Abstract
Study objectives
Renal hyperfiltration (RHF) has emerged as a novel marker of early renal damage in various conditions such as diabetes and metabolic syndrome. Aberrant sleep duration and excessive daytime napping may affect the development of chronic kidney disease (CKD). In this study, the association between sleep duration, daytime napping, and renal hyperfiltration was assessed.
Setting
This study was conducted in three communities in China.
Participants
A total of 16,119 community volunteers (5735 males and 10,384 females) aged 40–65 years without CKD were included for the study.
Methods and results
Participants with short sleep duration (<6 h/day) or long sleep duration (≥10 h/day) were at a significantly increased risk of renal hyperfiltration. The fully adjusted ORs (95% CI) were 2.112 (1.107, 4.031) and 2.071 (1.504, 2.853), respectively (P < 0.05). In addition, those who took naps longer than 1.5 h per day had a higher risk of renal hyperfiltration compared with those without napping (OR 1.400, 95% CI 1.018–1.924). Further joint analysis indicated that participants with long sleep duration (≥10 h/day) had a more than twofold increased risk of RHF regardless of nap status compared with those who slept 8–9 h per day without daytime napping. The association between sleep duration or daytime napping and RHF could not be explained by the influence of sleep quality. Additional subgroup analysis showed long sleep duration (≥9 h/day) and long daytime napping (≥1.5 h) were associated with an increased risk of RHF among individuals with good sleep quality.
Conclusion
Sleep duration less than 6 h/day or more than 10 h/day and long daytime napping tend to be associated with an increased risk of renal hyperfiltration in middle-aged general population, and this relationship was independent of diabetes, hypertension, obesity, or poor sleep quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past decades, emerging evidence showed that chronic sleep loss and sleep disorders represent as an increasing public burden adversely affecting metabolic health. According to recent epidemiological studies, short sleep duration, long sleep duration, impaired sleep quality, and irregular sleep wake patterns have been associated with the prevalence and adverse outcome of multiple metabolic disorders such as obesity [1], diabetes [2, 3], hypertension [4, 5], cardiovascular disease [6, 7], as well as osteoporosis [8]. On the other hand, there is abundant evidence indicating that sleep-related disturbances including obstructive sleep apnea (OSA), restless leg movements, and periodic leg movements are highly prevalent in chronic kidney disease (CKD) population as compared with general population [9–12]. OSA has been showed to be independently associated with the risk of death from any cause and cardiovascular events of patients on peritoneal dialysis or hemodialysis [13]. However, the association between sleep disorders and the development of CKD remains unclear. Limited data from single-center studies showed that sleep duration and OSA may be risk factors affecting the development of proteinuria and CKD [14]. Recently, Yamamoto et al. reported that short sleep duration, especially five or fewer hours, was a predictor of proteinuria [15]. The underlying mechanisms linking sleep disturbances and progression of CKD may be through activation of the sympathetic nervous system, the renin–angiotensin–aldosterone (RAAS) system, endothelial dysfunction, and inflammation. In addition, the impact of OSA on renal hemodynamics may be another important mechanism that contributes to the CKD progression. Kinebuchi et al. showed that renal hyperfiltration (RHF) could be reversed after institution of continuous positive airway pressure (CPAP) therapy in patients with OSA, which implicates the potential association between renal hyperfiltration and sleep disorders [16].
RHF has been considered as a marker of early renal damage in pre-diabetes and pre-hypertension [17]. High metabolic risk was associated with glomerular hyperfiltration before overt manifestations of cardiovascular disease [18]. The long-term clinical implication of RHF as a predictor of all-cause mortality has been investigated in a recent study; Park et al. observed a significant association between age-adjusted, sex-adjusted, muscle mass-adjusted, and history of diabetes and/or hypertension medication-adjusted RHF and higher all-cause and cardiovascular mortality in a large apparently healthy adult population after a median follow-up period of 12.4 years [19]. Additionally, it has been reported that some lifestyle factors such as smoking and physical activity may be associated with RHF [20, 21]. Therefore, to further explore the risk factors associated with renal hyperfiltration may reveal novel modifiable factor to improve renal heath.
No study has yet evaluated the association between sleep duration and renal hyperfiltation. In this study, we aim to examine the association between sleep duration, daytime napping, and glomerular hyperfiltration
Materials and methods
Setting and study population
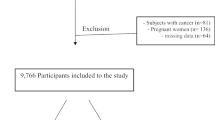
This is a population-based cross-sectional study designed to clarify the risk factors for cardiometabolic diseases. The details of the study have been described previously [8, 22]. Between June 2011 and January 2012, the health screenings were performed in three areas of China including Chongming, Ningde, and Wuyishan. Among the randomly invited participants aged 40–65 years old, we excluded 222 screenings with history of renal disease, albuminuria (urinary albumin creatinine ratio > 30 mg/g or positive dipstick protein), eGFR < 60 ml/min per 1.73 m2. A total of 16,119 participants (5735 males and 10,384 females) were included in the analysis. The study protocol was approved by the Ethics Committee of Fujian Provincial Hospital, and written informed consent was obtained from each participant.
Questionnaire
Information about the participant’s sleep duration, daytime napping time, medication for diabetes and/or hypertension, and the history of smoking, alcohol consumption, physical activity, diabetes, and hypertension was obtained using a self-reported, structured questionnaire by a trained interviewer as previously described [23]. All participants had been examined by a trained physician to have no systematic edema. Self-reported sleep duration and daytime napping time were ascertained by the following questions: (1) how many hours of sleep do you usually get at night, (2) how many minutes do you usually nap at noon, and (3) do you usually snore during sleep? The nocturnal sleep time and nap time were recorded in half hour and 15-min increments, respectively. Sleep duration was defined as the sum of nighttime sleep and daytime napping duration and was divided into six categories as <6, 6–7, 7–8, 8–9, 9–10, or ≥10 h, and daytime napping duration was classified into four categories (0, 0–1, 1–1.5, and ≥1.5 h). Respondents had four options in response to snoring: often (at least three to five times a week), sometimes (one to two times a week), never, and not sure. The answer of often was considered as snoring. Three self-reported options were provided to describe sleep quality: (1) good, (2) poor, and (3) need medication. Sleep difficulties could be rated by the number of days with sleep problem on a five-point scale ((1) none, average 1 day/month; (2) minor, average 1–3 days/month; (3) moderate, average 4–7 days/month; (4) severe, average 8 days/month; and (5) very severe, medication help for sleep). Good sleep was defined as level 1 or 2, poor sleep as level 3 or 4, and need medication as level 5. Smoking status and alcoholic consumption were classified into three categories: never, past (previous consumption but had quit for more than 6 months), and current (current consumption for more than 6 months). Leisure physical activity was divided into four categories: never, low-intensity (such as walking), moderate-intensity (such as jogging, playing table tennis, and Tai Chi), and high-intensity (such as playing basketball, swimming, and running) exercises, lasting 10 min or more at a time. The last three categories were recorded as having leisure time physical activity. Occupation activity was categorized into six groups: none, light (such as office worker), moderate (such as assembly line worker), intense (such as mounter and truck man), and severe intense (such as steelworker, farmer, and foundryman) according to the type of activity performed. The last five groups were regarded as having occupation activity.
Measurements and definition
Blood samples were drawn after a 12-h overnight fast. BP was measured using an automated BP measurement device (Omron Healthcare, Kyoto, Japan) after resting for at least 20 min in a sitting position, and the last two readings were averaged. Body mass index (BMI) was calculated by dividing weight (in kilograms) by height (in meters) squared. Fasting glucose, triglycerides, total cholesterol, low-density lipoprotein, and high-density lipoprotein were measured using an autoanalyzer. The diagnosis of diabetes was based on American Diabetes Association criteria [24], and hypertension was diagnosed according to Eighth Joint National Commission recommendation [25]. Dyslipidemia was defined as previously suggested [8]. Serum creatinine was measured using the modified Jaffe reaction with a Beckman Analyzer (Beckman Instruments Inc., CA, USA). The serum creatinine measurement was not standardized to isotope dilution mass spectrometry, and we reduced the serum creatinine levels by 5% as previously proposed [19]. eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation on the basis of serum creatinine. For the present analysis, RHF was defined as an absolute GFR >97.5th percentile of the whole participants after adjusted for sex, age, weight, height, and the use of angiotensin-converting enzyme inhibitor (ACE inhibitor) or angiotensin II receptor blockers (ARBs) as previously suggested [20, 26]. Briefly, all subjects >97.5th percentile in the distribution of residuals were selected from a multiple linear regression analysis where logarithm-transformed eGFR was used as a dependent variable and sex, the use of ACE inhibitors or ARB, logarithm-transformed age, and BMI as independent variables.
Statistical analyses
SPSS 17.0 for windows (SPSS Inc., Chicago, IL) was used for statistical analysis. P < 0.05 (two sided) was considered statistically significant. All normally distributed continuous variables were presented as mean ± SD, and categorical variables were as percentages. Differences between categories were compared with one-way ANOVA, χ 2, and nonparametric tests as appropriate. Multiple logistic regression models were used to analyze the odds ratio and 95% CIs of RHF associated with sleep duration or daytime napping, respectively, after adjustment for potential confounders, such as age, BMI, gender, smoking status, alcohol consumption, physical activity, occupation activity, diabetes, hypertension, dyslipidemia, as well as snoring. For the analysis of sleep duration, those with 8–9 h of sleep per day were used as the reference group because it has been recommended as the optimal duration for adults over 18 years. In the analysis of napping, subjects without napping were used as the reference group. Additional logistic models were used to analyze difference between poor and good sleep quality. Finally, a joint analysis by combining categories of sleep duration with daytime napping in the regression model was conducted to analyze the interaction between day napping and sleep duration using subjects with no napping and 8–9 h of sleep duration as the referent. Those who with sleep duration less than 8 h or 0–1.5 h of daytime napping were grouped together, respectively, because of the small sample size.
Results
After excluding 222 individuals with eGFR < 60 ml/min/1.73 m2 or treatment for self-reported kidney diseases, a total of 16,119 participates aged 40–65 years, including 5735 males and 10,384 females, were included in the analysis. The mean age of the total population was 52.4 ± 6.8 years; self-reported sleep duration was 8.4 ± 1.3 h, and daytime nap duration was 0.5 ± 0.6 h. The 198 men and 205 women with hyperfiltration had mean eGFRs of 114.9 (range 113.9–115.8) and 115.5 (range 114.5–116.5) ml/min/1.73m2, compared with 96.8 and 97.2 ml/min/1.73m2 for men and women with normal filtration.
The general characteristics of the participants in this study according to different categories of sleep duration are shown in Table 1. Compared with those who slept 8–9 h per day, participants who slept less than 6 h were more likely to be older and underweight and to have higher proportion of dyslipidemia, while those with sleep duration longer than 10 h were more likely to be current smokers, of male gender, to have higher systolic blood pressure, and less leisure time activities. In addition, both short and long sleep durations were significantly associated with higher proportions of current drinkers and more work activities. Furthermore, no difference in self-reported medications, particularly antihypertensive agents, antidiabetic agents, and cardiovascular medications, was observed across all sleep categories. The proportion of hypolipidemic treatment was higher in long sleep group (>10 h) than that of control group. For antihypertensive agents, there was no difference of the use of diuretics, ACEI/ARB, beta-blocker, or calcium channel blocker (CCB) among different sleep categories.
Table 2 summarizes the participants’ characteristics by nap habits. Napping during the day was reported by 2672 of 5733 men (46.6%) and 4177 of 10,380 women (40.3%). Those who took naps were more likely to be men than those who did not nap. Individuals who took naps were more likely to be smokers, current drinkers, and to have more work activities, more snoring, and higher prevalence of dyslipidemia. The proportion of RHF was also higher among the daytime napping individuals than those who did not nap. The above associations were all stronger for those who napped for longer time periods (≥1.5 h). In addition, the percentages of CCB use and hypolipidemic or antidiabetic treatment were higher in participants with daytime napping than those without napping.
The association between sleep durations and RHF was analyzed with a multivariate logistic regression adjusted for possible confounding variables, such as age, sex, smoking, alcohol intake, diabetes, hypertension, dyslipidemia, BMI, systolic BP, diastolic BP, fasting glucose, leisure time, and occupation activities; ORs of RHF across sleep or napping categories are showed in Table 3. Participants reporting short sleep (<6 h) and long sleep (≥10 h) had significantly higher risks of RHF in all three models (P < 0.05), although the magnitude of association attenuated in models 2 and 3. After adjustment for all confounders (model 3), as compared with reference, fully adjusted ORs (95% CI) of <6 h/day and ≥10 h/day were 2.112 (1.107–4.031, P = 0.023) and 2.071 (1.504–2.853, P < 0.001), respectively. On the other hand, compared with the participants who did not take naps, those who took naps longer than 1.5 h per day were significantly associated with greater odds of RHF (P = 0.037). After adjusting for all the above confounding variables, fully adjusted OR (95% CI) of 1.5 h/day was 1.400 (1.018, 1.924, P = 0.034). Further adjustment for medications and treatments for comorbid conditions did not attenuate the association between RHF and sleep duration or naps (Table S2).
The interaction of daytime napping and sleep duration on the risk of RHF is shown in Fig. 1. There was a statistically significant overall interaction between hours of sleep duration and daytime napping on RHF (P = 0.012). However, the relationship between daytime napping and RHF depended on the hours of sleep duration. Among those who reported no napping, both short (<8 h) and long (≥10 h) hours of sleep duration were associated with higher risks of RHF. Daytime napping further increased the risk of RHF among participants with sleep duration longer than 10 h per day. Overall, participants with no napping and 8–9 h of sleep per day had the lowest risk of RHF, whereas individuals who napped ≥1.5 h during the day and slept ≥10 h per day had the highest risk. The fully adjusted OR (95% CI) was 2.732 (1.690, 4.416) (P < 0.001).
Joint analysis on total sleep duration and day napping in relation to the risk of renal hyperfiltration. Multivariate ORs for renal hyperfiltration were adjusted for age, sex, BMI, diabetes, hypertension, dyslipidemia, smoking status, alcohol consumptions, snoring, leisure time physical activity, and occupation activity. Asterisk denotes result statistically different from 8 to 9 h of sleep duration per day without daytime naps (P < 0.05)
To further investigate whether the associations between sleep duration, daytime napping, and RHF varied with sleep quality, subgroup analysis was performed among the participants reported good or poor sleep quality, respectively (Table 4). Among participants with good sleep quality, those who slept 9–10 h/day and more than 10 h/day had higher fully adjusted ORs (OR 1.462, 2.241, 95% CI 1.043–2.049 and 1.585–3.170, P = 0.027 and P < 0.001) as compared with the reference group. Additionally, those who with daytime napping more than 1.5 h per day were associated with a higher risk of RHF compared with those who reported no napping. The OR (95% CI) was 1.507 (1.072, 2.120) after adjustment of the above covariates (P = 0.019). However, for those with poor-quality sleep, individuals who slept 9–10 h per day had a lower risk of RHF compared with the reference. No significant association was observed between daytime napping and the risk of RHF.
Discussion
In this study, we indentified an association between sleep duration, daytime napping, and the incidence of RHF in a large apparently healthy middle-aged population. Participants with short sleep duration (<6 h/day) or long sleep duration (≥10 h/day) are at a significantly increased risk of RHF, even after accounting for a variety of confounding or explanatory variables. In addition, our results showed a higher risk of RHF among those who took naps longer than 1.5 h per day compared with those without napping. Further joint analysis indicated that participants with long sleep duration (≥10 h/day) had a more than twofold increased risk of RHF regardless of nap status compared with those who slept 8–9 h per day without daytime napping. Importantly, the association between sleep duration or daytime napping and RHF could not be explained by the influence of sleep quality. Additional subgroup analysis showed that long sleep duration (≥9 h/day) and long daytime napping (≥1.5 h) were associated with an increased risk of RHF among individuals with good sleep quality. On the other hand, medication that passes through the blood–brain barrier has been reported to have potential effect on sleep pattern, and antihypertensive therapy may contribute to relieve sleep problems. In this study, the association between RHF and sleep duration or napping was independent of medications taken by the participants.
Although it has been documented that excessive daytime sleepiness and sleep disorders are common in patients with end-stage renal disease and may contribute to the augmented cardiovascular event in dialysis patients [27, 28], as well as transplant recipients [29], whether they also play a role in the development and progression of earlier stages of CKD remains unknown. Recently, Yamamoto et al. performed a retrospective cohort study among employees of the Osaka University in Japan to identify that short sleep duration, especially five or fewer hours, was a significant predictor of proteinuria, even adjusting for multiple clinically relevant metabolic and lifestyle factors [15]. However, due to the large number of participants in this cohort who have shorter sleep duration, it is unclear whether longer sleep duration may be associated with the development of CKD. Although a cross-sectional study using survey data from the National Health Interview Survey (NHIS) showed that an increased incidence of self-reporting kidney disease was observed in those with short and long sleep duration, the self-reported CKD in this study could lead to misclassification of the disease status [30]. On the other hand, although the association between CKD and RHF remains obscure, the clinical consequence of RHF has been ascertained in a recent study by Park et al. which suggested that RHF may be associated with increased all-cause mortality in an apparently healthy population [19]. RHF has been shown to be associated with rapid decline in eGFR in diabetic or nondiabetic CKD [31–33] and may predict microalbuminuria in metabolic risk factors associated CKD [34]. Therefore, as an early stage of CKD, RHF may share common pathophysiology with CKD in the association with cardiovascular disease and mortality. To date, no study has yet reported the association between sleep duration, daytime napping, and RHF. To address this issue, we conducted a cross-sectional study in a large middle-aged Chinese population. As habitual napping is often considered as a “healthy” lifestyle in China, daytime napping is prevalent (42.5%) in this population The mean sleep duration and daytime napping were 8.4 ± 1.3 and 0.5 ± 0.6 h, respectively, which rendered a statistically meaningful analysis to assess an association between aberrant sleep duration, napping, and RHF.
Our study showed that sleep duration <6 or ≥10 h per day was associated with an increased risk of RHF, which is consistent with the results of previous prospective studies reporting a U-shaped-like association between sleep duration and the development of diabetes, hypertension, metabolic syndrome, and cardiovascular diseases [2, 4, 6, 35]. Therefore, the association between aberrant sleep duration or daytime napping and RHF may in part confounded by the indirect effect of sleep disorders on metabolic syndrome-associated disorders, such as diabetes, obesity, and hypertension. Although impaired fasting glucose is an established risk factor for RHF [26], in this study, fasting glucose levels were not associated with any change in the odds of RHF. Likewise, there was no association between systolic blood pressure and hyperfiltration. Thus, it is unlikely that hyperglycemia or hypertension mainly contribute to RHF in this study.
Loss of muscle mass and obesity-related disorders might also contribute to the association between sleep disturbance and RHF. BMI was independently associated with RHF. In addition, muscle mass was positively correlated with RHF independent of BMI. In this study, adjustment for BMI or BMI categories modestly attenuated but did not eliminate the relationship between aberrant sleep duration or daytime napping and RHF. On the other hand, in a prospective study on nonhypertensive nondiabetic OSA patients, Chou et al. reported that severe OSA may positively correlate with RHF and the progression of CKD [13]. In particular, daytime napping is correlated with OSA that, in turn, increases the risk of diabetes [36, 37]. Thus, we then investigated the prevalence of snoring in this population. There was no significant interaction between snoring and RHF after adjustment of all the confounders, and the association between daytime napping and hyperfiltration remained significant among those without snoring (Table S1). The positive correlation between RHF and daytime napping could not be fully explained by snoring. Furthermore, poor sleep quality such as depression and insomnia may also be correlated with daytime napping or sleep duration and the risk of diabetes and cardiovascular disease [38, 39]. The participants were further divided according to self-reported sleep quality, and the association between long sleep duration with RHF remained significant in the subjects with good sleep quality. The relationship between short sleep duration and RHF was not significant probably due to the insufficient size of study subjects. In addition, because of the potential compensating effects on sleep deprivation, we included daytime napping in the calculation of whole day sleep duration. Hence, poor sleep quality could not be the main driver of the increased RHF in the present study.
The direct mechanism behind hyperfiltration in aberrant sleep duration or long daytime napping remains unclear. A potential explanation that connects aberrant sleep duration and excessive daytime napping with RHF could be systemic inflammation and oxidative stress, which is evident in a recent study reporting that several factors related to inflammation, oxidative stress, and antioxidant status such as GGT, carotenoids, uric acid, and vitamin D may contribute to the relationship between sleep duration and cardiometabolic health [40]. In addition, sleep deprivation may induce an increase in peripheral white blood cell count and serum IL-6, CRP levels [41–43]. Furthermore, sleep disturbance may also induce activation of RAAS system, systemic endothelial dysfunction, and auotonomic nervous system, which may subsequently induce RHF [11]. Therefore, RHF may share the common pathophysiologic mechanisms with metabolic syndrome-associated disorders.
The major strengths of our study include its large sample of the general population from three areas of China, detailed epidemiologic profiles, and thorough statistical analyses. However, the current study also has several limitations. First, the results might be biased because GFR was estimated using serum creatinine-based (CKD-EPI) equation rather than direct measurement. Therefore, in the current study, RHF was defined after adjustment for age, sex, BMI, and ACEI/ARB treatment. Nevertheless, it does not completely avoid overestimating GFR, especially in participants with muscle waste. Second, information on sleep duration and daytime napping relies on self-reports, which could lead to recall bias and misclassification. In addition, self-report snoring and sleep quality tend to be highly subjective. Thus, further studies with more precise measures of OSA and sleep quality index such as Epworth and Pittsburg Sleepiness Scale are needed to confirm that the association stands independent of other sleep disturbances. Third, we could not evaluate the effect of some underlying disease such as proteinuria in this general population. Fourth, although we have adjusted for several confounders that could explain the relationship between RHF and aberrant sleep duration or daytime napping, it is possible that residual factors not captured in this study had not been accounted in our analysis. Finally, the cross-sectional design limits causal inference. Follow-up studies are required to examine the long-term effect of sleep disturbance on proteinuria and incidence of CKD in this population.
In summary, our study is novel in demonstrating the associations between sleep duration, daytime napping, and RHF in middle-aged apparently healthy population, and this relationship was independent of diabetes, hypertension, obesity, or poor sleep quality. Whether aberrant sleep duration or excessive daytime napping could be the common modifiable risks shared by RHF or CKD with cardiometabolic diseases warrants further investigation.
Reference
Patel SR, Hayes AL, Blackwell T, Evans DS, Ancoli-Israel S, Wing YK, Stone KL (2014) The association between sleep patterns and obesity in older adults. Int J Obes 38(9):1159–1164. doi:10.1038/ijo.2014.13
Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H (2010) Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care 33(1):78–83. doi:10.2337/dc09-1143
Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB (2003) A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26(2):380–384
Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D (2006) Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 47(5):833–839. doi:10.1161/01.HYP.0000217362.34748.e0
Wang Q, Xi B, Liu M, Zhang Y, Fu M (2012) Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res 35(10):1012–1018. doi:10.1038/hr.2012.91
Stone KL, Ewing SK, Ancoli-Israel S, Ensrud KE, Redline S, Bauer DC, Cauley JA, Hillier TA, Cummings SR (2009) Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc 57(4):604–611. doi:10.1111/j.1532-5415.2008.02171.x
Leng Y, Wainwright NW, Cappuccio FP, Surtees PG, Hayat S, Luben R, Brayne C, Khaw KT (2014) Daytime napping and the risk of all-cause and cause-specific mortality: a 13-year follow-up of a British population. Am J Epidemiol 179(9):1115–1124. doi:10.1093/aje/kwu036
Chen G, Chen L, Wen J, Yao J, Li L, Lin L, Tang K, Huang H, Liang J, Lin W, Chen H, Li M, Gong X, Peng S, Lu J, Bi Y, Ning G (2014) Associations between sleep duration, daytime nap duration, and osteoporosis vary by sex, menopause, and sleep quality. J Clin Endocrinol Metab 99(8):2869–2877. doi:10.1210/jc.2013-3629
Plantinga L, Lee K, Inker LA, Saran R, Yee J, Gillespie B, Rolka D, Saydah S, Powe NR (2011) Association of sleep-related problems with CKD in the United States, 2005-2008. Am J Kidney Dis 58(4):554–564. doi:10.1053/j.ajkd.2011.05.024
Hanly PJ, Pierratos A (2001) Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med 344(2):102–107. doi:10.1056/NEJM200101113440204
Turek NF, Ricardo AC, Lash JP (2012) Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis 60(5):823–833. doi:10.1053/j.ajkd.2012.04.027
Tang SC, Lam B, Ku PP, Leung WS, Chu CM, Ho YW, Ip MS, Lai KN (2006) Alleviation of sleep apnea in patients with chronic renal failure by nocturnal cycler-assisted peritoneal dialysis compared with conventional continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 17(9):2607–2616. doi:10.1681/ASN.2005090936
Chou YT, Lee PH, Yang CT, Lin CL, Veasey S, Chuang LP, Lin SW, Lin YS, Chen NH (2011) Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant 26(7):2244–2250. doi:10.1093/ndt/gfq821
Tsioufis C, Thomopoulos C, Dimitriadis K, Amfilochiou A, Tsiachris D, Selima M, Petras D, Kallikazaros I, Stefanadis C (2008) Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. Am J Kidney Dis 52(2):285–293. doi:10.1053/j.ajkd.2008.05.001
Yamamoto R, Nagasawa Y, Iwatani H, Shinzawa M, Obi Y, Teranishi J, Ishigami T, Yamauchi-Takihara K, Nishida M, Rakugi H, Isaka Y, Moriyama T (2012) Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis 59(3):343–355. doi:10.1053/j.ajkd.2011.08.032
Kinebuchi S, Kazama JJ, Satoh M, Sakai K, Nakayama H, Yoshizawa H, Narita I, Suzuki E, Gejyo F (2004) Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci (Lond) 107(3):317–322. doi:10.1042/CS20040074
Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S (2012) Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 27(5):1821–1825. doi:10.1093/ndt/gfr651
Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, Sattar N, Zukowska-Szczechowska E, Dominiczak AF (2007) Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 71(8):816–821. doi:10.1038/sj.ki.5002160
Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ (2015) Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol 26(6):1426–1433. doi:10.1681/ASN.2014010115
Maeda I, Hayashi T, Sato KK, Koh H, Harita N, Nakamura Y, Endo G, Kambe H, Fukuda K (2011) Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol 6(10):2462–2469. doi:10.2215/CJN.00700111
Melsom T, Mathisen UD, Eilertsen BA, Ingebretsen OC, Jenssen T, Njolstad I, Solbu MD, Toft I, Eriksen BO (2012) Physical exercise, fasting glucose, and renal hyperfiltration in the general population: the Renal Iohexol Clearance Survey in Tromso 6 (RENIS-T6). Clin J Am Soc Nephrol 7(11):1801–1810. doi:10.2215/CJN.02980312
Qiu C, Chen H, Wen J, Zhu P, Lin F, Huang B, Wu P, Lin Q, Lin Y, Rao H, Huang H, Liang J, Li L, Gong X, Peng S, Li M, Chen L, Tang K, Chen Z, Lin L, Lu J, Bi Y, Ning G, Chen G (2013) Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab 98(4):1612–1621. doi:10.1210/jc.2012-2919
Huang C, Guo C, Nichols C, Chen S, Martorell R (2014) Elevated levels of protein in urine in adulthood after exposure to the Chinese famine of 1959-61 during gestation and the early postnatal period. Int J Epidemiol 43(6):1806–1814. doi:10.1093/ije/dyu193
Standards of medical care in diabetes--2015: summary of revisions (2015). Diabetes Care 38 Suppl:S4. doi:10.2337/dc15-S003
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311(5):507–520. doi:10.1001/jama.2013.284427
Melsom T, Mathisen UD, Ingebretsen OC, Jenssen TG, Njolstad I, Solbu MD, Toft I, Eriksen BO (2011) Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 34(7):1546–1551. doi:10.2337/dc11-0235
Tang SC, Lam B, Yao TJ, Leung WS, Chu CM, Ho YW, Ip MS, Lai KN (2010) Sleep apnea is a novel risk predictor of cardiovascular morbidity and death in patients receiving peritoneal dialysis. Kidney Int 77(11):1031–1038. doi:10.1038/ki.2010.76
Masuda T, Murata M, Honma S, Iwazu Y, Sasaki N, Ogura M, Onishi A, Ando Y, Muto S, Shimada K, Kario K, Kusano E, Asano Y (2011) Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol Dial Transplant 26(7):2289–2295. doi:10.1093/ndt/gfq756
Molnar MZ, Novak M, Mucsi I (2009) Sleep disorders and quality of life in renal transplant recipients. Int Urol Nephrol 41(2):373–382. doi:10.1007/s11255-009-9527-z
Salifu I, Tedla F, Pandey A, Ayoub I, Brown C, McFarlane SI, Jean-Louis G (2014) Sleep duration and chronic kidney disease: analysis of the national health interview survey. Cardiorenal Med 4(3–4):210–216. doi:10.1159/000368205crm-0004-0210
Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM (2015) Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with type 1 diabetes. Nephrol Dial Transplant 30(10):1706–1711. doi:10.1093/ndt/gfv121
Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW (2012) Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 8(5):293–300. doi:10.1038/nrneph.2012.19
Moriya T, Tsuchiya A, Okizaki S, Hayashi A, Tanaka K, Shichiri M (2012) Glomerular hyperfiltration and increased glomerular filtration surface are associated with renal function decline in normo- and microalbuminuric type 2 diabetes. Kidney Int 81(5):486–493. doi:10.1038/ki.2011.404
Palatini P, Mormino P, Dorigatti F, Santonastaso M, Mos L, De Toni R, Winnicki M, Dal Follo M, Biasion T, Garavelli G, Pessina AC (2006) Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int 70(3):578–584. doi:10.1038/sj.ki.5001603
Arora T, Jiang CQ, Thomas GN, Lam KB, Zhang WS, Cheng KK, Lam TH, Taheri S (2011) Self-reported long total sleep duration is associated with metabolic syndrome: the Guangzhou Biobank Cohort Study. Diabetes Care 34(10):2317–2319. doi:10.2337/dc11-0647
Masa JF, Rubio M, Perez P, Mota M, de Cos JS, Montserrat JM (2006) Association between habitual naps and sleep apnea. Sleep 29(11):1463–1468
Tasali E, Mokhlesi B, Van Cauter E (2008) Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133(2):496–506. doi:10.1378/chest.07-0828
Phillips B, Mannino DM (2007) Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med 3(5):489–494
Tasali E, Leproult R, Spiegel K (2009) Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis 51(5):381–391. doi:10.1016/j.pcad.2008.10.002
Kanagasabai T, Ardern CI (2015) Contribution of inflammation, oxidative stress, and antioxidants to the relationship between sleep duration and cardiometabolic health. Sleep
Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT (1994) Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest 93(5):1930–1939. doi:10.1172/JCI117184
Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP (1999) Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 84(8):2603–2607. doi:10.1210/jcem.84.8.5894
Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM (2004) Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43(4):678–683. doi:10.1016/j.jacc.2003.07.050
Acknowledgments
Funding: Chinese Medical Association Foundation and Chinese Endocrine Society (12020240314), National Natural Science Foundation of China (81270874, 81570706, 81200538), Natural Science Foundation of Fujian Province (2011J06012, 2014J05083), Provincial Health and Family Planning Commission of Fujian Province (2013-ZQN-ZD-3), National Clinical Research Center for Metabolic Diseases (2013BAI09B13), and the National Key New Drug Creation and Manufacturing Program of Ministry of Science and Technology (2012ZX09303006-001) provided financial support in the form of researcher funding. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure
The authors declare that they have no other relevant financial interests.
Additional information
Miao Lin, Qing Su, Junping Wen, Shichao Wei, and Jin Yao contributed to the study equally.
The current work was performed at Fujian Provincial Hospital, Fujian Medical University.
Rights and permissions
About this article
Cite this article
Lin, M., Su, Q., Wen, J. et al. Self-reported sleep duration and daytime napping are associated with renal hyperfiltration in general population. Sleep Breath 22, 223–232 (2018). https://doi.org/10.1007/s11325-017-1470-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1470-0