studies are shown in Fig. 7. Random-effects model exhibited that the BCAA supplementation did not significantly improve LDH levels (WMD − 10.2, 95% CI − 40.1 to 19.75; p = 0.5; heterogeneity I2 = 78.9%, p = 0.001). Also, there was an insufficient number of trials in the subgroup classification for subgroup analysis. The levels of CK were also analyzed. Concerning the heterogeneity between trials, random-effects model was calculated (Table 2). Also, subgroup analysis was performed. In the subgroup of the short duration of BCAA supplementation, no effect on CK levels was found (WMD − 1.96, 95% CI − 6 to 2.07; p = 0.34), but a significant reduction was seen in the long duration subgroup (WMD − 34.69, 95% CI − 55.9 to − 13.4; p = 0.001). Therefore, the reduction effects of BCAA supplementation are only related to trials with a long duration of BCAA supplementation.
Sensitivity analysis and risk of publication bias
After leaving out the studies (leave-one-out approach), results did not show significant changes (data not shown). Funnel plot for lactate as important fatigue substance was generated to evaluate the risk of publication bias (Fig. 9). The Eggers test was not observed to be significant for any of outcome variables.
Discussion
In this meta-analysis, the effect of BCAA supplementation on central fatigue, fatigue substances (lactate and ammonia), energy metabolites (glucose and free fatty acids), and muscle soreness substances (LDH and CK) was evaluated. The results of this meta-analysis suggest that BCAA supplementation had a beneficial effect on lactate, FFA, glucose, ammonia, and CK; however, no beneficial effects of BCAAs on central fatigue and LDH were observed. The assessment of publication bias and the sensitivity analysis revealed solid evidence that supports BCAAs as a potentially effective nutritional supplement for sport fatigue.
Branched chain amino acids (BCAA; leucine, isoleucine, and valine) are the most oxidized types of amino acids that are catabolized in the skeletal muscle and augment muscle regeneration by suppressing endogenous post-exercise muscle protein degradation [2, 49]. Insufficient essential post-exercise amino acids may delay stimulating skeletal muscle protein synthesis. Therefore, the popularity of protein and amino acid supplementation for athletes is growing [7, 49]. Nutritional strategies were designed to decrease 5-HT-mediated fatigue. Carbohydrate sports drinks are frequently used for people who practice sports. The increased ratio of free tryptophan (f-Trp) was shown by consumption of carbohydrate sports drinks; whereas, BCAA supplementation decreases the ratio of free tryptophan (f-Trp) compared to sports drinks [23].
Fatigue during exercise is related to both peripheral and central fatigue factors. Several biochemical factors were identified as causes of peripheral fatigue including depletion of phosphocreatine, depletion of muscle glycogen, and accumulation of protons. However, the factors related to central fatigue are less known [3]. In this meta-analysis, five studies can be pooled and assessed to analyze central fatigue. These studies evaluated central fatigue with different scales. Therefore, the standardized mean differences (SMD) were calculated. BCAAs resulted in no improvement in fatigue sensation compared to control group. Several studies that evaluated the effect of BCAAs on sport fatigue were excluded. These studies reported different scales which they did not have suitable condition for pooling, for example, Gualano et al. [26] and Watson et al. [34] used exercise time to exhaustion, Dudgeon et al [49] used repetition to fatigue and Matsumoto et al [13] used a visual analog scale (VAS) for assessment of peripheral fatigue.
This meta-analysis measured the lactate and ammonia levels, with an aim to determine the effect of BCAA supplementation on fatigue substances. Lactate is considered to reflect the anaerobic glucose metabolism during exercise. Lactate is the most important barometer known to affect the limitation factors of muscle activity and muscle fatigue [8]. This meta-analysis confirms that BCAAs had a positive influence on lactate levels. Several studies suggested that the high ammonia can be highly toxic to the brain, and can deteriorate muscle function [2, 6, 28, 63,64,65]. BCAA supplementation can lead to suppression of endogenous skeletal muscle protein breakdown, and can lead to detoxification of ammonia to glutamine (GLN) in skeletal muscle [10, 11]. The results of this meta-analysis demonstrate a beneficial effect of BCAA supplementation on ammonia levels. Although BCAA had no decreasing effect on serum levels of ammonia in the BCAA group, it prevented increasing ammonia in this group.
The liver’s activity of releasing glucose and mobilizing stored glycogen increases during exercise. However, ingestion of BCAA helps to supply additional energy. BCAA is taken up by the muscles and is oxidized. As a result, glucose release from the liver is reduced, and consequently, the glucose level in the blood is decreased. The evidence indicated that ingestion of BCAA can prevent exercise performance from deteriorating which is caused by depletion of the liver glycogen stores [2, 66]. The result of this meta-analysis indicated that short-term BCAA supplementation had a reduction effect on glucose levels. However, long-term BCAA ingestion did not decrease the levels of glucose. Pooled results from these studies confirmed the beneficial effect of BCAA supplementation on glucose levels. In addition, this review confirms that BCAA supplementation had a beneficial effect on FFA levels. Decreasing levels of FFA during exercise are presumably the result of the insulinogenic effect of ingestion of the BCAA mixture [34]. Additionally, increasing levels of FFA during exercise can increase the amount of free tryptophan that enters the brain [5]. The current evidence confirms that BCAA supplementation before exercise can decrease the FFA levels and consequently can reduce sensation of fatigue.
This review did not demonstrate any beneficial effect of BCAA supplementation on LDH levels. LDH as an indicator of muscle soreness plays an important role in production of lactate in muscle cells. The levels of LDH and CK play an important role in providing skeletal muscle energy metabolism for muscle activity. CK and LDH serve as global markers for muscle damage [13]. This review confirms that BCAA supplementation had a positive influence on CK levels. Meanwhile, the beneficial effect of BCAA supplementation is related to trials with a long-term BCAA supplementation. The result of this meta-analysis suggested that ingestion of BCAA following chronic supplementation can decrease serum levels of CK. Of the eligible publications that were included, a limited number of studies were performed on women. A significant gender effect is demonstrated in serum levels of CK [67]. Furthermore, the response of LDH and CK relies on where the site of muscle cell damage happened [68].
This is the first systematic review and meta-analyses that specifically evaluated the effect of BCAA supplementation on sport fatigue. This meta-analysis has certain limitations. This study is limited to English language trials. Also, a limited number of studies included in this meta-analysis were performed on women, and most studies were performed on men. Included studies in this review measured each outcome variable at multiple follow-up times, for example, before exercise and immediately (15 min, 30 min, 1 h, 2 h, 3 h, 24 h, 72 h and 96 h) after exercise for both intervention and control groups. Because of different follow-up times for each outcome variable among trials, this review focused on outcome variables reported before and immediately after exercise. In addition, the variation in dosage and mixture of BCAAs (leucine, isoleucine, and valine), different repetition per day of BCAA and different BCAA manufacturers were observed among trials. Also, included studies in this review reported acute or chronic BCAA supplementation. The protocols of BCAA supplementation were different among studies. BCAAs were applied before exercise and continued during and after exercise or not continued during and after exercise. These factors may contribute to the incompatibility between findings. Subgroup analysis was performed. However, available data were insufficient to perform subgroup meta-analysis for several outcome variables. In this review, high heterogeneity and inconclusive findings were observed. However, the effect of heterogeneity was minimized by selecting a random-effects model and the source of heterogeneity was found by subgroup analysis in possible.
Conclusion
This meta-analysis suggests that BCAA supplementation had no statistically significant effect on the feeling of fatigue; however, it leads to a beneficial effect on fatigue substances, which subsequently leads to a favorable effect on some muscle damage substances and some energy metabolites. Therefore, the ingestion of the BCAA ingestion can play a beneficial role in enhancement of the exercise performance. Further well-designed randomized controlled trials may be necessary to improve our knowledge regarding of BCAA ingestion on exercise performance.
References
Coombes JS, McNaughton LR (2000) Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J Sports Med Phys Fitness 40(3):240–246
Kim D-H, Kim S-H, Jeong W-S, Lee H-Y (2013) Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J Exerc Nutr Biochem 17(4):169–180
Blomstrand E (2006) A role for branched-chain amino acids in reducing central fatigue. J Nutr 136(2):544 s–7 s
Davis JM. Carbohydrates (1995) Branched-chain amino acids, and endurances: the central fatigue hypothesis. Int J Sport Nutr 5(s1):S29–S38
Greer BK, White JP, Arguello EM, Haymes EM (2011) Branched-chain amino acid supplementation lowers perceived exertion but does not affect performance in untrained males. J Strength Cond Res 25(2):539–544
Portier H, Chatard JC, Filaire E, Jaunet-Devienne MF, Robert A, Guezennec CY (2008) Effects of branched-chain amino acids supplementation on physiological and psychological performance during an offshore sailing race. Eur J Appl Physiol 104(5):787–794
Shimomura Y, Kobayashi H, Mawatari K, Akita K, Inaguma A, Watanabe S et al (2009) Effects of squat exercise and branched-chain amino acid supplementation on plasma free amino acid concentrations in young women. J Nutr Sci Vitaminol 55(3):288–291
Matsumoto K, Takashige K, Hamada K, Tsujimoto H, Mitsuzono R (2009) Branched-chain amino acid supplementation increases the lactate threshold during an incremental exercise test in trained individuals. J Nutr Sci Vitaminol 55(1):52–58
MacLean D, Graham T, Saltin B (1994) Branched-chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. Am J Physiol Endocrinol Metab 267(6):E1010–E1022
MacLean DA, Graham TE, Saltin B (1996) Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J Physiol Lond 493(3):909–922
Maclean DA, Graham TE. (1993) Branched-chain amino-acid supplementation augments plasma, ammonia responses during exercise in humans J Appl Physiol 74(6):2711–2717
Koba T, Hamada K, Sakurai M, Matsumoto K, Hayase H, Imaizumi K et al (2007) Branched-chain amino acids supplementation attenuates the accumulation of blood lactate dehydrogenase during distance running. J Sports Med Phys Fitness 47(3):316
Matsumoto K, Koba T, Hamada K, Sakurai M, Higuchi T, Miyata H (2009) Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J Sports Med Phys Fit 49(4):424–431
Sheikholeslami-Vatani D, Ahmadi S (2016) Effect of oral branched-chain amino acid supplementation prior to resistance exercise on metabolic hormones, plasma amino acids, and serum indices of muscle damage in the recovery period. Top Clin Nutr 31(4):346–354
Rahimi MH, Shab-Bidar S, Mollahosseini M, Djafarian K. (2017) Branched chain amino acid supplementation and exercise induced muscle damage in exercise recovery: a meta-analysis of randomized clinical trials. Nutrition 42:30–36
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controll Clin Trials 17(1):1–12
Fedorov S. (2008) GetData graph digitizer. http://www.getdata-graph-digitizer.com
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controll Clin Trials 7(3):177–188
Higgins J, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj 315(7109):629–634
Blomstrand E, Hassmen P, Ek S, Ekblom B, Newsholme EA (1997) Influence of ingesting a solution of branched-chain amino acids on perceived exertion during exercise. Acta Physiol Scand 159(1):41–49
Cheuvront SN, Carter R, Kolka MA, Lieberman HR, Kellogg MD, Sawka MN (2004) Branched-chain amino acid supplementation and human performance when hypohydrated in the heat. J Appl Physiol 97(4):1275–1282
Wisnik P, Chmura J, Ziemba AW, Mikulski T, Nazar K (2011) The effect of branched chain amino acids on psychomotor performance during treadmill exercise of changing intensity simulating a soccer game. Applied physiology, nutrition, and metabolism = Physiologie appliquee. Nutrition et metabolisme 36(6):856–862
Mittleman KD, Ricci MR, Bailey SP (1998) Branched-chain amino acids prolong exercise during heat stress in men and women. Med Sci Sports Exerc 30(1):83–91
Gualano AB, Bozza T, Lopes De Campos P, Roschel H, Dos Santos Costa A, Luiz Marquezi M et al (2011) Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J Sports Med Phys Fit 51(1):82–88
Blomstrand E, Hassmen P, Ekblom B, Newsholme EA (1991) Administration of branched-chain amino acids during sustained exercise–effects on performance and on plasma concentration of some amino acids. Eur J Appl Physiol Occup Physiol 63(2):83–88
Struder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K. Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Horm Metab Res = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1998;30(4):188–94
Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G et al. (2010) Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr Exerc Metab 20(3):236–44 (Internet)
Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN (2012) Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr 9(1):20
Blomstrand E, Saltin B (2001) BCAA intake affects protein metabolism in muscle after but not during exercise in humans. Am J Physiol Endocrinol Metab 281(2):E365–E374
Bigard AX, Lavier P, Ullmann L, Legrand H, Douce P, Guezennec CY (1996) Branched-chain amino acid supplementation during repeated prolonged skiing exercises at altitude. Int J Sport Nutr 6(3):295–306
Greer BK, Woodard JL, White JP, Arguello EM, Haymes EM (2007) Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. Int J Sport Nutr Exerc Metab 17(6):595–607
Watson P, Shirreffs SM, Maughan RJ (2004) The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur J Appl Physiol 93(3):306–314
Tang F-C (2006) Influence of branched-chain amino acid supplementation on urinary protein metabolite concentrations after swimming. J Am Coll Nutr 25(3):188–194
Carli G, Bonifazi M, Lodi L, Lupo C, Martelli G, Viti A (1992) Changes in the exercise-induced hormone response to branched chain amino acid administration. Eur J Appl Physiol Occup Physiol 64(3):272–277
De Palo E, Gatti R, Cappellin E, Schiraldi C, De Palo C, Spinella P (2001) Plasma lactate, GH and GH-binding protein levels in exercise following BCAA supplementation in athletes. Amino acids 20(1):1–11
Apró W, Blomstrand E. (2010) Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70S6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol (Oxford, England) 200(3):237–48. (Internet)
Fouré A, Nosaka K, Gastaldi M, Mattei J-P, Boudinet H, Guye M et al (2016) Effects of branched-chain amino acids supplementation on both plasma amino acids concentration and muscle energetics changes resulting from muscle damage: a randomized placebo controlled trial. Clin Nutr 35(1):83–94
De P, Metus P, Gatti R, Previti O, Bigon L, De P. (1993) Branched chain amino acids chronic treatment and muscular exercise performance in athletes: A study through plasma acetyl-carnitine levels. Amino Acids, 4(3):255–266
Watanabe S, Inaguma A, Bajotto G, Sato J, Kobayashi H, Mawatari K et al (2007) Effects of branched-chain amino acid (BCAA) supplementation before and after exercise on delayed-onset muscle soreness (DOMS) and fatigue. Faseb J 21(5):A331
Schena F, Guerrini F, Tregnaghi P, Kayser B (1992) Branched-chain amino acid supplementation during trekking at high altitude. The effects on loss of body mass, body composition, and muscle power. Eur J Appl Physiol Occup Physiol 65(5):394–398
Knechtle B, Mrazek C, Wirth A, Knechtle P, Rust CA, Senn O et al (2012) Branched-chain amino acid supplementation during a 100-km ultra-marathon—a randomized controlled trial. J Nutr Sci Vitaminol 58(1):36–44
Kephart WC, Wachs TD, Mac Thompson R, Mobley CB, Fox CD, McDonald JR et al (2016) Ten weeks of branched-chain amino acid supplementation improves select performance and immunological variables in trained cyclists. Amino Acids 48(3):779–789
Gee TI, Deniel S (2016) Branched-chain aminoacid supplementation attenuates a decrease in power-producing ability following acute strength training. J Sports Med Phys Fit 56(12):1511–1517
Ganzit GP, Benzio S, Filippa M, Goitra B, Severin B, Gribaudo CG (1997) Effects of oral branched-chain amino acids supplementation in bodybuilders. Med Dello Sport 50(3):293–303 (Internet)
Areces F, Salinero JJ, Abian-Vicen J, Gonzalez-Millan C, Gallo-Salazar C, Ruiz-Vicente D et al (2014) A 7-day oral supplementation with branched-chain amino acids was ineffective to prevent muscle damage during a marathon. Amino Acids 46(5):1169–1176
Hassmén P, Blomstrand E, Ekblom B, Newsholme EA (1994) Branched-chain amino acid supplementation during 30-km competitive run: mood and cognitive performance. Nutrition (Burbank, Los Angeles County. Calif) 10(5):405–410
Dudgeon WD, Kelley EP, Scheett TP (2016) In a single-blind, matched group design: branched-chain amino acid supplementation and resistance training maintains lean body mass during a caloric restricted diet. J Int Soc Sports Nutr 13:1
Blomstrand E, Ek S, Newsholme EA (1996) Influence of ingesting a solution of branched-chain amino acids on plasma and muscle concentrations of amino acids during prolonged submaximal exercise. Nutrition 12(7–8):485–490
Blomstrand E, Andersson S, Hassmen Pa, Ekblom B, Newsholme E (1995) Effect of branched-chain amino acid and carbohydrate supplementation on the exercise-induced change in plasma and muscle concentration of amino acids in human subjects. Acta Physiol 153(2):87–96
Varnier M, Sarto P, Martines D, Lora L, Carmignoto F, Leese G et al (1994) Effect of infusing branched-chain amino acid during incremental exercise with reduced muscle glycogen content. Eur J Appl Physiol Occup Physiol 69(1):[26–31 (Internet)
Pitkanen HT, Oja SS, Rusko H, Nummela A, Komi PV, Saransaari P et al (2003) Leucine supplementation does not enhance acute strength or running performance but affects serum amino acid concentration. Amino Acids 25(1):85–94
Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ et al (2011) Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr 94(3):809–818
Nelson AR, Phillips SM, Stellingwerff T, Rezzi S, Bruce SJ, Breton I et al (2012) A Protein-Leucine Supplement Increases Branched-Chain Amino Acid and Nitrogen Turnover But Not Performance. Med Sci Sports Exerc 44(1):57–68
Crowe M, Weatherson J, Bowden B. (2006) Effects of dietary leucine supplementation on exercise performance. Eur J Appl Physiol 97(6):664–672. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/412/CN-00561412/frame.html (Internet)
van Hall G, Raaymakers JS, Saris WH, Wagenmakers AJ (1995) Ingestion of branched-chain amino acids and tryptophan during sustained exercise in man: failure to affect performance. J Physiol 486(Pt 3):789–794
Mikulski T, Dabrowski J, Hilgier W, Ziemba A, Krzeminski K (2015) Effects of supplementation with branched chain amino acids and ornithine aspartate on plasma ammonia and central fatigue during exercise in healthy men. Folia Neuropathol 53(4):377–386
Chen IF, Wu HJ, Chen CY, Chou KM, Chang CK (2016) Branched-chain amino acids, arginine, citrulline alleviate central fatigue after 3 simulated matches in taekwondo athletes: a randomized controlled trial. J Int Soc Sports Nutr 13:28
Chang CK, Chang Chien KM, Chang JH, Huang MH, Liang YC, Liu TH (2015) Branched-chain amino acids and arginine improve performance in two consecutive days of simulated handball games in male and female athletes: a randomized trial. PLoS One 10(3):e0121866
Lagarde D, Batejat D (1995) Disrupted sleep-wake rhythm and performance: Advantages of modafinil. Military Psychol 7(3):165
Borg G. (1990) Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 16:55–8
Wagenmakers A, Coakley J, Edwards R (1990) Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle’s disease. Int J Sports Med 11(S 2):S101–S113
Suarez I, Bodega G, Fernandez B (2002) Glutamine synthetase in brain: effect of ammonia. Neurochem Inter 41(2–3):123–142
Dasarathy S, Mookerjee RP, Rackayova V, Thrane VR, Vairappan B, Ott P et al (2017) Ammonia toxicity: from head to toe? Metab Brain Dis 32(2):529–538
Assenza A, Bergero D, Tarantola M, Piccione G, Caola G (2004) Blood serum branched chain amino acids and tryptophan modifications in horses competing in long-distance rides of different length. J Anim Physiol Anim Nutr 88(3-4):172–177
Stupka N, Lowther S, Chorneyko K, Bourgeois J, Hogben C, Tarnopolsky M (2000) Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol 89(6):2325–2332
Maughan RJ, Gleeson M (2010) The biochemical basis of sports performance. Oxford University Press, Oxford
Acknowledgements
The authors are indebted to all the researchers whom we cited in this review for their significant and valuable research.
Funding
This work was financially supported by a grant (97s32) from Vice-Chancellor for Research Affairs of Ahvaz Jundishapur University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hormoznejad, R., Zare Javid, A. & Mansoori, A. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: a systematic review with meta-analysis. Sport Sci Health 15, 265–279 (2019). https://doi.org/10.1007/s11332-019-00542-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-019-00542-4