Abstract
Background
F2-isoprostanes are considered to be a reliable standard biomarker of oxidative stress in vivo because they are not influenced by the intake of lipids in the diet, and they are chemically stable molecules and easily detected. This study aimed to test the hypothesis that 8-isoprostane level is a useful marker to valuate the severity of pediatric obstructive sleep apnea (OSA).
Methods
Sixty-five children with sleep-disordered breathing (SDB) (mean age 5.9 ± 2.0 years; 63.1 % males) were recruited. The urine sample for the measurement of 8-isoprostane was collected the morning after the polysomnographic recording. Children were divided into two groups according to their apnea–hypopnea index (AHI).
Results
Urinary 8-isoprostane levels positively correlated with the sleep clinical record score (r = 0.38, p = 0.002) and AHI (r = 0.24, p = 0.05) and negatively correlated with age (r = −0.36, p = 0.003) and body surface area (r = −0.38, p = 0.002). Urinary 8-isoprostane levels were significantly higher in the group with AHI of ≥5 events (ev)/h than in the group with AHI of <5 ev/h (p < 0.01).
Conclusions
Urinary 8-isoprostane may be used as a specific inflammatory marker to predict the severity of OSA; this method has the advantage of being noninvasive and easy to use in both compliant and noncompliant children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by prolonged partial upper airway obstruction or intermittent complete obstruction (obstructive apnea) that disrupts normal ventilation during sleep and normal sleep patterns [1]. OSA may affect children of all ages, though the highest incidence is between 2 and 6 years of age [2]. The prevalence of OSA in children ranges from 1.2 to 5.7 % [1].
The repeated episodes of hypoxia and reoxygenation that cause ischemia–reperfusion events are currently believed to promote the production of reactive oxygen species and oxidative stress. Oxidative stress may lead to the development of endothelial dysfunction, systemic inflammation (increased IL-8, TNF-α, 8-isoprostane), increased adhesion molecule expression in leukocytes (ligand for CD40), and lipid peroxidation. Consequently, oxidative stress may promote atherogenesis and vascular dysfunction in both children and adults with OSA [3–7].
F2-isoprostanes are formed in situ from fatty acid esterified in membrane phospholipids induced by free radicals, whereas classic prostaglandins are formed by PGH synthase isozymes from free arachidonic acid [8–10]. Isoprostanes are released from membrane phospholipids in response to cellular activation, presumably through a phospholipase A2. They may circulate either freely or as esters in phospholipids. The factors that regulate the release of endogenous isoprostanes from cell membranes and interconversion between the free and esterified forms have yet to be fully understood [11]. F2-isoprostanes are considered to be a reliable standard biomarker of oxidative stress in vivo because they are not influenced by the intake of lipids in the diet, and they are chemically stable molecules and easily detected [12, 13]. These properties allow the molecule to be detected not only in plasma and exhaled breath condensate but also in urine, which thus offers the possibility of noninvasive measurements even in noncollaborative patients.
In a recent study conducted on adults, Monneret et al. showed that the urinary 8-isoprostane levels were significantly higher in severe OSA patients than in controls and that the mean carotid intima-media thickness positively correlated with urinary 8-isoprostane concentrations [14]. Studies on children have yielded contradictory results; when Biltagi et al. examined oxidative stress in children with OSA by analyzing exhaled concentrations of 8-isoprostane and IL-6, they observed that concentrations increased according to the severity of the disease [15]. By contrast, Hawley et al. did not observe any correlation between urine F2-isoprostane levels and the severity of SDB in children [11].
The aim of this study was to investigate whether urinary concentrations of 8-isoprostane (also called 8-iso-PGF2α, 8-epi-PGF2α, 15-F2t-isoprostane, or iPF2α-III) could be used as a marker of severity of pediatric OSA in a group of children in whom OSA was diagnosed by means of a validated sleep clinical record (SCR) and by polysomnography[16].
Methods
Study subjects
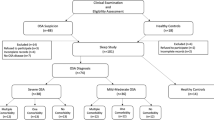
Between May 2012 and May 2013, we consecutively enrolled 65 children who were referred to our pediatric sleep center (Rome, Italy) for habitual snoring, apnea, or restless sleep, as reported by their parents. Exclusion criteria were as follows: epilepsy, acute or chronic cardiorespiratory or neuromuscular diseases, dysmorphism, chronic inflammatory diseases, major craniofacial abnormalities, chromosomal syndromes, previous treatment for OSAS, and age under 3 years on account of poor compliance. Standard informed consent was obtained from the parents of each child before enrolment in the study.
Polysomnography
All the subjects were evaluated in our pediatric sleep center for one full-night polysomnography (PSG) after a night of adaptation in the hospital. Standard overnight PSG recordings were performed using a Grass Heritage polygraph. The variables recorded included a six-channel electroencephalogram (frontal, central temporal, and occipital, referred to the contralateral mastoid), an electrooculogram (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to A1), a submental electromyogram, and an electrocardiogram (one derivation). Chest and abdomen movements were measured by strain gauges. Oronasal airflow was recorded using a thermocouple; nasal pressure was recorded by means of a nasal cannula. Arterial oxygen saturation (SaO2) was monitored using a pulse oximeter.
Total sleep time was subdivided into 30-s epochs, and sleep stages were scored according to the standard criteria of the American Academy of Sleep Medicine (AASM) [17]. We considered a clinical cutoff value of AHI ≥ 5 for moderate–severe OSAS [18, 19].
Evaluation of patients
A detailed personal and family history was obtained for all the participants, and a clinical examination was performed. We calculated the body mass index (BMI), the BMI percentile, and the body surface area (BSA) in all the children [20]. According to the literature, which defines subjects as obese when they have a BMI of ≥95th percentile, the sample was divided in two groups: nonobese and obese children [21].
We filled out the SCR, which combines the patient’s history and clinical items, for each child [16]. The first part considers the nose, oropharynx, dental, and skeletal occlusion. Tonsillar hypertrophy was graded according to a standardized scale ranging from 0 to 4. Tonsillar size was graded as follows: 1+, medial borders of tonsils lateral to or extending to the pillars; 2+, medial borders of tonsils lateral to or extending to the lateral uvular margins; 3+, medial borders of tonsils medial to the lateral uvular margins; and 4+, includes “kissing” tonsils, which meet at the midline [22]. Grades 3 and 4 were considered as positive. The position of the palate was graded according to the Friedman classes, with classes 3 and 4 being considered as positive [23]. The second part is based on the Brouillette questionnaire [24], while the last part examines the possible presence of symptoms of inattention and hyperactivity by means of the attention deficit hyperactive disorder rating scale [25], adapted to the Italian population [26].
Urinary 8-isoprostane level measurement
The urine sample was collected the morning after the polysomnographic recording.
In order to avoid oxidation of the compound, 0.005 % butyl hydroxy toluene (10 μl of 5 mg/ml solution in ethanol per 1 ml sample) was added to each sample, which was then quickly stored at −80 °C until analysis. The concentration of 8-isoprostane was measured using an enzyme immunoassay (Cayman Chemical Co., Ann Arbor, MI, USA) and is corrected for creatinine levels.
The antiserum used in this assay displays a cross-reactivity of 100 % with 8-isoprostane, of 0.16 % with prostaglandin D2 and of 0.02 % with prostaglandin E2.
Intra- and inter-assay coefficients of variation were 4.9 and 4.7 %, respectively.
Statistical analysis
The normality of the data distribution was assessed by the Kolmogorov–Smirnov test. Values were expressed as arithmetic means ± standard deviation (SD) for normally distributed data or as a median and interquartile range (IR) for nonparametric data. The Mann–Whitney test was used to compare impaired nonparametric data, while Student’s t test was used for normal data. The χ 2 was used to compare categorical variables. The Pearson (normal data) or Spearman (nonparametric data) tests were used to assess any correlations between variables.
A multiple linear regression analysis (stepwise method) was performed with 8-isoprostane as the dependent variable against age, sex (female = 0, male = 1), BMI percentile, AHI, mean and minimal SaO2, SCR score, severity of disease (subjects with AHI < 5 ev/h = 0, subjects with AHI ≥ 5 ev/h = 1), and BSA as potentially explanatory (independent) variables. A p value of <0.05 was considered statistically significant.
Results
The sample was composed of 65 children (mean age 5.9 ± 2.0 years, 63.1 % males). Children were divided into two groups according to their AHI. Primary snoring/mild OSA subjects (n = 28, 43.1 %, mean age 6.2 ± 2.0 years) had an AHI of <5 ev/h, while moderate/severe OSA subjects (n = 37, 56.9 %, mean age 5.6 ± 2.1) had an AHI of ≥5 ev/h. The children’s anthropometric, clinical parameters, polysomnographic respiratory variables and 8-isoprostane levels are shown in Table 1. There was no difference in sex, age, or clinical parameters between the two groups. The BMI percentile was higher in the group with AHI ≥ 5 ev/h than in the group with AHI < 5 ev/h (p < 0.01). There was no significant difference in the urinary 8-isoprostane levels between male and female patients: 0.73 (0.59–0.97) vs 0.99 (0.66–1.49) ng/mg creatinine, p = 0.062. Urinary 8-isoprostane levels were significantly higher in the group with AHI ≥ 5 ev/h than in the group with AHI < 5 ev/h, (p < 0.01) (Table 1).
No differences emerged between the two groups in the SCR (p > 0.05). The SCR differentiates snorers from children with OSA and, as expected, the OSA children in our sample had a higher score (Tables 2 and 3) in part 2 of the Sleep Clinical Record (structural and inflammatory items).
Urinary 8-isoprostane levels positively correlated with the SCR score (r = 0.38, p = 0.002; Fig. 1) and AHI (r = 0.24, p = 0.05; Fig. 2) and negatively correlated with age (r = −0.36, p = 0.003; Fig. 3) and body surface (r = −0.38, p = 0.002, Fig. 4). No correlation emerged between 8-isoprostane and the mean and minimal SaO2, or the BMI percentile AHI positively correlated with the SCR score (r = 0.26, p = 0.04).
The AHI was higher in the nonobese group than in obese group. The nonobese children with AHI ≥ 5 ev/h were shorter than those in the other groups. Urinary 8-isoprostane levels were significantly higher in the nonobese group with AHI ≥ 5 ev/h than in the nonobese group with AHI < 5 ev/h (p = 0.001) (Table 4).
The multiple linear regression analysis (stepwise) with 8-isoprostane values as a dependent variable and age, sex, BMI percentile, AHI, mean and minimal SaO2, SCR score, severity of OSA, and body surface area as independent variables yielded a three-variable model (r = 0.53, r 2 = 0.28). This model included the SCR score (t = 2.93, p = 0.005), severity of disease (t = 2.32, p = 0.02), and body surface area (t = −2.16, p = 0.04) (Table 5).
Discussion
Our study demonstrates that urinary concentrations of 8-isoprostane may be used as a marker of inflammation in pediatric OSA to predict the severity of disease. The results of our study suggest that the increase of urinary 8-isoprostane levels and that of the severity of OSA might indirectly be expressed by the SCR score [16]. The SCR score is a validated instrument adopted to screen OSA which combines clinical examination and patient history and is able to provide a better overview of a child compared to the analysis of the single items taken alone.
The correlation between 8-isoprostane levels and the severity of disease is confirmed by the fact that urinary 8-isoprostane levels were higher in children with moderate/severe OSA than in those with mild OSA. Our results are in keeping with those reported in a previous study by Biltagi et al. conducted on the breath condensate of children with OSA [15]. By contrast, Hawley and coauthors did not find any association between F2-isoprostane metabolites and PSG values in children with SDB of varying severity [11]. A possible explanation for this discrepancy between the studies may be that the patient sample investigated by Hawley et al. was composed of 47 children with OSA, fewer of whom (7 out of 47 children) had a moderate–severe form of OSA than in our patient sample.
When we divided the sample not only according to the severity but also to the BMI percentile, 8-isoprostane levels remained high only in nonobese children with more severe disease. This finding highlights the fact that 8-isoprostane is a marker of inflammation that is related to the severity of the disease rather than to the degree of obesity. Moreover, it suggests that exposure to intermittent hypoxia during the night might trigger lipid peroxidation. Episodes of intermittent hypoxia and associated episodes of intermittent reoxygenation may result in the activation of inflammatory genes and in the production of reactive oxygen species (ROS) that increase IL-8, TNF-α, IL-6, and 8-isoprostane levels [27, 28]. This hypothesis is in keeping with the results of a study by Monneret conducted on nonobese adults, in which 8-isoprostane levels correlated with the severity of the pathology [14]. Our hypothesis is further supported by the study conducted by Del Ben showing that 8-isoprostane levels in obese adult patients decrease significantly after treatment with CPAP for 6 months, in the absence of changes in the BMI [29].
When we investigated the possibility of using 8-isoprostane as a predictor of severity, the linear regression analysis identified the SCR score, the severity of disease, and the BSA as the most significant predictors of increased urinary 8-isoprostane levels in children with OSA (Table 5). Levels of 8-isopostane were found to be associated with body surface area and the children’s age, with the highest levels being detected in younger subjects with a reduced body surface area (Table 4).
Since there was no correlation with mean and minimal SaO2, other variables dependent on oxidative stress, such as sleep fragmentation, may explain the increase in 8-isoprostane. In the study conducted by Del Ben et al. [29] on 138 adults with OSA, the authors found that 8-isoprostane correlated with the AHI, though not with the mean SaO2. By contrast, in their study conducted on a smaller population of adults with OSA [14], Monneret et al. reported a correlation between 8-isoprostane and both the AHI and the mean SaO2.
In conclusion, our results indicate that urinary 8-isoprostane levels may be considered a specific inflammatory marker of the severity of OSA that can be assessed noninvasively and can thus easily be adopted for both compliant and noncompliant children. The measurement of urinary 8-isoprostane, combined with administration of the SCR, may thus, we believe, be recommended as a valid and inexpensive diagnostic tool for pediatric OSA. Further studies on larger populations of children are warranted to confirm our results.
References
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Sheldon SH, Spruyt K, Ward SD, Lehmann C, Shiffman RN, American Academy of Pediatrics (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130:576–584
Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, Fedok F, Vlasic V, Graff G (2009) Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep 32:731–736
Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E (2007) Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation 116:2307–2314
Dyugovskaya L, Lavie P, Lavie L (2002) Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 165:934–939
Gozal D, Kheirandish-Gozal L (2008) Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 177:369–375
Kim J, Hakim F, Kheirandish-Gozal L, Gozal D (2011) Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol 178:465–474
Lavie L, Vishnevsky A, Lavie P (2004) Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27:123–128
Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ 2nd (1992) Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A 89:10721–107215
Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ 2nd (1990) A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase free radical catalyzed mechanism. Proc Natl Acad Sci U S A 87:9383–9387
Nourooz-Zadeh J (2008) Key issues in F2-isoprostane analysis. Biochem Soc Trans 36:1060–1065
Hawley Montgomery-Downs HE, Krishna J, Roberts LJ 2nd, Gozal D (2006) Urinary F2-isoprostane metabolite levels in children with sleep-disordered breathing. Sleep Breath 10:211–215
Roberts LJ, Morrow JD (2000) Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 4:505–513
Richelle M, Turini ME, Guidoux R, Tavazzi I, Métairon S, Fay LB (1999) Urinary isoprostane excretion is not confounded by the lipid content of the diet. FEBS Lett 459:259–262
Monneret D, Pepin JL, Godin-Ribuot D, Ducros V, Baguet JP, Levy P, Faure P (2010) Association of urinary 15-F2t-isoprostane level with oxygen desaturation and carotid intima-media thickness in nonobese sleep apnea patients. Free Radic Biol Med 48:619–625
Biltagi MA, Maguid MA, Ghafar MA, Farid E (2008) Correlation of 8-isoprostane, interleukin-6 and cardiac functions with clinical score in childhood obstructive sleep apnoea. Acta Paediatr 97:1397–1405
Villa MP, Paolino MC, Castaldo R, Vanacore N, Rizzoli A, Miano S, Pozzo MD, Montesano M (2013) Sleep clinical record: a help to rapid and accurate diagnosis of paediatric sleep disordered breathing. Eur Respir J 41:1355–1361
Iber C, Ancoli-Israel S, Chesson AL, Quan SF (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL (1992) Normal polysomnographic values for children and adolescents. Am Rev Respir Dis 146:1235–1239
Uliel S, Tauman R, Greenfeld M, Sivan Y (2004) Normal polysomnographic respiratory values in children and adolescents. Chest 125:872–878
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93:62–66
Barlow SE, Expert committee (2007) Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120:S164–S192
Brodsky L (1989) Modern assessment of tonsils and adenoids. Pediatr Clin North Am 36:1551–1569
Friedman M, Ibrahim H, Joseph NJ (2004) Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope 114:454–459
Brouilette R, Hanson D, David R, Klemka L, Szatkowski A, Fernbach S, Hunt C (1984) A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr 105:10–14
DuPaul GJ, McGoey KE, Eckert TL, VanBrakle J (2001) Preschool children with attention-deficit/hyperactivity disorder: impairments in behavioral, social, and school functioning. J Am Acad Child Adolesc Psychiatry 40:508–515
Marzocchi GM, Cornoldi C (2000) Una scala di facile uso per la rilevazione dei comportamenti problematici dei bambini con Deficit di Attenzione e Iperattività. Psicologia Clinica dello Sviluppo 4:43–64
Minoguchi K, Yokoe T, Tanaka A, Ohta S, Hirano T, Yoshino G, O’Donnell CP, Adachi M (2006) Association between lipid peroxidation and inflammation in obstructive sleep apnoea. Eur Respir J 28:378–385
Ryan S, Taylor CT, McNicholas WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112:2660–2667
Del Ben M, Fabiani M, Loffredo L, Polimeni L, Carnevale R, Baratta F, Brunori M, Albanese F, Augelletti T, Violi F, Angelico F (2012) Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med 12:36
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villa, M.P., Supino, M.C., Fedeli, S. et al. Urinary concentration of 8-isoprostane as marker of severity of pediatric OSAS. Sleep Breath 18, 723–729 (2014). https://doi.org/10.1007/s11325-013-0934-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-013-0934-0