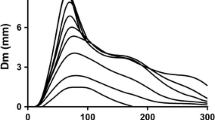
Abstract
Purpose
The review aimed to provide a wider overview on the new application fields of MMG signal. A particular emphasis on measurements reliability and sensitivity was also given.
Methods
Five electronic databases were searched for eligible studies published between 2000 and 2014. Two authors assessed selected articles. Several domains (sensor types, participants’ characteristics, experimental protocols, investigated muscle/s, measured parameters, and main results) were extracted for analysis. From a total of 1326 citations, 170 were selected for evaluation and 111 studies were identified.
Results
From the analysis of the literature it resulted that MMG signal (a) has a high level of reliability, especially for the parameters calculated during isometric contractions; (b) can be used to examine muscle mechanical activation and motor unit recruitment strategies under several types of exercise paradigms; (c) is influenced by the mechanical characteristics of cross-bridges and series elastic components, and may provide deeper insights into their behaviour under several physiological models; (d) could be a useful biomarker for triggering orthosis or multifunction access devices, and for the evaluation of patients presenting alterations in muscle function.
Conclusions
The MMG approach has been proficiently applied in several fields ascribable to both exercise physiology and clinical settings. This approach can provide deeper insights into muscle mechanical behaviour under several physiological models and for the evaluation of patients with altered muscle function.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
In the last decades, a growing body of literature focused on the use of mechanomyography (MMG) as a means to study non-invasively skeletal muscle mechanical activity. MMG signal is detectable at the skin surface during the dimensional changes of active muscle fibres that generate pressure waves due to voluntary or evoked contractions. It has been suggested that this signal may reflect the three main physiological phenomena of the mechanical aspects of muscle contraction, including (a) the gross lateral movement of the contracting muscle at the beginning of muscle contraction, (b) the subsequent vibrations at the resonance frequency of the muscle, and (c) the dimensional changes in the active muscle fibres [1, 2]. Electromyography (EMG), as the conventional modality to monitor skeletal muscle electrical activity, is limited in providing sufficient information on muscle mechanical behaviour [3], focusing only on the electrical aspects (neural control) of muscle function [4]. EMG signal is also not sufficiently suitable to quantify electrically evoked dynamic muscle actions [5]. The exploration of complementary paradigm that is sensitive to the muscle mechanical activities and devoid of inherent electrical noise such as MMG is, therefore, needed. The classical works on muscle sound, the former term to indicate the MMG signal, revealed that its properties, i.e. vibrations and dimensional changes of the active fibres, could be used to get more insights into the mechanical counterpart of muscle contraction [6–12].
The term MMG was suggested during the CIBA Foundation Symposium in 1995 [13] to overcome the terminological confusion (phonomyogram, acoustic myogram, vibromyography, soundmyogram, muscle sound, etc.) caused also by the habit of identifying the phenomenon with other explanations than its mechanical origin [2, 6, 14].
The pressure waves transmitted from the active muscle fibres to the skin surface can be detected by specific transducers to record muscle surface oscillations related to motor units’ (MUs) mechanical activity [2, 15]. Several types of transducers can be used, among which piezoelectric contact sensors (PIZ), microphones (MIC), laser distance sensors (LDS), electret condenser microphones (ECM), and accelerometers (ACC), which is largely the most used sensor for MMG detection.
Usually, the MMG signal is analysed in time domain by calculating the root mean square (RMS), the averaged rectified value (ARV) of the signal and/or the maximum displacement during the transient phase of contraction (p–p), and in frequency domain by determining the mean frequency (MPF or MF), and/or the median frequency (MNF) of the power spectrum density distribution. Altogether, these parameters provide information about the neuromuscular strategies adopted by the contracting muscle to activate and modulate the force output during contraction (see Fig. 1).
The application fields of MMG are numerous and increasing: they span from the assessment of muscle function during isometric or dynamic contractions under different physiological conditions, such as fatigue [16–18], muscle temperature manipulation [19, 20], stretching [21–23], ageing [24], training [25, 26], to the analysis of the effects of rehabilitation programs [27, 28], the development of prosthesis [29], and/or the use of the MMG as a triggering signal [30].
From the pioneering proposal of Hufschmidt [31], a novel and interesting application field that is taking place in the last years is the use of an EMG, MMG, and force signals combined approach as a tool to partition the electrical and the mechanical events underpinning the electromechanical delay during muscle contraction (EMD) and relaxation (R-EMD) phase [19, 32–37].
Despite the utilization and the applicability of MMG are becoming even more frequent and widespread, only four review articles related to MMG [38–41] are actually present in the literature. They all focus on particular aspects of MMG application, such as the examination of MMG amplitude and frequency responses [41], the assessment of MU recruitment strategies [38, 41], and the evaluation of muscle function [39, 40].
The present review aimed to provide a wider overview on the new and different application fields (from basic muscle physiology to sports and rehabilitation), in which the MMG signal is employed. A particular emphasis on the reliability and sensitivity of the MMG signal analysis will be also given.
Methods
Article searching
A comprehensive literature search for MMG and skeletal muscle was performed on the electronic databases PubMed, Scopus, Web of Science, Embase and Google Scholar for relevant articles published from January 2000 to October 2014. The key words used were mechanomyography, mechanomyogram, mechanomyographic, MMG, skeletal muscle, muscle function, muscle assessment and rehabilitation. The combination among key words with #AND and #OR was performed. Only papers written in English were considered during databases searching. Journal articles, conference proceedings and clinical reports were included for potentially eligible studies. Moreover, the reference list of all the articles was carefully checked.
Study selection
Article titles and abstracts identified by the search were screened for potential relevance. The full text of all potentially relevant studies was reviewed to determine if it fulfilled the eligible criteria. Articles describing a theoretical or practical use of MMG in the field of muscle contraction physiology and rehabilitation were included. Two authors (EC and SR) screened independently the results of the electronic searches to select potentially relevant citations according to the criteria defined above. The published works meeting the most relevant criteria were included. Studies without proper data presentation, with unclear or vague protocol description, and without an in-depth discussion were excluded from this review.
Data extraction
Two authors (EC and SR) analysed the articles individually using an MS-Excel structured data extraction form purposely created for this review. Extracted data were compared and discussed by these two authors before being included in the final pile of reviewed papers. Information extracted from each article included: (a) type of sensor, (b) participants characteristics, (c) experimental protocols, (d) investigated muscle/s, (e) measured and calculated parameters, and (f) main results.
Validity assessment
Three authors (EC, SR and FE) analysed the data extracted from the potentially relevant articles. After the analysis, only the information extracted from the most relevant studies (those organized with proper data presentation, clearly verified selection of protocols, and through demonstration of research methodologies) was discussed, to reduce risk of bias.
Quantitative data synthesis
All the articles were divided in three main areas: (a) reliability, (b) muscle physiology, and (c) rehabilitation. Articles in the muscle physiology area were further divided into: (a) muscle contraction under isometric (in vivo and in isolated muscle) and dynamic conditions, (b) MMG utilization in different physiological models (fatigue, stretching and different training modalities), and (c) the use of MMG in EMD partitioning. A description of these studies is presented in tables in the Results section (Tables 1, 2, 3, 4, 5, 6, 7, 8, 9).
Results
Flow of included studies and their characteristics
The comprehensive literature search returned 111 articles (Fig. 2). Of these, 7 met the inclusion criteria for “reliability”, 88 for “muscle physiology”, and 16 for “rehabilitation”. Within “muscle physiology”, 29 articles were included in “isometric contraction” (23 in vivo and 6 concerning isolated muscle), 20 were included in “dynamic contraction”, 20 in “fatigue”, 8 in “stretching”, four in “different training” and 7 in “electromechanical delay”.
Among the 111 relevant studies, 102 studies were peer-reviewed journal articles and 9 studies were conference papers.
Reliability of MMG signal
In accordance with Munro [42], reliability values were consider as very high if Intraclass Correlation Coefficient (ICC) was >0.90, high if between 0.70 and 0.89 and moderate if between 0.50 and 0.69. Table 1 includes the studies on the reliability of the MMG signal under isometric [33, 43–45] and dynamic contractions [46–48]. All the investigations used the ACC as sensor. With the exception of Stock et al. [48], who investigated the reliability of the MMG signal of the pectoralis major and of the triceps brachii muscles, all the other studies focused on lower limbs muscles, in particular the quadriceps and gastrocnemius muscles.
Cè et al. [33] reported a high intersession intraclass correlation coefficient (ICC) and a low standard error of measurement (SEM%) of the maximum MMG displacement during the muscle relaxation phase (R-MMG p–p) after a tetanic stimulation. Ryan et al. [43] found a lack of consistency and some degree of inter-individual variability between MMG amplitude and isometric torque. MMG mean power frequency (MPF) and isometric torque relationship were best fit with linear models for both the low-strength and high-strength groups. Al-Zahrani et al. [44] reported moderate to strong ICC levels with high small detectable differences (SDDs) in MMG RMS and MF versus torque relationship, both before and after a fatiguing protocol. The authors, given the not very high between-day reliability, suggested caution in using MMG RMS and MF and their time behaviour in assessing muscle fatigue. Similar conclusions were also supported by Herda et al. [45], who found comparable levels of reliability of the amplitude and the MPF of the MMG signal.
Under dynamic condition [46, 48], reported a lack of reliability of the relationship between MMG amplitude and force in quadriceps, pectoralis major and triceps brachii muscles. The authors concluded that when using MMG in the clinical setting, dynamic muscle actions do not appear to be appropriate for assessing training-induced changes in muscle function. Lastly, Armstrong et al. [47] showed moderate ICC values of the MMG signal detected by high-resolution triaxial ACC assessed on quadriceps muscle during repetitive single-leg balance test. The authors concluded that MMG can provide precise information on postural balance, and may have application in evaluating postural control and stability.
MMG in muscle physiology
All the studies concerning muscle physiology are divided into sub-groups and reported in Tables 2, 3, 4, 5, 6, 7, 8. Among the 88 studies, 49 used an ACC as sensor to detect the MMG signal, 28 used a PIZ, 6 an MIC, 3 used an LDS and 2 used an ECM. As far as the investigated muscle is concerned, 33 studies over 88 were conducted on the elbow flexors muscles (biceps brachii and brachioradialis muscles), the quadriceps muscle was analysed in 25 studies, the plantar flexors muscles (the whole triceps surae or a single component) were investigated in 22 studies, 4 on the triceps brachii muscle, 2 on the upper trapezius, tibialis anterior and first digital interosseum muscles, and only 1 on the finger flexors, pectoralis major, erector spinae and biceps femoris muscles.
MMG during isometric contraction “in vivo”
Five studies observed a strong relationship between force output and MMG amplitude and frequency content up to a certain % MVC [24, 49–53]. On one hand, the MMG RMS, which provides information about the number of active MUs [2], has been reported to have a curvilinear shape with the increase in force output, reaching a plateau or even a clear decrease that differ from muscle to muscle: the more the investigated muscle is composed by slow twitch MUs, the earlier the plateau appears. Moreover, a difference in RMS and an earlier plateau in the RMS/% MVC relationship were reported in the elderly [24], by the gender of the investigated subjects [53], and at different joint angles at which the muscle is contracted [54]. On the other hand, the relationship between MMG signal frequency content and force output is mostly linear [53]. With these characteristics, MMG permitted to study the adaptations of neuromuscular activation strategies induced by several conditions, such as climbing [49] or resistance and aerobic training [55]. Also different physiological models, such as potentiation [56], muscle pain [57], hypoxia [58], hypothermia [59], muscle relaxation [60, 61], while overimposing an electrical twitch stimulation during maximum voluntary contraction [62], or during spinal anaesthesia [63] could be also investigated.
MMG during isometric contraction in isolated muscle
The articles concerning the MMG signal during isometric contraction in isolated muscle are presented in Table 3. All the six studies catalogued within this area, used muscles from a mammalian model (four studies used rat’s muscle and two cat’s muscles). Five of them analysed the gastrocnemius muscle [8, 64–67], while the other investigated the tibialis anterior muscle [68].
In their investigation, Orizio et al. [64] demonstrated that the force and MMG frequency response can be modelled by a critically damped second-order system with two real double poles and a pure time delay. This allowed the authors to conclude that MMG could be a reliable tool to investigate the muscle frequency response during stimulated isometric contraction, and that different components of the muscle mechanical model may specifically affect the force or MMG signals.
In a further study, Orizio et al. [67] observed that the rate of MMG increase during the transient phase of muscle contraction is always higher than that of force, while during the relaxation phase, the force decrease is much faster than the MMG. A counterclockwise hysteresis in the graph with the relative force exerted vs the relative MMG amplitude relationship was reported. Authors concluded that during relaxation more force is developed for the same muscle surface displacement.
Hsieh et al. [68] used MMG signal to investigate the paired-pulse transcranial magnetic stimulation inhibitory phenomenon in awaked rats, and then applied differential pharmacology to test the hypothesis that long-interval cortical inhibition is mediated by the GABAA receptor. Their results demonstrated predictable latency discrepancy between the motor-evoked potential and the evoked MMG. With pharmacological testing, time course observations showed that paired-pulse transcranial magnetic stimulation-MMG inhibition was acutely reduced following pentylenetetrazole (GABAA antagonist), acutely enhanced after pentobarbital (GABAA agonist) and then recovered to pre-treatment baseline after 1 h. Their findings support the application of the paired-pulse transcranial magnetic stimulation-MMG technique for measuring the cortical excitability in awaked rats and provide the evidence that GABAA receptor contributes to long-interval paired-pulse cortical inhibition. They concluded that paired-pulse transcranial magnetic stimulation-MMG appears to be a well-tolerated biomarker for measuring GABAA-mediated cortical inhibition in rats.
In their investigations, Bichler and coworkers [8, 65, 66] described the shape and the amplitude of the MMG signal during muscle contraction and relaxation at different stimulation frequencies. They observed that MMG onset was coincident with the beginning of the increase in tension during single twitch and tetanically evoked stimulations, followed by a weaker MMG signal accompanying the fused tetanus phase [8]. During contraction and relaxation, MMG was characterized by the reverse direction of the first extreme phase, positive and negative, respectively. MMG amplitude was correlated with both the tension increase and the velocity of tension increase during both stimulation modalities [65]. The strongest type II MUs (fast fatigable) generated the MMG signal of the highest amplitude. Lastly, MMG p–p was directly correlated with the increase in twitch tension during the potentiation phase and with the decrease of twitch tension after a fatigue test, respectively [66].
MMG during dynamic contraction
Table 4 presents the analysed articles concerning the MMG signal during dynamic contractions. Ten over twenty studies used an ACC to detect MMG signal [46, 69–77], nine used a PIZ [78–85], and one used an MIC [86]. Ten articles focused on the quadriceps muscle, seven investigated the biceps brachii muscle, two were centred on the triceps brachii muscle, while pectoralis major, first digital interosseum and forearm flexors muscles were analysed in just one study.
The investigations mainly focused on the relationship between MMG signal and force output during concentric isotonic [48, 69, 74, 76] and isokinetic contraction [78, 81–83], as well as eccentric isokinetic contraction [76, 80, 84, 85]. Altogether, authors observed that MMG amplitude increases linearly in both isotonic and isokinetic contraction until a certain muscle-dependent threshold that could be identified to be about the 80 % 1-RM and 240°/s for isotonic and isokinetic contraction, respectively. Then, MMG amplitude reaches a plateau or even decreases. MMG amplitude was reported to be higher during concentric than eccentric contractions [71].
Two investigations evaluated the relationship between MMG amplitude and excess post-exercise oxygen consumption after incremental cycle ergometer exercise [70], and between the metabolic request and MMG responses during continuous exercise at critical power determined from the 3-min all-out test [75]. Malek et al. [70] evidenced significant time-constant values for excess post-exercise oxygen consumption and MMG amplitude for quadriceps muscles during the 60-min post-exercise recovery period. These results suggested that excess post-exercise oxygen consumption after exercise could not be exclusively attributed to elevated activity of the working muscles. Bergstrom et al. [75] found no changes in MMG amplitude or MPF over time against an increase in oxygen uptake and heart rate.
Three studies demonstrated no changes in MMG signal properties during dynamic contractions as a consequence of muscle compression [72], change in muscle oxygen tension [77], and muscle dehydration [79].
MMG analysis and the effects of training
Four studies evaluated the effect of different training modalities on muscle performance utilizing MMG signal analysis (Table 5). All works used a PIZ to detect MMG signal. Two studies analysed the quadriceps muscle [26, 87], while the other two analysed the biceps brachii [88] and the plantar flexors muscles [25], respectively.
Evetovich et al. [87] examined the effects of concentric isokinetic leg extension training on MMG MPF in a group of 21 young men. Training consisted in six sets of leg extensions, 3 days per week, for 12 weeks at an angular velocity of 90°/s. The authors found an increase in peak torque but no changes in the MMG MPF from the vastus lateralis muscle over the 12-week training period. They explained these results with training-induced changes in muscle stiffness that could affect the MMG signal, and/or adaptations in other quadriceps muscles than the investigated vastus lateralis.
Esposito et al. [26] verified the hypothesis that isokinetic training could induce even in the elderly changes in EMG and MMG parameters, compatible with functional changes in the MU pool. In a group of ten sedentary males, aged from 62 to 78 years., the surface EMG and MMG were recorded from the vastus lateralis muscle during isometric contractions at 20, 40, 60, 80 and 100 % MVC, before and after 12 weeks of isokinetic actions. Training sessions consisted of six series of ten repetitions, each at an angular velocity of 2.09 and 4.19 rad/s, twice a week). The authors found increases in MVC and cross-sectional area determined by magnetic resonance imaging (CSA), MVC/CSA ratio, EMG RMS and MMG MF at the highest workloads. Altogether, these findings indicated that (a) isokinetic training can improve muscle size and performance even in the elderly, and (b) EMG and MMG changes may be compatible with a retrieval of the fast twitch fibre MUs.
Ebersole et al. [88] evaluated the effects of unilateral, isometric training of the forearm flexors on strength, MMG and EMG responses of the biceps brachii in the trained and untrained limb at three joint angles. A group of seventeen young women was involved in a unilateral training program consisting in 3–5 sets of 8/6-s repetitions at 80 % MVC, 3 times per week, for 8 weeks. The authors found an increase in arm circumference as well as maximum isometric strength in the trained limb at all three joint angles. There were, however, no changes in MMG or EMG amplitude in the trained or untrained limb. These findings indicated that the increased strength might have been due to factors associated with hypertrophy, independent of neural adaptations in the biceps brachii. Furthermore, hypertrophy may have had counteractive effects on the MMG signal that could be responsible for the lack of training-induced changes in the MMG amplitude.
Cramer et al. [25] assessed the effects of 3 days of isokinetic resistance training combined with 8 days of creatine monohydrate supplementation on peak torque, mean power output, acceleration time, and EMG and MMG signals of the vastus lateralis muscle during maximal concentric isokinetic leg extension actions. A group of 25 young men were randomly assigned to either the creatine or the placebo group. Training consisted of three sets of 10 repetitions at 150°/s, performed on day 3, 5 and 7. The authors reported an increase in EMG and MMG MDF and in peak torque, with a decrease in acceleration time in both groups.
MMG and fatigue
The investigations on the assessment of the fatigue phenomenon through MMG signal analysis are reported in Table 6.
Eleven studies over twenty used an ACC to detect MMG signal [2, 16–18, 89–94], seven used a PIZ [82, 95–100], and two utilized an MIC [101, 102].
These articles focused mainly on the modifications of MMG signal (in particular by calculating MMG RMS and MF or MPF) after isometric voluntary [2, 90, 92–95, 97, 100, 102] and electrically evoked contractions [16, 17], or dynamic contractions [82, 89, 91, 96, 98]. With the only exception of Camic et al. [89] and Orizio et al. [2], increases in MMG amplitude accompanied by decreases in MMG signal frequency content were reported in all the other investigations under both isometric and dynamic conditions. Interestingly, Gobbo et al. [16] and Cè et al. [17] highlighted strong correlations between the peak torque and the p–p of the MMG signal during the transient phase of muscle contraction and relaxation. These correlations suggested that MMG amplitude of these two phases might be strictly correlated with the transient of Ca2+ release and re-uptake occurring during muscle contraction and relaxation, respectively [16, 17].
Two studies [101, 103] aimed to evaluate the use of MMG as a biomarker to evidence the occurrence of the anaerobic threshold during incremental cycling exercise until maximum effort, with different results: Kimura et al. [101] found an abrupt change in MMG signal coinciding with the ventilatory threshold, suggesting that the MMG could be used practically to retrieve the fatigue-related changes in muscle mechanical properties during cycle exercise. On the other hand, Zuniga et al. [103] retrieved no correlations between MMG signal and ventilatory threshold, indicating that gas exchange and neuromuscular fatigue thresholds may demarcate different exercise intensity domains.
Lastly, Hendrix et al. [18], aiming to compare the mean torque levels derived from the critical torque and MMG MPF fatigue threshold (MPFFT) during isometric forearm flexion muscle actions, found no correlations between the two parameters.
MMG and stretching effects
The articles aimed to evaluate the acute effects of stretching on muscle force output are summarized in Table 7. Six investigations over eight used an ACC to detect MMG signal, whereas the other two studies used a PIZ. Plantar flexors muscles were analysed in five investigations [21, 23, 104–106], while the others focused on quadriceps muscle [107], biceps femoris [108], and biceps brachii [22].
In their investigations, Esposito et al. [21, 105], Ce et al. [104] and Longo et al. [23] found a decrease in MMG p–p after passive stretching coupled to a decrement of evoked twitch and tetanic force. The authors stated that such decrements were ascribable to mechanical modifications of the muscle–tendon unit (stretching-induced decrease in stiffness), which altered the transmission of force from muscle to the tendon insertion point. These modifications have been shown to persist for at least 2 h [21]. Interestingly, MMG RMS during the force plateau phase of tetanic stimulation, increased after stretching, recovering its pre-stretch value within 15 min. The authors explained this finding suggesting that during the plateau phase both series elastic components and contractile elements reach their isometric condition, during which MMG is mainly influenced by contractile and parallel viscoelastic elements. Thus, the stretching-induced decrease in MTU stiffness should lead to an increase in MMG amplitude. Evidence of this mechanism was provided by Longo et al. [23], who reported an inverse correlation between MMG RMS (calculated during torque plateau phase) and stiffness (calculated at muscle–tendon unit and tendon level).
Similar decreases in muscle force output evaluated under concentric isokinetic condition with a concomitant increase in MMG amplitude were also reported by other investigations [22, 106–108]. They all found a stretching-induced reduction in MVC and single twitch after passive stretching of plantar flexor and quadriceps muscles, without significant increases in MMG amplitude. It was suggested that other mechanisms than muscle–tendon unit stiffness, such as force reduction, might have played a major role in determining MMG amplitude.
MMG and EMD components
The investigations that used MMG signal to assess the EMD during the contraction and/or the relaxation phase of muscle activation are summarized in Table 8. To this purpose, MMG signal was detected by ACC from the gastrocnemius medialis muscle [32, 35, 36], and biceps brachii muscle [19, 34, 37]. Four over seven articles analysed the contraction phase and three focused on the muscle relaxation phase [32–34].
Esposito et al. [35] assessed the effects of a bout of passive stretching on both the electrochemical and mechanical contributors to EMD, and their relative recovery time course. They found a stretching-induced increase in both EMD components, with different recovery times. Indeed, while the “electrochemical” component recovered within 15 min, the mechanical contribution (as well as the total EMD duration) remained lengthened for the entire recovery period, suggesting that stretching had effects of short duration at the electrochemical level, but more persisting effects on muscle–tendon unit viscoelastic characteristics.
Sasaki et al. [37] quantified the contribution of the electrochemical and mechanical processes of EMD in the human biceps brachii muscle over a wide range of elbow joint angles, finding that the time for electrochemical process was independent of joint angle, while the time for mechanical process and the total EMD duration were significantly greater at the most extended joint positions.
Cè et al. [19] evaluated the effects of muscle temperature and fatigue on the electrochemical and mechanical EMD components. Fatigue lengthened both of them while muscle cooling affected only the electrochemical processes of EMD.
Similarly, Rampichini et al. [36] found a fatigue-induced increase in all EMD contributors under electrically evoked, tetanic conditions. Reliability of the measurements and calculations were found to be from high to very high.
Lastly, Cè et al. [32–34] assessed for the first time during muscle relaxation the duration of the electrochemical and the mechanical contributors to R-EMD. The effects of fatigue on single R-EMD components were also determined. They found a lengthening of all R-EMD contributors after fatigue, with a main effect mainly on the mechanical component.
MMG in the rehabilitation field
The investigations aimed to assess the use of MMG for the evaluation of muscle activation in patients with different neuromuscular disorders, or the effects of particular rehabilitation programs, are reported in Table 9. Fourteen over sixteen studies detected the MMG signal by ACC and the other two investigations used an MIC. Six investigations analysed the quadriceps muscle, four the biceps brachii muscle, two the diaphragm muscle, and one on the masticatory muscles, soleus, pronator teres, wrist extensors and flexors, and rectus abdominis muscles, respectively. Five studies focused on patients with spinal cord injuries [109–113], two on patients with Parkinson’s disease [27, 114] and with chronic obstructive pulmonary disease [115, 116], one study on patients with myopathy [117]. The other six studies were conducted on healthy, able-body participants [20, 28, 30, 118–120].
Huang et al. [111] reported that patients presenting spasticity (patients with either spinal cord injuries or stroke) exhibited a larger H reflex/M-wave ratio and MMG amplitude than able-bodies controls. Moreover, MMG amplitude correlated with functional impairments. The authors concluded that spastic hypertonia involved an atypical increase in motoneuronal excitability and muscle mechanical properties, while impairment of functional performance and daily activity was attributable primarily to altered mechanical properties of a spastic muscle.
Some authors [109, 110, 112, 113] tested the best profile for functional electrical stimulation of the quadriceps muscle allowing raising the knee from 90° to 40° of flexion in a group of patients with spinal cord injuries. They found that a functional electrical stimulation protocol set to 1 kHz pulse frequency, with a 200 μs active pulse duration and a burst frequency of 50 Hz was the most effective. Higher MMG signal amplitude in patients than controls with no differences between rectus femoris and vastus lateralis muscles was also reported.
Marusiak et al. [27, 114] found no differences in MMG activity of agonist and antagonist muscles and in peak torque in patients with Parkinson’s disease during the “on” phase of their medication. On the other side, without pharmacological support, patients with Parkinson’s disease exhibited higher MMG amplitude and a lower median frequency of the MMG signal in the biceps brachii muscle. The authors concluded that, since MMG was not affected by physiological postural tremor and can depict differences between parkinsonians and controls, it might be a valuable tool for neuromuscular assessment in this scenario.
Sarlabous et al. [115] found a high positive correlation between the maximum inspiratory pressure developed in a respiratory cycle and MMG amplitude of the left and right hemi-diaphragm, and a negative correlation between the maximum inspiratory pressure and the maximum frequency of the MMG signal spectrum. These correlations were more evident in patients with severe chronic obstructive pulmonary disease. Torres et al. [116] found that the slope of the evolution of the MMG amplitude parameters, as the load increases during the respiratory test, has positive correlations with the Tiffenau index (% forced expiratory volume/forced vital capacity). Altogether, these authors suggested that the information provided by MMG signal could be used to evaluate the respiratory effort, muscular function and efficiency in patients with chronic obstructive pulmonary disease.
Lamraoui et al. [118] reported that, although detection of cough effort by MMG presents lower performances than EMG detection, mostly in terms of cough anticipation, MMG might be a useful tool in detecting cough event because of its better portability.
Ng et al. [117] reported a significant positive correlation between the post-activation potentiation evaluated with force signal and that assessed by MMG, with lower values in patients with non-dystrophic myopathies. They concluded that post-activation potentiation assessed by MMG could be used as an index of muscle contractility.
Kawakami et al. [119] reported correlations between the needle EMG signal of the lateral pterygoid muscle and the MMG signal during different mandibular positions, suggesting that the activity of this muscle could be evaluated by MMG signal recorded in the external ear canal.
Alves, Chau [120] investigated the discriminability of multiple hand motions using multichannel forearm MMG. In a group of nine able-bodied participants, MMG signals from six sites were differentiated among eight classes of forearm muscle activity with high level of accuracy. These results suggest that, with additional research, MMG may become a usable control signal for multifunction access devices.
Nolan, dePaor [30] described a system to provide a communication and control tool for disabled people, triggered by MMG signal, which can be detected and processed by the computer. A decision is made as to whether the muscle is contracted or relaxed based on the amplitude of the processed signal. If the computer decides that the muscle is contracted, a software switching action is performed. This switching action is used to control a software alphabet board that the disabled person can use to spell out messages.
Tian et al. [28] investigated the effects of age-related sarcopenia on the time- and frequency-domain properties of lower extremity muscles’ MMG activities. Compared to the young subjects, the elderly had significantly less lean thigh volume, absolute and relative maximal force and power. While the MMG RMS of the young subjects increased with testing intensities, in the elderly it increased only from 45 to 60 %, but levelled off from 60 to 75 % of 1-RM. These observations could be explained by the fact that neuromuscular performance and changes in MU activation pattern may result from age-related sarcopenia. The authors suggested that MMG could be used as an important means to study muscle contraction in age-related sarcopenia.
Mitchell et al. [20] examined the effects of pulsed shortwave diathermy on intramuscular temperature and MMG of the vastus lateralis muscle. They found that intramuscular temperature, as well MMG amplitude and MF increased during the MVC with the greatest increases observed for the diathermy group. During ramp contractions, MMG amplitude and MF increased at all percentages of MVC for the diathermy group only. The authors concluded that diathermy treatments may decrease musculotendinous stiffness, but not absolute strength or motor control strategies that influence force production.
Discussion
The present review aimed to provide a wider overview on the new and different application fields (from basic muscle physiology to sports and rehabilitation), in which the MMG signal can be employed, giving also particular emphasis on the reliability of the measurements.
The analysis of the literature showed that MMG, besides its well-established use to investigate muscle activation and MU recruitment strategies under different types of exercise paradigms, (a) has a high level of reliability, in particular during isometric contraction; (b) is influenced by the mechanical characteristics of cross-bridges and SEC behaviour during contraction; and (c) in the rehabilitation field, could be a useful biomarker for triggering orthosis or multifunction access devices, and for the assessment of patients with alterations in muscle function.
Reliability of MMG signal
Contradictory findings on MMG signal reliability emerged from the analysis of the literature: while moderate to high reliability values were reported for both time- and frequency-domain MMG parameters under voluntary isometric contraction [33, 43–45], during dynamic contraction the measurements reliability appears less evident [46, 48]. This aspect should be taken into consideration when planning experimental protocols in which repeated MMG measurements on different days or sessions are required.
The use of MMG to assess muscle activation and training effects
MMG signal has been widely used to assess MU recruitment strategies under both static and dynamic conditions. Recently, MMG signal was also acquired during incremental aerobic exercise, with the aim to correlate MMG variables with some cardiopulmonary and metabolic parameters related to the anaerobic threshold [70, 75]. Whereas correlations between MMG signal amplitude and frequency content and the level of force output were reported under both isometric [24, 49–53, 64] and dynamic contractions [48, 69, 74, 76], the use of MMG signal to evaluate muscle activation during aerobic tasks has been less investigated [70, 75].
Irrespective of muscle contraction regime (static or dynamic), MMG amplitude increases with force until a certain level, which for isometric contraction depends strictly from muscle dimension and fibres composition, spanning from 60 to 100 % MVC. In dynamic contractions, it occurs near the 80 % 1-RM for concentric and 240°/s for isokinetic contractions. After this “threshold”, MMG amplitude reaches a plateau or even decreases [48, 78, 121]. On the other side, MMG frequency content increases linearly with force output [41, 53]. The typical behaviour of MMG amplitude and frequency content with respect to force output, allowed investigators to apply this approach to evaluate the effects of different interventions and/or training protocols on muscle activation and MU recruitment strategies [24, 26, 48–51, 53, 56, 64, 69, 74, 76, 89, 122].
As far as aerobic exercise is concerned, only the relationship between MMG and excess post-exercise oxygen consumption (EPOC) after incremental cycle ergometer exercise [70], and between the metabolic request and MMG responses during continuous exercise at critical power determined from the 3-min all-out test [75] was evaluated. The first study reported a significant correlation between EPOC and MMG amplitude. The second investigation found no changes in MMG amplitude and MPF during a 3-min all-out cycling exercise performed at critical power. The use of MMG signal as a biomarker to evaluate muscle activation during aerobic tasks is still to be fully examined in depth.
MMG signal for fatigue assessment
Studies concerning the use of MMG as a biomarker for fatigue assessment, in particular at peripheral level, were mainly performed during sustained contractions under both static and dynamic conditions. Generally, a decrease in MMG amplitude, coupled with a downshift of its frequency content, was reported. Under a physiological point of view, these findings indicate that the fatigue-induced modifications in MU recruitment and firing rate (two events typically involved in fatigue) can be investigated by MMG. In particular, some authors found the MMG signal to be more sensitive to fatigue than EMG [82, 96]. Indeed, MMG amplitude and MPF were reported to change because of fatigue during isokinetic concentric contractions more noticeably than EMG parameters [82, 96]. Interestingly, MMG p–p, which represents the maximum displacement of the muscle belly during the on- (muscle contraction) and off-phase (muscle relaxation), has been shown to be strongly correlated with the force signal and its rate of development, both before and after fatigue [16, 17]. These correlations, in particular between MMG p–p and the second derivative of force development, and between R-MMG p–p and the second derivative of force relaxation, led the authors to suggest that these two MMG parameters may reflect the transient of Ca2+ uptake and re-uptake.
As a novelty, two investigations [101, 103] evaluated the use of MMG to mark the occurrence of the anaerobic threshold during incremental cycle exercise. In the light of the contradictory results, this peculiar MMG approach in detecting anaerobic threshold still needs to be fully explored.
MMG signal for stretching effects assessment
A correlation between MMG amplitude and MTU stiffness has been often hypothesized [1, 12, 21, 22, 123–126]. The “axiom” underpinning this hypothesis was that a reduction in MTU stiffness should yield to a greater slack when the series elastic components lengthen due to the shortening of contractile elements. Therefore, a less stiff MTU would lead to wider oscillations of muscle surface and, in turn, to an increase in MMG amplitude during the on-phase of contraction and during the subsequent force plateau.
Passive stretching is largely used in sport and rehabilitation practice to improve joint range of motion (ROM) [127]. Acute passive stretching has been shown to reduce MTU stiffness [128, 129]. Recently, Longo et al. [23], through an ultrasound and MMG approach, provided experimental evidence that, after the administration of a bout of passive stretching, the reduction in MTU stiffness was significantly correlated with the increase in MMG amplitude. Explanation was given that in this scenario contractile and elastic elements are allowed to oscillate to a higher extent, thus increasing MMG amplitude. Under a practical point of view, the correlation between MTU stiffness and MMG amplitude indicates that MMG may represent a reliable and effective means to evaluate MTU stiffness in all those muscles and under those circumstances where a direct measure of this parameter is not possible or difficult to obtain.
MMG signal for EMD and R-EMD assessment
Traditionally, EMD and R-EMD have been calculated only through the EMG and force signals. Recently, a combined EMG–MMG and force signals approach permitted to partition EMD and R-EMD in different sub-components that may reflect the duration of the mainly electrochemical and mechanical events underpinning the two latencies [17, 19, 32, 35–37]. However, it should be taken into account that, due to temporal/spatial limitations inherent in recording electrical phenomena and lateral accelerations from the surface of the skin, and to elasticity and viscosity of non-contractile tissues, a precise endpoint for the electrochemical mechanisms and a starting point for the mechanical events cannot be precisely determined [34].
With this approach, the effects of different joint angles [37], muscle temperature manipulation [19], peripheral fatigue [19, 32, 36], and passive stretching [35] were assessed on the different components included in EMD and R-EMD. Moreover, the analysis of MMG signal, in particular during the relaxation phase provides more insights into the mechanical behaviour of cross-bridges and the series elastic components toward their return to a pre-contraction condition, giving rise to a new overall delay during relaxation, namely R-DelayTOT [32].
MMG signal in the rehabilitation field
Given the information provided by MMG about neuromuscular activation and MTU behaviour, its application in the rehabilitation area is growing. From this review, two main application fields emerged: (a) the use of MMG signal as an alternative to EMG in evaluating muscle functionality in different physio-pathological models (chronic obstructive pulmonary disease [115, 116]; spinal cord injury [109–113]; Parkinson’s disease [27, 114], and myopathy [117]); and (b) its employment as a biomarker to trigger orthosis or devices devoted to ameliorate patients self-sufficiency [30, 120]. As far as the first application is concerned, MMG signal permits to discriminate differences in muscle activation between patients and controls. Although the sensitivity of MMG is not significantly higher than EMG, in the light of the lack of influence of the change in the skin impedance due to sweating and its better portability [29, 118, 130], some authors consider the MMG as a reliable alternative method in assessing neuromuscular activation and MTU behaviour in those conditions where the EMG cannot be easily detected. In consideration of the second point, the use of MMG as a trigger to activate or deactivate some devices represents to date a promising area of investigation. Actually, only few studies used MMG to this purpose, but with interesting results [30, 120].
Nolan, dePaor [30] investigated the possibility to use MMG as a trigger signal to activate a software alphabet board that the disabled person can use to spell out messages. Alves, Chau [120] investigated the discriminability of multiple hand motions using multichannel forearm MMG. Further investigations aiming at evaluating the use of MMG as a control signal for multifunction access devices, may be strongly recommended.
Conclusions
MMG parameters have a high level of reliability when they are calculated during isometric contractions. They can be used to examine the level of muscle activation and MU recruitment strategies, and provide information on the mechanical behaviour of cross-bridges and SEC during contraction. An MMG approach can be useful in different types of exercise paradigms and it may provide deeper insights into muscle mechanical behaviour under several physiological models. Moreover, MMG could be a useful biomarker in the rehabilitation field for triggering orthosis or multifunction access devices, and for the evaluation of patients with alterations in muscle function.
References
Barry DT, Cole NM (1990) Muscle sounds are emitted at the resonant frequencies of skeletal muscle. Ieee T Bio-Med Eng 37(5):525–531. doi:10.1109/10.55644
Orizio C, Gobbo M, Diemont B, Esposito F, Veicsteinas A (2003) The surface mechanomyogram as a tool to describe the influence of fatigue on biceps brachii motor unit activation strategy. Historical basis and novel evidence. Eur J Appl Physiol 90(3–4):326–336. doi:10.1007/s00421-003-0924-1
Sasidhar S, Panda SK, Xu J (2013) A wavelet feature based mechanomyography classification system for a wearable rehabilitation system for the elderly. In: Biswas J, Kobayashi H, Wong L, Bessam A, Mounir M (eds) 11th International Conference on Smart Homes and Health Telematics, ICOST 2013, Springer, Singapore
Farina D, Merletti R (1985) Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol 96(4):1486–1495. doi:10.1152/japplphysiol.01070.2003
Braz GP, Russold M, Davis GM (2009) Functional electrical stimulation control of standing and stepping after spinal cord injury: a review of technical characteristics. Neuromodulation 12(3):180–190
Stokes MJ, Blythe M (2001) Muscle sounds in physiology, sports science and clinical investigation. Applications and history of mechanomyography. Medintel Monographs, Oxford
Allum JH, Dietz V, Freund HJ (1978) Neuronal mechanisms underlying physiological tremor. J Neurophysiol 41(3):557–571
Bichler E (2000) Mechanomyograms recorded during evoked contractions of single motor units in the rat medial gastrocnemius muscle. Eur J Appl Physiol 83(4–5):310–319
Gordon G, Holbourn AH (1948) The sounds from single motor units in a contracting muscle. J Physiol 107(4):456–464
Petitjean M, Maton B (1995) Phonomyogram from single motor units during voluntary isometric contraction. Eur J Appl Physiol O 71(2–3):215–222
Orizio C, Perini R, Diemont B, Maranzana Figini M, Veicsteinas A (1990) Spectral analysis of muscular sound during isometric contraction of biceps brachii. J Appl Physiol 68(2):508–512
Orizio C, Perini R, Veicsteinas A (1989) Muscular sound and force relationship during isometric contraction in man. Eur J Appl Physiol Occup Physiol 58(5):528–533
Investigating muscle sounds by mechanomyography. Discussion at: CIBA Foundation Symposium, 12 Dec 1995, London, England
Orizio C (1993) Muscle sound: bases for the introduction of a mechanomyographic signal in muscle studies. Crit Rev Biomed Eng 21(3):201–243
Orizio C, Gobbo M (2006) Mechanomyography. Wiley Encyclopedia of Biomedical Engineering. Wiley, Brescia, pp 1–23
Gobbo M, Ce E, Diemont B, Esposito F, Orizio C (2006) Torque and surface mechanomyogram parallel reduction during fatiguing stimulation in human muscles. Eur J Appl Physiol 97(1):9–15. doi:10.1007/s00421-006-0134-8
Cè E, Rampichini S, Limonta E, Esposito F (2013) Torque and mechanomyogram correlations during muscle relaxation: effects of fatigue and time-course of recovery. J Electromyogr Kinesiol 23(6):1295–1303. doi:10.1016/j.jelekin.2013.09.007
Hendrix CR, Housh TJ, Camic CL, Zuniga JM, Johnson GO, Schmidt RJ (2010) Comparing electromyographic and mechanomyographic frequency-based fatigue thresholds to critical torque during isometric forearm flexion. J Neurosci Methods 194(1):64–72. doi:10.1016/j.jneumeth.2010.07.006
Cè E, Rampichini S, Agnello L, Limonta E, Veicsteinas A, Esposito F (2013) Effects of temperature and fatigue on the electromechanical delay components. Muscle Nerve 47(4):566–576. doi:10.1002/mus.23627
Mitchell SM, Trowbridge CA, Fincher AL, Cramer JT (2008) Effect of diathermy on muscle temperature, electromyography, and mechanomyography. Muscle Nerve 38(2):992–1004
Esposito F, Limonta E, Ce E (2011) Time course of stretching-induced changes in mechanomyogram and force characteristics. J Electromyogr Kinesiol 21(5):795–802. doi:10.1016/j.jelekin.2011.07.012
Evetovich TK, Nauman NJ, Conley DS, Todd JB (2003) Effect of static stretching of the biceps brachii on torque, electromyography, and mechanomyography during concentric isokinetic muscle actions. J Strength Cond Res 17(3):484–488
Longo S, Cè E, Rampichini S, Devoto M, Limonta E, Esposito F (2014) Mechanomyogram amplitude correlates with human gastrocnemius medialis muscle and tendon stiffness both before and after acute passive stretching. Exp Physiol 99(10):1359–1369. doi:10.1113/expphysiol.2014.080366
Akataki K, Mita K, Watakabe M, Itoh K (2003) Mechanomyographic responses during voluntary ramp contractions of the human first dorsal interosseous muscle. Eur J Appl Physiol 89(6):520–525. doi:10.1007/s00421-003-0835-1
Cramer JT, Stout JR, Culbertson JY, Egan AD (2007) Effects of creatine supplementation and three days of resistance training on muscle strength, power output, and neuromuscular function. J Strength Cond Res 21(3):668–677. doi:10.1519/R-20005.1
Esposito F, Cè E, Gobbo M, Veicsteinas A, Orizio C (2005) Surface EMG and mechanomyogram disclose isokinetic training effects on quadriceps muscle in elderly people. Eur J Appl Physiol 94(5–6):549–557. doi:10.1007/s00421-005-1371-y
Marusiak J, Jaskolska A, Jarocka E, Najwer W, Kisiel-Sajewicz K, Jaskolski A (2009) Electromyography and mechanomyography of elbow agonists and antagonists in Parkinson disease. Muscle Nerve 40(2):240–248. doi:10.1002/mus.21250
Tian SL, Liu Y, Li L, Fu WJ, Peng CH (2010) Mechanomyography is more sensitive than EMG in detecting age-related sarcopenia. J Biomech 43(3):551–556. doi:10.1016/j.jbiomech.2009.09.034
Xie HB, Zheng YP, Guo JY (2009) Classification of the mechanomyogram signal using a wavelet packet transform and singular value decomposition for multifunction prosthesis control. Physiol Meas 30(5):441–457. doi:10.1088/0967-3334/30/5/002
Nolan Y, dePaor A (2004) The mechanomyogram as a channel of communication and control for the disabled. P Ann Int Ieee Embs 26:4928–4931
Hufschmidt A (1985) Acoustic phenomena in the latent period of skeletal muscle: a simple method for in vivo measurement of the electro-mechanic latency (EML). Pflugers Arch 404(2):162–165
Cè E, Rampichini S, Limonta E, Esposito F (2014) Fatigue effects on the electromechanical delay components during the relaxation phase after isometric contraction. Acta Physiol (Oxf) 211(1):82–96. doi:10.1111/apha.12212
Cè E, Rampichini S, Limonta E, Esposito F (2013) Reliability of the electromechanical delay components assessment during the relaxation phase. Physiol J 2013:1–7. doi:10.1155/2013/517838
Cè E, Rampichini S, Venturelli M, Limonta E, Veicsteinas A, Esposito F (2014) Electromechanical delay components during relaxation after voluntary contraction: reliability and effects of fatigue. Muscle Nerve. doi:10.1002/mus.24466
Esposito F, Limonta E, Ce E (2011) Passive stretching effects on electromechanical delay and time course of recovery in human skeletal muscle: new insights from an electromyographic and mechanomyographic combined approach. Eur J Appl Physiol 111(3):485–495. doi:10.1007/s00421-010-1659-4
Rampichini S, Ce E, Limonta E, Esposito F (2014) Effects of fatigue on the electromechanical delay components in gastrocnemius medialis muscle. Eur J Appl Physiol 114(3):639–651. doi:10.1007/s00421-013-2790-9
Sasaki K, Sasaki T, Ishii N (2011) Acceleration and force reveal different mechanisms of electromechanical delay. Med Sci Sports Exerc 43(7):1200–1206. doi:10.1249/MSS.0b013e318209312c
Malek MH, Coburn JW (2012) The utility of electromyography and mechanomyography for assessing neuromuscular function: a noninvasive approach. Phys Med Rehabil Clin N Am 23(1):23–32. doi:10.1016/j.pmr.2011.11.005
Islam MA, Sundaraj K, Ahmad RB, Ahamed NU (2013) Mechanomyogram for muscle function assessment: a review. PLoS One 8(3):e58902. doi:10.1371/journal.pone.0058902
Ibitoye MO, Hamzaid NA, Zuniga JM, Abdul Wahab AK (2014) Mechanomyography and muscle function assessment: A review of current state and prospects. Clin Biomech (Bristol, Avon). doi:10.1016/j.clinbiomech.2014.04.003
Beck TW, Housh TJ, Johnson GO, Cramer JT, Weir JP, Coburn JW, Malek MH (2007) Does the frequency content of the surface mechanomyographic signal reflect motor unit firing rates? A brief review. J Electromyogr Kinesiol 17(1):1–13
Munro B (1997) Statistical methods for health care research. 3rd Edn. Lippincott, New York
Ryan ED, Cramer JT, Housh TJ, Beck TW, Herda TJ, Hartman MJ (2007) Inter-individual variability in the torque-related patterns of responses for mechanomyographic amplitude and mean power frequency. J Neurosci Methods 161(2):212–219. doi:10.1016/j.jneumeth.2006.11.007
Al-Zahrani E, Gunasekaran C, Callaghan M, Gaydecki P, Benitez D, Oldham J (2009) Within-day and between-days reliability of quadriceps isometric muscle fatigue using mechanomyography on healthy subjects. J Electromyogr Kinesiol 19(4):695–703. doi:10.1016/j.jelekin.2007.12.007
Herda TJ, Ryan ED, Beck TW, Costa PB, DeFreitas JM, Stout JR, Cramer JT (2008) Reliability of mechanomyographic amplitude and mean power frequency during isometric step and ramp muscle actions. J Neurosci Methods 171(1):104–109. doi:10.1016/j.jneumeth.2008.02.017
Stock MS, Beck TW, Defreitas JM, Dillon MA (2010) Linearity and reliability of the mechanomyographic amplitude versus dynamic torque relationships for the superficial quadriceps femoris muscles. Muscle Nerve 41(3):342–349. doi:10.1002/mus.21491
Armstrong WJ, McGregor SJ, Yaggie JA, Bailey JJ, Johnson SM, Goin AM, Kelly SR (2010) Reliability of mechanomyography and triaxial accelerometry in the assessment of balance. J Electromyogr Kinesiol 20(4):726–731. doi:10.1016/j.jelekin.2010.02.002
Stock MS, Beck TW, DeFreitas JM, Dillon MA (2010) Linearity and reliability of the mechanomyographic amplitude versus concentric dynamic constant external resistance relationships for the bench press exercise. J Strength Cond Res 24(3):785–795. doi:10.1519/JSC.0b013e3181cc22f1
Esposito F, Limonta E, Ce E, Gobbo M, Veicsteinas A, Orizio C (2009) Electrical and mechanical response of finger flexor muscles during voluntary isometric contractions in elite rock-climbers. Eur J Appl Physiol 105(1):81–92. doi:10.1007/s00421-008-0877-5
Ryan ED, Beck TW, Herda TJ, Hartman MJ, Stout JR, Housh TJ, Cramer JT (2008) Mechanomyographic amplitude and mean power frequency responses during isometric ramp vs. step muscle actions. J Neurosci Methods 168(2):293–305. doi:10.1016/j.jneumeth.2007.10.010
Akataki K, Mita K, Watakabe M, Ito K (2002) Age-related change in motor unit activation strategy in force production: a mechanomyographic investigation. Muscle Nerve 25(4):505–512
Miyamoto N, Oda S (2003) Mechanomyographic and electromyographic responses of the triceps surae during maximal voluntary contractions. J Electromyogr Kinesiol 13(5):451–459. doi:10.1016/s1050-6411(03)00058-0
Nonaka H, Mita K, Akataki K, Watakabe M, Itoh Y (2006) Sex differences in mechanomyographic responses to voluntary isometric contractions. Med Sci Sports Exerc 38(7):1311–1316. doi:10.1249/01.mss.0000227317.31470.16
Miyamoto N, Oda S (2005) Effect of joint angle on mechanomyographic amplitude during unfused and fused tetani in the human biceps brachii muscle. Eur J Appl Physiol 95(2–3):221–228. doi:10.1007/s00421-005-1359-7
Herda TJ, Housh TJ, Fry AC, Weir JP, Schilling BK, Ryan ED, Cramer JT (2010) A noninvasive, log-transform method for fiber type discrimination using mechanomyography. J Electromyogr Kinesiol 20(5):787–794. doi:10.1016/j.jelekin.2010.01.004
Miyamoto N, Fukunaga T, Kawakami Y (2009) Evidence for intermuscle difference in postactivation potentiation in the human triceps surae: a mechanomyographic study. Muscle Nerve 39(5):703–706. doi:10.1002/mus.21320
Madeleine P, Arendt-Nielsen L (2005) Experimental muscle pain increases mechanomyographic signal activity during sub-maximal isometric contractions. J Electromyogr Kinesiol 15(1):27–36. doi:10.1016/j.jelekin.2004.06.006
Esposito F, Orizio C, Parrinello G, Veicsteinas A (2003) Chronic hypobaric hypoxia does not affect electro-mechanical muscle activities during sustained maximal isometric contractions. Eur J Appl Physiol 90(3–4):337–343. doi:10.1007/s00421-003-0922-3
Kimura T, Hamada T, Massako Ueno L, Moritani T (2003) Changes in contractile properties and neuromuscular propagation evaluated by simultaneous mechanomyogram and electromyogram during experimentally induced hypothermia. J Electromyogr Kinesiol 13(5):433–440. doi:10.1016/s1050-6411(03)00062-2
Orizio C, Celichowski J, Toscani F, Calabretto C, Bissolotti L, Gobbo M (2013) Extra-torque of human tibialis anterior during electrical stimulation with linearly varying frequency and amplitude trains. J Electromyogr Kinesiol 23(6):1375–1383. doi:10.1016/j.jelekin.2013.07.008
Jaskolska A, Kisiel K, Brzenczek W, Jaskolski A (2003) EMG and MMG of synergists and antagonists during relaxation at three joint angles. Eur J Appl Physiol 90(1–2):58–68. doi:10.1007/s00421-003-0859-6
Ohta Y, Shima N, Yabe K (2009) In vivo behaviour of human muscle architecture and mechanomyographic response using the interpolated twitch technique. J Electromyogr Kinesiol 19(3):e154–e161. doi:10.1016/j.jelekin.2008.01.004
McKay WP, Lett B, Chilibeck PD, Daku BL (2009) Effects of spinal anesthesia on resting metabolic rate and quadriceps mechanomyography. Eur J Appl Physiol 106(4):583–588. doi:10.1007/s00421-009-1054-1
Orizio C, Baratta RV, Zhou BH, Solomonow M, Veicsteinas A (2000) Force and surface mechanomyogram frequency responses in cat gastrocnemius. J Biomech 33(4):427–433
Bichler E, Celichowski J (2001) Mechanomyographic signals generated during unfused tetani of single motor units in the rat medial gastrocnemius muscle. Eur J Appl Physiol 85(6):513–520
Bichler E, Celichowski J (2001) Changes in the properties of mechanomyographic signals and in the tension during the fatigue test of rat medial gastrocnemius muscle motor units. J Electromyogr Kinesiol 11(6):387–394
Orizio C, Gobbo M, Veicsteinas A, Baratta RV, Zhou BH, Solomonow M (2003) Transients of the force and surface mechanomyogram during cat gastrocnemius tetanic stimulation. Eur J Appl Physiol 88(6):601–606. doi:10.1007/s00421-002-0765-3
Hsieh TH, Dhamne SC, Chen JJ, Pascual-Leone A, Jensen FE, Rotenberg A (2012) A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats. J Neurophysiol 107(3):966–972. doi:10.1152/jn.00690.2011
Malek MH, Coburn JW, Tedjasaputra V (2009) Comparison of mechanomyographic amplitude and mean power frequency for the rectus femoris muscle: cycle versus knee-extensor ergometry. J Neurosci Methods 181(1):89–94. doi:10.1016/j.jneumeth.2009.04.026
Malek MH, Coburn JW, Housh TJ, Rana S (2011) Excess post-exercise oxygen consumption is not associated with mechanomyographic amplitude after incremental cycle ergometry in the quadriceps femoris muscles. Muscle Nerve 44(3):432–438. doi:10.1002/mus.22089
Qi L, Wakeling JM, Ferguson-Pell M (2011) Spectral properties of electromyographic and mechanomyographic signals during dynamic concentric and eccentric contractions of the human biceps brachii muscle. J Electromyogr Kinesiol 21(6):1056–1063. doi:10.1016/j.jelekin.2011.08.011
Fu W, Liu Y, Zhang S, Xiong X, Wei S (2012) Effects of local elastic compression on muscle strength, electromyographic, and mechanomyographic responses in the lower extremity. J Electromyogr Kinesiol 22(1):44–50. doi:10.1016/j.jelekin.2011.10.005
Beck TW, Kasishke PR, 2nd, Stock MS, DeFreitas JM (2012) Neural contributions to concentric vs. eccentric exercise-induced strength loss. J Strength Cond Res 26(3):633–640. doi:10.1519/JSC.0b013e3182474296
Camic CL, Housh TJ, Zuniga JM, Russell Hendrix C, Bergstrom HC, Traylor DA, Schmidt RJ, Johnson GO (2013) Electromyographic and mechanomyographic responses across repeated maximal isometric and concentric muscle actions of the leg extensors. J Electromyogr Kinesiol 23(2):342–348. doi:10.1016/j.jelekin.2012.09.010
Bergstrom HC, Housh TJ, Zuniga JM, Traylor DA, Lewis RW Jr, Camic CL, Schmidt RJ, Johnson GO (2013) Mechanomyographic and metabolic responses during continuous cycle ergometry at critical power from the 3-min all-out test. J Electromyogr Kinesiol 23(2):349–355. doi:10.1016/j.jelekin.2012.11.001
Madeleine P, Bajaj P, Sogaard K, Arendt-Nielsen L (2001) Mechanomyography and electromyography force relationships during concentric, isometric and eccentric contractions. J Electromyogr Kinesiol 11(2):113–121
Vedsted P, Blangsted AK, Sogaard K, Orizio C, Sjogaard G (2006) Muscle tissue oxygenation, pressure, electrical, and mechanical responses during dynamic and static voluntary contractions. Eur J Appl Physiol 96(2):165–177. doi:10.1007/s00421-004-1216-0
Cramer JT, Housh TJ, Johnson GO, Ebersole KT, Perry SR, Bull AJ (2000) Mechanomyographic amplitude and mean power output during maximal, concentric, isokinetic muscle actions. Muscle Nerve 23(12):1826–1831
Evetovich TK, Boyd JC, Drake SM, Eschbach LC, Magal M, Soukup JT, Webster MJ, Whitehead MT, Weir JP (2002) Effect of moderate dehydration on torque, electromyography, and mechanomyography. Muscle Nerve 26(2):225–231. doi:10.1002/mus.10203
Perry-Rana SR, Housh TJ, Johnson GO, Bull AJ, Cramer JT (2003) MMG and EMG responses during 25 maximal, eccentric, isokinetic muscle actions. Med Sci Sports Exerc 35(12):2048–2054. doi:10.1249/01.MSS.0000099090.73560.77
Cramer JT, Housh TJ, Weir JP, Johnson GO, Berning JM, Perry SR, Bull AJ (2004) Gender, muscle, and velocity comparisons of mechanomyographic and electromyographic responses during isokinetic muscle actions. Scand J Med Sci Sports 14(2):116–127. doi:10.1111/j.1600-0838.2003.00317.x
Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, Malek MH (2004) Mechanomyographic amplitude and mean power frequency versus torque relationships during isokinetic and isometric muscle actions of the biceps brachii. J Electromyogr Kinesiol 14(5):555–564. doi:10.1016/j.jelekin.2004.03.001
Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, Malek MH (2004) Mechanomyographic and electromyographic time and frequency domain responses during submaximal to maximal isokinetic muscle actions of the biceps brachii. Eur J Appl Physiol 92(3):352–359. doi:10.1007/s00421-004-1110-9
Coburn JW, Housh TJ, Malek MH, Weir JP, Cramer JT, Beck TW, Johnson GO (2006) Mechanomyographic and electromyographic responses to eccentric muscle contractions. Muscle Nerve 33(5):664–671. doi:10.1002/mus.20509
Coburn JW, Housh TJ, Weir JP, Malek MH, Cramer JT, Beck TW, Johnson GO (2004) Mechanomyographic Responses of the Vastus Medialis to Isometric and Eccentric Muscle Actions. Med Sci Sports Exerc 36(11):1916–1922. doi:10.1249/01.mss.0000145449.14799.5f
Jaskolski A, Andrzejewska R, Marusiak J, Kisiel-Sajewicz K, Jaskolska A (2007) Similar response of agonist and antagonist muscles after eccentric exercise revealed by electromyography and mechanomyography. J Electromyogr Kinesiol 17(5):568–577. doi:10.1016/j.jelekin.2006.05.002
Evetovich TK, Housh TJ, Weir JP, Housh DJ, Johnson GO, Ebersole KT, Smith DB (2000) The effect of leg extension training on the mean power frequency of the mechanomyographic signal. Muscle Nerve 23(6):973–975
Ebersole KT, Housh TJ, Johnson GO, Perry SR, Bull AJ, Cramer JT (2002) Mechanomyographic and electromyographic responses to unilateral isometric training. J Strength Cond Res 16(2):192–201
Camic CL, Housh TJ, Zuniga JM, Traylor DA, Bergstrom HC, Schmidt RJ, Johnson GO (2013) Mechanomyographic and electromyographic responses during fatiguing eccentric muscle actions of the leg extensors. Med Sci Sport Exer 45(5):95
Madeleine P, Farina D, Merletti R, Arendt-Nielsen L (2002) Upper trapezius muscle mechanomyographic and electromyographic activity in humans during low force fatiguing and non-fatiguing contractions. Eur J Appl Physiol 87(4–5):327–336. doi:10.1007/s00421-002-0655-8
Kawczynski A, Nie H, Jaskolska A, Jaskolski A, Arendt-Nielsen L, Madeleine P (2007) Mechanomyography and electromyography during and after fatiguing shoulder eccentric contractions in males and females. Scand J Med Sci Sports 17(2):172–179. doi:10.1111/j.1600-0838.2006.00551.x
Madeleine P, Farina D (2008) Time to task failure in shoulder elevation is associated to increase in amplitude and to spatial heterogeneity of upper trapezius mechanomyographic signals. Eur J Appl Physiol 102(3):325–333. doi:10.1007/s00421-007-0589-2
Tarata MT (2003) Mechanomyography versus electromyography, in monitoring the muscular fatigue. Biomed Eng Online 2:3
Blangsted AK, Vedsted P, Sjogaard G, Sogaard K (2005) Intramuscular pressure and tissue oxygenation during low-force static contraction do not underlie muscle fatigue. Acta Physiol Scand 183(4):379–388. doi:10.1111/J.1365-201x.2005.01411.X
Weir JP, Ayers KM, Lacefield JF, Walsh KL (2000) Mechanomyographic and electromyographic responses during fatigue in humans: influence of muscle length. European J Appl Physiol 81(4):352–359. doi:10.1007/S004210050054
Perry-Rana SR, Housh TJ, Johnson GO, Bull AJ, Berning JM, Cramer JT (2002) MMG and EMG responses during fatiguing isokinetic muscle contractions at different velocities. Muscle Nerve 26(3):367–373. doi:10.1002/mus.10214
Beck TW, Housh TJ, Fry AC, Cramer JT, Weir JP, Schilling BK, Falvo MJ, Moore CA (2007) The influence of muscle fiber type composition on the patterns of responses for electromyographic and mechanomyographic amplitude and mean power frequency during a fatiguing submaximal isometric muscle action. Electromyogr Clin Neurophysiol 47(4–5):221–232
Ebersole KT, Malek DM (2008) Fatigue and the electromechanical efficiency of the vastus medialis and vastus lateralis muscles. J Athl Train 43(2):152–156. doi:10.4085/1062-6050-43.2.152
Søgaard K, Blangsted AK, Jørgensen LV, Madeleine P, Sjøgaard G (2003) Evidence of long term muscle fatigue following prolonged intermittent contractions based on mechano- and electromyograms. J Electromyogr Kinesiol 13(5):441–450. doi:10.1016/s1050-6411(03)00075-0
Madeleine P, Jorgensen LV, Sogaard K, Arendt-Nielsen L, Sjogaard G (2002) Development of muscle fatigue as assessed by electromyography and mechanomyography during continuous and intermittent low-force contractions: effects of the feedback mode. Eur J Appl Physiol 87(1):28–37. doi:10.1007/s00421-002-0578-4
Kimura T, Fujibayashi M, Tanaka S, Moritani T (2008) Mechanomyographic responses in quadriceps muscles during fatigue by continuous cycle exercise. Eur J Appl Physiol 104(4):651–656. doi:10.1007/s00421-008-0816-5
Yang ZF, Kumar DK, Arjunan SP (2009) Mechanomyogram for identifying muscle activity and fatigue. In: Proceedings of the Annual Internationa Conference of the IEEE Engineering in Medicine and Biology Society, EMBC 2009, Minneapolis, MN, USA. Sept 2009, pp 408–411
Zuniga JM, Housh TJ, Camic CL, Hendrix CR, Schmidt RJ, Mielke M, Johnson GO (2010) A mechanomyographic fatigue threshold test for cycling. Int J Sports Med 31(9):636–643. doi:10.1055/s-0030-1255112
Ce E, Paracchino E, Esposito F (2008) Electrical and mechanical response of skeletal muscle to electrical stimulation after acute passive stretching in humans: a combined electromyographic and mechanomyographic approach. J Sports Sci 26(14):1567–1577. doi:10.1080/02640410802277429
Esposito F, Ce E, Rampichini S, Veicsteinas A (2009) Acute passive stretching in a previously fatigued muscle: electrical and mechanical response during tetanic stimulation. J Sports Sci 27(12):1347–1357. doi:10.1080/02640410903165093
Herda TJ, Ryan ED, Smith AE, Walter AA, Bemben MG, Stout JR, Cramer JT (2009) Acute effects of passive stretching vs vibration on the neuromuscular function of the plantar flexors. Scand J Med Sci Spor 19(5):703–713. doi:10.1111/j.1600-0838.2008.00787.x
Cramer JT, Housh TJ, Weir JP, Johnson GO, Coburn JW, Beck TW (2005) The acute effects of static stretching on peak torque, mean power output, electromyography, and mechanomyography. Eur J Appl Physiol 93(5–6):530–539
Herda TJ, Cramer JT, Ryan ED, McHugh MP, Stout JR (2008) Acute effects of static versus dynamic stretching on isometric peak torque, electromyography, and mechanomyography of the biceps femoris muscle. J Strength Cond Res 22(3):809–817. doi:10.1519/JSC.0b013e31816a82ec
Krueger-Beck E, Scheeren EM, Nogueira-Neto GN, Button VLSN, Nohama P (2010) Mechanomyographic response during fes in healthy and paraplegic subjects. 2010 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc):626–629. doi:10.1109/Iembs.2010.5627274
Krueger-Beck E, Scheeren EM, Nogueira-Neto GN, Button VLSN, Nohama P (2010) Optimal FES parameters based on mechanomyographic efficiency index. 2010 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc):1378–1381. doi:10.1109/Iembs.2010.5626735
Huang CY, Wang CH, Hwang IS (2006) Characterization of the mechanical and neural components of spastic hypertonia with modified H reflex. J Electromyogr Kinesiol 16(4):384–391. doi:10.1016/j.jelekin.2005.09.001
Scheeren EM, Nogueira-Neto GN, Krueger-Beck E, Button VLSN, Nohama P (2010) Investigation of muscle behavior during different functional electrical stimulation profiles using mechanomyography. 2010 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc):3970–3973. doi:10.1109/Iembs.2010.5627986
Krueger E, Scheeren EM, Nogueira-Neto GN, Button VLDN, Nohama P (2012) A new approach to assess the spasticity in hamstrings muscles using mechanomyography Antagonist Muscular Group. 2012 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc):2060–2063
Marusiak J, Jaskolska A, Kisiel-Sajewicz K, Yue GH, Jaskolski A (2009) EMG and MMG activities of agonist and antagonist muscles in Parkinson’s disease patients during absolute submaximal load holding. J Electromyogr Kinesiol 19(5):903–914. doi:10.1016/j.jelekin.2008.03.003
Sarlabous L, Torres A, Fiz JA, Gea J, Martinez-Llorens JM, Jane R (2009) Evaluation of the respiratory muscular function by means of diaphragmatic mechanomyographic signals in COPD patients. Ieee Eng Med Bio:3925–3928. doi:10.1109/Iembs.2009.5333536
Torres A, Sarlabous L, Fiz JA, Gea J, Martinez-Llorens JM, Morera J, Jane R (2010) Noninvasive measurement of inspiratory muscle performance by means of diaphragm muscle mechanomyographic signals in COPD patients during an incremental load respiratory test. 2010 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc):2493–2496. doi:10.1109/Iembs.2010.5626618
Ng AR, Arimura K, Akataki K, Mita K, Higuchi I, Osame M (2006) Mechanomyographic determination of post-activation potentiation in myopathies. Clin Neurophysiol 117(1):232–239. doi:10.1016/j.clinph.2005.09.014
Lamraoui H, Bonvilain A, Robain G, Mozer P, Moreau-Gaudry A, Cinquin P, Gumery PY, Basrour S (2010) Rectus Abdominis Electromyography and Mechanomyography Comparison for the Detection of Cough. 2010 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc):6502–6505. doi:10.1109/Iembs.2010.5627369
Kawakami S, Kodama N, Maeda N, Sakamoto S, Oki K, Yanagi Y, Asaumi J, Maeda T, Minagi S (2012) Mechanomyographic activity in the human lateral pterygoid muscle during mandibular movement. J Neurosci Methods 203(1):157–162. doi:10.1016/j.jneumeth.2011.09.026
Alves N, Chau T (2009) Classification of the mechanomyogram: its potential as a multifunction access pathway. Ieee Eng Med Bio:2951–2954. doi:10.1109/Iembs.2009.5332490
Ebersole KT, O’Connor KM, Wier AP (2006) Mechanomyographic and electromyographic responses to repeated concentric muscle actions of the quadriceps femoris. J Electromyogr Kinesiol 16(2):149–157. doi:10.1016/j.jelekin.2005.05.005
Stock MS, Beck TW, Defreitas JM, Dillon MA (2010) Relationships among peak power output, peak bar velocity, and mechanomyographic amplitude during the free-weight bench press exercise. J Sports Sci 28(12):1309–1317. doi:10.1080/02640414.2010.499440
Smith DB, Housh TJ, Johnson GO, Evetovich TK, Ebersole KT, Perry SR (1998) Mechanomyographic and electromyographic responses to eccentric and concentric isokinetic muscle actions of the biceps brachii. Muscle Nerve 21(11):1438–1444
Barry DT (1987) Acoustic signals from frog skeletal muscle. Biophys J 51(5):769–773. doi:10.1016/S0006-3495(87)83403-3
Evetovich TK, Housh TJ, Johnson GO, Smith DB, Ebersole KT, Perry SR (1998) Gender comparisons of the mechanomyographic responses to maximal concentric and eccentric isokinetic muscle actions. Med Sci Sports Exerc 30(12):1697–1702
Beck TW, Housh TJ, Fry AC, Cramer JT, Weir JP, Schilling BK, Falvo MJ, Moore CA (2009) A wavelet-based analysis of surface mechanomyographic signals from the quadriceps Femoris. Muscle Nerve 39(3):355–363. doi:10.1002/Mus.21208
Taylor DC, Dalton JD Jr, Seaber AV, Garrett WE Jr (1990) Viscoelastic properties of muscle-tendon units. The biomechanical effects of stretching. Am J Sports Med 18(3):300–309
Nakamura M, Ikezoe T, Takeno Y, Ichihashi N (2011) Acute and prolonged effect of static stretching on the passive stiffness of the human gastrocnemius muscle tendon unit in vivo. J Orthop Res 29(11):1759–1763. doi:10.1002/jor.21445
Morse CI, Degens H, Seynnes OR, Maganaris CN, Jones DA (2008) The acute effect of stretching on the passive stiffness of the human gastrocnemius muscle tendon unit. J Physiol (Lond) 586(1):97–106
Martin MYE (2009) MMG sensor for muscle activity detection-low cost design, implementation and experimentation [Master’s Dissertation]. Massey University, Auckland
Youn W, Kim J (2010) Estimation of elbow flexion force during isometric muscle contraction from mechanomyography and electromyography. Med Biol Eng Comput 48(11):1149–1157
Chen X, Zheng YP, Guo JY et al (2012) Sonomyographic responses during voluntary isometric ramp contraction of the human rectus femoris muscle. Eur J Appl Physiol 112(7):2603–2614
Madeleine P, Tuker K, Arendt-Nielsen L et al (2007) Heterogeneous mechanomyographic absolute activation of paraspinal muscles assessed by a two-dimensional array during short and sustained contractions. J Biomech 40(12):2663–2671
Shima N, Rice CL, Ota Y et al (2006) The effect of post-activation potentiation on the mechanomyogram. Eur J Appl Physiol 96(1):17–23
Oshita K, Yano S (2010) Asymmetry of force fluctuation in knee extension. Int J Sports Med 31(5):342–346
Krueger E, Scheeren EM, Nogueira-Neto GN et al (2011) Correlation between mechanomyography features and passive movements in healthy and paraplegic subjects. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp 7242–7245
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cè, E., Rampichini, S. & Esposito, F. Novel insights into skeletal muscle function by mechanomyography: from the laboratory to the field. Sport Sci Health 11, 1–28 (2015). https://doi.org/10.1007/s11332-015-0219-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-015-0219-z