Abstract
Purpose
To evaluate the association of recently diagnosed obstructive sleep apnea (OSA) with TNF-α and IL-6 and to measure the effect of short-term continuous positive airway pressure (CPAP) therapy on these markers.
Methods
A prospective, open-label, controlled trial was conducted among patients referred for diagnostic polysomnography (PSG). After PSG, patients were divided into 3 groups: OSA intervention group (N = 21), OSA control untreated group (N = 19), and non-OSA control group (N = 24). IL-6 and TNF-α levels were measured at baseline and 1 month after intervention. Repeated measures (RM) ANOVA and ANCOVA were used to compare the three groups regarding changes in TNF-α and IL-6 levels by analyzing between-subject and within-subject effects as a function of time and adjusting for significant covariates.
Results
At baseline, IL-6 (p = 0.05) and TNF-α (p = 0.04) were significantly higher in the OSA patients than in the non-OSA controls. There was no effect of time either on the TNF-α (p = 0.069) or IL-6 (p = 0.717) after 1 month of CPAP. No interaction effect between group and time was found for either TNF-α (p = 0.240) or IL-6 (p = 0.552) after 1 month of CPAP. There was neither a group effect nor an interaction effect between group and time for either IL-6 or TNF α after adjusting for age, BMI, neck circumference, and AHI.
Conclusion
This study showed increases in proinflammatory state as illustrated by plasma TNF-α and IL-6 levels among recently diagnosed OSA patients, but there were no changes in these inflammatory markers following 1-month CPAP therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common disorder that affects nearly 1 billion people worldwide [1, 2]. It is characterized by recurrent episodes consisting of the absence or reduction of airflow due to upper airway collapse during sleep, despite ongoing efforts to breathe. Increasing evidence suggests that OSA is associated with cerebrovascular and cardiovascular diseases and metabolic derangements [3, 4]. Several physiological and biochemical alterations that underlie these comorbidities appear to be the result of periodic nocturnal hypoxemia-hypercapnia and increased intrathoracic pressure swings, along with frequent arousals with consequent fragmentation of sleep and emergence of excessive daytime sleepiness (EDS) [3, 4]. Among the systemic alterations of OSA, systemic inflammation with increased inflammatory markers, endothelial dysfunction, and derangement in autonomic nervous system homeostasis are prominently present [5]. Indeed, McNicholas and Bonsignore [6] labeled inflammatory pathways relative to intermittent hypoxia in sleep apnea as an important mechanistic contributor to the evolution of atherogenesis and subsequent vascular disease.
A large number of measurable biomarkers are used as indices for systemic inflammation, including C-reactive protein (CRP), fibrinogen, and proinflammatory cytokines [7, 8]. In particular, significant elevations in serum levels of tumor necrosis factor-alpha (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) have been observed in patients with OSA [9, 10]. Steiropoulos et al. [11] reported that TNF-α elevation in OSA patients was not due to the confounding effects of obesity, as obese OSA patients showed higher TNF-α levels than BMI-matched controls, thereby supporting an independent role of OSA in promoting inflammation. The same group followed up a cohort of 52 OSA patients for 6 months and found a selective reduction in soluble and cellular immune response factors in patients who adhered to continuous positive airway pressure (CPAP) therapy when compared with non-adherent patients, thus providing further evidence for an ongoing activation of systemic immunity in OSA [12].
However, studies focusing on inflammatory markers in OSA have yielded conflicting results. In a recent summary of the evidence Kheirandish-Gozal and Gozal showed that although there was an overall tendency for a majority of positive findings, i.e., increased TNF-α and IL-6 and evidence of reversibility with treatment among all published studies till 2019, there was still a significant proportion of such studies that failed to identify significant changes in the circulating levels of these cytokines [10]. Another report by Yokoe et al [7] evidenced a positive effect of CPAP therapy in reducing CRP and IL-6 levels, while another 4-week study failed to demonstrate any effect of CPAP therapy on IL-6 or IFN-gamma plasma concentrations in moderate-severe OSA patients [13]. Furthermore, in the Multicentre Obstructive Sleep Apnoea Interventional Cardiovascular (MOSAIC) trial, there were no consistent changes observed in plasma IL-6, IL-10, CRP or TNF-α levels, regardless of CPAP usage [14]. In another study that measured the levels of IL-6 and TNF-α in 33 OSA patients versus 24 matched controls and evaluated the impact of CPAP therapy, there were no differences in the baseline levels of IL-6 or TNF-α, and no clear effect of CPAP therapy on the mean level of these cytokines was demonstrated [15].
These overall mixed and somewhat contradictory findings overall hamper our understanding of the translational evidence regarding the association of OSA with inflammation, and the potentially beneficial impact of CPAP therapy in reducing the inflammatory status in OSA patients. Hence, we conducted this study to evaluate the association of OSA with two classical proinflammatory markers, TNF-α and IL-6, and to evaluate the effect of short-term CPAP therapy.
Materials and methods
Design and setting
This prospective, open-label controlled trial was conducted at the Sleep Medicine Research Center (SMRC) of King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia, from April 2018 to May 2019.
Population
This study included all patients who were referred to the SMRC for diagnostic polysomnography (PSG) due to clinical symptoms and overall suspicion of OSA. Patients who were previously diagnosed with OSA or those with an antecedent history of OSA treatment were excluded. Other exclusion criteria included all patients who presented with conditions that may affect the inflammatory markers, such as a history of neuromuscular disorders, infectious diseases, rheumatic diseases, immunological diseases, tumors, peripheral vascular diseases, coagulation disorders, liver or kidney diseases, psychogenic disorders, cigarette smoking in the past 6 months, diabetes mellitus, history of injury or surgery in the past 3 months, recent use (past 3 months) of antibiotics, hormones, corticosteroids, immune suppressors, cytotoxins, or free radical scavengers, and other cases characterized by chronic hypoxia, such as chronic obstructive pulmonary disease, obesity hypoventilation syndrome, interstitial lung diseases, and congestive heart failure.
Procedures
Initial assessment
Standard medical history collection and a physical examination were performed for all patients referred to SMRC for a sleep study. Demographic and anthropometric data, including age, sex, BMI, and neck circumference, were recorded. Neck circumference was measured at the cricothyroid level. Blood pressure was measured by electronic sphygmomanometers that self-adapt to arm circumference. Sleepiness was evaluated using the Epworth Sleepiness Scale (ESS) [16]. Beyond the initial assessment, patients were assessed for eligibility to participate in the study by ruling out the exclusion criteria. The study objectives and procedure were presented and explained to eligible patients who were invited to voluntarily participate by signing an informed consent form of the study that was approved by the local IRB (Reference Number 178–18). A semi structured case report form was used to collect data from the participants, and PSG was scheduled according to the patient’s convenience and schedule availability.
Polysomnography (PSG)
All participants underwent an overnight PSG using a standard montage of electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), and electrocardiogram (ECG) signals, together with pulse oximetry and airflow as measured using combined oronasal thermistors. Thoracic cage and abdominal motion were recorded by inductive plethysmography. Apneas, hypopneas, and sleep states were scored according to the recommendations and guidelines of the American Academy of Sleep Medicine (AASM) [17].
Group allocation
According to the PSG results, patients were classified into 3 groups. The first group included those with confirmed OSA of moderate to severe degree according to AASM definition [18], i.e., apnea hypopnea index (AHI) of 15 or greater, who accepted treatment with CPAP (intervention group). The second group included patients with OSA who refused CPAP treatment (OSA control group). The third group included those without OSA, i.e., AHI below 5 (Non-OSA control group) (Fig.1).
Baseline proinflammatory marker measurement
On the day after PSG (day 0), fasting blood samples were collected between 8 and 9 am for all patients to measure serum levels of proinflammatory markers. Blood samples were collected from peripheral veins into 5 ml tubes without any added anticoagulation factors, and sera were separated and stored at −80 °C immediately. Commercial ELISA testing was carried out to measure IL-6 and TNF-α according to the kit inserts (Abcam, UK.) (Human IL-6 Elisa Kit - ab178013 and human TNF-α Elisa Kit -ab181421). Samples were assayed in duplicate, and human IL-6 and TNF-α assays exhibited a 1.6 pg/ml and 4.32 pg/ml sensitivity, respectively. All serum samples and reagents were prepared at room temperature. The standards and samples were added to the appropriate wells and antibodies (capture antibody and detector antibody) and incubated at room temperature. After the wells were washed several times, TMB substrate was added to each well and incubated at room temperature. Subsequently, the stop solution was added to each well, and the final color was recorded. The results were read by an ELISA reader (INOVA; USA). Afterwards, the results were calculated from the standard curve for each sample to indicate the serum levels of IL-6 and TNF-α.
CPAP therapy
Each patient with OSA underwent a night study in the sleep laboratory for CPAP titration according to the AASM guidelines [19]. Those who accepted CPAP therapy (intervention group) were started on the CPAP device at the optimal pressure on the consecutive day.
Follow-up and endpoint
The three groups were followed for 1 month following PSG, including weekly visits to the SMRC to assess for any changes in medications or events that may affect the level of the inflammatory markers, as well as ensuring adherence of the intervention group to CPAP treatment using data from memory cards of the CPAP devices [20]. Acceptable adherence was defined as the minimum CPAP use of >/= 4 h/70% nights. All patients in the intervention group must meet the acceptable adherence definition otherwise would be excluded. At the end of the 1-month follow-up (day 30), fasting morning blood samples were drawn to monitor levels of the proinflammatory biomarkers using the same method described above.
Statistical methods
Statistical analysis was performed with the Statistical Package for Social Sciences version 23.0 for Windows (SPSS Inc., Chicago, IL, USA). Repeated measures (RM) ANOVA and ANCOVA were used to compare the three groups regarding changes in TNF-α and IL-6 levels by analyzing between-subject and within-subject effects as a function of time after adjusting for significant covariates, including age, BMI, neck circumference, and AHI. Further, post hoc pairwise comparisons were performed using the Bonferroni correction. IL-6 and TNF-α were log transformed for the normality requirement in the repeated measures ANOVA; however, serum levels and graphical presentations are shown for original (non-log transformed) values for ease of interpretation. Additionally, paired t tests were used to compare baseline and 1-month follow-up levels of TNF-α and IL-6 within each group separately. A p value of < 0.05 was considered to reject the null hypothesis. Binary logistic regression was used to determine factors associated with OSA at baseline.
Results
Participant characteristics
Participant characteristics are summarized in Table 1. There were no significant differences across the three groups regarding sex distribution (p = 0.367), systemic blood pressure (p = 0.269 and p = 0.086 for systolic and diastolic pressures, respectively), and ESS scores (p = 0.433). However, age (p < 0.01), BMI (p = 0.017), neck circumference (p = 0.001), and AHI (p < 0.01) differed significantly across the three groups. Although, the intervention group was slightly older than the untreated group (mean [SD] age = 51.43 [21.78] years versus 43.10 [11.02] years with p value of 0.045), the AHI was not significantly higher (mean [SD] = 40.13 [26.29] versus 32.98 [20.25] with p value of 0.69).
OSA: associated factors at baseline
Binary logistic regression analysis was used to identify associated conditions of OSA at baseline. The model with nine independent variables, i.e., sex, age, BMI, neck circumference, systolic BP, diastolic BP, ESS score, IL-6, and TNF-α, was significant in comparison with the model with only intercepts; χ2(9, N = 64) = 37.59, p < .001. The model with these predictors explained 62.3% of the variance in the classification of OSA with an accuracy of 85.2% (sensitivity = 89.2% and specificity = 79.2%). Increasing age was associated with OSA classification (adjusted odds ratio (AOR) = 1.15, 95% confidence interval (CI) 1.04–1.26).
Baseline difference in inflammatory markers in control (non-OSA) and OSA cases
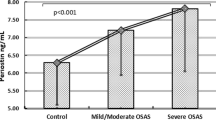
Serum IL-6 and TNF-α levels were significantly higher in the OSA patients (combined group of those on treatment and those who declined treatment) than in the non-OSA controls (t(60.14) = −1.97, p = 0.05; t(62) = −2.08, p = 0.04, for log IL-6 and log TNF-α, respectively) (Fig. 2).
TNF-α level: between-subject and within-subject effects
Repeated measures ANOVA showed no effect on mean TNF-α levels over time (F(1, 61) = 3.438, p = 0.069) or time*group interaction (F(2, 61) = 1.461, p = 0.240). Further, no significant increase in TNF-α level was revealed on post hoc pairwise comparison with the Bonferroni correction between pretest and post-test (6.494 vs 6.695 pg/ml, p = 0.061). On the other hand, TNF-α significantly increased in the non-OSA control group from baseline to the 1-month follow-up (4.833 vs 5.177, respectively, paired t test: p = 0.047) (Fig. 3).
TNF-α level: between-subject and within-subject effects after adjustment for the covariates
As age, BMI, neck circumference, and AHI were significantly different across the three groups, a multifactor ANCOVA was carried out, including these four factors as covariates. This adjusted model showed no time effect (F(1, 57) =0.232, p = 0.632) or time*group interaction effect (F(2, 57) = 0.581, p = 0.562) on the mean TNF-α levels. Additionally, no interaction effect was seen for time with any of the covariates, i.e., age, BMI, neck circumference, and AHI, on the mean TNF-α levels.
IL-6 level: between-subject and within-subject effects
Similar to TNF-α, RM ANOVA showed no time effect (F(1, 61) = 0.132, p = .717) or time*group interaction effect (F(2, 61) = 0.600, p = .552) on the mean IL-6 levels. No significant increase in IL-6 levels was observed on post hoc pairwise comparison with the Bonferroni correction between pretest and post-test (5.459 vs 4.650 pg/ml, p = 0.717). Further, paired analysis showed no significant baseline to 1-month follow-up variation in IL-6 levels in any of the three groups (Fig. 4).
IL-6 level: between-subject and within-subject effects after adjustment for the covariates
As age, BMI, neck circumference and AHI were significantly different across the three groups, a multifactor ANCOVA was carried out, including these four factors as covariates. The adjusted RM ANOVA model showed no time effect (F(1, 57) = 3.713, p = .059) or time*group interaction effect (F(2, 57) = 0.118, p = 0.889) on the mean IL-6 levels.
Differences in IL-6 and TNF-α levels between OSA groups at baseline: age, BMI, neck circumference, and AHI-adjusted comparison
There was no significant difference in the IL-6 level between the two OSA groups at baseline after adjustment for age, BMI, neck circumference, and AHI (F (2, 57) = 0.73, p = 0.49). However, TNF-α differed significantly between the two OSA groups at baseline after adjustment for age, BMI, neck circumference, and AHI (F (2, 57) = 11.07, p < .01).
Discussion
This open-label prospective study investigated the association of OSA with serum levels of TNF-α and IL-6 and the effect of 1-month CPAP therapy on their levels among recently diagnosed patients. Our findings support the association of OSA with increased levels of proinflammatory markers (Fig. 2). However, there was no effect of month-long CPAP therapy on the levels of inflammatory markers. There was no significant change in TNF-α or IL-6 observed in patients who benefited from 1 month of well-conducted CPAP therapy compared with those who refused the treatment.
Serum IL-6 and TNF-α levels were significantly higher in the OSA patients (combined group of those on treatment and those who declined treatment) than in the non-OSA controls (U = 329.5, p = .04; U = 250.0, p < .01, respectively). Our findings of higher levels of inflammatory markers in OSA patients than in non-OSA controls are similar to previous reports [10, 21] . The association of OSA with markers of systemic inflammation constitutes a major topic of interest, as this association is potentially relevant to the OSA pathophysiology and comorbidity, notably cardiovascular and cerebrovascular diseases. Furthermore, a meta-analysis by Nadeem et al. [21] demonstrated higher levels of systemic inflammatory markers, including CRP, TNF-α, IL-6, IL-8, intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), and selectins, among patients with OSA compared with control subjects. Another meta-analysis by Wang et al. [22] evidenced higher levels of CRP among 736 patients with moderate-severe OSA than among 424 healthy controls, and the standardized mean difference was 0.58. Additionally, the study demonstrated impaired endothelial function and increased arterial stiffness among OSA patients. Such observations may reflect some of the putative inflammatory pathways underlying the development of cardiovascular diseases in OSA patients [22].
A comprehensive list of criteria was implemented to exclude different types of comorbidities that might have confounded the results in our study. Furthermore, multiple linear regression analysis was used to analyze the association of inflammatory markers, i.e., IL-6 and TNF-α, as dependent variables and obesity indices, such as BMI and neck circumference, as independent variables. No such associations emerged between obesity indices and inflammatory markers. Therefore, this implies that the results of this study as related to group differences as well as the effect of 1-month-long CPAP on inflammatory markers are relatively free from the confounding effects of obesity and other comorbidities. Several studies have reported that such an association between OSA and systemic inflammation is confounded by the effect of obesity, highlighting the proinflammatory state induced by excess adiposity and aggravated by intermittent hypoxia [23]. Other researchers advocated the synergistic effect of obesity and other risk factors, such as hypertension, in inducing chronic inflammation in OSA patients. An early study by Ryan et al. [24] compared the levels of CRP in morbidly obese patients with severe OSA with those in three other BMI-matched groups including non-OSA patients, mild-to-moderate OSA, and severe OSA. The results showed significantly higher levels of CRP in morbidly obese patients with severe OSA than in the other three groups, while no statistically significant differences were detected between the three other groups. Further, multivariate models showed that BMI was independently associated with CRP levels [24]. The implication of obesity in chronic inflammation among OSA patients is believed to be the consequence of OSA-related intermittent hypoxia on visceral adipose tissue, leading to the development of a proinflammatory phenotype of adipocytes that upregulate and secrete proinflammatory adipokines and may involve other systemic inflammatory pathways, notably IL-6, vascular endothelial growth factor and leptin [25, 26].
The present study showed no effect of 1 month of CPAP therapy on TNF-α or IL-6. However, in a meta-analysis of a total of 23 nonrandomized studies, Baessler et al. [27] analyzed the effect of CPAP therapy on TNF-α (9 studies, 209 patients), IL-6 (8 studies, 165 patients), and CRP (14 studies, 771 patients). Despite the heterogeneity of the results, the authors reported mean decreases of 1.14 pg/ml, 1.01 pg/ml and 0.14 mg/dl in TNF-α, IL-6, and CRP, respectively, as an effect of CPAP therapy [27]. Notably, the CPAP therapy duration was ≥ 3 months in most studies included in this meta-analysis, which is longer than that in the present study. Thus, the absence of an effect in our study may be due to the short CPAP therapy duration. Furthermore, another meta-analysis by Xie et al. [28], which pooled 1985 OSA patients from 35 studies, analyzed the effect of CPAP therapy on 4 inflammatory markers, including TNF-α (12 studies), IL-6 (16 studies), IL-8 (3 studies), and CRP (24 studies). The results showed standardized mean differences for TNF-α (0.48), IL-6 (0.30), IL-8 (0.65), and CRP (0.45) from pre- to post-CPAP therapy. Furthermore, adjusted analysis demonstrated that longer CPAP therapy duration (3 months or longer) along with improved adherence (at least 4 h per night) was associated with beneficial effects on proinflammatory markers [28]. This supports the hypothesis that the absence of an effect in our study may be due to the relatively short duration of CPAP therapy, the latter likely insufficient to allow for significant reductions in TNF-α and IL-6. Indeed, consistent with our findings, the MOSAIC trial showed no effects of a 6-month CPAP therapy on TNF-α, IL-6, IL-10, and CRP among 391 patients with moderate OSA [14]. Given the longer CPAP duration in that trial (6 months), such observations may suggest a selective CPAP effect on inflammatory markers among patients with severe or advanced OSA. Furthermore, a systematic review of randomized controlled trials assessed the impact of CPAP treatment on cardiometabolic biomarkers such as catecholamines, sympathetic activity of catecholamine metabolites, and oxidative stress, as well as proinflammatory and coagulation biomarkers. The study evidenced a strong and rapid reduction in sympathetic activity as an effect of CPAP among OSA patients, whereas no significant effects on systemic and vascular inflammation biomarkers or their metabolites were apparent in the included studies [29].
The present study has some limitations. The small sample size may potentially induce type II error. At baseline, significant anthropometric differences were observed between the three groups (Table 1). On average, patients from the intervention group were 8 years older; however, there was no significant difference in the AHI level at baseline between the two OSA groups with p value of 0.69. Given that group allocation was not randomized, but depended on the patient’s acceptance or refusal of treatment, these findings may suggest the presence of a selection bias. Further, the presence of significant differences in TNF-α levels after adjustment for age, BMI, neck circumference, and AHI at baseline may imply issues of selection bias.
In conclusion, our open-label controlled trial showed the presence of a proinflammatory state among patients recently diagnosed with OSA, as reflected by significant increases in TNF-α and IL-6. However, no significant changes in TNF-α or IL-6 levels occurred after 1 month of adherent CPAP therapy compared with untreated OSA patients. Further controlled trials comparing risk factor-matched treated and untreated groups are warranted to address the effect of CPAP therapy on chronic inflammation among OSA patients.
Change history
28 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11325-022-02605-2
References
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MS, Morrell MJ, Peppard PE (2019) Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 7(8):687–698
Wali SO, Abalkhail B, Krayem A (2017) Prevalence and risk factors of obstructive sleep apnea syndrome in a Saudi Arabian population. Annals of thoracic medicine 12(2):88–94
Lee W, Nagubadi S, Kryger MH, Mokhlesi B (2008) Epidemiology of obstructive sleep apnea: a population-based perspective. Expert review of respiratory medicine 2(3):349–364
Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM (2009) Burden of sleep apnea: rationale, design, and major findings of the Wisconsin sleep cohort study. WMJ: official publication of the State Medical Society of Wisconsin 108(5):246–249
Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK (2000) Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 102(21):2607–2610
McNicholas W, Bonsignore T, The Management Committee of EU COST ACTION B26 (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanism and research priorities. Eur Respir J 29:156–178
Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M (2003) Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107(8):1129–1134
Maeder MT, Strobel W, Christ M, Todd J, Estis J, Wildi K et al (2015) Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem 48(4–5):340–346
Ciftci TU, Kokturk O, Bukan N, Bilgihan A (2004) The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 28(2):87–91
Kheirandish-Gozal L, Gozal D (2019) Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci 20(3):459
Steiropoulos P, Papanas N, Nena E, Antoniadou M, Serasli E, Papoti S & Tsara V (2010) Inflammatory markers in middle-aged obese subjects: does obstructive sleep apnea syndrome play a role? Mediators of inflammation, 2010
Steiropoulos P, Kotsianidis I, Nena E, Tsara V, Gounari E, Hatzizisi O, Kyriazis G, Christaki P, Froudarakis M, Bouros D (2009) Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep 32(4):537–543
Kohler M, Ayers L, Pepperell JC, Packwood KL, Ferry B, Crosthwaite N et al (2009) Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax 64(1):67–73
Stradling JR, Craig SE, Kohler M, Nicoll D, Ayers L, Nunn AJ, Bratton DJ (2015) Markers of inflammation: data from the MOSAIC randomised trial of CPAP for minimally symptomatic OSA. Thorax 70(2):181–182
Ünüvar Doğan F, Yosunkaya Ş, Kuzu Okur H, Can Ü (2014) Relationships between obstructive sleep apnea syndrome, continuous positive airway pressure treatment, and inflammatory cytokines. Sleep disorders 2014:1–6
Johns MW (1991) Sleep disorders unit. a new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep 14(6):540–545
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV (2017) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications: version 2.3. American Academy of sleep medicine, Darien, IL
American Academy of Sleep Medicine (2014) International classification of sleep disorders. American Academy of sleep medicine, Darien
Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, Parthasarathy S, Quan SF, Rowley JA, Positive Airway Pressure Titration Task Force of the American Academy of Sleep Medicine (2008) Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 04:157–171
Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M et al (2013) An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med 188(5):613–620
Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R (2013) Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 09:1003–1012
Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, Wei Y (2015) Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: an updated meta-analysis and metaregression of 18 studies. J Am Heart Assoc 4(11):e002454
Unnikrishnan D, Jun J, Polotsky V (2015) Inflammation in sleep apnea: an update. Reviews in Endocrine and Metabolic Disorders 16(1):25–34
Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT (2007) Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax 62(6):509–514
Trayhurn P, Wang B, Wood IS (2008) Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100(2):227–235
Ryan S (2017) Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol 595(8):2423–2430
Baessler A, Nadeem R, Harvey M, Madbouly E, Younus A, Sajid H, Naseem J, Asif A, Bawaadam H (2013) Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers-a meta-analysis. J Inflamm 10(1):13
Xie X, Pan L, Ren D, Du C, Guo Y (2013) Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 14(11):1139–1150
Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Launois S, Borel AL, Levy P, Pepin JL (2015) Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: a systematic review from sham CPAP randomized controlled trials. Sleep Med Rev 21:23–38
Acknowledgments
We are grateful to Dr. Afnan Hafiz and Dr. Ibrahim Zakaria for their help in the data collection process. Authors would also like to thank Mr. Badrudin Banjar and Mrs. Ofelia El-Assal from the Immunology Lab, King Abdulaziz University Hospital for their help in collecting and analyzing blood samples. Finally, we would like to acknowledge the great efforts done by Mrs. Walaa Abuzahra, Research Coordinator, Sleep Medicine and Research Center, in coordinating all study procedures.
Funding
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia under grant no. (KEP-2-140-39). The authors, therefore, acknowledge and appreciate DSR technical and financial support.
Author information
Authors and Affiliations
Contributions
SW, JM, FH, DG involved in planning for the study including the research question, methodology and data collection. FS, HM collected data and were involved in planning and supervised the work. DM processed the experimental data, performed the statistical analysis, drafted the manuscript and designed the figs. JM performed the measurements and processed the collected data. SW, JM, DM, DG aided in interpreting the results and worked on the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
None.
Availability of data and material
All research data available at the Sleep Medicine and Research Center, King Abdulaziz University Hospital, Jeddah, Saudi Arabia and will be retained for a period of 5 years.
Ethics approval
Reference number 178–18
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wali, S.O., Al-Mughales, J., Alhejaili, F. et al. The utility of proinflammatory markers in patients with obstructive sleep apnea. Sleep Breath 25, 545–553 (2021). https://doi.org/10.1007/s11325-020-02149-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02149-3